Abstract
This study at Indian Sundarbans, identifies metal tolerant mangroves with phytoremediation potential by understanding the affect of metal pollution on the community structure of this estuarine ecosystem. Community study indicates that polluted site has lower relative density of the true mangrove and four metal tolerant species (Cryptocoryne ciliata, Heliotropium currasavicum, Hemarthria altissima and Avicennia officinalis) are predominant with 72 % of relative density. Biodiversity indices (Simpson’s index of Dominance and Diversity, Shannon-Weiner index) indicate reduction in diversity and increase in dominance of metal tolerance species at the polluted site. The cluster/components originating after cluster and principal component analysis shows that Cr, Pb, Cd and Ni are metal pollutants of anthropogenic origin. Metal accumulation study is conducted on these four species after assessing the status of metal pollution in their rhizospheres using Ecological risk index, Geoaccumulation index, Enrichment factor and Contamination factor. Bioaccumulation factor emphasized that C. ciliata has a high potential for extracting Cd, Cr and Pb. Bioconcentration factor of Cr is high for A. officinalis, C. ciliata (potentially invasive) and H. altissima (invasive species) whereas translocation factor indicates Cd, Ni and Zn can be translocated to the aerial part of these plants. In addition, H. altissima also transfer Cr and Pb to their aerial parts. This study concluded that C. ciliata could be used as phytoextracter for Cd, Cr and Pb in metal contaminated mangrove ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are coastal forests found in sheltered estuaries, along riverbanks and lagoons in the tropics and subtropics. These are uniquely adapted plant groups showing anatomical, morphological and physiological adaptations to persist in coastal and tidal flats subjected to high salinity stress (Giri et al. 2014). These imitable floral assemblage not only sustain the amphibious ecosystem, but also render numerous economic and environmental services like sequestering carbon (100 tons of CO2 per hectare), stabilizing the soil particles to control soil erosion, act as a hatchery for aquatic fauna and stands as a first wall of defense against natural disasters like tsunami or cyclones (Donato et al. 2011; Dasgupta and Shaw 2015).
The mangrove forests of South and Southeast Asia are facing escalating risk from habitat modification and fragmentation due to environmental and anthropogenic reasons. In an estimate, it is argued that mangroves are disappearing at an alarming rate of 2 % per year, which is higher than any other endangered ecosystems like coral reefs or tropical rain forest (Duke et al. 2007; Alongi 2008). Transboundary forest of Sundarbans, spread over 1 million Ha of which 60 % is in Bangladesh and 40 % in India, are also at significant risk from exposure to pollution from effluent discharge and diversion of land for aquaculture activities (DasGupta and Shaw 2015). It has been apparent from past researches that there is a reduction of 4.38 % of mangrove forest in the Indian part of the Sunderbans between 1999 and 2010 due to these unsustainable anthopogenic practices (Giri et al. 2014).
Mangrove sediment acts as the sink of heavy metal and several researchers are working on the effect of anthropogenic pollutants on the mangrove sediments and halophytes (Defew et al. 2005; Lacerda et al. 1992; Tam and Wong 2000; Natesan et al. 2014). Heavy metal is foremost in imparting deleterious effect to this fragile ecosystem due to their potential to get into the food chain and getting bio-magnified in the process (Defew et al. 2005). Higher accumulation of metals in mangroves is due to the unique physico-chemical properties of the asphyxiated mangrove sediment and elevated quantity of organic matter (Harbison 1986; Silva et al. 1990; Tam and Wong 2000; Defew et al. 2005). This is an important reason behind studying metal accumulation in mangroves in order to identify plants with phytoremediation potential. The mangroves that are in close proximity to the urban developmental areas are exposed to high loads of toxic heavy metals (Defew et al. 2005; Tam and Wong 2000). Moreover mangroves are mostly found in coastal/estuarine areas where it remains in close vicinity to the human habitations exposing to the pollutants released due to anthropogenic activities. The presence of elevated concentration of heavy metals and metalloids especially cadmium (Cd) and lead (Pb) have an enzyme disruptive deleterious effect on the native flora as they replace essential metals in pigments and creates reactive oxygen species (Babula et al. 2008). So mangrove patches exposed to heavy metal pollution have a selection pressure that permits only metal tolerants, bioaccumulators or excluders to proliferate in this stressed environment which could affect the community composition of the halophytes and associative flora.
Metal extraction methods and protocols are highly debated in bioaccumulation studies. Particular extraction methods can extract different form of metals in different mineralogical and soil types (Nirel and Morel 1990; Peijnenburg et al. 2007). Generally CaCl2, Ca(NO3), NH4Ac, Mg-salts, BaCl2 are used as mild extractants; HCl, Sodium dithionite (hyposulphite), Na2S2O4 as reductants; EDTA–DTPA are strong chelating agents; strong acids like H2SO4, HNO3 are also used for metal extraction purposes from sediment matrix but there is no common consensus on the specific metal extraction methodology and reagents in toxicological studies (Peijnenburg et al. 2007). EDTA–DTPA extractable metal is assumed to be the bioavailable part of the total metal and used for some bioaccumulation-translocation studies. Maiti and Jaiswal (2013) have used DTPA, EDTA and aqua regia to separately calculate the bioaccumulation/translocation factors in Typha latifolia, Fimbristylis dichotoma, Amaranthus defluxes, Saccharum spontaenum and Cynodon dactylon found near a thermal power plant in India. But both the extractants (EDTA and DTPA) are found most suitable for calcareous soil to extract “plant-available fractions” (Peijnenburg et al. 2007; Das and Maiti 2008).
Total metal is sometime extracted with varied acid mixtures along with HF to break the silicate lattices for the toxicity studies (Bannerjee et al. 2015). Pseudo-total metal concentration is another extraction method popularly used for evaluating metal concentrations and consequent uptake in higher plants where HF is not used to break the plant unavailable silicate lattice bound heavy metals (MacFarlane et al. 2007). Again bioaccumulation and translocation of heavy metals in rice and maize is studied by Kumar et al. (2015), by analyzing the pseudo-total metal concentration in the soil using HNO3 and HCLO4 in a ratio of 5:1. Pseudo-total metal concentration is also evaluated by Kumar and Maiti (2015), in the cromite-asbestos waste to assess the pollution levels in the area of Chaibasa, India. MacFarlane et al. (2003), have estimated the bioaccumulation of metals in Avicennia marina using HNO3 and H2O2 as an extractant. Zhang et al. (2013), have used 6 ml HNO3 and 1.5 ml HCLO4 to extract pseudo-total metal from 0.2 g of sediment sample to study the bioaccumulation of the metals in the aquatic food chain. MacFarlane et al. (2007) extensively reviewed different methods for metal extraction from sediments and reported that if microwave digester is used (Marchand et al. 2006), method of extraction using HCl + HNO3 combination gives good result. Marchand et al. (2006) have estimated pseudo-total metal of the sediment samples collected from mangrove patches dominated by Avicennia germinalis and associated with other mangroves like Laguncularia racemosa, Acrostichum aureum and Rhizophora mangale at the coastline of French Guiana. Microwave digester is envisaged to be more appropriate for metal extraction studies for better reproducibility and to improve the effectiveness (Ianni et al. 2001; Peijnenburg et al. 2007). This same protocol is adapted in our study to understand the metal contamination in the same ecosystem type for bioaccumulation-translocation studies in mangrove flora of Indian Sundarbans.
In the present study, the areas of Sundurban reserve receiving the discharge of sewage and industrial effluent from Calcutta Metropolitan Corporation (CMC) and tannery industries were selected to screen the tolerant mangrove species according to their degree of heavy metal accumulation. Past researches emphasized that principal component analysis (PCA) and hierarchical cluster analysis (HCA) can be effectively used to identify the polluting metals in the sediment. These methodologies also have been widely used as a tool in interpreting polluting metals (Ghannem et al. 2014; Haris and Aris 2015). Zhang et al. (2013) studied seasonal and spatial dynamics of trace elements in water and sediment from Pearl River Estuary, South China by using PCA and HCA. Similarly, Ogwueleka (2014) used HCA and PCA to identify pollution sources at Kaduna River in Niger State, Nigeria. Hejabi et al. (2011) studied the metal pollution status in water and sediments in the Kabini River, Karnataka, India. Possible sources of heavy metal contamination in lagoons and canal water in Dhaka, Bangladesh have been elucidated by Bhuiyan et al. (2011). Kumar et al. (2015) have used PCA to identify two factors, of which, one denotes the anthropogenic pollutants (Cu, Cd, Pb and Zn) in the surface sediments of Gulf of Kachchh mangrove ecosystem. Singh et al. (2004) used multivariate statistics to evaluate the temporal and spatial variations in water quality in the Gomti River, India.
HCA identifies the similarity–dissimilarity between variables and group them into distinct classes, which give a recognizable pattern. Factor analysis namely PCA is a powerful and popular technique used to reduce dimensionality of the dataset with a large number of interrelated variables. This reduction is possible by transforming the data into new variables called Principle Components (PCs) by orthogonal rotation to get a meaningful groups.
Pollution indexes like Contamination Factors (C f ), Geo-accumulation Index (I geo) along with multivariate statistics are widely used to evaluate the pollution in soil and water bodies. I geo index compares the concentration of a particular pollutant with the background concentration. An et al. (2009) used I geo index to evaluate heavy metals and polychlorinated biphenyls pollution level in the sediment of Yangtze River estuary (China) and found As and Cd pollution is due to the anthropogenic factors. Manjunatha et al. (2001), used I geo values to shows moderate to high pollution of Hg and Cd in the drainage basin of Karwar, in the south west coast of India.
Ecological Risk Index (ERI) has also been a popular method to estimate the level of impact of the polluting heavy metals in the natural ecosystem. Several researchers have used this method to enumerate the impact of metal pollution on the sensitive ecosystem. El-Said and Youssef (2013) have assessed the ecotoxicological impact of heavy metals in the mangrove sediment of the red sea, Egypt. Rahman et al. (2014) have elucidated the potential ecological risk of heavy metal contamination in sediment and water bodies around Dhaka, Bangladesh using ERI.
Identification of metal tolerant species from plant communities affected by metal pollution is a popular method for screening species with phytoremediation potential. Freitas et al. (2004) have identified that Juncus efesus, J. conglomeratus and Scirpus holoschoenus can accumulate Pb and As in high concentration at São Domingos mine in the south east of Portugal, after studying the effect of metal pollution on plant community structure. Hernández and Pastor (2008) have assessed the effect of metal pollution on the grassland biodiversity of abandoned metal mines in the Sierra de Guadarrama, Spain using biodiversity indices, and concluded that Zn has the greatest effect on biodiversity followed by Cd, Cu and Pb. Similar ecological region exposed to varied level of pollution stress may effect the succession pattern, dominance and community structure of the plants favoring pollution tolerant species in stressed areas and normal assemblage in the pollution free area. In this paper it is hypothesized that metal pollution must have selection pressure on a similar community, that would affect the abundance of the species. So, this process could be used to identify/screen pollutant tolerant mangroves by comparing a pollution-affected community with a non-polluted site.
Mangrove plants can bioaccumulate metals from contaminated soils. A plant’s ability to accumulate metals from soils is estimated using the Bioconcentration factor (BCF), which is the ratio of metal concentration in the roots to that in soil (Maiti and Nandini 2006; Yoon et al. 2006). A plant’s ability to translocate metals from the roots to the shoots is measured using the Translocation factor (TF), which is defined as the ratio of metal concentration in the shoots to the roots (Kumar and Maiti 2014). Different species have shown different potential for metal accumulation. Metal concentration is usually higher in mangrove roots than the aerial parts. But effect of metal pollution on mangrove community structure is rarely dealt in past works and this gap is addressed in this current study. Along with that a screening method is also addressed in this work that identifies plants with metal accumulating/tolerating potential in a dynamic ecosystems using simple community level ecological study.
The main aims of the present work are: (i) to study changes in mangrove community structure due to heavy metal pollution by comparing the community structure of a metal polluted site with a control site, (ii) to ascertain the pollution status of the metal affected part in contrast to a control site using geoaccumulation index (I geo), contamination factor (C f ) and enrichment factor (ErF), (iii) to identify metal pollution and their possible sources by using PCA and HCA, (iv) to identify the species that can tolerate highest degree of ecological risk due to heavy metal pollution by using ERI and plant community study, and (v) bioaccumulation potential of the four metal tolerant species are compared using BCF, BAF (Bioaccumulation factor) and TF to screen out mangroves with phytoextraction potential.
Methodology
Study area
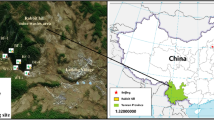
Mangrove community exposed to the municipal sewage and tannery effluent has been selected in the study area to understand the effects of heavy metal pollution on mangrove community structure. The polluted site, Ghushighata (22°31′25.43″N; 88°41′41.71″E) situated in the vicinity of the Kulti River (part of Bidya River) receiving large quantity of domestic sewage and industrial effluent of Calcutta Municipal Corporation (CMC) with the brackish water of Bidya River. Control site was selected amidst the undisturbed mangrove forest (Jharkhali) not influenced by anthropogenic activities (22°00′32.02″N; 88°42′48.23″E) (Fig. 1a–c).
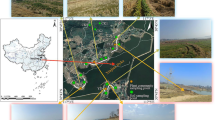
The structure of mangroves community thriving at the high pollution stress zone has a fragmented population of mangrove flora as shown in Fig. 2a. The structure of mangrove community in control site, which is devoid of anthropogenic pollution, is centrally located at the downstream of the Bidya River (Fig. 2b).
Biodiversity assessment
Total 14 quadrate plots (size: 10 m × 10 m) were randomly selected in the natural mangrove patches, out of which, 7 were in polluted site affected by anthropogenic disturbances and 7 plots in control site (devoid of any anthropogenic disturbance). For the assessment of metal accumulation in sediment and their bioaccumulation in mangrove species, 1000 m stretch of Bidya River from the discharge point of effluents was selected. The effluents from CMC and tannery industries at Bantala is released into the Bidya River at Kulti lock gate (up-stream of Bidya River) was taken as the polluted site, affected by the elevated metal pollutions. Bidya River is a tide fed river and it’s down-stream has the unpolluted pristine conserved mangrove patches at Jharkhali, taken as a control site for this study. Mangrove propagules float towards up-stream side by tides and established a community at the riverbanks bathed by tidal waters at the vicinity of the Kulti lock gate. So 7 sites were randomly selected at the polluted site to access the condition of biodiversity exposed to pollution. In contrast, the unpolluted Jharkhali site (control site) is selected for assessing mangrove biodiversity not affected by metal pollution by laying 7 random quadrates. Mangrove habitat is said to show a distinct zonation between 0 and 150 m from the river level to High Tide Level (HTL) and plants association responds to soil quality (Ellison and Fransworth 1993; Duke et al. 1998). However research study of Ellison et al. (2000), at Bangladesh part of Sundarbans, have reported the association of plants resulted due to the random colonization rather than any distinct zonation as believed earlier and study showed that there is no actual zonation in mangroves at any scale specially in respect to Sundarbans. Ellison et al. (2000) also opined that the previous concept of mangrove association in the ecosystem has been based on ‘a priori’ qualitative assumption of zonation without any scientific basis. As the colonization is random and there is no zonation, then the changes in plant abundance would be due to the changes in soil quality parameters and in response to level of pollution in the region, which form the basis of our hypothesis. Still to keep the sampling method unbiased, representative forest community types have been selected along the riverbank between high tide level (HTL) and low tide level (LTL), where the mangrove community lies within 20 m from the riverbank. As the main objective of this study was to screen out mangroves with highest potential of metal tolerance, sites were selected in such a way as to get access to the most polluted part of the ecosystem.
As most of the plants/saplings were within diameter range of <4 cm and sporadic in nature, individual plant of each species was counted for the assessment biodiversity and abundance. The relative density (%) of mangrove species, biodiversity indices (Simpson’s index of Dominance and Diversity, Shannon-Weiner index) were calculated to access the community structure and health of biodiversity along with the heavy metal pollution gradient. Relative density is an estimate of the numerical strength of a species in relation to all the individuals of all the species defined as;
The distribution or dispersion of a individual species is generally estimated as percentage occurrence and defined as;
Both the estimators give an idea of the status of a particular species in relation to the whole community, which is used in this study to understand the changes in distribution of the plants.
Similarity in biodiversity between polluted and control sites can be calculated by Sorenson coefficient of community (CC) (Smith and Smith 2012). This a widely used index to study the similarity of two communities based on the presence/absence of a particular species, calculated as:
where, C is the common species in both the patches (i.e., polluted and control site), S1 is the number of species in polluted site, S2 is the number of species in control site.
Sediment sampling and analysis
Sediment samples were collected by using the Ekman grab sampler (15 cm × 15 cm). The surface sediment (0–30 cm) was collected from the central part of the grab sample in the rhizospheric zone of the plants, showing highest abundance. Seven rhizospheric sediment samples were collected for each plant species for the analysis of pesudototal metal concentration. The pH and electrical conductivity (EC) of the sediment were measured in a soil–water suspension (1:1.5; soil:deionised water) using a pH meter (Cyberscan 510) and conductivity meter (EI 601) respectively (Maiti 2013). The collected sediment samples were air-dried, crushed in a porcelain mortar, sieved through a nylon sieve (pore size 0.45 mm) and kept in polypropylene air tight zip bags for further analysis. Organic carbon (OC) was determined by the rapid dichromate oxidation method (Walkley and Black 1934) and total nitrogen by Kjeldhal method (Jackson 1973).
Digestion of sediment for metal analysis
A 10 ml volume of a mixture of HCl (37–40 %; Rankem), HNO3 (69–71 %; Rankem) and H2O (1:1:1) was added to 100 mg of sediment sample (Marchand et al. 2006). For analysis of Pb, similarly 100 mg of sieved sample was digested with 3 % HNO3 acid (Banerjee et al. 2016). The mixture was placed in a Teflon vessels, previously washed with concentrated nitric acid. Samples were digested in a Microwave Digester (Model: ETHOS One, Italy) at 100 % power with pressure set at 120 psi for 25 min (two cycles), and overall digestion time was 50 min. This was used to estimate the pseudo-metal concentration of the sediment samples. Blank acid mixture was digested in the same way. The same process of digestion was used to digest estuarine sediment certified reference material (LGC 6137) and analyzed using a flame atomic absorption spectrophotometer (FAAS-GBC Avanta, Australia) at the most sensitive resonance wavelength, respective to each element. Digested samples were warmed with 1 % HNO3, filtered (Whatman #42 filter, pore size 2.5 l µm), made up the volume up to 100 ml and stored in polyethylene bottles at 4 °C before analysis at FAAS. Standard Reference Materials (AccuTrace, AccuStandard Inc, USA) were used for the calibration and calibration coefficients were maintained at ≥0.99. The FAAS detection limits of metals are as follows: Cd = 0.0004 mg l−1, Cr = 0.003 mg l−1, Ni = 0.004 mg l−1, Mn = 0.0015 mg l−1, Ni = 0.009 mg l−1, Pb = 0.01 mg l−1 and Zn = 0.0005 mg l−1. Accuracy of the measurements were checked by analysis of an estuarine sediment certified reference material (LGC 6137). Analytical results obtained for reference materials differed by less than 12 %.
Metal analysis in plants
For total metal analysis in mangrove plants, whole plants were collected from each sampling sites effected by metal pollution and that have the highest abundance of the sampled species in the quadrats. Mangrove specimens were incised using acid washed knife into root and shoot, before processing for metal analysis. Samples were washed several times with distilled water and oven dried at 80 °C until a constant weight was achieved. Samples (0.5 g) were digested using HNO3 (69–71 %; RANKEM) and HClO4 (71–73 %; RANKEM) (5:1; v/v) and made up the volume up to 100-ml with 1 % HNO3. Metal concentrations in all the samples were analyzed using flame atomic absorption spectrophotometer (FAAS-GBC Avanta, Australia). Quality assurance (QA) and analytical quality control (AQC) were done using certified reference materials (CRMs): NIST SRM 1547 (peach leaves). Analytical results obtained for reference materials differed by less than 18 %. Inter batch variations were monitored by repeated analysis of selected samples in various analytical batches (less than 10 % relative variation).
Calculation of metal pollution indices
-
(i)
Translocation factor (TF) and bioconcentration factor (BCF) values are used to calculated metal pollution indices. For each species, seven rhizosphere sediment samples were collected (i.e., seven replicates/species). The mean values of these seven replicates are taken for calculation of metal indices. The significant difference between mean values is tested using Duncan’s multiple range test p < 0.05. Bioconcentration factor is defined as:
$${\text{BCF = }}\frac{\text{Metal concentration in Root}}{\text{Metal concentration in Soil}}\quad \left( {\text{Maiti and Jaiswal 2008; Alloway 2010}} \right)$$Translocation Factor (TF) is defined as:
$${\text{TF = }}\frac{\text{Metal concentration in Shoot}}{\text{Metal concentration in Root}}\quad \left( {\text{Maiti and Jaiswal 2008; Alloway 2010}} \right)$$Liu et al. (2007) defined Bioaccumulation factor (BAF) as
$${\text{BAF = }}\frac{{{\text{Metal concentration in Plant}}\left( {{\text{root}}{\kern 1pt} { + }{\kern 1pt} {\text{shoot}}} \right)}}{\text{Metal concentration in Soil}} \quad \left( {{\text{Maiti and Jaiswal 2008; Liu et al}} . {\text{ 2007; Alloway 2010}}} \right)$$-
(ii)
Degree of Contamination of heavy metals
-
(ii)
The contamination factor (\(C_{f}^{{^{i} }}\)) is obtained from a ratio between the measured concentration of the heavy metals in rhizospheric sediment and the pre-industrial reference value for the same metal (Hakanson1980; Rahman et al. 2014). The degree of contamination is defined as the sum of all contamination factors (Table 1).
where C i is the concentration of heavy metal in sediment, \(C_{n}^{i}\) is the standard pre-industrial reference level (in mg/kg): The C f is the ratio of heavy metal concentration in the sediment to the average shell value of earth crust (Hakanson 1980). For background concentration of metals, the average shale values for different metals are used for Pb 20 mg/kg, Mn = 850 mg/kg, Ni 68 mg/kg, Cd 0.3 mg/kg, Cr 90 mg/kg and Zn 95 mg/kg (Turekian and Wedepohl 1961). Hakanson (1980) proposed a method to assess the potential ecological risk for areas under special conservation attention. According to this method, the potential ecological risk factor (\(E_{r}^{i}\)) of a single element can be assessed with a formulae incorporating the toxic response of each metals as given in Table 1.
\(C_{f}^{{^{i} }}\) is contamination factor and T i is the toxic response factor of ith element which is as follows; Pb = 5, Cd = 30, Cr = 2, Zn = 1, Ni = 5, and Mn = 1 (Hakanson 1980). ERI is the summation of the ecological risk factors (Table 1).
This index shed light into the potential of pollution and used to assess the tolerance of different plants for different ecological risk.
The C f is the ratio of heavy metal concentration in the sediment to the average shell value of earth crust (Hakanson 1980). For background concentration of metals, the average shale values for different metals are used for Pb 20 mg/kg, Ni 68 mg/kg, Cd 0.3 mg/kg, Cr 90 mg/kg, Zn 95 mg/kg and, Mn 850 mg/kg (Turekian and Wedepohl 1961). Similarly, geoaccumulation index (I geo) is the assessment of contamination by comparing the current and past concentrations originally used with bottom sediment and were determined using Muller’s equation as given in Table 1 (Ruilian et al. 2008; Banerjee et al. 2016). Both the factors are single, easy to apply and quantitative index that can be applied without considering the grain size and natural geochemical variability. Enrichment factor (ErF) has also calculated by comparing the value of the polluted soil with the control site, to find out how many times, the polluted site have more metal concentration than the control site (Table 1) (Singh et al. 2010).
Statistical analysis
Statistical analyses were carried out to interpret the result by using Data Analysis Package of MS Excel 2007 and SPSS 16 (SPSS Inc. Chicago, USA). Descriptive statistics like one way ANOVA was used to access the variance between the means of a analyzed parameter, between the sampling sites and where significant F value is observed, difference between individual means were tested using DMRT (Duncan’s Multiple Range Test) at the 5 % level of significance. ANOVA is conducted between the rhizospheres of polluted sites (4 species) and the control sites with n = 7 and degree of freedom = 4. This help us to test whether the means of the analyzed parameters are same or different. Metal associations were tested with one tailed Pearson test to get a matrix of correlations between the parameters. HCA was performed on normalized dataset of heavy metals to group similar metal clusters using Pearson coefficient as distance and between group by linkage methods. PCA was applied on the metal concentrations in the sediment to obtain groups of similar metals (Bhuiyan et al. 2011; Rahman et al. 2014). Normalized variables (original variables) were transformed into the rotated components to extract significant principal components (PC) by suppressing the contribution of variables with minor significance, after Kaiser–Meyer–Olken (KMO) test that determines the appropriateness of data reduction through PCA analysis. Furthermore, these PC’s were subjected to varimax rotation with loading coefficients (>0.1) to generate PC factors/groups. There is always a debate between the uses of orthogonal versus oblique rotation methods. Orthogonal rotation classifies the components/factors with the assumption that they are unrelated to each other. But in case of metal clusters unrelated components are easy to read, so varimax orthogonal rotation was used in the current study. Out of several methods, multivariate statistical analyses with PCA, Factor Analysis (FA) and unsupervised pattern recognition (i.e., HCA) are popularly used in source identification of the pollutant (Srivastava et al. 2011; Magesh et al. 2011; Bhuiyan et al. 2011; Ogwueleka 2014; Rahman et al. 2014).
Result and discussion
Effect of anthropogenic pollution on community composition
A total of 24 species were found in polluted and control sites, out of which 33 % are mangrove associates, only one is an invasive saline grass and rest were true mangrove varieties (Table 2). In the polluted sites only 16 species were found, of which relative density of three species, namely C. ciliata (26.2 %), H. altissima (21.83 %) and H. currasavicum (20.58 %) accounts for 69 % of the total recorded species. In contrast, the control site has 24 species and none have a relative density more than 10 % which is also evident from the relative density distribution graph of species between control and polluted site (Fig. 3). True mangrove and endangered species of rhizophoraceae family like B. cylindrica, B. sexangula, R. mucronata and endangered mangrove timber species of Meliaceae family like X. granatum and X. molluccensis was totally absent in the polluted sites (Fig. 3).
Xylocarpus spp, generally grows in areas with balanced flow of fresh water, so it is a possibility that the pollutant laden effluent is counterproductive for the proliferation of the species in the polluted site (Aston and Macintosh 2002). Again the edaphic sub-climax mangrove palm (P. paludosa) was also not recorded in the polluted site. Similarly, salinity tolerant pioneer species S. maritima was also absent in polluted site. The true mangrove varieties recorded in the polluted site also have a low frequency and relative density in contrast with the control site. True mangrove varieties like Bruguiera gymnorrhiza shows 14 % frequency in the polluted site in contrast to 100 % in the control. Same is also true for other Rhizophoraceae (true mangroves) members like Ceriops tagal and Ceriops decandra shows 43 % frequency in polluted site, where as 100 % in the control site. The control site showed predominance of true mangrove varieties having maximum abundance of P. coarctata–S. maritima (pioneer species). Tides prominently effect the colonization of the river banks bringing propagules and nourishing the seedlings, so metal pollution would act as a stressor in this succession process excluding the stress sensitive species. There is a maximum distribution of the seedlings (<1 m height) and saplings (>1 m height) in the river bank showing a high diversity. Avicennia spp, is the only tree species with considerable girth (15–35 cm) and height (10–20 m) was found in the metal polluted areas. Other tree species encountered in the site were E. agallocha, A. marina and S. caseolaris but their girth range between 10 and 20 cm.
Many hypothesis are used to understand the changes in forest community structure in response to environmental factors that include geomorphological factors, climatic variability, physio-anatomical variations due to changing abiotic parameters, biotic factors such as herbivory, predation, parasitism and animal plant interactions (Aston and Macintosh 2002; Smith and Smith 2012). Two mangrove associates (C. ciliata and H. currasavicum) and invasive saline red swamp grass (H. altissima) have proliferated growth and dominance in the pollution effected meta-populations, whereas healthy patches shows a decreased dominance of these species. C. ciliata is also potentially invasive for mangrove ecosystem (Biswas et al. 2007). H. altissima has been one of the foremost invaders that replace the coastal mangroves patches subjected to reduced tidal inundation (McCartney 2009). But as this species was observed to be proliferating in the riverbank, there could be a possibility that it has adapted to metal pollution stress.
Avicennia spp have tolerance to different concentrations of heavy metal pollution. A. marina roots and leaves have the potential for bioaccumulation of Pb, Zn, Cu and it’s roots could be used as metal pollution indicator in soil (MacFarlane et al. 2003). A. officinalis was also found in the polluted site with higher relative density than the control site and have a frequency of 86 % in polluted area unlike 57 % in the control patch. So we analyzed the metal accumulation of the mangrove tree along with three abundant herbs at polluted site. Growth of A. officinalis in close proximity of the bank is shown in Fig. 2a. We identified four species that may have metal tolerant trait, which were the three herb namely C. ciliata, H. altissima, H. currasavicum and one true mangrove tree species of A. officinalis.
Effect of metal pollution on mangrove biodiversity
The effect of metal pollution on mangrove biodiversity was studied by using Simpson’s index of dominance, Shannon-Weiner index and Similarity index. Species diversity is reciprocal to the dominance, so increase in dominance reduces the diversity. While comparing the two patches, it was observed that the dominance is more in the polluted patches and so have lesser diversity (Table 3). The polluted patches have larger dominances (D = 0.16) because only three species (C. ciliata, H. altissima and H. currasavicum) contributed 69 % of dominance value, and Simspon diversity was found lower (0.84) compared to the control site (0.953). Similarly, Shannon-Winner value for polluted site was found lesser (2.03) compared to control site (2.7). Dominant species generally achieve their majority in the ecosystem at the expense of the non-competitive species (Smith and Smith 2012). Sorenson similarity index shows a similar mangrove patch with a value of 0.8 between the two sampling points (polluted and control). As Sundarbans is a prograding delta and heavily influenced by tidal regime, succession is dynamic in this delta (Comeaux et al. 2012).
Physiochemical characteristics of the rhizospheric sediment
Metal and associated parameters of the rhizospheric sediments from vegetation plots with maximum species abundance of the concerned flora are given in Table 4. All the analyzed parameters show a significant difference in means when subjected to ANOVA, between rhizosphere of different plants and control site. pH is lower in all the polluted rhizospheric samples unlike the control site. Low soil EC value of the rhizospheres is due to its distance from the sea, and more influx of fresh water. Organic carbon (OC) and plant available N (Av N), is also lower in the polluted sites indicating a stressed ecosystem with lesser activity of the detritus food chain. Amongst the four plants, A. officinalis rhizospheric sediment has the highest OC and Av N may be because of the decay of its leaves from higher canopy. Cr was found in elevated concentration in all the rhizospheric soil and it was observed that a concentration of 75–100 mg/kg of soil can have toxic effects on plants and in case of Pb the permissible limit was reported for the uncontaminated soil is <20 mg/kg (Kabata-Pendias and Pendias 2001; Alloway 2013). Cd concentration generally ranges between 0.1 and 1.0 mg/kg in unpolluted soil, whereas the polluted rhizospheres have concentration above this limit unlike the control site (Alloway 2013). Mean metal concentrations in the C. ciliata rhizospheric soil follow a order of Mn > Cr > Zn > Ni > Pb > Cd. Mean metal concentrations in rhizospheric soil of A. officinalis, H. altissima and H. currasavicum follows an order of Cr > Mn > Zn > Ni > Pb > Cd. Jharkhali, the control site devoid of anthropogenic pollutants shows Mn > Zn > Ni > Cr > Pb > Cd order of mean metal concentration. Control site have lower concentration of metals except Mn, which is showing similarity with Heliotropium sp and Avicennia sp rhizosphere in polluted site. The reason can be a anthropogenic independent cause of the presence of these two metals in the ecosystem. Zn have a similar trend with other rhizospheres because of it’s geogenic origin, which would be clearly understood in later statistical treatment of the data using PCA and HCA. EC values are higher in control site because of its proximity to Bay of Bengal and the tidal currents bring saline water from the seas.
The metal concentrations of the four polluted rhizospheres and the control site are subjected to Pearson correlation test to find out relations between analyzed parameters elucidated in Table 5. Variables are ten in number and number of samples are 70 (n = 7 × 10 = 70). This gives an insight into the correlation of the parameters in the total environment combining both the polluted and control sites. This will also indicates whether the association is anthropogenic or geogenic ones. Here Cd shows weak correlation with Ni, while strong correlation with Cr and Pb could indicates a similarity of the source of these metals. Reduction in pH increases the mobility of Cd ions hence Cd shows a negative significant correlation with pH (Alloway 2013). Cd also inhibits the microbial activity due to its cytotoxicity so negative significant correlation was observed in the matrix. Organic carbon (OC) has negative correlation with most of the metals (Cd, Cr—significant high correlation, Ni—significant low correlation). OC has an inverse proportionality with metal ions as it binds/adsorb the mobile cations making it less available and mobile (Alloway 2013). Cd, Cr, Mn, Pb, Ni have a negative correlation trend with pH and EC mainly because acidic pH increase the mobility of metal ions, whereas increase in light metals (Na, K) can competitively exclude the availability of metal ions in the soil-capillary water phase. Positive significant correlation of Pb with Ni, Cd and Cr indicates a similar origin of the metals in the sediment. The reason is that at lower pH the metallic cations are more mobile and shows more activity. EC shows a negative significant correlation with Cr. Metals have an increased adsorption rate as pH rises, because of reduced competition with H+ ions and increase in negative surface potential (Alloway 2013). As pH rises there is a hydrolysis to CrOH2+, indicating a negative correlation of Cr-pH in the Pearson matrix (Fischer et al. 2007). As microbial activity is negatively affected by decreasing pH as well as in increasing pH, thus OC and N showed a positive significant correlation.
Identifying the anthropogenic pollutants (heavy metal)
The paradox of locating the source of pollution and to ascertain whether the metal pollution is geogenic or anthropogenic in origin, can be assertained by the component and cluster analysis (Ogwueleka 2014). The dendrogram (between groups) obtained by using HCA with Pearson correlation distance, shows the pattern of metals present in the polluted rhizospheres of the four investigates species (Fig. 4).
There are distinct clusters of Cr, Pb and Cd in one joined by Ni and Mn–Zn in another cluster. The former is showing anthropogenic origin, while the later is geogenic origin. The other soil parameters like pH, EC, OC, N were in a separate cluster.
The Kaiser–Meyer–Olkin (KMO) test is used to determine the appropriateness of data reduction through PCA analysis. The KMO test also measures of sampling adequacy while Bartlett’s test of spericity indicates the strength of correlation among variables. In this study the KMO test result was found above 0.78 and significant Bartlett’s test (significance = 0.001) showed that data reduction can be achieved through PCA. Therefore, PCA test was performed with varimax orthogonal rotation. Here ten variables (pH, EC, OC, N, Cd, Cr, Mn, Ni, Pb and Zn) from four polluted rhizosphres and one-control sites were analyzed through PCA. Seven samples (n = 7) of each variable are taken from each sites (rhizospheres and control site). So in total there are 350 cases. Here four rhizospheric sediment data and that of the control site are taken together for PCA analysis. The reason is that in this study the aggregation of the metals in both the polluted and control sites are considered together to understand their association in same ecosystem (mangrove) with two different stress levels (metal contaminated and uncontaminated). From this it would be evident that the metals with geogenic origin has same trends in both the sites so would come together in the components after orthogonal rotation and same is true for anthropogenic pollutants. In this multivariate analysis identifying the origin of the metals in the ecosystem is the foremost objective. The rotated explanation of the total variations clearly indicate that two components can be solved out of this data reduction and exploratory methods as they cumulatively explained a variation of 75.6 % of the total variance (Table 6). The rotated solution in accordance with the dendogram clusters, which showed three clear groups: Pb, Cr, Cd and Ni in one group, Zn and Mn in another group, whereas other associative soil parameters like OC, pH, EC and available N in the third group (Fig. 5).
The former group was of anthropogenic origin. Major anthropogenic sources are industrial sources like tannery industry accounts for the Cr (used for tanning hides), Pb (present in low quality diesel/petrol fossil fuels) and Cd–Ni (battery, industrial sources, tannery effluent) (Alloway 2013; Mwinyihija 2010). Zn has a geogenic origin in Sundarban waters and as Mn is associated with Zn in the dendrogram and PCA components it could be presumed that both are of the same origin (Guhathakurta and Kaviraj 2000).
Contamination indices
Contamination factor (C f ), shows that there is medium contamination of Cd in rhizospheres of Cryptocoryne sp (1.5) and Hemarthria altissima (2.0) as shown in Fig. 6a. Cd concentration is moderate in rhizospheres of A. officinalis and H. currasavicum. Cr pollution is medium in all the investigated rhizospheres (1.8–2.0) at the polluted site. Rest of the metals (Ni, Zn, Mn and Pb) has no pollution pressure in all the sites. Control site of Jharkhali shows no pollution so substantiated its selection as a reference site in this current study.
I geo is the geoaccumulation index that compares the contamination of a particular metal against a background value normalized by a factor of 1.5 to minimize the lithographic effect and the Igeo values of the metals for the five sites are given in Fig. 6b. The C. ciliata rhizosphere is uncontaminated to moderately contaminate with Cd (1.7), whereas other rhizospheres are moderately contaminated (2.1–2.7). Cr also shows an uncontaminated to moderately contamination in the four polluted rhizospheres (0.3). Control site shows no contamination for all of these metals.
Enrichment factor (ErF), compares the contamination level of the pollutants with a reference control site. Here the Jharkhali site is selected as a control site owing to it’s pristine conserved mangrove patches, lower level of metals in the rhizospheres and as evident from previous indices like I geo and C f . The comparison between the four polluted rhizosphere shows that C. ciliata have Cd level three times of the control, almost four times for both H. altissima and A. officinalis and highest five time for H. currasavicum. Cr contamination in all the rhizospheres ranges around eight times to the control soil and Pb ranges around two times. These three anthropogenic metals (Pb, Cd and Cr) are heavily polluting the Ghushighara site as evident from the enrichment factors.
The assessment of the potential ecological risk of the heavy metal contamination was used as a diagnostic index for water pollution control purposes as a result of the increasing content of heavy metals in sediments that could threaten ecological health (Hakanson1980; Rahman et al. 2014). The Ecological risk factor for individual metals and ERI is given in Table 7. ERI of rhizospheres show a descending trend of: H. currasavicum > A. officinalis > H. altissima > C. ciliata > control site (Jharkhali). But individual Ecological risk factor (E r) highlights the tolerance of the plants to individual metal. In respect Cr all the species have near equal tolerance. A. officinalis showed a higher tolerance to Pb and H. currasavicum to Cd. The E r value of Cd range between 149 and 286 in polluted sites whereas the control site has a value of 50. Here C. ciliata shows Cd pollution in “Considerable Potential Ecological Risk” category and rhizosphere of A. officinalis, H. currasavicum and H. altissima falls under “High Potential Ecological Risk” as per E r values. The Cd pollution in the polluted site may be due to industrial pollution from tannery industry (Mwinyihija 2010). A. officinalis shows highest Pb in their rhizosphere followed by H. altissima and H. currasavicum. But all these anthropogenic pollutants showed higher Ecological risk in the polluted site than the control site indicating anthropogenic impact. In control site ERI value falls under the category of “Low Ecological Risk” whereas H. currasavicum shows “Considerable Ecological Risk” and rest of the polluted sites are in “Moderate Ecological Risk” category.
Several reasons are there for the bioavailability of the naturally derived metals in the sediment core and foremost amongst is the deposition rate, which is dependent on the hydrology of the river (Fernandes et al. 2014). Metal pollution have a far reaching effect on the fragile biodiversity of the region, as metals have a potential to enter into the food chain and get biomagnified (Zhang et al. 2014; Bannerjee et al. 2015). Pb is known to cause toxicity in the people especially children (Alloway 2013). Again Cd have a high bioavailability potential and owing to it’s lesser phytotoxicity, is accumulated in higher levels in plant tissues, which can come into the terrestrial food chain through the process of grazing. Cr has toxic properties to flora and fauna alike. Toxicity of Cr is expressed as xeromorphic foliage, development of large root system, dwarfism, plagiotropism, glabrescence or pubescence, glaucescence and erythrism in plants (Alloway 2013).
Bioaccumulation of metals in the plants
Numerous researches shed light into the fact that the root system of the plants can impart change in their rhizospheric soil by changing pH or exuding H2CO3 or any other means that can affect the bioavailability of the metals. The metal concentration in root and shoot/leaves components of the four plants namely A. officinalis, C. ciliata, H. currasavicum and H. altissima is shown in Fig. 7. None of the species are hyperaccumulators of any metals (Alloway 2013). Hyperacumulators are generally accumulate more than 100 mg kg−1 of Cd, 1000 mg kg−1 of Co, Cu, Ni, Pb and 10,000 mg kg−1 of Mn, Zn in their aboveground body parts (KabataPendias and Pendias 2001).
Root portions of C. ciliata have the highest Cr concentration than any other plants followed by A. officinalis. But the ability of these plants to tolerate heavy metal pollutants could be useful for phytostabilization. Both bioconcentration factors (BCF) and translocation factors (TF) have a wide use to estimate a plant’s potential for phytoremediation purpose (Yoon et al. 2006). Enrichment occurs when a contaminant taken up by a plant is accumulated in the plant instead of getting degraded. For phytoextraction, the translocation of heavy metals to the easily harvestable plant parts, i.e. shoot is required. By comparing BAF, BCF and TF, the ability of different plants of taking up metals from soils and translocating them to the shoots, can be compared (Fig. 8). Tolerant plants restrict the soil–root and root–shoot transfers, and therefore have much less metal concentration in their biomass, while hyperaccumulators actively take up and translocate metals into their aboveground biomass (Yoon et al. 2006; Maiti and Jaiswal 2008).
The most important sources of Cd pollution are metal industry, plastics, tannery industry and sewers (Aksoy et al. 2005; Mwinyihija 2010). In this study it was found that Cd showing high bioconcentration in all the investigated plant species except H. currasavicum. The later species can tolerate highest Ecological risk in respect to Cd but is showing limited transmission to root. Elevated CO2 can also induce more uptake of Cd by increasing the root growth and hence capture more Cd in the root part as observed in poplars and willows (Wang et al. 2012). Glutathione linked phytocheletins are generally responsible for Cd tolerance in many plant species and yeast (Clemens et al. 2002). So deficiency in the same can restricts the inactivation of Cd and sensitivity to this metal. But H. currasavicum was seen to proliferate in the rhizosphere with highest Cd risk yet shows low bioconcentration factor (<1) so can be an excluder for this metal. Cd can easily taken up by the plants through apoplastic and symplastic transport to higher plant parts via vascular tissues and have low toxicity to macrophytes than other pollutants (Alloway 2013; Clemens et al. 2002). A. officinalis and H. currasavicum show a low TF for Cd substantiating the exclusion of this metal in the root parts. Ma et al. (2011), reported a high bioaccumulation (bioenrichment) of Cd in the aerial in the aquatic plant Erigeron bonariensis growing in the Nanjing section of Changjiang River, China.
Cryptocoryne ciliata have the high BCF in respect to Cd, Cr, Pb whereas have high TF value for Cd, Zn, Mn, Pb. Research studies have proved that many heavy metals/metalloids are transported to aerial plant parts by binding ligands, especially sulphur ligands and organic acids (Babula et al. 2008). Otherwise metals are restricted to the vacuolar parts hence restricting its upward transport. Cd has an affinity for Ni–Zn enzyme, therefore very mobile in higher plants as evident from our study. Past researches have revealed that glandular tissues of halophytes can secrete toxic metals, such as Cd, Zn, Pb which can accumulate in their salt glands or trichomes (Manousaki and Kalogerakis 2011). Many salt tolerant extreme halophytic species like Salicornia brachiata, can bioaccumulate Cd and the catalase activity was markedly increased due to contamination of Cd and Ni (Sharma et al. 2010).
Cr is a toxic metal that is available in elevated concentration in all the rhizosphere of polluted sites. Accumulation of Cr has been observed in some aquatic and floating plants, namely Eichhornia crassipes (Babula et al. 2008). Cr rarely gets transported to the leaves and leaves vascular bundles (Alloway 2013), so above ground biomass of the investigated plants shows lesser translocation of Cr. Cr toxicity resulted in stunted growth, poorly developed root system, discolored leaves, leaf chlorosis and it is observed that in uncontaminated soil Cr concentration in the plant tissues rarely exceeds 0.2 mg kg−1 due to it's relative immobility in soil, water and plant (Alloway 2013). But none of the investigated flora shows such defects in their morphology. Only H. altissima (red swamp grass, an invasive species) is an exception showing TF factor >1. This tolerance of H. altissima to the Cr toxicity may be responsible for proliferation of invasive grass in the mangrove patches exposed to metal pollution. Though Pb is rarely mobile in soil–water interface due to its strong affinity to soil particles hence shows low bioavailability, however, it is bioconcentrated in C. ciliata. Mangroves like A. corniculatum also have BCF values >1, but previous researches reported translocation to higher plant parts is rare (Zhou et al. 2011).
Bioaccumulation factor (BAF) shows that C. ciliata have potential to be used as phytoextractors for Cd (BAF >16), Pb (BAF >5) and Cr (BAF >2). Ni and Mn also have BCF >1 for H. currasavicum. H. altissima shows higher BCF values for Pb and Cd. TF of Ni is higher than 1 in A. officinalis, C. ciliata and H. altissima. Cr is slightly soluble in soil–water phase and is not generally taken up by the macrophytes like Pb which is also strongly bound with the soil particles. Cd and Zn are more mobile in plants and has a greater affinity to be transported to the vegetative parts (Kabata-Pendias and Pendias 2001). Ni and Zn also less toxic owing to its presence in several key plant enzymes. Generally Ni is easily translocated to the aerial parts of the plants owing to its affinity in many biochemical pathways of the plant. Hydrogenases, carbon mono-oxide dehydrogenase, methyl coenzyme M reductase are few key plant enzymes dependent on Ni for their proper functioning (Alloway 2013). C. ciliata (Araceae amphibious member with waxy leaves) has highest tolerance to most of the pollutants. Anatomical and physiological process of the plant may be responsible for the same. As seen in E. crassipes, the presence of few unique anatomical adaptations of the aquatic plant like presence of aerenchyma, is responsible for elevated tolerance, which can also be the case for C. ciliata.
Conclusion
This study shows that three anthropogenic heavy metal pollutants namely Cd, Pb and Cr affect the mangrove site exposed to industrial, municipal and tannery effluent. Here the concentration of Cr coming from tannery industries is above the permissible limit and warrants immediate attention. I geo , C f and ErI shows that control site is devoid of pollution pressure. ERI and Er values indicate that all the four species (H. currasavicum, A. officinalis, H. altissima and C. ciliata) have equal tollerence in respect to Cr stress, whereas A. officinalis is tolerent to Cd. Control site (Jharkhali) is devoid of pollution pressure, having a mangrove community structure different from the stressed region. True mangrove varieties are replaced by stress tolerent halophytes and invasive flora in the polluted site. Diversity is also lesser in the polluted site than the pristine mangrove patches of the control site with higher dominance of stress tolerant species. Three herbs namely C. ciliata, H. altissima and H. currasavicum were seen to proliferate in these metal stressed polluted mangrove fragments. A. officinalis, a true mangrove tree species, was also present in higher abundance at that polluted patch. Here C. ciliata (Araceae family) shows high metal extraction potential in respect to Cr, Cd and Pb. Other plants have different levels of tolerance to the pollutants. H. currasavicum have the potential to tolerate highest Ecological risk but shows low uptake level for the metals. It is also observed that metal tolerant mangrove associates and invasive flora was excluding the true mangrove members as evident from low diversity and high dominance in the stressed mangrove community in respect to the control site that is away from the anthropogenic industrial activities. This work also highlights that by understanding the affect of pollution on fragile ecosystem and studying the plant community structure, metal tolerant species with phytoremediation potential could be screened out or identified. So this methodology can be employed in similar habitats in other parts of the globe to find out pollutant extracting/tolerating plant species.
References
Aksoy A, Demirezen D, Duman F (2005) Bioaccumulation, detection and analyses of heavy metal pollution in Sultan Marsh and its environment. Water Air Soil Pollut 164(1–4):241–255
Alloway BJ (2013) Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Environ Pollut 22 (Springer, Dordrecht). doi:10.1007/978-94-007-4470-7_12
Alongi DM (2008) Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuar Coast Shelf Sci 76(1):1–13
An Q, Wu Y, Wang J, Li Z (2009) Heavy metals and polychlorinated biphenyls in sediments of the Yangtze river estuary, China. Environ Earth Sci 59:363–370
Aston EC, Macintosh DJ (2002) Preliminary assessment of the plant diversity and community ecology of Sematan mangrove forest, Sarawak, Malaysia. For Ecol Manag 166(1):111–129
Babula P, Adam V, Opatrilova R, Zehnalek J, Havel L, Kizek R (2008) Uncommon heavy metals, metalloids and their plant toxicity: a review. Environ Chem Lett 6(4):189–213
Banerjee S, Maiti SK, Kumar A (2015) Metal contamination in water and bioaccumulation of metals in the planktons, molluscs and fishes in Jamshedpur stretch of Subarnarekha River of Chotanagpur Plateau, India. Water Environ J 29(2):207–213
Banerjee S, Kumar A, Maiti SK, Chowdhury A (2016) Seasonal variation in heavy metal contaminations in water and sediments of Jamshedpur stretch of Subarnarekha river, India. Environ Earth Sci 75 (3):1–12
Bhuiyan MAH, Suruvi NI, Dampare SB, Islam MA, Quraishi SB, Ganyaglo S, Suzuki S (2011) Investigation of the possible sources of heavy metal contamination in lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environ Monit Assess 175(1–4):633–649
Biswas SR, Choudhury JK, Nishat A, Rahman MM (2007) Do invasive plants threaten the Sundarbans mangrove forest of Bangladesh? For Ecol Manag 245:1–9
Clemens S, Palamgren MG, Krämer U (2002) A long way ahead: understanding and engineering plant metal accumulation. Trends Plant Sci 7(7):309–315
Comeaux RS, Allison MA, Bianchi TS (2012) Mangrove expansion in the Gulf of Mexico with climate change: implications for wetland health and resistance to rising sea levels. Estuar Coast Shelf Sci 96:81–95
Das M, Maiti SK (2008) Comparison between availability of heavy metals in dry and wetland tailing of an abandoned copper tailing pond. Environ Monit Assess 137(1–3):343–350
Dasgupta R, Shaw R (2015) An indicator based approach to assess coastal communities’ resilience against climate related disasters in Indian Sundarbans. J Coast Conserv 19(1):85–101
Defew LH, Mair JM, Guzman HM (2005) An assessment of metal contamination in mangrove sediments and leaves from Punta Mala Bay, Pacific Panama. Mar Pollut Bull 50(5):547–552
Donato CD, Kauffman JB, Murdiarso D, Kurnianto S, Stidham M, Kanninen M (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci. doi:10.1038/NGEO1123
Duke N, Ball M, Ellison J (1998) Factors influencing biodiversity and distributional gradients in mangroves. Glob Ecol Biogeogr Letters 7(1):27–47
Duke NC, Meynecke JO, Dittmannetal S (2007) A world without mangroves? Science 317(5834):41–42
Ellison AM, Farnsworth EJ (1993) Seedling survivorship, growth, and response to disturbance in Belizean Mangal. Am J Bot 80(10):1137–1145
Ellison AM, Mukherjee BB, Karim A (2000) Testing patterns of zonation in mangroves: scale dependence and environmental correlates in the Sundarbans of Bangladesh. J Ecol 88(5):813–824. doi:10.1046/j.1365-2745.2000.00500.x
El-Said GF, Youssef DH (2013) Ecotoxicological impact assessment of some heavy metals and their distribution in some fractions of mangrove sediments from Red Sea, Egypt. Environ Monit Assess 185(1):393–404
Fernandes MC, Nayak GN, Pande A, Volvoikar SP, Dessai DRG (2014) Depositional environment of mudflats and mangroves and bioavailability of selected metals within mudflats in a tropical estuary. Environ Earth Sci 72:1861–1875
Fischer L, Brummer GW, Barrow NJ (2007) Observations and modelling of the reactions of 10 metals with goethite: adsorption and diffusion processes. Eur J Soil Sci 58:1304–1315
Freitas H, Prasad MNV, Pratas J (2004) Plant community tolerant to trace elements growing on the degraded soils of São Domingos mine in the south east of Portugal: environmental implications. Environ Int 30(1):65–72
Ghannem N, Gargouri D, Sarbeji MM, Yaich C, Azri C (2014) Metal contamination of surface sediments of the Sfax-Chebba coastal line, Tunisia. Environ Earth Sci 72:3419–3427
Giri S, Mukhopadhay A, Hazra S, Mukherjee S, Roy D, Ghosh S, Ghosh T, Mitra D (2014) A study on abundance and distribution of mangrove species in Indian Sundarban using remote sensing technique. J Coast Conserv 18(4):359–367
Guhathakurta H, Kaviraj A (2000) Heavy metal concentration in water, sediment, shrimp (Penaeus monodon) and mullet (Liza parsia) in some brackish water ponds of Sunderban, India. Mar Pollut Bull 40(11):914–920
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(8):975–1001
Harbison P (1986) Mangrove muds: a sink or source for trace metals. Mar Pollut Bull 17(6):246–250
Haris H, Aris AZ (2015) Distribution of metals and quality of intertidal surface sediment near commercial ports and estuaries of urbanized rivers in Port Klang, Malaysia. Environ Earth Sci 73:7205–7218
Hejabi AT, Basavarajappa HT, Karbassi AR, Monavari SM (2011) Heavy metal pollution in water and sediments in the Kabini River, Karnataka. India. Environ Monit Assess 182(1–4):1–13
Hernández A, Pastor J (2008) Relationship between plant biodiversity and heavy metal bioavailability in grasslands overlying an abandoned mine. Environ Geochem Health 30(2):127–133
Ianni C, Ruggieri N, Rivaro P, Frache R (2001) Evaluation and comparison of two selective extraction procedures for heavy metal speciation in sediments. Anal Sci 17:1273–1278
Jackson ML (1973) Soil chemical analysis. Prentice Hall, New Delhi
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. CRC Press, London
Kumar A, Maiti SK (2014) Translocation and bioaccumulation of metals in Oryza sativa and Zea mays growing in chromite-asbestos contaminated agricultural fields, Jharkhand, India. Bull Environ Contam Toxicol 93(4):434–441
Kumar A, Maiti SK (2015) Assessment of potentially toxic heavy metal contamination in agricultural fields, sediment, and water from an abandoned chromite-asbestos mine waste of Roro Hill, Chaibasa, India. Environ Earth Sci 74(3):1–17
Kumar G, Kumar M, Ramanathan AL (2015) Assessment of heavy metal contamination in the surface sediments in the mangrove ecosystem of Gulf of Kachchh, West Coast of India. Environ Earth Sci 74:545–556
Lacerda ID, Fernandez MA, Calazans CF, Tanizaki KF (1992) Bioavailability of heavy metals in sediments of two coastal lagoons in Rio de Janeiro, Brazil. Hydrobiologia 228(1):65–70
Laude R (1996) Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76:5–13
Liu W-X, Shen L-F, Liu J-W, Wang Y-W, Li S-R (2007) Uptake of toxic heavy metals by rice (Oryza sativa L.) cultivated in the agricultural soil near Zhengzhou City, People’s Republic of China. B Environ Contam Toxic 79(2):209–213
Ma H, Hua L, Ji J (2011) Speciation and phytoavailability of heavy metals in sediments in Nanjing section of Changjiang River. Environ Earth Sci 64(1):185–192
MacFarlane GR, Pulkownik A, Burchett MD (2003) Accumulation and distribution of heavy metals in the grey mangrove, Avicennia marina (Forsk.)Vierh.: biological indication potential. Environ Pollut 123(1):139–151
MacFarlane GR, Koller CE, Blomberg SP (2007) Accumulation and partitioning of heavy metals in mangroves: a synthesis of field-based studies. Chemosphere 69(9):1454–1464
Magesh NS, Chandrasekar N, Roy DV (2011) Spatial analysis of trace element contamination in sediments of Tamiraparani estuary, southeast coast of India. Estuar Coast Shelf Sci 92(4):618–628
Maiti SK (2013) Ecorestoration of the coalmine degraded lands. Springer, New York. doi:10.1007/978-81-322-0851-8
Maiti SK, Nandhini N (2006) Bioavailability of metals in fly ash and their bioaccumulation in naturally occurring vegetation. Environ Monit Assess 116 (1–3):263–273
Maiti SK, Jaiswal S (2008) Bioaccumulation and translocation of metals in the natural vegetation growing on fly ash lagoons: a field study from Santaldih thermal power plant, West Bengal, India. Environ Monit Assess 136:355–370
Manjunatha BR, Balakrishna K, Shankar R, Mahalingam TR (2001) Geochemistry and assessment of metal pollution in the soils and river components of a monsoon dominated environment near Karwar, southwest coast of India. Environ Geol 40:1462–1470
Manousaki E, Kalogerakis N (2011) Halophytes—an emerging trend in phytoremediation. Int J Phytoremediat 13(10):959–969
Marchand C, Lallier-Verges E, Baltzer F, Alberic P, Cossa D, Baillif P (2006) Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Mar Chem 98(1):1–17
McCartney M (2009) Living with dams: managing the environmental impacts. Water Policy 11:121–139
Mwinyihija M (2010) Main pollutants and environmental impacts of the tanning industry. In: Ecotoxicological diagnosis in the tanning industry. Springer, New York, pp 17–35
Natesan U, Kumar MM, Deepthi K (2014) Mangrove sediments a sink for heavy metals? An assessment of Muthupet mangroves of Tamil Nadu, southeast coast of India. Environ Earth Sci 72(4):1255–1270
Nirel PMV, Morel FMM (1990) Pitfalls of sequential extractions. Water Res 24(8):1055–1056
Ogwueleka TC (2014) Assessment of the water quality and identification of pollution sources of Kaduna River in Niger State (Nigeria) using exploratory data analysis. Water Environ J 28(1):31–37
Peijnenburg WJ, Zablotskaja M, Vijver MG (2007) Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotox Environ Safe 67(2):163–179
Rahman MS, Saha N, Molla AH (2014) Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci 71(5):2293–2308
Ruilian YU, Xing YUAN, Yuanhui ZHAO, Gongren HU, Xianglin TU (2008) Heavy metal pollution in intertidal sediments from Quanzhou Bay. China. J Environ Sci 20(6):664–669
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–656
Sharma A, Gontia I, Agarwal PK, Jha B (2010) Accumulation of heavy metals and its biochemical responses in Salicornia brachiata, an extreme halophyte. Mar Biol Res 6(5):511–518
Silva CAR, Lacerda LD, Rezende CE (1990) Heavy metal reservoirs in a red mangrove forest. Biotropica 22:339–345
Simpson EH (1949) Measurement of diversity. Nat 163:688
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India)—a case study. Water Res 38(18):3980–3992
Singh R, Singh DP, Kumar N, Bhargava SK, Barman SC (2010) Accumulation and translocation of heavy metals in soil and plants from fly ash contaminated area. J Environ Biol 31(4):421–430
Smith TM, Smith RL (2012) Elements of ecology, 3rd edn. Pearson, Glenview
Srivastava PK, Mukherjee S, Gupta M, Singh SK (2011) Characterizing monsoonal variation on water quality index of River Mahi in India using geographical information system. Water Qual Expo Health 2(3–4):193–203
Tam NFY, Wong WS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environ Pollut 110(2):195–205
Turekin KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72(2):175–192
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:29–38
Wang R, Dai S, Tang S, Tian S, Song Z, Deng X, Ding Y, Zou Y, Smith DL (2012) Growth, gas exchange, root morphology and cadmium uptake responses of poplars and willows grown on cadmium-contaminated soil to elevated CO2. Environ Earth Sci 67(1):1–13
Yoon J, Cao X, Xao Q, Ma LQ (2006) Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ 368(2):456–464
Zhang D, Zhang X, Tian L, Ye F, Huang X, Zeng Y, Fan M (2013) Seasonal and spatial dynamics of trace elements in water and sediment from Pearl River Estuary, South China. Environ Earth Sci 68(4):1053–1063
Zhang G, Pan Z, Hou X, Wang X, Li X (2014) Distribution and bioaccumulation of heavy metals in food web of Nansi Lake, China. Environ Earth Sci 73(5):2429–2439
Zhou YW, Peng YS, Li XL, Chen GZ (2011) Accumulation and partitioning of heavy metals in mangrove rhizosphere sediments. Environ Earth Sci 64:799–807
Acknowledgments
The first author is indebted to Indian School of Mines and MHRD, India to provide fellowship (Regestration no: 2013DR0015) and Department of Environmental Science and Engineering, for providing necessary laboratory facilities for this study. We acknowledge the support provided by Mr. T.K. Sinha (senior technical assistant) at the department during the laboratory analysis of the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chowdhury, A., Maiti, S.K. Identification of metal tolerant plant species in mangrove ecosystem by using community study and multivariate analysis: a case study from Indian Sunderban. Environ Earth Sci 75, 744 (2016). https://doi.org/10.1007/s12665-016-5391-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-016-5391-1