Abstract
Background
FOXF2 is a member of the forkhead box (FOX) family of transcription factors. FOXF2 plays an important role in several tumors but its expression and role in hepatocellular carcinoma (HCC) remains unknown.
Methods
Using immunohistochemistry, western blot, and real-time polymerase chain reaction, we analyzed FOXF2 expression in 295 clinicopathologically characterized HCC cases. Using RNA interference (RNAi), we investigated the effects of FOXF2 depletion on tumor cell behavior in vitro. Statistical analyses were used to determine associations between FOXF2 levels, tumor features, and patient outcomes.
Results
FOXF2 downregulation was observed in HCC tissues (p < 0.001) compared with peritumorous tissues, and its expression levels were closely correlated with overall survival and recurrence-free survival (p = 0.023 and 0.006, respectively) in patients with HCC. RNAi-mediated silencing of the FOXF2 gene in the MHCC-97H cell line significantly promoted proliferation and anti-apoptosis.
Conclusions
The results of the present study indicate that FOXF2 may serve as a prognostic biomarker for HCC and may be a promising target in the treatment of patients with HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hepatocellular carcinoma (HCC) is one of the most common types of malignant tumors and the third leading cause of cancer-related death globally. More than 700,000 new HCC cases were diagnosed in 2008,1,2 with an age-adjusted incidence of 16 cases per 100,000 residents worldwide.3 Despite recent improvements in surgery and chemotherapy, the prognosis for HCC remains grim;4 therefore, there is a pressing requirement to identify new prognostic biomarkers and therapeutic targets for HCC.
The forkhead box (FOX) family of transcription factors are characterized by a highly conserved DNA-binding domain and tissue-specific expression patterns, which play important roles in the regulation of cell growth and differentiation, embryogenesis, and tissue development.5–9 Recent studies have shown that several members of the FOX family of transcription factors are alternatively expressed in cancers, correlate with tumor progression and metastasis, and are especially linked to the biological characteristics of breast cancer.10,11 As a member of the FOX family, FOXF2 can downregulate Wnt5a, which plays important roles in carcinogenesis. Moreover, Nik et al. have recently shown that FOXF2 can prevent adenoma formation by inhibiting Wnt signaling.12 In addition, expression of FOXF2 is decreased in prostate cancer, and downregulation of FOXF2 expression always indicates progressive tumor type.13 FOXF2 is a target gene of miR-301, which acts as a crucial oncogene in breast cancer.14 Furthermore, Kong et al. have proven that decreased FOXF2 messenger RNA (mRNA) expression indicates early-onset metastasis and poor prognosis for breast cancer patients with histological grade II tumor;15 However, the role of FOXF2 in HCC has not been described.
In the present study, we investigated the expression of FOXF2 in HCC using human HCC tissue samples and cell lines, and then assessed the association between HCC expression and HCC outcome after resection. Moreover, we performed RNA interference (RNAi)-mediated gene silencing of FOXF2 in HCC cells to reveal the role of FOXF2 in HCC proliferation and apoptosis in vitro.
Materials and Methods
Patients and Tissue Samples
Overall, 295 HCC tumor tissues and peritumorous tissues were obtained from patients with HCC undergoing curative resection at the Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, from March 2008 to September 2010. Diagnosis was confirmed by postoperative pathological analysis. Patients with a history of malignancy and those who had previously received anticancer therapy were excluded from the present study. No patient had detectable distant metastases at surgery. All patients were staged according to the TNM staging system of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC), 7th edition. The clinicopathological characteristics of patients were retrieved from the medical records and summarized in Table 1. Follow-up data were obtained by telephone, letter, and the outpatient clinical database. All patients were followed from the date of initial surgery until either death or the closing date of this study (30 April 2014). Recurrence was detected in 183 patients (62.0 %) at the last follow-up examination, and 46 patients (32.2 %) died due to HCC-related disease. The mean follow-up time was 40.3 months (range 1–67 months). The study was approved by the Ethical Committee of the Second Military Medical University, and written informed consent was obtained from each patient.
Immunohistochemistry
Formalin-fixed, paraffin-embedded primary HCC tissues were collected from the 295 patients mentioned above. Hematoxylin and eosin (HE) slides from these patients were viewed under a light microscope by a pathologist and 4-μm-thick tissue sections were cut from corresponding blocks containing representative tumor regions. After deparaffinization with dimethylbenzene, the tissue sections were rehydrated through 100, 95, 90, 80, and 70 % ethanol. After three washes in phosphate-buffered saline (PBS), the slides were boiled in antigen retrieval buffer containing 0.01 mol/L citrate antigen retrieval (pH 6.0) in the pressure cooker, and then rinsed in peroxidase quenching solution (Invitrogen, Carlsbad, CA, USA) to block endogenous peroxidase. The sections were then incubated with a specific antibody against FOXF2 (Santa Cruz biotechnology, Inc., CA, USA) [1:200, R&D Systems] at 4 °C overnight and then with a broad spectrum second antibody (Invitrogen) at 37 °C for 20 min. After three washes, the visualization signal was developed with A Invitrogen Histostain Plus kit and counterstained with hematoxylin.
The intensity and extent of FOXF2 immunostaining were evaluated for all samples under double-blinded conditions. In brief, the percentage of positive staining was scored as 0 (0–9 %), 1 (10–20 %), 2 (21–50 %), 3 (51–75 %), or 4 (76–100 %), and the intensity was scored as 0 (no staining), 1 (weak staining), 2 (moderate staining), or 3 (dark staining). The total score was calculated as the product of intensity and extent, ranging from 0 to 12. The expression level of FOXF2 was divided into negative (score 0) and positive (score 1–12) staining.
Cell Lines
Human hepatic cell line L02, low metastatic potential human HCC cell line PLC (obtained from the Cell Bank of the Chinese Academy of Sciences, Shanghai, China), and MHCC-97H cells (human HCC cell lines with high metastatic potential, established at the Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, China) were maintained in Dulbecco’s modified Eagle’s medium containing 10 % fetal calf serum, 100 U/ml penicillin, and 50 mg/ml streptomycin. The cells were harvested in the logarithmic phase of growth for use in the experiments outlined below.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted using TRIzol solution (Invitrogen) according to the manufacturer’s recommended instructions. Reverse transcription was performed in a 20 ml reaction system with 2 mg of total RNA that had been treated with Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase to synthesize first-strand complementary DNA (cDNA) [Promega, Madison, WI, USA] according to the manufacturer’s recommendations, followed by cDNA amplification, as previously described. The primer sequences that were used for reverse transcription polymerase chain reaction (RT-PCR) for FOXF2 were:5′-TGCACTCCAGCATGTCCTCCTA-3′, 5′-CGCTAGCTGAGGGATGGAAAGA-3′ and 5′-ACCTCTCAGTGGGACTGCCCCGTTA-3′.
Western Blot
Total protein was collected using the Total Protein Extraction Kit (KeyGen, Nanjing, China) and 30 mg of protein per lane was separated using 12 % sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The membrane was blocked in 5 % skim milk for 2 h and then incubated with a specific antibody against FOXF2 (Santa Cruz biotechnology, Inc., CA, USA) for 12 h. In addition, β-actin (Abcam, Cambridge, UK) on the same membrane was used as a loading control. The band densities of specific proteins were quantified after normalization to the density of β-actin.
Small Interfering RNA-Mediated FOXF2 Gene Silencing
Expression of human FOXF2 was knocked down, as previously described, using small interfering RNA (siRNA) duplexes targeting the following sequence: 5′-CACGCGGATAGCAGTAAGC-3′. Negative control siRNAs 5′-UUCUCCGAACGUGUCACGUTT-3′ targeting unknown mRNA sequences were used as controls. All of the siRNAs were synthesized by GenePharma (Shanghai, China). A BLAST search of the human genome verified that the selected sequences were specific for the target genes. Cells in the exponential growth phase were plated in six well plates at a density of 0.531 cells/ml, cultured for 24 h and transfected with 1 mg of siRNA in reduced serum medium (OPTI-MEM-I; Invitrogen) once they had reached 30–50 % confluence according to the manufacturer’s recommended protocol. Fluorescein (FAM)-labeled negative control siRNA was used to visualize the transfection efficiency.
In Vitro Proliferation Assays
MHCC-97H was seeded at 3000 cells per well in 96-well plates, and were cultivated in the supernatant of siRNA treated with Lp2000 (Invitrogen). The Cell Counting Kit (CCK)-8 assay was used to determine the relative viability of cells according to the manufacturer’s instructions. This procedure was repeated at indicated times when the cells were cultivated in the corresponding supernatant.
Apoptosis Assay
The cells were harvested and resuspended in 500 μl of binding buffer. 5 μl of annexin V-FITC solution and 10 μl propidium iodide (PI) (1 μg/ml) were added to these cells for 30 min away from the light. Apoptosis was detected on a flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions.
Statistical Analysis
SPSS Statistics software, version 19.0 (IBM Corporation, Armonk, NY USA) was used to conduct all statistical analyses. Differences among the categorical variables were analyzed using a Chi-square analysis, and quantitative variables were analyzed using the paired Wilcoxon signed-rank test or unpaired t-test. Univariate and multivariate Cox proportional hazards analyses were used to assess the effects of various factors on prognosis. A Kaplan–Meier analysis was used to assess survival, and log-rank tests were used to compare patient survival between subgroups. All p-values were two-sided, and p < 0.05 was considered to be statistically significant.
Results
Decreased Expression of FOXF2 Protein in Hepatocellular Carcinoma (HCC) Tissues
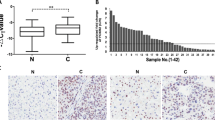
To clarify the underlying role of FOXF2 in HCC progression, we first examined the expression levels of FOXF2 using immunohistochemistry (IHC) in 295 tumor tissues and peritumor tissues. We found that FOXF2 expression was significantly downregulated in the tumor tissues compared with the matched adjacent peritumorous tissues (Fig. 1a, b; p < 0.001). To verify the results obtained using IHC, we detected FOXF2 expression in four HCC tissues and their matched adjacent peritumorous tissues using western blot analyses (Fig. 1d). The results also revealed decreased FOXF2 expression in tumor compared with peritumorous tissues. Real-time PCR was then applied in 50 tumor tissues and paired peritumorous tissues, and FOXF2 mRNA levels were also found to be downregulated in tumor tissues (Fig. 1c). Furthermore, we determined that FOXF2 was expressed in two HCC cell lines and one normal cell line using western blot analyses. Consistent with the results obtained in the tissue samples, higher FOXF2 levels were detected in the normal cell line (LO2) than in the HCC cell lines (PLC and MHCC-97H) (Fig. 1d).
FOXF2 expression in HCC tissues and cell lines. a FOXF2 expression analyses in tumorous tissues and peritumorous tissues using IHC (magnification ×200 and ×400). b FOXF2 expression in 295 tumor tissues is shown compared with paired peritumorous tissues using a paired Wilcoxon signed-rank test. Differential FOXF2 expression between tumor and peritumorous tissues was verified using c real-time PCR, and d western blot. d Differential FOXF2 expression was analyzed in different cell lines using western blot. HCC hepatocellular carcinoma, IHC immunohistochemistry, PCR polymerase chain reaction, mRNA messenger RNA
Decreased FOXF2 Expression was Correlated with Tumor Progression and Poor Prognosis in HCC Patients
Correlations between FOXF2 expression and clinicopathologic characteristics were analyzed using the χ 2 test. As summarized in Table 1, significant correlations were found between FOXF2 expression and two parameters, including tumor size (p = 0.049) and differentiation grade (p = 0.007). However, there were no statistical associations between FOXF2 expression and the remaining parameters, such as age and sex (p > 0.05).
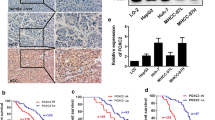
Univariate and multivariate Cox regression analyses were then used to assess the association between FOXF2 expression and outcome in HCC patients. In the univariate analysis (Table 2), tumor size, tumor number, TNM stage, and FOXF2 downregulation were significantly correlated with poor overall survival (OS) [p = 0.020, p < 0.001, p < 0.001, and p = 0.023, respectively] and recurrence-free survival (RFS) [p < 0.001, p < 0.001, p < 0.001, and p = 0.006, respectively] in HCC patients. The six factors that were significantly associated with outcome (p < 0.05) in the univariate analysis were then subjected to a multivariate analysis, which revealed that tumor number, TNM stage, and FOXF2 downregulation were independent prognostic factors for OS (p = 0.002, p < 0.001, and p = 0.046, respectively) and RFS (p = 0.001, p < 0.001 and p = 0.01, respectively) in HCC patients (Table 2). Moreover, the Kaplan–Meier curve and log-rank test also indicated that FOXF2 downregulation was associated with poorer outcome in HCC patients (Fig. 2a, b).
FOXF2 Silencing Promoted HCC Cell Proliferation and Induced Apoptosis In Vitro
We investigated the role of FOXF2 in the proliferation and apoptosis of HCC cells in vitro. siRNA transfection was employed to knockdown FOXF2 expression in MHCC-97H cells, which qualified high metastatic potential. The effects of siRNA transfection on FOXF2 expression were confirmed using western blot analyses. The amount of FOXF2 protein, normalized by β-actin was obviously reduced compared with that in the negative control cells (Fig. 3a). Furthermore, proliferation assays revealed that silencing FOXF2 expression promoted the proliferation of the MHCC-97H cells (Fig. 3b), and flow cytometry analysis showed that the upregulation of FOXF2 induced apoptosis in the MHCC-97H cell line (Fig. 3c).
FOXF2 silencing promoted HCC cell proliferation and anti-apoptosis in vitro. a Western blotting was used to verify knock-down of FOXF2 expression in MHCC-97H cells by siRNA transfection. b CCK-8 assay showing that interference of FOXF2 expression promoted the proliferation of the MHCC-97H cells. c Results are expressed as the percentage of the number of apoptosis cells compared with the total number of cells. HCC hepatocellular carcinoma, siRNA small interfering RNA, CCK Cell Counting Kit
Discussion
In the present study, we investigated FOXF2 expression in a series of 295 HCC tissues and three HCC cell lines. The results revealed that expression levels of FOXF2 were lower in HCC tissues than paired peritumorous tissues, as indicated by IHC and validated using western blot and real-time PCR. Association analyses then revealed that FOXF2 downregulation was significantly associated with larger tumor size and poor differentiation. Taken together, these results indicated that FOXF2 may play an important role in HCC progression. The multivariate analysis indicated that FOXF2 expression may be an independent prognostic factor in HCC.
As a member of the FOX family, FOXF2 plays an important role in inhibiting cancer development, and downregulation of FOXF2 has been observed in several malignancies.5,16 It has been reported that FOXF2 expression is downregulated in human prostate and breast cancer. Moreover, downregulation of FOXF2 promotes cancer growth and progression, and predicts poor prognosis in patients.14,17 In addition, loss of the Foxf2 gene has been shown to promote intestinal adenoma formation and growth in a murine model.18 In line with these findings, we found that FOXF2 expression was markedly downregulated in HCC tissues and FOXF2 upregulation induced apoptosis in HCC cells. Furthermore, low FOXF2 expression was associated with poor prognosis in HCC patients. These data collectively suggest that FOXF2 may serve as an independent prognostic factor and a potential therapeutic target for HCC. However, our study suggests that FOXF2 could serve as a prognostic marker for resected early-stage tumors, but further study is needed to prove this in advanced tumors.
The molecular mechanisms by which FOXF2 inhibits cancer development remains incompletely clear. It has been reported that FOXF2 can inhibit Wnt signaling, an important pathway for cancer initiation and progression.12 Moreover, previous studies have demonstrated that FOXF2 is the target of several oncogenic molecules. Shi et al. have shown that FOXF2 is a target gene of miR-301, which acts as a crucial oncogene in breast cancer, and miR-301 overexpression was associated with an increased risk of nodal or distant relapse, and mediated proliferation in many tumors.14,19,20 Moreover, Aitola et al. demonstrated FOXF2 is a target of Hedgehog signaling in many organs, including the intestine, and inhibition of Hedgehog signaling results in epithelial over proliferation and formation of ectopic crypts, whereas constitutive activation of the same pathway leads to stem cell depletion.21 Hirata et al. reported that MicroRNA-182-5p promotes cell invasion and proliferation by downregulating the FOXF2, RECK, and MTSS1 genes in human prostate cancer.17 However, the underlying mechanisms of how FOXF2 is downregulated in HCC need further investigation.
Conclusions
Our data suggest that FOXF2 might play an important role in the regulation of growth in human HCC. The downregulation of FOXF2 was demonstrated to be a novel prognostic marker for HCC. Our data also revealed that FOXF2 inhibited HCC cell proliferation and induced HCC cell apoptosis. FOXF2 can be a novel prognostic marker in HCC. Future investigations are needed to explore the underlying mechanisms of FOXF2 in HCC.
References
A comment on ‘global activity of cancer registries and cancer control and cancer incidence statistics in Korea’. J Prev Med Public Health. 2008;41(3):208–209; author reply 209–11.
Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–917.
Bruix J, Sherman M. American association for the study of liver diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–2.
Katoh M, Katoh M. Human FOX gene family (review). Int J Oncol. 2004;25(5):1495–500.
Badve S, Turbin D, Thorat MA, et al. FOXA1 expression in breast cancer: correlation with luminal subtype A and survival. Clin Cancer Res. 2007;13(15 Pt 1):4415–21.
Lo PK, Lee JS, Liang X, et al. Epigenetic inactivation of the potential tumor suppressor gene FOXF1 in breast cancer. Cancer Res. 2010;70(14):6047–58.
Ray PS, Wang J, Qu Y, et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70(10):3870–6.
Wolf I, Bose S, Williamson EA, Miller CW, Karlan BY, Koeffler HP. FOXA1: growth inhibitor and a favorable prognostic factor in human breast cancer. Int J Cancer. 2007;120(5):1013–22.
Gong C, Fujino K, Monteiro LJ, et al. FOXA1 repression is associated with loss of BRCA1 and increased promoter methylation and chromatin silencing in breast cancer. Oncogene. doi:10.1038/onc.2014.421.
Liu J, Shen L, Yao J, et al. Forkhead box C1 promoter upstream transcript, a novel long non-coding RNA, regulates proliferation and migration in basal-like breast cancer. Mol Med Rep. 2015;11(4):3155–9.
Nik AM, Reyahi A, Ponten F, Carlsson P. Foxf2 in intestinal fibroblasts reduces numbers of Lgr5(+) stem cells and adenoma formation by inhibiting Wnt signaling. Gastroenterology. 2013;144(5):1001–11.
van der Heul-Nieuwenhuijsen L, Dits NF, Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 2009;103(11):1574–80.
Shi W, Gerster K, Alajez NM, et al. MicroRNA-301 mediates proliferation and invasion in human breast cancer. Cancer Res. 2011;71(8):2926–37.
Kong PZ, Yang F, Li L, Li XQ, Feng YM. Decreased FOXF2 mRNA expression indicates early-onset metastasis and poor prognosis for breast cancer patients with histological grade II tumor. PloS One. 2013;8(4):e61591.
Figueroa JD, Han SS, Garcia-Closas M, et al. Genome-wide interaction study of smoking and bladder cancer risk. Carcinogenesis. 2014;35(8):1737–44.
Hirata H, Ueno K, Shahryari V, et al. MicroRNA-182-5p promotes cell invasion and proliferation by down regulating FOXF2, RECK and MTSS1 genes in human prostate cancer. PloS One. 2013;8(1):e55502.
van den Brink GR, Rubin DC. Foxf2: a mesenchymal regulator of intestinal adenoma development. Gastroenterology. 2013;144(5):873–6.
Funamizu N, Lacy CR, Parpart ST, Takai A, Hiyoshi Y, Yanaga K. MicroRNA-301b promotes cell invasiveness through targeting TP63 in pancreatic carcinoma cells. Int J Oncol. 2014;44(3):725–34.
Cao G, Huang B, Liu Z, et al. Intronic miR-301 feedback regulates its host gene, ska2, in A549 cells by targeting MEOX2 to affect ERK/CREB pathways. Biochem Biophys Res Commun. 2010;396(4):978–82.
Aitola M, Carlsson P, Mahlapuu M, Enerback S, Pelto-Huikko M. Forkhead transcription factor FoxF2 is expressed in mesodermal tissues involved in epithelio-mesenchymal interactions. Dev Dyn. 2000;218(1):136–49.
Acknowledgment
This work was supported by Grant Number 81272522 from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, Z., Liu, J., Yu, X. et al. Loss of FOXF2 Expression Predicts Poor Prognosis in Hepatocellular Carcinoma Patients. Ann Surg Oncol 23, 211–217 (2016). https://doi.org/10.1245/s10434-015-4515-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4515-2