Abstract
Hydrogeochemistry of groundwater is important for sustainable development and effective management of the groundwater resource. Fifty-six groundwater samples were collected from shallow tube wells of the intensively cultivated southern part of district Bathinda of Punjab, India, during pre- and post-monsoon seasons. Conventional graphical plots were used to define the geochemical evaluation of aquifer system based on the ionic constituents, water types, hydrochemical facies and factors controlling groundwater quality. Negative values of chloroalkaline indices suggest the prevalence of reverse ion exchange process irrespective of the seasons. A significant effect of monsoon is observed in terms chemical facies as a considerable amount of area with temporary hardness of Ca2+–Mg2+–HCO3 − type in the pre-monsoon switched to Ca2+–Mg2+–Cl− type (18%) followed by Na+–HCO3 − type (14%) in the post-monsoon. Evaporation is the major geochemical process controlling the chemistry of groundwater process in pre-monsoon; however, in post-monsoon ion exchange reaction dominates over evaporation. Carbonate weathering is the major hydrogeochemical process operating in this part of the district, irrespective of the season. The abundance of Ca2+ + Mg2+ in groundwater of Bathinda can be attributed mainly to gypsum and carbonate weathering. Silicate weathering also occurs in a few samples in the post-monsoon in addition to the carbonate dissolution. Water chemistry is deteriorated by land-use activities, especially irrigation return flow and synthetic fertilisers (urea, gypsum, etc.) as indicted by concentrations of nitrate, sulphate and chlorides. Overall, results indicate that different natural hydrogeochemical processes such as simple dissolution, mixing, weathering of carbonate minerals locally known as ‘‘kankar’’ and silicate weathering are the key factors in both seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemistry of groundwater is an important factor determining its use for domestic, irrigation and industrial purposes. Interaction of groundwater with aquifer minerals through which it flows greatly controls the groundwater chemistry. Hydrogeochemical processes that are responsible for altering the chemical composition of groundwater vary with respect to space and time. The hydrogeochemical processes of the groundwater system help to obtain an insight into the contributions of rock/soil–water interaction and anthropogenic influences on groundwater. These geochemical processes are responsible for the spatiotemporal variations in groundwater chemistry (Matthess 1982; Kumar et al. 2006). Groundwater chemistry, in turn, depends on a number of factors, such as general geology, degree of chemical weathering of the various rock types, quality of recharge water and inputs from sources other than water–rock interaction (Domenico 1972; Hem 1985; Schuh et al. 1997; Toth 1984). Such factors and their interactions result in a complex groundwater quality (Hussein 2004).
In the arid and semi-arid regions of India, due to uncertainty of monsoon and scarcity of surface water, dependence on groundwater resource has increased tremendously in recent years. India supports more than 16% of the world’s population with only 4% of the world’s fresh water resources (Singh 2003). The total area cultivated in India using groundwater has increased from 6.5 million hectare in 1951 to 35.38 million hectare in 1993 (GWREC 1997; Kumar et al. 2007). About 98% of the total sown area in Punjab is irrigated, out of which 73% is irrigated by tube wells and 27% by canals (Statistical abstract of Punjab, 2008). Although some studies have been reported on chemical quality and hydrogeochemical processes operated in other districts of Punjab, very little work has been done in the southern part of Bathinda district.
Groundwater is being continuously exploited in the Bathinda district of Punjab to meet the demand for water supply and irrigation, due to insufficiency of surface water as no river flows through the district, it is essential to know the hydrogeochemical processes that take place in the aquifer system. Therefore, the main objective of this study was to identify the major hydrogeochemical processes that are responsible for groundwater chemistry in the study region.
Study area
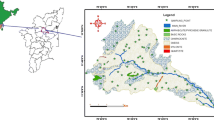
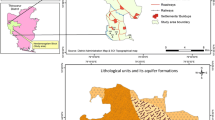
Bathinda district is in the southern part of Punjab State. It lies between 29°33′ and 30°36′ North latitude and 74°38′ and 75°46′ East longitude. It covers an area of 3,367 Sq. km. The Western Himalayas in the north and the Thar Desert in the south and southwest mainly determine the climatic conditions of Bathinda. The climate of Bathinda district can be classified as semi-arid and hot which is mainly dry except in rainy months and characterised by intensely hot summer and cold winter. Geologically the Bathinda district forms a part of Indo-Gangetic alluvium of Quaternary period (GSI 1976). The study area is underlain by an alluvial complex of fluviatile origin deposited by the ancestral tributaries of the Indus River system which include the ancient Satluj River (Wadia 1981). The alluvial complex consists of principally alternating beds of fine to medium sand, silt and clay. It is usually overlain by a thin layer of unstratified loam with lenses of organic matter. Concretions and nodules of calcium carbonate (kankar) are associated with fine-grained strata of the alluvium. The occurrence of kankar was observed beyond the depth of the water table as massive cover and nodular and granular beds. It has been found that there is no hard and fast rule regarding the association of kankar with a particular type of lithology (WRD 1982). Quaternary alluvial sediments consisting of fine to medium grained sand or sand and kankar with admixture of clay constitute the aquifer system of the area. In these soils, the basic cations are Ca2+ and Mg2+ and the distribution of calcium carbonate suggests different degrees of carbonate leaching (Sharma et al. 1998). Sand and silt fractions are dominated by primary quartz. Other minerals present in these fractions include muscovite, plagioclase and alkali feldspars, amphibole, biotite, anhydrite (gypsum) and chlorite. Illite, chlorite, and kaolinite have been identified in the clay fraction of these area soils (Sidhu and Gilkes 1977; Raj-Kumar et al. 2010). The aquifers in the area belong to the huge aquifer system of the lndo-Gangetic alluvial plain (Thomas et al. 1995).
Hydrogeology
The area is underlain by the Indus alluvium of the Quaternary age. The exploratory drilling in Indus basin also shows the presence of thick sandy aquifer zones with the intervening thin clay layers identifying it as one unified multi-aquifer system. Below the thick alluvial sediments, different types of basement rocks have been encountered. The area has both confined and unconfined aquifers (CGWB Report 2007; Gupta and Thakur 1989). All the aquifers were shallow and varied from 8 to 30 m. The mean annual rainfall in the district is 408 mm (2004–2008). Most of the rainfall occurs in four months of the southwest monsoon period from May to September (about 77.1%).
Materials and methods
Groundwater sampling campaigns were carried out during March 2007 (pre-monsoon) and October 2008 (post-monsoon). Twenty-eight water samples were collected during March 2007 (Fig. 1), and the same locations were again sampled in October 2008 to evaluate seasonal variations. Overall, 56 samples were collected for chemical analysis. Water samples were collected from different alluvial aquifers representing soil associations of Bathinda district (Sidhu et al. 1995). Southern parts of Bathinda district have 14 soil mapping units, and two water samples from each soil association were collected.
Duplicate water samples were collected in clean polyethylene bottles of 250 ml capacity. At the time of sampling, bottles were thoroughly washed 2–3 times with groundwater to be sampled. In the case of bore wells the water samples were collected after pumping for 10 min. This was done to remove groundwater stored in the well. One part of the water sample to be analysed for physical parameters and major anions was not acidified. The other part of the sample was acidified to make 1% in HNO3, producing a pH around 1.5 sufficient to stabilise trace metals prior to analysis on ICAP-AES. The samples were placed in a cooled ice box during transportation and stored refrigerated until analysis (at 5°C). The non-acidified water samples were analysed for pH, EC, total dissolved solids, bicarbonate and chloride as per the standard procedures given in American Public Health Association (APHA 1998) within 24 h of sampling. The reliability of pH analysis was checked after every five samples using standard buffer solutions of pH 7 and 9.2, whereas that of EC analysis was checked using standard EC solution provided by M/s Crison instruments SA having EC = 1413 μS cm−1. These samples were also analysed for nitrate and silica using UV/visible spectrophotometer; and fluoride by potentiometer method, using the F1052F solid-state membrane electrode in conjunction with K401 calomel electrode.
Laboratory analysis of major and minor cations such as Ca, Mg, Na, K, Mn, Zn and Fe were analysed on Thermo Electron Corporation’s Emission Spectrometer, iCAP 6300. The iCAP 6300 Duo model allows for simultaneous analysis of all elements from sub ppb to % level. The detection limits for Na, K, Ca, Mg, S, Fe and Mn on the iCAP duo emission spectrometer are 1.80, 0.60, 0.003, 0.010, 2.22, 0.25, and 0.07 ppb at 589, 766, 393, 279, 181, 260 and 258 nm, respectively. Overall, measurement reproducibility and precision for each analysis was less than 2%.
Results and discussion
Groundwater geochemistry
The chemical indices derived from hydrogeochemical parameters of pre- and post-monsoon groundwater samples of Bathinda district of Punjab and their computed values have been presented along with their standard deviation in Table 1. Groundwater was found to be alkaline in nature with an average pH value of 7.63 and 7.84 in the pre- and post-monsoon, respectively. A slight increase in pH was noticed in the post-monsoon season in comparison to pre-monsoon. Increase in pH in the post-monsoon season suggests that dissolution has been enhanced due to high interaction between soil and rainwater (Subramanian and Saxena 1983). The electrical conductivity (EC) ranged from 220 to 4,390 μS cm−1 with a mean of 2,120 μS cm−1 and from 672 to 4,840 μS cm−1 with a mean of 2,270 μS cm−1 for pre- and post-monsoon water samples, respectively. The total dissolved solids (TDS) ranged from 126 to 2,590 mg l−1 with a mean of 1,251 mg l−1 and from 396 to 2,856 mg l−1 with a mean of 1,340 mg l−1 for pre- and post-monsoon water samples, respectively. The increase of EC and TDS in post-monsoon season is on the higher side than the pre-monsoon season. Salts, which held back in the interstice or pores in clay while groundwater is evaporated or water table falls, get leached back to the groundwater during the rainy period. Further, the groundwater level in the study area is rising in the last 30 years (CGWR 2007). Hence, the post-monsoon waters have higher TDS and EC values as compared to the pre-monsoon season. Very high standard deviation in EC for both the pre- and post-monsoon seasons indicates the spatial variability of leaching and dilution with recharging rainfall water.
Dominant cations are in the order of Na+ > Ca2+ > Mg2+ > K+ in the Bathinda district of Punjab. Clear seasonal variations were observed both in minimum and maximum concentrations of these cations, but the difference in mean value is not significant except for K+. Despite the greater resistance of potassium silicate to weathering, ions are released during weathering but they seem to be used up in the formation of secondary minerals. The concentration of potassium at a few places is unusually very high, which may be due to salt patches present geogenically and fertiliser leaching or return flow.
Dominant anions are in the order of HCO3 − > SO4 2− > Cl− > NO3 −. Alkalinity is a measure of the ability of groundwater to neutralise acids to the equivalence point of carbonate or bicarbonate. Bicarbonate represents the major sources of alkalinity in these waters. Bicarbonates are higher in post-monsoon sampling periods indicating the contribution from carbonate weathering process. There is a slight variation observed in seasonal and spatial distribution of HCO3 − but very significant at certain locations, which may be due to mixing of geogenically present salt patches in the soil profile and due to rise in the water table (Raj-Kumar et al. 2010). Chloride and sulphate show large variation between minimum and maximum value and also express high standard deviation. This inference not only suggests that the water chemistry in the study region is heterogeneous but also reveals the influences of complex contamination sources and geochemical process. Table 1 also shows a variation in the mean values of chemical parameters between pre- and post-monsoon data. The mean values of EC and major ions are higher in post-monsoon data compared to the pre-monsoon data, which apparently suggest the enhancement of solute load in the aquifer.
Characterisation of hydrogeochemical data
Hydrochemical diagrams are aimed at facilitating interpretation of evolutionary trends, particularly in groundwater systems, when they are interpreted in conjunction with distribution maps and hydrochemical sections. An overall characterisation of hydrogeochemical data can be possible by knowing the hydrochemical facies of water, generally known as water type, using various plots such as Durov diagram (1948), tri-linear diagram (Piper 1944), Schoeller diagram (1965), Stiff diagram and Radial plot. Chadha (1999) has proposed a new diagram for geochemical classification of natural waters and interpretation of chemical data which is used in this paper. The proposed diagram is a modification of the Hill Piper diagram with a view to extend its applicability in representing water analysis in the simplest possible way. In the proposed diagram (Fig. 2), the difference in mill equivalent percentage between alkaline earths (Ca2+ + Mg2+) and alkali metals (Na+ + K+), expressed as percentage reacting values, is plotted on the x-axis, and the difference in mill equivalent percentage between weak acidic anions (CO3 2− + HCO3 −) and strong acidic anions (Cl− + SO4 2−) is plotted on the y-axis. The mill equivalent percentage differences between alkaline earths and alkali metals and between weak acidic anions and strong acidic anions would plot in one of the four possible sub-fields of the rectangle diagram. The rectangular field describes the overall character of the water. To define the primary character of water, the rectangular field is divided into eight sub-fields, each of which represents a water type: (1) Alkaline earths exceed alkali metals. (2) Alkali metals exceed alkaline earths. (3) Weak acidic anions exceed strong acidic anions. (4) Strong acidic anions exceed weak acidic anions. (5) Alkaline earths and weak acidic anions exceed both alkali metals and strong acidic anions, respectively. Such water has temporary hardness. The positions of data points in this domain represent Ca2+–Mg2+–HCO3 − water type. (6) Alkaline earths exceed alkali metals and strong acidic anions exceed weak acidic anions. Such water has permanent hardness and does not deposit residual sodium carbonate in irrigation use. The positions of data points in this domain represent Ca2+–Mg2+–Cl− type, Ca2+–Mg2+ dominant Cl−-type, or Cl− dominant Ca2+–Mg2+ type waters. (7) Alkali metals exceed alkaline earths and strong acidic anions exceed weak acidic anions. The positions of data points in this domain represent Na+–Cl−-type and Na+-SO4 2−-type Na+-dominant Cl− type, or Cl− dominant Na+-type waters. (8) Alkali metals exceed alkaline earths and weak acidic anions exceed strong acidic anions. The positions of data points in this region represent Na+–HCO3 −-type waters. Results of analyses are plotted on the Chadha diagram (Fig. 2) to test its applicability for geochemical classification of groundwater (Table 2) and to study hydrochemical processes of pre- and post-monsoon seasons. From Table 2, it is evident that there is significant increase (18%) in the permanent hardness, i.e., domain 6 (Ca2+–Mg2+–Cl− type) followed by 14% in domain 8 (Na+–HCO3 −-type) in the post-monsoon as compared to the pre-monsoon samples where 50% samples fall in domain 5 of temporary hardness group. Sodium-bicarbonate type waters deposit residual sodium carbonate in irrigation use and causes foaming problems. Twenty-one percent samples of both the seasons fall under the category of Na+–Cl− type; such water creates salinity problems both in irrigation and drinking purposes. These observations suggest that monsoons have strong effect on the quality of water which further deteriorates after the monsoon season.
Identification of hydrogeochemical process
Reactions between groundwater and aquifer minerals have a significant role on water quality, which are also useful to understand the genesis of groundwater (Cederstorm 1946). The hydrogeochemical data are subjected to various conventional graphical plots to identify the hydrogeochemical processes operating in aquifer region of study area. Some of the possible identified processes are explained below.
Ion exchange
Knowledge of the changes brought about in the chemical composition of the groundwater during its travel underground is essential (Sastri 1994). Control on the dissolution of undesirable constituents in waters is impossible during the subsurface runoff, but it is essential to know the various changes undergone by waters during the travel (Pojasek 1977; Johnson 1979). The ion exchange between the groundwater and its host environment during residence or travel can be understood by studying the chloroalkaline indices (CAI) (Schoeller 1965, 1967):
All values are expressed in meq l−1. When there is an exchange between Na+ or K+ with Mg2+ or Ca2+ in the groundwater, both the above indices will be positive and if there is a reverse ion exchange prevalent then both these indices will be negative (Schoeller 1965, 1967, 1977). The groundwater samples of the study area (Fig. 3) indicates that the reverse ion exchange process is dominant in both the seasons as 96% samples in pre-monsoon and 67% samples in the post- monsoon season have negative ratios of CA 1 and CA 2. Only 33% samples in the post-monsoon have positive ratios and exhibit the ion exchange process. Fisher and Mulican (1997) have explained that when reverse ion exchange is a significant geochemical process for controlling the composition of groundwater, the relationship between Na+–Cl− and Ca2+ + Mg2+–HCO3 −–SO4 2− should be linear with a slope of −1.0. Figure 4 apparently shows that most of the groundwater samples of the Bathinda followed a straight line (R 2 = 0.96 in pre-monsoon to 0.93 in post-monsoon) with a slope of −1.04, which indicates the occurrence of reverse ion exchange.
The plot of (Ca2+ + Mg2+) versus (SO4 2− + HCO3 −) will be close to the 1:1 line if the dissolutions of calcite, dolomite and gypsum are the dominant reactions in a system. Ion exchange tends to shift the points to right due to an excess of SO4 2− + HCO3 − (Cerling et al. 1989; Datta and Tyagi 1996; Fisher and Mulican 1997). If reverse ion exchange is the process, it will shift the points to the left due to a large excess of Ca2+ + Mg2+ over SO4 2− + HCO3 −. The plot of Ca2+ + Mg2+ versus SO4 2− + HCO3 − (Fig. 5) shows that all the groundwater samples of the southern part of Bathinda district falls left to the 1:1 line irrespective of the seasons which indicate that reverse ion exchange tends to dominant over ion exchange process in the study area.
The plot of m(Ca2+ + Mg2+) versus m(Cl−) (Fig. 6a) indicates that Ca2+ and Mg2+ do not increase with increasing salinity which further proved that the reverse ion exchange is the main process in the clay/weathered layer except in a few samples of the pre-monsoon season.
Carbonate weathering and dissolution
Calcium and magnesium are the dominant cations present in groundwater next to sodium in this region. Similarly, bicarbonate is also present in considerable amounts. The crystalline limestone, dolomitic limestone and kankar (the lime rich weathered mantle overlies carbonate rocks) are the major sources for carbonate in the area. The carbonates from these sources might have been dissolved and added to the groundwater system with recharging water during irrigation, rainfall or leaching and mixing processes. In Ca2+ + Mg2+ versus SO4 2− + HCO3 − scatter diagram (Fig. 5), the points falling along the equiline (Ca2+ + Mg2+ = SO4 2− + HCO3 −) suggest that these ions have resulted from weathering of carbonates and silicates (Datta and Tyagi 1996; Datta et al. 1996; Rajmohan and Elango 2004). Most of the points in this study fall in the Ca2+ + Mg2+ side, suggesting that carbonate weathering is the major hydrogeochemical process operating in this part of Bathinda district, irrespective of the season. Also, it indicates that excess of calcium and magnesium may be derived from other processes such as reverse ion exchange reactions.
If the ratio Ca/Mg = 1, dissolution of dolomite should occur, whereas a higher ratio is indicative of greater calcite contribution (Maya and Loucks 1995). Molar ratio of Ca/Mg > 2 indicates the dissolution of silicate minerals, which contribute calcium and magnesium to groundwater (Katz et al. 1998). The plot of Ca/Mg ratio of the groundwater samples of the study area reveals that the dominant process is the dissolution of calcite and dolomite that present in the alluvium material of the Bathinda (Fig. 6b). The points closer to the line (Ca/Mg = 1) indicate the dissolution of dolomite. In the present study, most of the groundwater samples found to be below 1 ratio indicate the calcite weathering as the most prominent process. However, 16% in the pre- and 10% samples in post-monsoon lie near the 1 ratio line revealing dolomite dissolutions. Only 6% samples in pre- and 3% in the post-monsoon lie above the ratio line 2 indicating the effect of silicate minerals. Presence of ‘‘kankar’’ carbonates in the alluvial sediments and occurrence of metamorphosed dolomite limestones could favour the weathering process. However, a possibility of calcite weathering by sulphuric acid is also there as indicated by Ca2+ and SO4 2− ratio. Sulphuric acid may be produced by SOx emission dissolved in rain from industrial sources (Bathinda district has a thermal plant for electricity generation).
In silicate terrain, if the calcium and bicarbonate in groundwater are solely originated from calcite, the equivalent ratio of dissolved Ca2+ and HCO3 − in the groundwater is 1:2, whereas from dolomite weathering, it is 1:4 (Garrels and Mackenzie 1971; Holland 1978). Similarly, if the calcium and sulphate in groundwater derived from dissolution of gypsum or anhydrite, then the Ca2+/SO4 2− ratio is almost 1:1 (Das and Kaur 2001). In Ca2+ versus HCO3 − scatter diagram (Fig. 6c), only three groundwater samples fall on the 1:1 line and most of them lie above the 1:1 equiline, indicating the contribution of calcite weathering on groundwater chemistry. Moreover, in Ca2+ versus SO4 2− scatter diagram (Fig. 6d), there are almost 50:50 distributions of the samples above and below the 1:2 eqiline. This also shows the dominance of calcite weathering rather than dolomite (Garrels and Mackenzie 1971). Furthermore, it indicates the presence of dissolution of gypsum in the Bathinda district.
The sources of Ca2+ and Mg2+ in groundwater can be estimated from the m (Ca2+ + Mg2+)/mHCO3 − ratio. As this ratio does not increase with salinity (Fig. 6e), Ca and Mg are added to solution at a lesser rate than HCO3 − which is evident as the majority of the samples of pre- and post-monsoon fall below one ratio. If Ca2+ and Mg2+ originate mainly from the dissolution of carbonates in the aquifer materials and from the weathering of accessory pyroxenes and amphibole minerals, this ratio would be about 0.5 (Sami 1992). Therefore, the abundance of Ca2+ + Mg2+ in groundwater of Bathinda can be attributed mainly to gypsum and carbonate weathering (Sharma et al. 1998; Kumar et al. 2007).
Silicate weathering
Silicate weathering may play a vital role in controlling the major ions chemistry of the groundwater, especially in hard rock aquifers (Mackenzie and Garrells 1965; Rajmohan and Elango 2004). Silicate weathering can be understood by estimating the ratio between Na+ + K+ and total cations (TZ+). The relationship between Na+ + K+ and total cations (TZ+) of the area revealed that most of the samples are plotted near the Na+ + K+ = 0.5TZ+ line (Fig. 6f). This observation indicates the involvement of silicate weathering in the geochemical processes, which contributes mainly sodium and potassium ions to the groundwater (Stallard and Edmond 1983; Sarin et al. 1989).
Moreover, weathering of soda feldspar (albite) and potash feldspars (orthoclase and microcline) is greatly responsible for the contribution of Na+ and K+ ions to groundwater. Feldspars are more susceptible for weathering and alteration than quartz in silicate rocks. The (Ca2+ + Mg2+)/HCO3 − ratio of more than 3 (Fig. 6e) of a few samples in the post-monsoon suggests that silicate weathering occurs in this region in addition to the carbonate dissolution.
Evaporation
The study region experiences dry and semiarid climatic condition, evaporation may also contribute in water chemistry. Gibbs (1970) suggested that a simple plot of TDS versus the weight ratio of Na+/(Na+ + Ca2+) could provide information on the relative importance of the major natural mechanisms controlling groundwater chemistry. Hence, Gibbs’ plot is employed in this study to understand and differentiate the influences of rock–water interaction, evaporation and precipitation on water chemistry. From the Gibbs plot (Fig. 7), the dominant process determining the water composition is evaporation. Evaporation of surface water and moisture in the unsaturated zone is the main process in the evolvement of groundwater chemical composition. Evaporation concentrates the remaining water and led to precipitation and deposition of evaporates that are eventually leached into the saturated zone. This is expected, as evaporation greatly increases the concentration of ions formed by chemical weathering, leading to high salinity and TDS. This is clearly reflected in the Gibbs plot, which indicates a trend towards evaporations in the pre-monsoon and rock/soil-dominated dissolution in the post-monsoon season.
Furthermore, sodium versus chloride ratio can be used to identify the evaporation process in groundwater. Evaporation will increase the concentration of total dissolved solids in groundwater; and the Na/Cl ratio remains the same, and it is one of the good indicative factors of evaporation. If evaporation is the dominant process, Na/Cl ratio should be constant when EC rises (Jankowski and Acworth 1997). Hence, the plot of Na+/Cl− versus EC would give a horizontal line, which would then be an effective indicator of concentration by evaporation and evapotranspiration. The EC versus Na/Cl scatter diagram of the groundwater samples (Fig. 8a) shows that the trend line is horizontal for pre-monsoon indicating that evaporation is the major process for this season’s samples. For post-monsoon samples trend line is inclined revealing that evaporation may not be the major geochemical process controlling the chemistry of groundwater or ion exchange reaction dominating over evaporation in the post-monsoon season.
Effect of land use on groundwater chemistry
The porous soils of the study area are characterised by high infiltration, low moisture retention and poor fertility due to limitation of organic matter. Groundwater is the main source for domestic and agricultural activities. Most of the study area is covered by agricultural lands and residents. Hence, groundwater contamination by irrigation return flow, fertiliser and farm manure application, leaching of soil mineralised nitrogen, domestic sewage, runoff and leached rainfall water, etc., is also an important issue on groundwater contamination in the study area. The concentrations of nitrate and chloride in the groundwater justify the effect of anthropogenic source of contamination (Table 1). The nitrate concentration varies from below detection limit (BDL) to 79 mg l−1 with a mean value of 30 mg l−1 in pre-monsoon and from BDL to 93 mg l−1 with a mean value of 37 mg l−1. However, six samples in pre-monsoon and 10 samples in post-monsoon exceed 50 mg l−1 permissible limit (WHO 1996) and are not fit for drinking, which may cause blue baby disease/methaemoglobinaemia in children and gastric carcinomas (Comly 1987; Johnson et al. 1987; Gilly et al. 1984; Kross et al. 1993). Also, as most of the study area is intensively irrigated and the nitrogen use efficiency is only 30–40%, therefore, higher concentration of nitrate in groundwater can be expected from the agricultural activities (Aulakh and Bijay-Singh 1997; Cassman et al. 2002). Similarly, chloride ranges from 4 to 153 mg l−1 in pre-monsoon with a mean value 61 mg l−1 and 28 to 696 mg l−1 in post-monsoon with a mean value of 237 mg l−1. Chloride content in pre-monsoon is well within the permissible limit (600 mg l−1) set by WHO for drinking water and only 4% samples in post-monsoon exceeds the permissible limit. High chloride concentration in groundwater may indicate pollutions by sewage, industrial wastes or saline water intrusions (Bertram and Balance 1998). Sulphate varies from 45 to 785 mg l−1 with a mean value of 273 mg l−1 in pre-monsoon and 39 to 887 mg l−1 with a mean value of 302 mg l−1 in post-monsoon. Sulphate maximum permissible limit is 400 mg l−1 and causes a laxative effect on humans, together with excess magnesium in groundwater. Twenty-five and 29% samples in pre- and post- monsoon samples exceed the MPL. This may result in gastrointestinal irritation in the human system.
Sulphate and chloride show strong correlation in post-monsoon sampling data only [Fig. 8b (Post-monsoon) R 2 = 0.76] and implies the effect of surface contamination, especially irrigation return flow since gypsum application is very common in the paddy field to improve the physical conditions of the soils.
Conclusions
The results suggest that dissolution, mixing, weathering of carbonate (locally known as ‘‘kankar’’) and of silicate minerals and reverse ion exchange are the hydrogeochemical processes which occurred during pre- and post-monsoon seasons. There is a significant effect of monsoon observed in terms of hardness and the significant amount of area with temporary hardness in the pre-monsoon switched to permanent hardness domain by the post-monsoon. There is 14% increase in the Na+–HCO3 − type water in the post-monsoon. Elevated sodium content in water usually deteriorates the physical conditions of the soil. Therefore, there is a need to shift the cropping pattern from paddy (high water requirement crop)–wheat to cotton (less water requirement)–wheat rotation. Nitrate, sulphate and chloride concentrations reveal that water chemistry is impaired by land-use activities, especially irrigation return flow, synthetic fertilisers (urea, gypsum, ammonium sulphate, etc.) and farm manures. Therefore, there is a need to monitor these types of changes on the long-term basis over a period of time.
References
APHA (1998) Standard methods for the examination of water and waste waters, 20th edn. APHA, Washington DC, USA
Aulakh MS, Bijay-Singh (1997) Nitrogen losses and fertilizer N use efficiency in irrigated porous soils. Nutr Cycl Agroecosys 47:197–212
Bertram J, Balance R (1998) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes, 1st edn. Taylor and Francis, USA., ISBN: 0419223207, pp 400
Cassman KG, Dobermann A, Walters D (2002) Agroecosystems, nitrogen-use efficiency, and nitrogen management. Ambio 31:132–140
Cederstorm DJ (1946) Genesis of groundwater in the coastal plain of Virginia. Environ Geol 41:218–245
Cerling TE, Pederson BL, Damm KLV (1989) Sodium-Calcium ion exchange in the weathering of shales: implications for global weathering budgets. Geology 17:552–554
CGWB Report (2007) Report on groundwater resources and development potentials of Bathinda district, Punjab
Chadha DK (1999) A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol J 7:431–439
Comly HH (1987) Cyanosis in infants caused by nitrates in well water. J Am Med Assoc 257:2788–2792
Das BK, Kaur P (2001) Major ion chemistry of Renuka lake and weathering processes, Sirmaur district, Himachal Pradesh, India. J Environ Geol 40:908–917
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater regime. J Geol Soc India 47:179–188
Datta PS, Bhattacharya SK, Tyagi SK (1996) 18O studies on recharge of phreatic aquifers and groundwater flow-paths of mixing in the Delhi area. Hydrogeol J 176:25–36
Domenico PA (1972) Concepts and models in groundwater hydrology. McGraw-Hill, New York
Durov SA (1948) Natural waters and graphical representation of their composition. Dokl Akad Nauk SSSR 59:87–90
Fisher RS, Mulican WF III (1997) Hydrochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the Northern Chihuahuan desert, Trans-Pecos, Rexas, USA. Hydrogeol J 10:455–474
Garrels RM, Mackenzie FT (1971) Evolution of sedimentary rocks. WW Norton, New York
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
Gilly G, Corrao G, Favilli S (1984) Concentrations of nitrates in drinking water and incidence of gastric carcinomas First descriptive study of the Piemonate Region, Italy. Sci Total Environ 34:35–37. doi:10.1016/0048-9697(84)90039-1
GSI (1976) Know your District Bathinda, Punjab—By Geological Survey of lndia, 1976
Gupta S, Thakur RS (1989) Report on hydrogeology of District Bathinda, Punjab State (F.S.P. 1989–93). Central Ground Water Board, North Western Region, Chandigarh
GWREC (1997) Report of groundwater resource estimation committee, Ministry of water resources, Government of India, New Delhi
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. USGS, Water Supply Paper, 2254:264
Holland HD (1978) The chemistry of the atmosphere and ocean. Wiley, New York
Hussein MT (2004) Hydrochemical evaluation of groundwater in the Blue Nile Basin, eastern Sudan, using conventional and multivariate techniques. Hydrogeol J 12:144–158. doi:10.1007/s10040-003-0265-5
Jankowski J, Acworth RI (1997) Impact of debris-flow deposits on hydrogeochemical process and the development of dry land salinity in the Yass River catchment, New South Wales, Australia. Hydrogeol J 5:71–88
Johnson CC (1979) Land application of waste—an accident waiting to happen. Groundwater 17(1):69–72
Johnson CJ, Bonrud PA, Dosch TL et al (1987) Fatal outcome of methemoglobinemia in an infant. J Am Med Assoc 257:2796–2797
Katz BG, Coplen TB, Bullen TD, Davis JH (1998) Use of chemical and isotopic tracers to characterize the interaction between groundwater and surface water in mantled Karst. Groundwater 35:1014–1028
Kross BC, Hallberg GR, Bruner R, Cherryholmes K, Johnson KJ (1993) The nitrate contamination of private well water in Iowa. Am J Public Health 83:270–272
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi. India J Environ Geol 50:1025–1039
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two agriculture dominated districts of Punjab, India. J Environ Geol 53:553–574
Mackenzie FJ, Garrells RH (1965) Silicates: reactivity with water. Sci J 1505:57–58
Matthess G (1982) The properties of groundwater. Wiley, New York, p 498
Maya AL, Loucks MD (1995) Solute and isotopic geochemistry and groundwater flow in the Central Wasatch Range, Utah. J Hydrol 172:31–59
Piper AM (1944) A graphic procedure in geochemical interpretation of water analyses. Trans Am Geophys Union 25:914–923
Pojasek RB (1977) Drinking water quality enhancement through protection. Ann Arbor Publishers Inc., Ann Arbor, p 614
Raj-Kumar, Balwinder-Singh, Hundal, HS, Kuldip-Singh, Yadwinder-Singh (2010) Geospatial quality of underground water of Punjab. Department of Soils, Punjab agricultural University, Ludhiana-141004, India, pp 72 + 26 figures
Rajmohan N, Elango L (2004) Identification and evolution of hydrogeochemical processes in the groundwater environment in an area of the Palar and Cheyyar River Basins, Southern India. Environ Geol 46:47–61
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern Cape, South Africa. J Hydro 139:27–48
Sarin MM, Krishnaswamy S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga-Brahmaputra river system: weathering process and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009. doi:10.1016/0016-7037(89)90205-6
Sastri JCV (1994) Groundwater chemical quality in river basins, hydrogeochemical facies and hydrogeochemical modeling. Lecture notes—refresher course conducted by school of Earth Sciences. Bharathidasan University, Thiruchirapalli
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO, pp 54–83
Schoeller H (1967) Geochemistry of groundwater—an international guide for research and practice, UNESCO
Schoeller H (1977) Geochemistry of groundwater. In: Brown RH et al (eds) Groundwater studies—an international guide for research and practice. UNESCO, Paris, pp 1–18
Schuh WM, Klinekebiel DL, Gardner JC, Meyar RF (1997) Tracer and nitrate movements to groundwater in the Norruem Great Plains. J Environ Qual 26:1335–1347
Sharma BD, Mukopadhyay SS, Sidhu PS (1998) Microtpographic controls on soil formation in the Punjab region, India. Geoderma 81:357–368
Sidhu PS, Gilkes RJ (1977) Mineralogy of soils developed on alluvium in the Indo-Gangetic plain (India). Soil Sci Soc Am J 41:1194–1201
Sidhu G, Walia C, Lal T, Rana K, Sehgal J (1995) Soils of Punjab for optimizing land use. Soils of India Series 4. National Bureau of Soils Survey and Land Use Planning, Nagpur, pp 1–75
Singh AK (2003) Water resources and their availability. In: Souvenir, national symposium on emerging trends in agricultural physics, Indian Society of Agrophysics, New Delhi, pp 18–29
Stallard RF, Edmond JM (1983) Geochemistry of Amazon, the influence of geology and weathering environment on the dissolved load. J Geophys Res 88:9671–9688
Statistical abstract of Punjab (2008) Government of Punjab, India
Subramanian V, Saxena K (1983) Hydrogeology of groundwater in Delhi region of India, Relation of water quality and quantity. In: Proceedings of the Hamberg symposium IAHS publication no. 146
Thomas A, Verma VK, Sood A, Litoria PK, Sharma PK, Ravindran KVl (1995) Hydrogeology of Talwandi Sabo Tehsil, Bathinda District (Punjab). J Indian Soc Remote Sen 23:47–56
Toth J (1984) The role of regional gravity flow in the chemical and thermal evolution of groundwater. In: Proceedings of the first Canadian/American conference on hydrogeology, Banff, Alta
Wadia DN (1981) Geology of India. Tata McGraw-Hill, New Delhi, p 508
WHO (1996) Water quality monitoring: a practical guide to the design and implementation of freshwater quality studies and monitoring programmes. E&FN Spon, London
WRD (1982) Hydrogeological studies in the area lying South of Satluz—By Hydrogeological Division, Water Resources Directorate (Govt. of Punjab). Chandigarh, June 1982
Acknowledgments
This research was supported by a grant given by the Government of Punjab, India, through NP-76 scheme. The authors would like to warmly thank Dr. James W. LaMoreaux (Editor-in-Chief) and anonymous reviewers for their valuable advice and suggestion to improve the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuldip-Singh, Hundal, H.S. & Dhanwinder-Singh Geochemistry and assessment of hydrogeochemical processes in groundwater in the southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci 64, 1823–1833 (2011). https://doi.org/10.1007/s12665-011-0989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-0989-9