Abstract
Located in a semi-arid climate, Çumra (Konya) plain is one of the most important agricultural regions in Turkey. Groundwater is the major source for agricultural, domestic, and other water-related activities due to the insufficiency of surface water. As groundwater is the only major source of water in this area, it is important to know the effects of geological formations and anthropogenic activities on groundwater chemistry. This study was carried out with the objective of identifying the hydrogeochemical characteristics and processes controlling the groundwater chemistry in Çumra Plain, Central Anatolia, Turkey. The study area is comprised of two main aquifers which are the semi-confined aquifer of the Neogene age and the unconfined aquifer of the Quaternary age. To identify the hydrogeochemical characteristics of groundwater in two aquifer systems and to understand the major factors and mechanisms controlling the groundwater chemistry, graphical plots, mineral saturations, and multivariate statistical analysis of chemical constituents in the groundwater were used. Study results show that groundwater is generally neutral to slightly alkaline in nature with a pH ranging from 6.67 to 8.10, and the dominance of ions is in the order of Ca2+ > Mg2+ > Na+ > K+ for the Neogene aquifer and HCO3 − > SO4 2− > Cl− > NO3 −; Mg2+ > Ca2+ > Na+ > K+ and SO4 2− > HCO3 − > Cl− > NO3 − for the Quaternary aquifer. While the chemical composition of groundwater in the Neogene aquifer is mainly controlled by water–rock interaction including dissolution of carbonates and gypsum, calcite precipitation, and de-dolomitization, the main geochemical processes in the Quaternary aquifer are reverse ion exchange, evaporation, dissolution of carbonates, gypsum and soil salts, calcite precipitation, and silicate weathering. The mechanism controlling groundwater chemistry in the Neogene aquifer is actually regulated by the geogenic processes (water–rock interaction) rather than by anthropogenic activities. However, the mechanism controlling groundwater chemistry in the Quaternary aquifer is regulated by both geogenic processes and anthropogenic activities. In addition, anthropogenic nitrogen pollution in the study area is currently not serious, but evaporation and leaching of soil salts due to anthropogenic activities increase the concentrations of ions in the Quaternary aquifer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater chemistry could reveal important information about the geological history of the aquifers and the suitability of groundwater for domestic, industrial, and agricultural purposes (Wen et al. 2008; Taheri Tizro and Voudouris 2008). Groundwater chemistry of a region is generally not homogeneous and controlled by various processes such as climate changes (García-Ruiz et al. 2011; Abrahão et al. 2015; Puntoriero et al. 2015), soil/rock–water interaction during recharge and groundwater flow (Matthess 1982; Montcoudiol et al. 2014; Ghesquière et al. 2015), dissolution and/or precipitation of mineral species, exchange reactions, degree of chemical weathering of the various rock types (Kumar et al. 2009; Rajesh et al. 2012; Ako et al. 2012; Su et al. 2013; Bozdağ and Göçmez 2013; Varol and Davraz 2014), anthropogenic activities such as overexploitation of groundwater resources and agriculture (Subramani et al. 2005; García-Garizábal et al. 2014; Redwan and Abdel Moneim 2015), and prolonged storage in the aquifer and so forth. (Hem 1985; Appelo and Postma 2005; Daniele et al. 2013). Additionally, detailed information about the geochemical evolution of groundwater can enhance our understanding of the hydrochemical systems, promoting sustainable development and effective management of groundwater resources (Wen et al. 2008; Rajesh et al. 2012). It is also necessary to understand the hydrogeochemical characteristics of the groundwater and its evolution under natural water circulation processes so as to utilize and protect water resources effectively and to predict the changes in groundwater environments (Guendouz et al. 2003; Wen et al. 2008; Taheri Tizro and Voudouris 2008). The previous researches in many parts of the world emphasized the importance of hydrogeochemical studies of groundwater (Moeller et al. 2007; Kumar et al. 2009; Su et al. 2013; Irfan and Said 2008; Zhang et al. 2011). Although the chemistry of groundwater has been extensively studied by many researchers in the past, most of these studies focused only on the hydrogeochemical characteristics of shallow unconfined aquifers where the depth of water table is generally lower than 20 m. For example, Al-Shaibani (2008) and Rajesh et al. (2012) evaluated the groundwater chemistry of shallow alluvial aquifers at different regions in western Saudi Arabia. Chae et al. (2004) assessed the hydrogeochemistry of a shallow alluvial groundwater system in an agricultural area in Korea using major ions and only Fe and Mn elements. Su et al. (2007) also assessed the chemistry of major ions of shallow groundwater to understand the groundwater evolution, to identify the predominant geochemical processes, and to determine the effects of anthropogenic factors on groundwater in Northwest China. Lastly, Marghade et al. (2012) studied the chemistry of major ions of shallow groundwater to understand the groundwater geochemical evolution in Nagpur City in India.
Çumra plain is a typical example of a semi-arid climate region where groundwater, the most important source of water supply due to insufficiency of surface water, is intensively exploited for human needs as a result of agricultural and demographic development. In the last two decades, groundwater level has dramatically dropped by about 10 m (Göçmez et al. 2008) due to the excessive abstraction. Despite the importance of groundwater in the study area, little is known about the natural processes and anthropogenic factors controlling the chemical composition of groundwater in the unconfined Quaternary and the semi-confined Neogene aquifers, with the latter serving as the higher groundwater potential. Few researchers have investigated the groundwater for different purposes in the study area and its surroundings yet these studies have not dealt with the hydrogeochemical characteristics of the groundwater in both aquifers (Bayarı et al. 2009a, b; Yavuz 2010; Bozdağ 2015). The lack of hydrogeochemical studies in such an area has led to restricted knowledge of variation in groundwater chemistry in cases when additional factors such as intense agriculture and overexploitation of groundwater resources may adversely affect the water quality. This paper aimed to investigate the main hydrogeochemical characteristics and to determine the processes including water–rock interactions, cation exchange, evaporation, and the impacts of agricultural activities affecting the chemistry of groundwater in two aquifer systems in Çumra plain, Central Anatolia. In this respect, this study is fundamental and can serve as a reference for the local government to improve and sustain the development of water resource management in the future.
Description of the study area
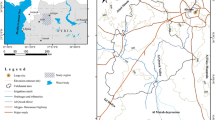
The study area is located southeast of Konya within the Konya Closed Basin, Central Anatolia, Turkey (Fig. 1). It lies between the north latitude of 37°22′–37°55′ and east longitude of 32°25′–33°01′ with an average area of 2500 km2. It is bordered by Abaz and Kızılkaya Mountains in the south and southwest and Bozdağ Mountain (the study area includes the Peynirlik Hill) in the northwest. The study area has a flat topography and the land surface elevation ranges mostly from 1000 to 1050 m except for the mountainous areas which has an elevation ranging from 1200 to 1499 m. The study area has a semi-arid climate with cold and snowy winters and moderate to hot summers. The 30-year (1983–2013) precipitation average in the region is 307.2 mm, evapotranspiration is approximately 288.5 mm, and the average temperature is 11.6 °C.
The study area is one of the most important agricultural areas in Turkey. The majority of the region, except for the mountainous parts, is covered with agricultural lands and residential areas (Fig. 1). Agricultural activities constitute the main source of income. There are more than 250 water wells in the region opened for irrigation by the State Hydraulic Works Department (DSI) and private companies. In addition, uncontrolled drilling of wells for irrigation has rapidly increased in recent years. The main crops cultivated in the region are sugar beet, sunflower, wheat, barley, corn, lentil, bean, tomato, potato, chickpea, alfalfa, oats, melon, and watermelon. Most of these products are in need of long-term irrigation, and groundwater proves to be the major source of irrigation water in the region.
Geologic and hydrogeologic settings
The geological units observed in the study area range from the Paleozoic to the Quaternary in age and include marble, crystalline limestone, ophiolitic mélange (serpentinite, quartzite, metasandstone, mudstone, and crystalline limestone and basalt blocks), marine carbonate origin (i.e., dolomite, dolomitic limestone, and limestone), conglomerate, sandstone, mudstone, gravel, sand, clay, gypsiferous clay, and silt. The geological map of the study area is illustrated in Fig. 1.
The Paleozoic-aged marbles appeared only in the Peynirlik Hill. The Upper Triassic–Lower Cretaceous deposits consist of limestone, crystalline limestone, and dolomite outcropping in Abaz Mountain and the hills south of the study area. The Upper Cretaceous-aged ophiolitic mélange is composed of serpentinite, quartzite, metasandstone, mudstone, and crystalline limestone, and basalt blocks. The Neogene units outcropping in the south of the region are represented by fractured and fissured limestone, dolomite, clayey limestone, marl, conglomerate, sandstone, gypsiferous clay, and gypsum (Hakyemez et al. 1992; Ulu et al. 1994; Bayari et al. 2009a; Yavuz 2010). The Quaternary deposits characterized by vertical and lateral heterogeneity and composed of conglomerate, sandstone, mudstone, gravel, gravely sandy mud, sand, clay, gypsiferous clay, and silt cover the rest of the study area. These deposits also include the gypsum mineral which increases in amount toward the northeast–east of the region (DSI 1975; Hakyemez et al. 1992).
As shown in the cross section (Fig. 2), two aquifers are identified in the study area: the Neogene and the Quaternary deposits. The Neogene aquifer is unconfined in the south and southwest of the study area while it is confined in rest of area where it is covered by Quaternary deposits (Fig. 1). The Neogene aquifer deposits are characterized by good permeability with a thickness varying between 200 and 400 m from the south to northeast–east (DSI 1975; Hakyemez et al. 1992). The depth from the land surface of the aquifer is about 5–10 m around the Çumra County and gradually gets deeper under the Quaternary units (DSI 1975). Transmissivity of the Neogene aquifer ranges from 800 to 3000 m2/day with the storage capacity of 10−3 to 10−1 (DSI 1975). The Quaternary groundwater is exploited from relatively permeable levels composed essentially of conglomerate, sandstone, and gravels. The thickness of the aquifer is variable and reaches up to 100–120 m in the northeast–east part of the study area (DSI 1975). The Neogene groundwater is exploited for agriculture, domestic, and drinking water needs mainly by boreholes. The Quaternary groundwater is exploited for only irrigation due to its poor quality.
Hydrogeological cross section of Fig. 1
The direction of groundwater flow in the study area is from southwest to northeast–east (Fig. 1). The depth of the wells ranges from 90 to 240 m in the Neogene aquifer and from 38 to 80 m in the Quaternary aquifer. The water level in all wells ranges from 20 to 60 m.
Materials and methods
Groundwater samples were collected from accessible and operational wells from both aquifers (Neogene and Quaternary) in August 2014. The regional distribution of the sampling sites is shown in Fig. 1. The samples were collected after 10 min of pumping and stored in acid-clean high-density polyethylene bottles. Polyethylene bottles (1 l volume) were carefully rinsed several times with the water from the well itself before sampling. The samples were filtered through a hand-held filter system using a 0.45-µm cellulose filter paper to remove suspended sediments. Physico-chemical parameters including electrical conductivity (EC) and pH were measured in the field using WTW-Multiline 340i Universal Meter. The samples for cation analyses were acidified with ultrapure HNO3 to reach pH < 2. The parameters were determined by the following standard and recommended analysis methods of APHA-AWWA-WPCF (1992). The cations were analyzed by inductively coupled plasma mass spectrophotometry (ICP-MS Enhanced) in the ACME Laboratory, Canada. Titrimetric methods were used for the determination of HCO3 and Cl. Also, SO4 was analyzed using spectrometry (Nova 60). Subsequently, NO3 was determined by spectrometry [WTW SpectroFlex (6600)] and the total dissolved solids (TDS) were determined by turbidimetry. Further accuracy of the chemical analysis was verified by calculating ion balance error which was generally within 5 %.
The Piper and Schoeller diagrams were used to investigate and identify the hydrogeochemical facies and the order of major ions abundance. Bivariate diagrams were used to evaluate the geochemical variations in the ionic concentrations in the groundwater. Also, in order to organize large data sets and to reveal the links between individual variables and various controlling factors, hydrogeochemical data were subjected to multivariate analytical techniques (Pearson correlation analysis) using the statistical software package (SPSS 15.0). The Gibbs diagram was used as reference to determine the mechanism controlling the groundwater chemistry in both aquifers. The PHREEQC code (Parkhurst and Appelo 1999) was used in saturation index (SI) calculations in order to evaluate the degree of equilibrium between water and respective mineral. Changes in saturation state are useful to distinguish in different stages of hydrochemical evolution and help to identify which geochemical reactions are important in controlling water chemistry (Coetsiers and Walraevens 2006). Additionally, the geochemical modeling program PHREEQC can be used in inverse modeling calculations in order to interpret geochemical processes and to detect cation-exchange process and mixing process, etc. In order to determine the cation-exchange processes controlling the water chemistry, an index of base exchange (Schoeller 1965) known as chloro-alkaline indices (CAI-I and CAI-II) was also calculated. Calculations are as follows [(Schoeller 1965); all values are expressed in meq/l];
Results and discussion
Physico-chemical parameters and hydrogeochemical facies in groundwater
Chemical analysis of major ions of groundwater samples from both aquifers is statistically summarized in Table 1. Overall, the groundwater of the study area shows neutral to slightly alkaline nature with a pH ranging from 6.4 to 8. On the one hand, The TDS value of the Neogene water samples ranges from 469 to 1473 mg/l with an average value of 675.8 mg/l. The samples collected in the Quaternary aquifer are, on the other hand, characterized by significantly higher TDS values, ranging from 861 to 4934 mg/l with an average value of 2010 mg/l. The TDS values of 85 % of the samples from the Neogene aquifer are lower than 750 mg/l. As it is shown in the histogram presented in Fig. 3, 7.5, 2.5, and 5 % of the rest of the samples have TDS values in the range of 750–1000, 1000–1250, and 1250–1500 mg/l, respectively. Despite the fact that the TDS values of all samples from the Quaternary aquifer are higher than 750 mg/l, 30 and 25 % of the samples predominantly have TDS values in the range of 1250–1500 and 1500–2000 mg/l, respectively. Low TDS values for the Neogene samples are related to the aquifer deposit that is mostly composed of limestone. The highest TDS values are observed downstream of the study region in the discharge area of the Quaternary aquifer (the samples Q1, Q2, Q4, Q11, Q12, and Q13). The Quaternary aquifer has high clay content. Salts, which are held back in the interstice or pores in clay while groundwater is evaporated or water table falls, are leached back into the groundwater during intense agricultural activities including agricultural runoff (Janardhan Raju 2007; Singh et al. 2011; Rao et al. 2013). Hence, the groundwater of the Quaternary aquifer has higher TDS values compared with the Neogene aquifer due to the greater affects of anthropogenic activities besides geochemical processes. The order of cation abundance for the Neogene aquifers is Ca2+ (60 %) > Mg2+ (26 %) > Na+ (13 %) > K+ (1 %), and the order of anion abundance is HCO3 − (67 %) > SO4 2− (18 %) > Cl− (13 %) > NO3 − (2 %). As for the Quaternary aquifer, the order of cation abundance is Mg2+ (44 %) > Ca2+ (32 %) > Na+ (22 %) > K+ (1 %), while the order of anion abundance is SO4 2− (37 %) > HCO3 − (33 %) > Cl− (29 %) > NO3 − (1 %). While the nitrate concentrations in water samples from the Neogene aquifer varied from 1.23 to 30.40 mg/l with an average value of 9.86 mg/l, samples from the Quaternary aquifer showed nitrate concentrations varying from 5.67 to 42.50 mg/l with an average value of 18.94 mg/l. The difference suggests that samples from the Quaternary aquifer have higher pollution potential than those of the Neogene aquifer because the former samples were extracted from shallower wells.
The data plot on the Piper diagram (Piper 1944) (Fig. 4) shows that the dominant hydrogeochemical facies is HCO3–Ca–Mg in the majority of the groundwater in the Neogene aquifer, with TDS less than 1000 mg/l, although the groundwater samples (samples N5, N13, N15, N17, N20, N22, N32, N34, N35, N37, and N38) show a tendency toward a HCO3–SO4–Ca–Mg or HCO3–SO4–Mg–Ca composition and their TDS values are up to 1473 mg/l due to dissolution of gypsum mineral in the aquifer (Fig. 5a). The majority of the groundwater samples taken from the Quaternary aquifer fall into no dominant zone in the cation facies, and a clear migration tendency from HCO3-zone to SO4-zone in the anion facies is observed (Fig. 4). The groundwater samples (Q1, Q2, Q4, Q11, Q12, and Q13) extracted in the east of the study area, which is also the discharge area, are predominantly SO4–Cl facies. The TDS value of these groundwater samples ranges from 2126 to 4934 mg/l with an average value of 3477 mg/l (Fig. 5b).
Sources of major ions and hydrogeochemical evolution
As seen in Figs. 4 and 5, Ca2+ and Mg2+ are the dominant cations and HCO3 − is the dominant anion in groundwater in the Neogene aquifer. Similarly, these ions are also present in considerable amounts in groundwater in the Quaternary aquifer. The limestone, dolomitic limestone, and dolomite, and carbonate rock fragments in the Quaternary aquifer are the major sources for carbonate in the area. The carbonates from these sources might have been dissolved and added to the groundwater system with recharging water during irrigation, rainfall, or leaching processes.
In (Ca2+ + Mg2+) versus HCO3 − diagram (Fig. 6a), samples representing the Neogene aquifer fall along carbonate dissolution line (1:1), suggesting that dissolution of carbonates is the main source of Ca2+ and Mg2+ (Stallard and Edmond 1983; Yangui et al. 2012). Despite the positive correlation (0.62) between (Ca2+ + Mg2+) and HCO3 − ions in the groundwater samples from the Quaternary aquifer, the majority of the samples are plotted far above the 1:1 line, which shows the occurrence of additional process or processes generating either an excess of Ca2+ + Mg2+ or a deficit of HCO3 −. As seen in Fig. 6b, samples belonging to both aquifers tend to plot along the 1:1 line which indicates that dissolution of carbonates and gypsum in both aquifers plays an important role in the evolution of the hydrochemistry of the study area. However, in most of the data points representing the Neogene aquifer, the small excess of (HCO3 − + SO4 2−) over (Ca2+ + Mg2+) may be derived from other processes such as ion-exchange reactions (Fisher and Mulican 1997; Kumar et al. 2006; Subba Rao and Surya Rao 2010). Furthermore, in most of the data points of the Quaternary aquifers, the excess of (Ca2+ + Mg2+) over (HCO3 − + SO4 2−) suggests that excess of calcium and magnesium might be derived from other processes such as reverse ion-exchange reactions and/or silicate weathering (Kumar et al. 2009). In addition, good positive correlations between Ca2+ and HCO3 − (0.82) and Ca2+ and SO4 2− (0.79), and positive correlations of Ca2+–Mg+2 (0.51), Mg2+–HCO3 − (0.65), and Mg2+–SO4 2− (0.67) in the groundwater of the Neogene aquifer (Table 2) support that the main source of these ions is dissolution of carbonates and gypsum in the aquifer (Al-Khashman 2007). In the Quaternary aquifer, Ca2+ showed positive correlation with Na+, Cl−, SO4 2−, and HCO3 − ions (0.65, 0.77, 0.72, and 0.63, respectively), and Mg2+ also showed good positive correlation with Na+, Cl−, SO4 2−, and HCO3 − ions (0.95, 0.90, 0.96, and 0.70, respectively) (Table 3), showing the dominance of the dissolution of minerals and subsequent ion-exchange processes (Drever 1997; Srivastava and Ramanathan 2008) in the Quaternary aquifer system (Table 3).
The (Ca2+ + Mg2+) versus TC+ (total cation) plot for the groundwater of the Neogene aquifer yields a closely linear trend (0.97) along the theoretical line (1:1), indicating that Ca2+ + Mg2+ accounts for most of the cations. In contrast, the trend (0.38) between Na+ + K+ and TC+ is poor (Fig. 7a). Additionally, the Ca2+ + Mg2+/HCO3 − ratio varies from 1.82 to 1.08 with an average value of 1.27. As previously stated, these results infer that the contribution of cations via dissolution of carbonate is more dominant in comparison with silicate weathering and/or ion exchange. In contrast to the Neogene aquifer, the average Ca2+ + Mg2+/HCO3 − and the Ca2+ + Mg2+/TC+ equivalent ratios of the samples representing the Quaternary aquifer are 3.23 and 0.76, respectively, suggesting that silicate weathering occurs in the Quaternary aquifer in addition to the dissolution of carbonate. Further, Ca2+ + Mg2+ versus TC+ plot (Fig. 7b) of groundwater samples have a linear spread along 0.7:1 (Ca2+ + Mg2+ = 0.7 TC+) (0.98) line, indicating that some of these ions are resulted from the weathering of silicate minerals (Kumar et al. 2009; Subramani et al. 2010). It can be concluded that the weathering of silicate minerals (biotite, chlorite, pyroxene, and amphibole minerals) in the host rocks and the rock fragments within the Quaternary aquifer is also a possible source of Mg2+ in the Quaternary aquifer. Also, in a plot of (Na+ + K+) versus TC+, the chemical data of the samples fall below the theoretical line (1:0.5) with a linear trend (0.93) (Fig. 7b). This leads to inference that the supply of these cations via silicate weathering and/or soil salts is significant (Stallard and Edmond 1983; Subba Rao 1998) in the Quaternary aquifer.
Scatter diagrams showing relationship between a the total cations (TC+) versus Ca + Mg and Na + K in the groundwater of the Neogene aquifer, b the total cations (TC+) versus Ca + Mg and Na + K in the groundwater of the Quaternary aquifer, c Na/Cl–Cl, d Na + K–Cl + SO4 e Ca + Mg–HCO3–SO4 versus Na–Cl, f Chloro-alkaline indices (CAI) of the Neogene samples, g Chloro-alkaline indices (CAI) of the Quaternary samples, h (Ca + Mg)–Cl
The ratios of Na+/Cl− for samples representing the Neogene and the Quaternary aquifers range from 0.5 to 1.4 with an average value of 0.98 and 0.44 to 2.29 with an average value of 0.82, respectively. A total of 65 % of samples in the Neogene aquifer and 95 % of samples in the Quaternary aquifer have lower Na+/Cl− ratio (Fig. 7c). Cl− content exceeding Na+ points at the ion-exchange process (Subramani et al. 2010; Dudeja et al. 2013; Li et al. 2013). It was also observed that the concentration of Na+ + K+ increased simultaneously with the increase of Cl− + SO4 2− concentration in the samples from both aquifers (0.76 for the Neogene and 0.98 for the Quaternary samples) (Fig. 7d). This is a result of the common source of Na+, K+, Cl−, and SO4 2− ions from the dissolution of soil salts in addition to the dissolution of gypsum, as also confirmed by Datta and Tyagi (1996), Subba Rao (2008), Sinivasamoorthy et al. (2008) and Subba Rao and Surya Rao (2010) in other regions. At the same time, quite good correlations between Ca2+ and Cl− (0.77), Mg2+ and Cl− (0.90), Na+ and Cl− (0.90), and SO4 2− and Cl− (0.82) in the Quaternary aquifer and positive correlations of Ca2+–Cl− (0.52), Mg2+–Cl− (0.56), Na+–Cl− (0.67), and SO4 2−–Cl− (0.80) in the Neogene aquifer support the leaching of soil salts (Prasanna et al. 2010; Esmaeili and Moore 2011) due to the irrigation return flows. Positive correlation between K+ and Cl− (0.68) in the Neogene aquifer, furthermore, suggests that dissolution of sylvite (KCl) mineral controls the K+ content. Bayarı et al. (2009a) also argued that dissolution of sylvite contributes slightly to the total ion content in the Neogene aquifer.
Ion exchange
In the study area, Ca2+ and Mg2+ were mainly provided by calcite, dolomite, and gypsum dissolution, but could be replaced with Na+ from a source other than halite. In order to determine the significance of cation exchange in controlling the water chemistry, a plot of Ca2+ + Mg2+–HCO3 −–SO4 2− versus Na+–Cl− was examined. The Na+–Cl− represents the amount of Na+ gained or lost relative to that provided by Cl− salt dissolution (mostly halite dissolution), while Ca2+ + Mg2+– (HCO3 − + SO4 2−) represents the amount of Ca2+ and Mg2+ gained or lost relative to that provided by carbonate or sulfate mineral dissolution. If cation exchange is a significant geochemical process for controlling the composition of groundwater, the relationship between these two parameters should be linear with a slope of −1.0 (Fisher and Mulican 1997; Tay 2012; Xiao et al. 2015). Similar results were observed for the Neogene and Quaternary groundwater samples (Fig. 7e). The diagram shows that the points representing the Neogene and Quaternary aquifers give a line with a slope of 0.83 and 0.99, respectively, suggesting the occurrence of reverse ion exchange in the study area.
Ion exchange occurring in this region is also verified by using an index of Base Exchange (Schoeller 1965) known as chloro-alkaline indices (CAI-I and CAI-II). When there is an exchange between Na+ and K+ in groundwater with Ca2+ and Mg2+ in the aquifer material, both indices are positive, indicative of reverse ion exchange. However, if the exchange takes place between Ca2+ and Mg2+ in groundwater with Na+ and K+ in the aquifer material, the indices will be negative, pointing at ion exchange (Aghazadeh and Mogaddam 2011; Li et al. 2013). It has been observed in the study area that most of the samples in the Neogene aquifer and all the samples in the Quaternary aquifer show positive ratios (Fig. 7f, g), confirming reverse ion-exchange reaction in which exchange takes place between Na+ and K+ in groundwater with Ca2+ and Mg2+ in the aquifer material. Despite all CAI-II values being positive, the CAI-I values of most samples from the Neogene aquifer are less than zero, suggesting that reverse ion-exchange reactions in the Neogene aquifer are weaker than those in the Quaternary aquifer. As also illustrated in Fig. 7h, especially in the Quaternary aquifer, salinity increases with an increase in Ca2+ + Mg2+, which may be stemming from reverse ion exchange in the clay/weathered layer (Rajmohan and Elango 2004) of the Quaternary aquifer.
Saturation index (SI)
Carbonate deposits (crystallized limestone, dolomite, and dolomitic limestone) represent the main deposits at mountain outcrops in the south–southwest of the study area. In addition, the Neogene aquifer is mostly composed of carbonate deposit and the Quaternary aquifer deposits include carbonate rock fragments. Besides, both aquifers contain gypsum. Along flow path, groundwater contacts with gypsum deposits. As gypsum has a higher solubility than calcite and conditions are appropriate for gypsum dissolution (negative medium saturation index, SIgyp: −1.66 and −0.91 in the Neogene and Quaternary aquifers, respectively), gypsum dissolution (CaSO4·2H2O + 2HCO3 → CaCO3 + SO4 + H2O + CO2) enhances calcium and sulfate concentrations (Fig. 8a). By further dissolution of gypsum and an increase in Ca concentration through common ion effect process, calcite is precipitated (Edmunds and Shand 2008) and therefore controls the amount of hydrogen carbonate in water (Helena et al. 1999). Bicarbonate reduction in water causes incongruent dissolution of dolomitic deposits in accordance with the reaction of Ca1−x Mg x CO3 → a Ca1−y Mg y CO3 + (x − ay) Mg2+ (1 − x − a − ay) Ca2+ (1 − a) CO3 2− where y < x (Edmunds and Shand 2008). The process is the replacement of dolomite by calcite, also called de-dolomitization. In the groundwater samples of the Neogene aquifer, the positive saturation index for calcite (SIc) shows that this mineral could potentially begin the precipitation (Fig. 8b). A slightly negative saturation index (SId) for dolomite marks the appropriate conditions for the dissolution of this mineral. These results infer that calcite precipitation and de-dolomitization compatible with the dissolution of gypsum are the other main hydrogeochemical reactions in the Neogene aquifer. In the groundwater of the Quaternary aquifer, calcite precipitation was continued compatible with the dissolution of gypsum (Fig. 8a, b). SId for dolomite suggested that the groundwater of the Quaternary aquifer was supersaturated with this mineral, but given dolomite’s sluggish precipitation kinetics (Morse and Arvidson 2002), precipitation did not likely occur. In other words, the solubility of magnesium carbonate is more than that of calcium carbonate and so magnesium carbonate is less likely to deposit. The high Mg2+/Ca2+ ratios (average mMg/Ca value is 1.6) in the groundwater of the Quaternary aquifer (Fig. 9) confirmed lack of dolomite precipitation. According to Schoeller (1964), the Mg2+/Ca2+ ratio is usually less than 1 in sedimentary rocks. However, the Mg2+/Ca2+ ratio is frequently high in the samples from the Quaternary aquifer since the evaporation process induces an increase in Mg2+ rather than Ca2+ concentrations in the course of migration pathways in the gypsum layers (Farid et al. 2013).
Groundwater evolution mechanisms
As the study area has a semi-arid climatic condition, evaporation may also contribute to water chemistry. Gibbs (1970), suggesting that the ratios of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3 −) as a function of TDS could provide information on the relative importance of the major natural mechanisms controlling groundwater chemistry (Naseem et al. 2010; Marghade et al. 2012). Hence, Gibbs’ plot is employed in this study to understand and differentiate the influences of rock–water interaction, evaporation, and precipitation on water chemistry. The majority of the samples from the Neogene aquifer fell in the category of water–rock interaction (Fig. 10), implying that rock–water interaction is apparently the dominant process influencing the groundwater hydrochemistry in the Neogene aquifer. The samples from the Quaternary aquifer were, on the contrary, plotted directly in and/or toward the evaporation zone (Fig. 10), which suggests evaporation is another dominant process determining the water composition. Evaporation observed in the samples from the Quaternary aquifer is likely to be caused by a slow infiltration process controlled by lower permeability due to high clay content in the aquifer materials. Furthermore, evaporation greatly increases the concentrations of ions formed by chemical weathering (dissolution), leading to higher salinity. Anthropogenic activities (agricultural fertilizers and irrigation return flows) also influence the evaporation by increasing Na and Cl, and thus TDS (Rao et al. 2013). When groundwater is used for irrigation, its recycling will rapidly increase the salinity of groundwater by repeated circulation in addition to evaporation (Fisher and Mulican 1997; Kraft et al. 1999; Subba Rao 1998, Duncan et al. 2008, Isidoro et al. 2006, Li et al. 2013). Hence, high TDS values in the Quaternary aquifer may be caused by anthropogenic activities (intensive and long-term irrigation) besides geochemical processes.
As mentioned earlier, groundwater is the major source for drinking, domestic, and agricultural purposes and the majority of the region except for the mountainous part is covered by agricultural lands and residential areas. Consequently, irrigation return flow, fertilizer and farm manure application, domestic sewage, etc., have important effects on groundwater contamination in the study area.
The nitrate concentration varies from 1.23 to 30.40 mg/l with a mean value of 9.86 mg/l in the Neogene aquifer and from 5.67 to 42.50 mg/l with a mean value of 18.94 mg/l in the Quaternary aquifer. The maximum concentrations of nitrate were found to be lower than the acceptable limits in the groundwater used for agricultural purposes, indicating that anthropogenic nitrogen pollution is currently not serious. However, overexploitation of groundwater resources can change local hydrodynamic conditions, which can in turn affect the relative concentrations of major and minor ions in the groundwater (Li et al. 2013; Xiao et al. 2015).
Conclusions
The analysis of the hydrochemical composition of groundwater in the study area has shown that the groundwater is generally neutral to slightly alkaline in nature with a pH ranging from 6.67 to 8.10. TDS of water samples show that Quaternary groundwater was observed to be more mineralized than Neogene groundwater. Most of the hydrochemical facies in the Neogene aquifer is HCO3–Ca–Mg type with minor HCO3–SO4–Ca–Mg or HCO3–SO4–Mg–Ca water types. The majority of groundwater samples in the Quaternary aquifer have no dominant cation, but the order of cation abundance is Mg2+ (44 %) > Ca2+ (32 %) > Na+ (22 %) > K+ (1 %) with a dominant hydrochemical type of HCO3–SO4, SO4–HCO3, and SO4–Cl water types.
Based on the geochemical evidence, chemical composition of the groundwater in the Neogene aquifer is largely controlled by water–rock interactions including dissolution of carbonates and gypsum, calcite precipitation, and de-dolomitization. The reverse ion exchange and dissolution of sylvite mineral also slightly control the concentration of calcium, magnesium, sodium, and potassium in the groundwater of the Neogene aquifer. In the Quaternary aquifer, however, reverse ion exchange, leaching soil salts, dissolution of carbonates and gypsum, calcite precipitation, and evaporation are the major hydrogeochemical processes responsible for the concentration of major ions in groundwater. Silicate weathering also contributes to increasing calcium and magnesium ions.
Although the study area is mostly covered by agricultural lands, the Gibbs plot demonstrated that the mechanism controlling groundwater chemistry in the Neogene aquifer is actually regulated by the geogenic processes (water–rock interaction) rather than anthropogenic activities. However, the mechanism controlling groundwater chemistry in the Quaternary aquifer is regulated by both geogenic processes (water–rock interaction) and anthropogenic activities such as intensive and long-term irrigation and irrigation return flows. Anthropogenic nitrogen pollution in the study area is currently not serious, yet evaporation and leaching of soil salts caused by anthropogenic activities increase the TDS in the Quaternary aquifer. For this reason, special care should be taken to protect this essential resource from negative impacts of anthropogenic activities.
Finally, dropping of groundwater level due to excessive abstraction for agriculture proves to be the major problem in Çumra plain. It is highly recommended that water-saving techniques such as spray irrigation and drip irrigation be applied in order to reduce the amount of groundwater used for irrigation and to improve and sustain the development of water resource management.
References
Abrahão R, García-Garizábal I, Merchán D, Causapé J (2015) Climate change and the water cycle in newly irrigated areas. Environ Monit Assess 187:22. doi:10.1007/s10661-014-4260-1
Aghazadeh N, Mogaddam AA (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environ Monit Assess 176:183–195
Ako AA, Shimada J, Hosono T, Ichiyanagi K, Nkeng GE, Eyong GET, Roger NN (2012) Hydrogeochemical and isotopic characteristics of groundwater in Mbanga, Njombe and Penja (Banana Plain)-Cameroon. J of Afr Earth Sci 75:25–36
Al-Khashman ÖA (2007) Study of water quality of springs in Petra region, Jordan: a 3-year follow-up. Water Resour Manage 21:1145–1163
Al-Shaibani AM (2008) Hydrogeology and hydrochemistry of a shallow alluvial aquifer, western Saudi Arabia. Hydrogeol J 16:155–165
APHA-AWWA-WPCF (1992) Standard methods for the examination of water and wastewater, 18th edn. APHA-AWWA-WPCF, USA
Appelo CAJ, Postma D (2005) Geochemistry, groundwater, and pollution, 2nd edn. Balkema, Amsterdam
Bayarı S, Özyurt N, Kilani S (2009a) Radiocarbon age distribution of grondwater in the Konya Closed Basin, central Anatolia, Turkey. Hydrogeol J 1:347–365
Bayarı S, Pekkan E, Özyurt N (2009b) Obruks, as giand collapse dolines caused by hypogenic karstification in the Central Anatolia, Turkey: analysis of likely formation processes. Hydrogeol J 17:327–345
Bozdağ A (2015) Combining AHP with GIS for assessment of irrigation water quality in Çumra Irrigation District (Konya), Central Anatolia, Turkey. Environ Earth Sci 73(12):8217–8236
Bozdağ A, Göçmez G (2013) Evaluation of groundwater quality in the Cihanbeyli basin, Konya, Central Anatolia, Turkey. Environ Earth Sci 69(3):921–937
Chae GT, Kim K, Yun ST, Kim KH, Kim SO, Choi BY, Kim HS, Rhee CW (2004) Hydrogeochemistry of alluvial groundwaters in an agricultural area: an implication for groundwater contamination susceptibility. Chemosphere 55:369–378
Coetsiers M, Walraevens K (2006) Chemical characterization of the Neogene Aquifer, Belgium. Hydrogeol J 14:1556–1568
Daniele L, Vallejo A, Corbella M, Molina L, Pulido-Bosch A (2013) Hydrogeochemistry and geochemical simulations to assess watererock interactions in complex carbonate aquifers: the case of Aguadulce (SE Spain). Appl Geochem 29:43–54
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater regime. J Geol Soc India 47:179–188
Drever JI (1997) Geochemistry of natural waters, 2nd edn. Prentice Hall, Eagle Wood Cliffs, p 388
DSI (General Directorate of State Hydraulic Works) (1975) Konya-Çumra-Karapınar Plain Hydrogeological Investigation Report, Ankara, p. 88
Dudeja D, Bartarya SK, Khanna PP (2013) Ionic sources and water quality assessment around a reservoir in Tehri, Uttarakhand, Garhwal Himalaya. Environ Earth Sci 69:2513–2527
Duncan RA, Bethune MG, Thayalakumaran T, Christen EW, McMahon TA (2008) Management of salt mobilization in the irrigated landscape. A review of selected irrigation regions. J Hydrol 351(1–2):238–252
Edmunds WM, Shand P (2008) Natural groundwater quality. Blackwell, Oxford
Esmaeili A, Moore F (2011) Hydrogeochemical assessment of groundwater in Isfahan province, Iran. Environ Earth Sci 67:107–120
Farid I, Trabelsi R, Zouari K, Abid K, Ayachi M (2013) Hydrogeochemical processes affecting groundwater in an irrigated land in Central Tunisia. Environ Earth Sci 68:1215–1231
Fisher RS, Mulican WF (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Chihuahuan desert, Trans-Pecos, Rexas, USA. Hydrogeol J 5(2):4–16
García-Garizábal I, José Gimeno M, Auque LF, Causapé J (2014) Salinity contamination response to changes in irrigation management. Application of geochemical codes. Span J Agric Res 12(2):376–387
García-Ruiz JM, López-Moreno JI, Vicente-Serrano SM, Lasanta-Martínez T, Beguería S (2011) Mediterranean water resources in a global change scenario. Earth Sci Rev 105(3–4):121–139
Ghesquière O, Walter J, Chesnaux R, Rouleau A (2015) Scenarios of groundwater chemical evolution in a region of the Canadian Shield based on multivariate statistical analysis. J Hydrol Reg Stud 4:246–266
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Göçmez G, Dıvrak BB, İş G (2008) Investigation of groundwater level change detection in Konya Closed Basin. Summary Report, WWF-Turkey, İstanbul, p. 18
Guendouz A, Moulla AS, Edmunds WM, Zouari K, Shand P, Mamou A (2003) Hydrogeochemical and isotopic evolution of water in the Complexe Terminal aquifer in the Algerian Sahara. Hydrogeol J 11:483–495
Hakyemez HY, Elibol E, Umut M, Bakırhan B, Kara İ, Dağıstan H, Metin T, Erdoğan N (1992) Geology of Konya-Cumra-Akören area: general directorate of mineral research and exploration comp. Report no: 42/24, Ankara. (in Turkish)
Helena BA, Vega M, Barrado E, Pardo R, Fernandez L (1999) A case of hydrochemical characterization of an alluvial aquifer influ-enced by human activities. Water Air Soil Pollut 112:365–387
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 3rd edn. U.S. Geological Survey Water-Supply Paper, 2254, 263
Irfan M, Said M (2008) Hydrochemical characteristics and the effects of irrigation on groundwater quality in Harran Plain, GAP Project, Turkey. Environ Geol 54:183–196
Isidoro D, Quílez D, Aragüés R (2006) Environmental impact of irrigation in La Violada District (Spain): I. Salt export patterns. J Environ Qual 35:766–775
Janardhan Raju N (2007) Hydrogeochemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, South India. Environ Geol 52:1067–1074
Kraft GS, Stites W, Mechenich DJ (1999) Impacts of irrigated vegetable agriculture on a Humid North-Central US. Sand plain aquifer. Groundwater 37(4):572–580
Kumar M, Ramanathan ALR, Kumar MSB (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039
Kumar M, Kumari K, Singh UK, Ramanathan AL (2009) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57:873–884
Li P, Wu J, Qian H (2013) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69:2211–2225
Marghade D, Malpe DB, Zade AB (2012) Major ion chemistry of shallow groundwater of a fast growing city of Central India. Environ Monit Assess 184:2405–2418
Matthess G (1982) The properties of groundwater. Wiley, New York
Moeller P, Rosenthal E, Geyer S, Guttman J, Dulski P, Rybakov M, Zilberbrand M, Jahnke C, Flexer A (2007) Hydrochemical processes in the lower Jordan valley and in the Dead Sea area. Chem Geol 239(1–2):27–49
Montcoudiol N, Molson J, Lemieux JM (2014) Groundwater geochemistry of the Outaouais Region (Québec, Canada): a regional-scale study. Hydrogeol J. doi:10.1007/s10040-014-1190-5
Morse JW, Arvidson RS (2002) The dissolution kinetics of major sedimentary carbonate minerals. Earth Sci Rev 58:51–84
Naseem S, Rafique T, Bashir E, Bhanger MI, Laghari A, Usmani TH (2010) Lithological influences on occurrence of high-fluoride groundwater in Nagar Parkar area, Thar Desert, Pakistan. Chemosphere 78:1313–1321
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (Version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. Water-Resources Investigations, Report 99-4259, Denver, Co, USA, p. 312
Piper AM (1944) A graphic procedure in the chemical interpretation of water analysis. Am Geophys Union Trans 25:914–928
Prasanna MV, Chidambaram S, Srinivasamoorthy K (2010) Statistical analysis of the hydrogeochemical evolution of groundwater in hard and sedimentary aquifers system of Gadilam river basin, South India. J King Saud Univ (Science) 22:133–145
Puntoriero ML, Cirelli AF, Volpedo AV (2015) Geochemical mechanisms controlling the chemical composition of groundwater and surface water in the southwest of the Pampean plain (Argentina). J Geochem Explor 150:64–72
Rajesh R, Brindha K, Murugan R, Elango L (2012) Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environ Earth Sci 65:1203–1213
Rajmohan N, Elango EL (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61
Rao GT, Rao VVSG, Rao YS, Ramesh G (2013) Study of hydrogeochemical processes of the groundwaters in Ghatprabha river sub-basin, Bagalkot District, Karnataka, India. Arab J Geosci 6(7):2447–2459
Redwan M, Abdel Moneim AA (2015) Factors controlling groundwater hydrogeochemistry in the area west of Tahta, Sohag, Upper Egypt. J Afr Earth Sci. doi:10.1016/j.jafrearsci.2015.10.002
Schoeller H (1964) La classification geochimique des eaux. General assembly of Berkeley 1963, vol 4. I.A.S.H Publication, Gentbrugge, Belgium, pp 16–24
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO Water Resources Series 33:44–52
Singh AK, Hundal HS, Singh D (2011) Geochemistry and assessment of hydrogeochemical processes in groundwater in the southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci 64:1823–1833
Sinivasamoorthy K, Chidambaram S, Prasanna MV, Vasanthavihar M, John Peter A, Anandhan P (2008) Identification of major sources controlling groundwater chemistry from a hard rock terrain-a case study from Mettur taluk, Salem District, Tamilnadu, India. J Earth Sys Sci 117(1):49–58
Srivastava SK, Ramanathan AL (2008) Geochemical assessment of groundwater quality in vicinity of Bhalswa landfill, Delhi, India, using graphical and multivariate statistical methods. Environ Geol 53:1509–1528
Stallard RF, Edmond JM (1983) Geochemistry of Amazon, the influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Su YH, Feng Q, Gao-Feng Z, Sı JH, Zhang YW (2007) Identification and Evolution of Groundwater Chemistry in the Ejin Sub-Basin of the Heihe River, Northwest China. Pedosphere 17(3):331–342
Su C, Wang Y, Pan Y (2013) Hydrogeochemical and isotopic evidences of the groundwater regime in Datong Basin, Northern China. Environ Earth Sci 70:877–885
Subba Rao N (1998) Groundwater quality in crystalline terrain of Guntur district, Andhra Pradesh, Visakhapatnam. J Sci 2(1):51–54
Subba Rao N (2008) Factors controlling the salinity in groundwaters from a part of Guntur district, Andhra Pradesh, India. Environ Monit Assess 138:327–341
Subba Rao N, Surya Rao P (2010) Major ion chemistry of groundwater in a river basin: a study from India. Environ Earth Sci 61(4):757–775
Subramani T, Elango L, Damodarasamy SR (2005) Groundwater quality and its suitability for drinking and agricultural use in Chithar River Basin, Tamil Nadu, India. Environ Geol 47:1099–1110
Subramani T, Rajmohan N, Elango L (2010) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ Monit Assess 162:123–137
Taheri Tizro A, Voudouris KS (2008) Groundwater quality in the semi-arid region of the Chahardouly basin, West Iran. Hydrol Process 22(6):3066–3078
Tay CK (2012) Hydrochemistry of groundwater in the Savelugu-Nanton District, Northern Ghana. Environ Earth Sci 67:2077–2087
Ulu Ü, Bulduk AK, Ekmekçi E, Karakaş M, Öcal H, Abbas A, Saçlı L, Taşkıran MA, Adır M, Sözeri Ş, Karabıyıkoğlu M (1994) Geology of İnlice-Akkise and Cihanbeyli-Karapinar area: General Directorate of Mineral Research and Exploration Comp. Rap. No: 9720, Ankara. (in Turkish)
Varol S, Davraz A (2014) Assessment of geochemistry and hydrogeochemical processes in groundwater of the Tefenni plain (Burdur/Turkey). Environ Earth Sci 71:4657–4673
Wen XH, Wu YQ, Wu J (2008) Hydrochemical characteristics of groundwater in the Zhangye Basin, Northwestern China. Environ Geol 55(8):1713–1724
Xiao J, Jin ZD, Wang J, Zhang F (2015) Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. Quatern Int 380–381:237–246
Yangui H, Zouari K, Rozanski K (2012) Hydrochemical and isotopic study of groundwater in Wadi El Hechim-Garaa Hamra basin, Central Tunisia. Environ Earth Sci 66:1359–1370
Yavuz S (2010) Hydrogeological parameters used in determining karstic features of Konya-Karapınar Basin (in Turkish). MSc Thesis, Institute of Natural and Applied science, Çukurova University, Adana, Turkey, p. 84
Zhang L, Song X, Jun X, Yuan R, Zhang Y, Liu X, Han D (2011) Major element chemistry of the Huai River basin, China. Appl Geochem 26:293–300
Acknowledgments
This study was financially supported by Selcuk University Scientific Research Projects (BAP) (Project no. 11401151) (Konya, Turkey).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bozdağ, A. Assessment of the hydrogeochemical characteristics of groundwater in two aquifer systems in Çumra Plain, Central Anatolia. Environ Earth Sci 75, 674 (2016). https://doi.org/10.1007/s12665-016-5518-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5518-4