Abstract
We previously identified a correlation between estrogen receptor alpha (ERα) and the candidate tumour suppressor gene Forkhead Box P1 (FOXP1), whose nuclear protein expression in breast tumours was associated with improved patient survival. However, the expression pattern of FOXP1 in normal breast tissue is more reminiscent of the second receptor, ERβ, which has an emerging role as a tumour suppressor in breast cancer and critically may underlie the ability of some ERα-negative tumours to respond to tamoxifen. In a series of 283 breast cancers, in which ERα-positive tumours were treated with tamoxifen, the nuclear expression of ERβ correlated significantly with ERα (p = 0.004), low-tumour grade (p = 0.008) and nuclear FOXP1 (p = 0.01). High-grade tumours exhibited significantly more cytoplasmic ERβ than the low-grade tumours (p = 0.006). Regression analysis demonstrated that FOXP1 expression was most closely related to nuclear ERβ (p = 0.021). Neither, nuclear or cytoplasmic ERβ expression demonstrated prognostic significance. FOXP1 is not estrogen regulated and silencing FOXP1 expression, using siRNA, did not affect ERα, ERβ or progesterone receptor expression, suggesting ER and FOXP1 co-expression may reflect a common regulatory mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forkhead Box P1 (FOXP1) belongs to the winged helix/forkhead group of transcription factors and plays an essential role in normal development [1]. FOXP1 is differentially expressed at both the mRNA and protein level in breast cancer and a wide range of solid tumours [2, 3]. The FOXP1 gene itself maps to chromosome 3p14.1, a region that shows loss of heterozygosity (LOH) in many solid tumour types, including breast cancer [4] where allelic loss at 3p is also associated with increasing tumour grade [5]. Furthermore certain embryonic tissues from the Foxp1 knockout mouse are reported to have increased proliferation and reduced apoptosis [1], consistent with the hypothesis that FOXP1 may act as a tumour suppressor gene.
Forkhead Box P1 expression is significantly associated with that of estrogen receptor alpha (ERα) in breast tumours and the absence of tumoural FOXP1 protein expression is associated with a significantly shorter relapse-free patient survival [3]. Furthermore, a correlation between nuclear FOXP1 and ERα was also observed in a study of early stage I endometrial adenocarcinoma, where loss of nuclear expression was the most striking observation [6]. In prostate cancer, nuclear FOXP1 expression is positively correlated with that of the androgen receptor [7]. These observations indicate that there is a strong relationship between the loss of FOXP1 and hormone receptor expression in tumours derived from hormonally responsive tissues. However, as neither estrogen [3], nor androgens [7] have been found to regulate FOXP1 expression, it appears that FOXP1 itself is not a direct target that is regulated by these receptors.
Estrogens have a key role in the development and progression of breast cancer and their effects are mediated by two hormonally responsive transcription factors, ERα and ERβ. Although ERα is an established prognostic indicator in breast cancer, and is a primary target for endocrine therapy [8], the value of ERβ remains to be clarified. In studies so far, increased expression of ERβ is associated with ERα and progesterone receptor (PgR) protein expression, improved patient survival and increased sensitivity of the tumour to endocrine therapy, reviewed in [9], and [10]. Thus, supporting the emerging hypothesis that ERβ is a tumour suppressor. However, interestingly the ERβ gene expression profile is distinct from that of the ERα signature and it has been proposed that ERβ expression is not simply a surrogate for ERα in ERα-negative tumours and that it may affect growth and proliferation through a different set of downstream target genes [11].
It has been reported that 5–10% of patients with ERα-negative breast tumours respond to tamoxifen [12, 13]. Although this may be partly due to the vagaries of testing, it is possible since ERβ expression is an independent marker for favourable prognosis after adjuvant tamoxifen treatment within ERα-negative but not ERα-positive patients [11], that signalling may occur through this alternate pathway. Therefore, assessing the level of ERβ expression, particularly in ERα-negative patients, may potentially become a valuable prognostic tool that may help identify additional patients that will benefit from endocrine therapies.
The nuclear myoepithelial expression of FOXP1 in normal breast tissue, together with staining of the ductal epithelium, endothelial cells, occasional stromal cells and some lymphocytes is more reminiscent of the pattern described for ERβ than ERα [14]. Given the clinical relevance of both ERβ and FOXP1 expression in breast cancer and their correlation with ERα, in the current study we have investigated whether there is a relationship between FOXP1 and ERβ in this malignancy.
Methods
Patients and tissue microarrays
Whole sections from histologically normal tissue from a patient without breast cancer were obtained from those undergoing breast reduction surgery. The microarrayed breast carcinomas represent a consecutive series of patients from the referral population of a regionally based cancer service. Tissues were obtained from patients undergoing surgery at The John Radcliffe Hospital (Oxford, UK) and the study was approved by the Local Ethics committee (C02.216). For this previously described invasive series [3] tumours were treated by mastectomy (n = 70) or lumpectomy (n = 213), axillary node sampling with node status confirmed histologically. Grading was carried out according to the modified Bloom and Richardson method [15]. For all ERα_positive patients, tamoxifen was prescribed as adjuvant treatment regardless of age or any other prognostic factors. In patients <50 years, adjuvant cyclophosphamide, methotrexate and 5-fluorouracil was administered if tumours were node positive, or ERα-negative and/or ≥3 cm. Patients ≥50 years with ERα-negative, node-positive tumours also received cyclophosphamide, methotrexate and 5-fluorouracil. For patients with invasive tumours, the median follow up was 7.3 years (range, 0.2–11.3 years) during which there were 100 relapses and 71 deaths. Invasive cases were biopsied from 1990 to 1995. The end of the follow-up period was September 2004.
Immunohistochemical labelling
All paraffin-embedded tissues were stored at 4 °C. Tissues were dewaxed followed by antigen retrieval by microwaving in 50 mM Tris/2 mM EDTA (pH 9.0). Immunostaining was carried out using the mouse anti-ERβ monoclonal antibody 14C8 (Abcam, Cambridge, UK) at a 1/75 dilution for 90 min, followed by detection using the DAKO Envision™ system (Dako Ltd., Cambridgeshire, UK). The FOXP1 staining method performed using our JC12 monoclonal antibody and nuclear scoring system has been described previously [3]. The stained arrays were counterstained with hematoxylin (Gill’s No.2; Sigma-Aldrich, Gillingham, UK) and mounted in Aquamount (VWR International, Leicestershire, UK). The level of neoplastic cell ERβ nuclear intensity was scored as negative = 0, with increasing intensity from 1 to 3. Nuclear % was scored as negative if no cells stained positive, 1 = 1–10% positivity, 2 = 11–50%, 3 = 51–80%, 4 = 81–100%.
Western blotting
Nuclear extracts were prepared using the Panomics Nuclear extraction kit according to the manufacturer’s instructions (Panomics, CA, USA). Proteins were resolved by SDS-PAGE and transferred to Hybond nitrocellulose membrane (GE Healthcare, Amersham, UK). Primary antibodies were applied overnight at 4°C using the following dilutions: Anti-FOXP1: clone JC12 (“in house” hybridoma supernatant) 1/10, anti-ERα: clone 6F11 (Novocastra, Newcastle, UK) 1/100, anti-ERβ: clone EMRO2 (Novocastra) 1/100, anti-βActin: clone AC-15 (Abcam) 1/10,000, anti-TBP: clone 1TBP18 (Abcam) 1/2,000. The membrane was washed and incubated with 1/2,000 goat anti-mouse HRP secondary antibody (Dako). Antigen/antibody complexes were detected using ECL reagent (GE Healthcare).
FOXP1 silencing by siRNA
MCF-7 cells were cultured in a 24-well plate in standard RPMI (Cambrex, UK) containing FCS and glutamine but no antibiotics. Cells were then transfected using 16.2 μl/4 wells of a stock 20 μM siRNA duplex (FOXP1 DNA target sequence 5′-AAGAAACCACAGGCAACAATC-3′, or standard non-silencing control) (Qiagen, Crawley, UK) in a final volume of 235 μl/well of oligofectamine in Opti-MEM according to the manufacturer’s protocol (Invitrogen, CA, USA). After 5 h 500 μl of RPMI medium containing FCS and glutamine but no antibiotics was added to each well. Next day cells were trypinised and two wells that had been transfected with the same siRNA were pooled into one well of a six-well plate. The following day the transfection was repeated using an equivalent siRNA concentration. After 48 h cells were harvested and prepared for Western blotting as described above.
Statistical analyses
The ERβ scores for both staining intensity and the percentage of positive tumour cells were added together to give a maximum score of 7. A cut off of seven for nuclear expression and six for cytoplasmic expression was used to define two approximately equal size groups of patients for subsequent statistical analyses. The Chi-square test was used to test for differences between categorical variables and the log rank test for differences in survival with the Cox proportional hazard model for independence. Survival was measured from the first day of diagnosis to the time of death or the time of last follow-up. All tests were performed using STATA package 8.1 (STATA corporation, TX, USA).
Results
Expression of FOXP1, ERα and ERβ in breast cancer cell lines
Western blotting was used to investigate the expression of FOXP1, ERα and ERβ proteins in a panel of commonly studied breast cancer cell lines (Fig. 1). The ER-positive and negative cell lines showed the expected expression pattern for ERα which was present only in the ER-positive lines. In contrast, ERβ and FOXP1 were detectable in all of the cell lines. The smaller FOXP1 proteins detectable in three cell lines are likely to reflect the expression of alternatively spliced isoforms. In breast cancer cell lines there was more common co-expression of FOXP1 and ERβ (5/5) than FOXP1 and ERα (3/5).
Relationship between ERβ, FOXP1 and ERα expression
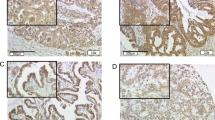
Staining of serial sections of normal breast tissue from the same patient with antibodies to ERα, ERβ and FOXP1 demonstrated that the nuclear expression pattern of ERα in ductal epithelium was more restricted than observed for the other two markers (Fig. 2a–c). Both nuclear FOXP1 and ERβ were expressed in myoepithelial cells and endothelial cells in addition to the ductal epithelium, with additional cytoplasmic expression being commonly observed for ERβ. Tumours exhibited variable levels of ERβ positivity, including both nuclear and cytoplasmic staining. Exclusively cytoplasmic ERβ expression was observed in a minority of tumours (Fig. 2d), the nuclear positivity of the surrounding tissue indicating that this was not a technical artefact. While some tumours exhibited both strong nuclear and cytoplasmic ERβ expression (Fig. 2e), others expressed higher levels in the nucleus (Fig. 2f).
Immunohistochemical detection of a FOXP1, b ERβ and c ERα in serial sections from the same normal breast tissue. ERβ expression in breast tumours; including d, an exclusively cytoplasmic tumour in which nuclear labelling of tumour infiltrating lymphocytes was observed; e a tumour with strong cytoplasmic and nuclear reactivity and f a tumour with predominantly nuclear expression
Correlation of ERβ protein expression with FOXP1 and clinicopathological variables
Statistical analysis of the nuclear expression data (Table 1) showed a significant correlation with the increased expression of nuclear ERβ by low-grade tumours (p = 0.008). Consistent with findings in other series, nuclear ERβ expression was also positively correlated to that of ERα (p = 0.004). FOXP1 expression was significantly positively associated with nuclear ERβ (p = 0.01) but not with cytoplasmic ERβ (p = 0.34) (Tables 1, 2, respectively). High-grade tumours expressed significantly more cytoplasmic ERβ than the low-grade tumours (p = 0.006). Neither, nuclear nor cytoplasmic ERβ expression had any prognostic significance in terms of either relapse-free or overall patient survival.
Because of the close correlation between nuclear expression levels of the two estrogen receptors and tumour grade, regression analysis was performed to determine whether FOXP1 expression was more closely related to any one of these variables. FOXP1 expression was most closely related to nuclear ERβ (p = 0.021), rather than ERα or tumour grade.
Effects of FOXP1 targeted siRNA on ER expression and function
We have previously reported that FOXP1 does not appear to be hormonally regulated by either androgens or estrogen [3, 7]. An alternative hypothesis was that FOXP1 could regulate the expression and/or activity of the estrogen receptor(s). To investigate this possibility, FOXP1 protein expression was silenced in MCF-7 cells by transfection with a small interfering RNA (siRNA), targeting an exon that is common to the FOXP1 isoforms containing the forkhead DNA binding domain. FOXP1 silencing at the protein level was successful (Fig. 3) and this was also confirmed by immunohistochemistry (data not shown). However, silencing FOXP1 had no effect on the expression of ERα or ERβ or on the expression levels of the PgR, one of their target genes.
Discussion
The functional interrelationship between the two estrogen receptors is now so compellingly established, that neither can be studied in isolation. Studies of mouse knockouts have demonstrated non-redundant roles for ERα and ERβ [16] and these receptors have also been demonstrated to have opposing regulatory action on target gene promoters, particularly those involved in cellular proliferation [17]. As expression of the FOXP1 forkhead transcription factor has been shown to correlate with that of ERα and with improved prognosis in breast tumours [3], a natural progression was to study its relationship with ERβ.
A panel of five well-characterised breast cancer cell lines showed more frequent co-expression of FOXP1 and ERβ than co-expression of FOXP1 and ERα. Western blotting, using an anti-C-terminal antibody, identified the expression of smaller FOXP1 isoforms that are likely to derive from the previously reported alternative splicing of this gene [2]. The role of these smaller isoforms is of future interest in breast cancer, as recurrent viral integration within the second foxP1 coding exon in a model of avian nephroblastoma has been reported to generate an N-terminally deleted protein that may contribute to oncogenic transformation [18].
In this series of 283 invasive breast tumours nuclear ERβ expression was positively correlated with that of ERα, low-tumour grade and there was no impact of ERβ on survival. This is consistent with results from previous studies, reviewed in [19]. Cytoplasmic staining for ERβ was commonly observed and exhibited significantly increased levels in high-grade tumours. Our findings are consistent with data reported at a recent scientific meeting, where cytoplasmic ERβ, either alone or in combination with nuclear positivity, was associated with decreased overall survival [20]. The biological role for cytoplasmic ERβ is unclear, however, the established roles of phosphorylation, DNA binding and interaction with coregulatory proteins in modulating ER function provide potential opportunities for functional differences based on subcellular localisation [21]. Nuclear FOXP1 expression was significantly positively associated only with nuclear ERβ (p = 0.01) and not with cytoplasmic ERβ expression. This finding is consistent with the tumour suppressor roles proposed for both nuclear FOXP1 and nuclear ERβ their correlation with low tumour grade in breast cancer and their roles in transcription regulation [3, 10]. Regression analysis suggested that the relationship between nuclear FOXP1 and ERβ was more significant than that originally identified with ERα [3].
We have previously reported that the relationship between FOXP1 and the estrogen receptors does not appear to be through hormonal regulation of FOXP1 expression, at least in the MCF-7 cell line [3]. An alternative hypothesis was that FOXP1 might regulate ER expression levels or activity. However, silencing of FOXP1 protein expression using siRNA did not reveal any effect on the expression of ERβ, ERα or their ability to regulate the expression of the PgR in the MCF-7 cell line. Another possibility is that FOXP1 and the estrogen receptors could share a common mechanism of gene regulation. Promoter hypermethylation is one such mechanism that is reported to silence the expression of both estrogen receptors in breast cancers [22–25]. However, treatment of the FOXP1-negative MDA-MB-435 melanoma cell line with the methylation inhibitor 5-AZA- 2′deoxycytidine did not restore FOXP1 protein expression (GJB, unpublished data).
Given the clinical relevance of both FOXP1 and ERβ expression for patients with breast cancer, further studies are warranted to investigate the as yet unidentified mechanisms that determine the association between the nuclear expression levels of these two proteins in breast tumours. In particular, functional studies are needed to address the biological role of FOXP1 in breast cancer and determine whether it does indeed act as a tumour suppressor.
References
Wang B, Weidenfeld J, Lu MM et al (2004) Foxp1 regulates cardiac outflow tract, endocardial cushion morphogenesis and myocyte proliferation and maturation. Development 131(18):4477–4487
Banham AH, Beasley N, Campo E et al (2001) The FOXP1 winged helix transcription factor is a novel candidate tumor suppressor gene on chromosome 3p. Cancer Res 61(24):8820–8829
Fox SB, Brown P, Han C et al (2004) Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor alpha and improved survival in primary human breast carcinomas. Clin Cancer Res 10(10):3521–3527
Bieche I, Lidereau R (1995) Genetic alterations in breast cancer. Genes Chromosomes Cancer 14(4):227–251
Martinez A, Walker RA, Shaw JA et al (2001) Chromosome 3p allele loss in early invasive breast cancer: detailed mapping and association with clinicopathological features. Mol Pathol 54(5):300–306
Giatromanolaki A, Koukourakis MI, Sivridis E et al (2006) Loss of expression and nuclear/cytoplasmic localization of the FOXP1 forkhead transcription factor are common events in early endometrial cancer: relationship with estrogen receptors and HIF-1alpha expression. Mod Pathol 19(1):9–16
Banham AH, Boddy J, Launchbury R et al (2007) Expression of the forkhead transcription factor FOXP1 is associated both with hypoxia inducible factors (HIFs) and the Androgen receptor in prostate cancer but is not directly regulated by Androgens or hypoxia. Prostate 67(10):1091–1098
Ali S, Coombes RC (2002) Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer 2(2):101–112
Murphy LC, Watson PH (2006) Is oestrogen receptor-beta a predictor of endocrine therapy responsiveness in human breast cancer? Endocr Relat Cancer 13(2):327–334
Speirs V, Walker RA (2007) New perspectives into the biological and clinical relevance of oestrogen receptors in the human breast. J Pathol 211(5):499–506
Gruvberger-Saal SK, Bendahl PO, Saal LH et al (2007) Estrogen receptor beta expression is associated with tamoxifen response in ERalpha-negative breast carcinoma. Clin Cancer Res 13(7):1987–1994
McGuire WL (1975) Current status of estrogen receptors in human breast cancer. Cancer 36(2):638–644
(1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351(9114):1451–1467
Speirs V, Skliris GP, Burdall SE et al (2002) Distinct expression patterns of ER alpha and ER beta in normal human mammary gland. J Clin Pathol 55(5):371–374
Elston C (1987) Grading of invasive carcinoma of the breast. In: Page D, Anderson T (eds) Diagnostic histopathology of the breast. Churchill Livingstone, Edinburgh, pp 300–311
Couse JF, Korach KS (1999) Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20(3):358–417
Liu MM, Albanese C, Anderson CM et al (2002) Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem 277(27):24353–24360
Pajer P, Pecenka V, Kralova J et al (2006) Identification of potential human oncogenes by mapping the common viral integration sites in avian nephroblastoma. Cancer Res 66(1):78–86
Speirs V, Carder PJ, Lane S et al (2004) Oestrogen receptor beta: what it means for patients with breast cancer. Lancet Oncol 5(3):174–181
Speirs V, Parker M, Green A et al (2006) Progress towards unlocking the secrets of oestrogen receptor beta in breast cancer. Breast Cancer Res 8(Suppl 2):P24
Heldring N, Pike A, Andersson S et al (2007) Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87(3):905–931
Skliris GP, Munot K, Bell SM et al (2003) Reduced expression of oestrogen receptor beta in invasive breast cancer and its re-expression using DNA methyl transferase inhibitors in a cell line model. J Pathol 201(2):213–220
Zhao C, Lam EW, Sunters A et al (2003) Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene 22(48):7600–7606
Kim SJ, Kim TW, Lee SY et al (2004) CpG methylation of the ERalpha and ERbeta genes in breast cancer. Int J Mol Med 14(2):289–293
Rody A, Holtrich U, Solbach C et al (2005) Methylation of estrogen receptor beta promoter correlates with loss of ER-beta expression in mammary carcinoma and is an early indication marker in premalignant lesions. Endocr Relat Cancer 12(4):903–916
Acknowledgements
This study was supported by funding from the Breast Cancer Campaign, Cancer Research UK, Tenovus and the Leukaemia Research Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bates, G.J., Fox, S.B., Han, C. et al. Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptorβ in primary invasive breast carcinomas. Breast Cancer Res Treat 111, 453–459 (2008). https://doi.org/10.1007/s10549-007-9812-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-007-9812-4