Abstract
This study investigated the effects of heavy metals on the species diversity of the Xinjian Dyke Wetland, an ecosystem where reclaimed farmlands are being transformed back into wetlands through the introduction of indigenous plants. The sources of soil heavy metals were analyzed, and correlation analyses were conducted to assess the relationships between heavy metal content and biodiversity indices. The results indicated that (1) the mean contents of Hg, Cd, Cu, Zn, As, Cr, and Pb were higher than the control values, with the content of Hg, Cd, Cu, and Zn exceeding the national standard; (2) the soil heavy metals mainly came from pesticides, chemical fertilizer, transportation, sewage irrigation, and the soil matrix; and (3) Hg and As were not significantly correlated with the diversity indices, but there was a highly positive correlation for Cu, Cr, and Pb, and a significant negative correlation for Zn and Cd. Collectively, our findings indicated that heavy metals have different effects on the plant species diversity inXinjian Dyke reconstruction area. The ecological restoration of wetlands from reclaimed farmlands should reasonably increase tolerant species and maximize the ecological niche differentiation of the species. Moreover, functionally redundant species should not be planted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Caizi Lake wetland is an important freshwater ecosystem located in the middle and lower reaches of the Yangtze River, which is an important wintering habitat for Anser fabalis and Cygnus columbianus along the migration route from East Asia to Australia, as well as for East Asian endangered species such as Grus monacha and the Ciconia boyciana (An et al. 2021). Therefore, strengthening the protection of the Caizi Lake wetland is critical for the conservation of the ecosystem and species diversity. The Yangtze-to-Huaihe Water Diversion Project was launched in December 2016 and the Caizi Lake is an important node lake. The project carries water northward through the Caizi Lake, with 60% of the total pilotage volume and more than 85% of the spontaneous pilotage volume flowing through the lake. After the completion of the project, the diversity of wetland plants and the survival of migratory birds in the tidal flat would be impacted as the water level rises and the wetland area decreases (Li et al. 2019a, b). In the face of the current reduction of wetland area and wetland destruction, the Chinese government has mandated the restoration of wetlands from farmlands to promote the protection of wetlands and the habitat of migratory birds. Particularly, the conversion of farmlands to wetlands in the Caizi Lake wetland should be prioritized.
Species diversity indices serve as indicators of the community structure and function and are therefore used as sensitive indicators of wetland community structure, function, migratory bird habitats, and ecosystem stability (Zheng et al. 2019). Understanding the species diversity of wetland vegetation is helpful to assess the community type, community succession stage, habitat quality, and ecosystem stability (Garbisu et al. 2020). Particularly, this study focuses on the species diversity of reclaimed farmlands that are being converted back into wetlands. Our findings indicated that the soil heavy metal content in the study area exceeded the screening value established by the Chinese GB15618-2018 standard (GB 15618–2018 n.d.). Therefore, pesticides and heavy metal stress could affect the complexity and specificity of regional plant diversity (Yang et al. 2020). Environmental pollutants such as pesticides and heavy metals are important factors to consider when planning the restoration of the Caizi Lake ecosystem. Therefore, it is important to understand the distribution and source of heavy metals in degraded or reduced farmlands, as well as to explore the influence on species diversity and plant tolerance mechanisms. Most studies on soil heavy metals have focused on biological effectiveness (Cyriac et al. 2021; Zuran et al. 2019), heavy metal toxicity (Matayoshi et al. 2021; Yang et al. 2021), and ecological risk to individuals (Ekengele et al. 2017; Tóth et al. 2016). Moreover, studies on group effects largely focus on the community diversity of microbes, aquatic organisms, animals, and mine scrap lands (Deng et al. 2021; Jia et al. 2019; Yang et al. 2020). However, few studies have evaluated the influence of heavy metals on the diversity of plant communities in degraded or reduced farmlands. Therefore, this study evaluates wild hygrophytes in Xinjian Dyke in the Caizi Lake National Wetland Park and discusses the PMF source apportionment of heavy metals and its influence on the species diversity of the plant community. This study thus provides a reference for the ecological restoration of reclaimed farmlands for the Yangtze-to-Huaihe Water Diversion Project.
Materials and methods
Study area
The Caizi Lake wetland is a typical silted shallow freshwater lake wetland located in a subtropical monsoon climate region, with abundant rainfall and a pleasant climate. The wetland is located in the north bank of the Yangtze River, at the junction between Yixiu District, Zongyang, and Tongcheng County, Anqing City, Anhui Province (117°01′–117°10′E, 30°45′–30°56′N), with an average elevation of 9.1 m. The flat water area of the wetland is 16,667 hm2, the wet water area is 24,230 hm2, and the dry water area is 14,520 hm2 (Gao et al. 2011). An analysis of the plant and bird species during the soil and plant sampling in this study revealed that there are 402 species of plants and 315 species of birds in Caizi Lake. The species and number of wintering waterbirds have been stable for many years, with 30–40 species and more than 20,000 individuals (Wang et al. 2018).
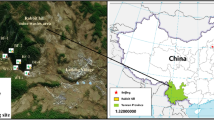
Xinjian Dyke is a restoration and reconstruction area that is less than 1 km away from the migratory bird gathering point. In view of the impact of the Yangtze-to-Huaihe Water Diversion Project on the habitat reduction of migratory birds, it is necessary to return farmland to wet. But more than 85% of the district is occupied by farmland, and rice, wheat, and vegetables were planted on it (Fig. 1); therefrom, the lots not occupied by human activities present narrowly transects and only four which are distributed in the river highlands and basically have no spatial heterogeneity. The damage of human activities is very serious in Xinjian Dyke. Wild plant communities are distributed along the benchland in a band-like plaque in land-lake ecotones. Wild vegetation species have distinct seasonal characteristics with fewer species before June, increasing from June to September, and decreasing again to only a single or a few species after October. Generally, very few species dominate each plaque.
Soil sampling and determination
Soil samples were collected in February 2021 in four 200 m × 20 m strip zones (No. YD1, YD2, YD3, YD4) (Fig. 1) locating on land and water alternating zones because of the large area of farmland and marsh restriction in this study. Moreover, three sampling sites were arranged in the central region of each strip zone, resulting in a total of 12 soil sample sites. Meanwhile, three sample sites were selected in the north forestland free of exogenous pollution as a control (CK) (Fig. 1). Soil samples with a 20-cm depth were collected with a columnar soil sampler. Each sampling site was collected three times and mixed, and 2 kg of fresh samples was gathered through a quad method (Sun et al. 2021). The samples were then naturally air-dried for testing after removing impurities such as animal and plant residues. Next, 100 g of sample was passed through a 200-mesh nylon sieve and digested by HCl-HNO3-HF-HClO4 solution in a screw-cap polytetrafluoroethylene digestion vessel. The samples were sent to a certified laboratory to determine heavy metal concentration after they were pretreated with 0.45-μm microporous filter membrane. Concentrations of Cd, Cr, Pb, Cu, As, and Zn were determined using an inductively coupled plasma-atomic emission spectrometry (Agilent 7900 ICP-MS, Agilent, USA) with the lowest detection limit of 0.07 mg/kg, 2 mg/kg, 2 mg/kg, 0.5 mg/kg, 0.6 mg/kg and 7 mg/kg, respectively, according to HJ 803–2016, that of Hg was determined using a hydride generation-atomic fluorescence spectrometry (HG-AFS, HD-CG10 made in Shandong Holder, China) with the lowest detection limit of 0.002 mg/kg according to HJ/T 166 -2004. The pH of the soil was measured with a soil-to-deionized water ratio of 1:2.5 and a minimum pH value display unit of 0.01 (Seven Compact™, Mettler-Toledo, Switzerland).
Plant diversity survey
The survey was conducted from October 11 to 14, 2021. A tidal flat survey method was adopted to estimate community type and species richness based on the geographical and topographical characteristics of the study area. Soil samples can be taken every 3 years according to HJ/T 166 -2004. Through the analysis on the local development status and 5-year plan, the major pollution sources are basically unchanged in the first and last 5 years. The soil data measured in this study can reflect the situation in last recent years. Based on this, plant samplings were selected in the same four strip zones as soil sampling. The plant community was distributed along the strips, and 1 m × 1 m quadrats were set according to the distribution characteristics of the plants in the strip zone. Furthermore, 10 m × 10 m quadrats were set for plots with shrubs to statistically analyze the species composition (GB T 30363-2013 n.d.; HJ 710.1.11–2014 n.d.), abundance, coverage, and dominant species of herbaceous and shrub communities (Xie et al. 2014). Scattered and artificially grown plants were not evaluated in this study. Twelve representative wild plant communities were investigated. The strip of each type of community is illustrated in Fig. 2.
Calculation and statistical analysis
PMF model
Positive matrix factorization (PMF) models are relatively advanced receptor models that are often used in ecological assessment. Particularly, this approach has been widely recognized and applied due to its low dependence on source spectrum information. In this model, the original matrix (Xij) is decomposed into the source component spectrum matrix (fkj), sharing rate matrix (gik), and a residual matrix (eij) (Sofowote et al. 2008). The calculation process does not require inputs from the source fingerprint, as shown in Eq. (8):
where Xij is the content matrix in the receptor and represents the content of element j in soil sample i (mg·kg−1); p is the number of factors (i.e., generating sources); gik is the factor contribution matrix, representing the content of j element in source k (mg·kg−1); fkj is the spectral matrix of the factor component, representing the content of j element in the k component of the source (mg·kg−1); eij is the residual matrix, which is calculated according to the definition of the objective function.
The minimum value of the objective function Q in the PMF model is then calculated as shown in Formula 2:
where n represents the number of samples, m represents the number of species, and Uij represents the uncertainty of the j species in sample i.
If the heavy metal content is less than or equal to the method detection limit (MDL), the uncertainty (UNC) calculation formula is shown in Formula 3:
If the heavy metal content is greater than MDL, the calculation formula of UNC is shown in Formula 4:
where c is the species content (mg·kg−1), RSD is the relative standard deviation, and MDL is the given limit of species detection (mg·kg−1).
Importance value
The importance value (IV) is a comprehensive quantitative indicator reflecting the role and position of a certain species in the forest community (GB T 30,363–2013), which is calculated based on various growth forms:
where,
Relative abundance = a plant’s abundance/sum of all plants in the sample plot × 100%
Relative dominance = a plant’s dominance/sum of dominance values of all plants in the sample plot × 100%
Relative frequency = a plant’s frequency/sum of frequency values of all plants in the sample plot × 100%
Plant diversity indices
Plant diversity is frequently characterized using the Shannon–Wiener, Margalef, and Simpson indices (Mulya et al. 2021, GB/T 30363-2013 n.d.).
where H is the Shannon–Wiener index; S is the number of species present in the community; Pi = ni /N, where ni is the number of individuals of a species and N is the total number of individuals of all species in the community. DM is the Margalef index; DS is the Simpson index.
Statistical analyses
The ArcGIS software was used to draw the spatial distribution map of heavy metal content and plant communities. Heavy metal source analysis was conducted using the EPA PMF 5.0 software. Pearson correlation analysis and regression analysis (RA) were conducted using SPSS 21.0. The standard deviation is shown as an error bar on the graph, and significance thresholds were set at P<0.05 (significant) and P<0.01 (highly significant). The plant species in this study were named according to the global biodiversity information facility (GBIF).
Results
Distribution characteristics and source analysis of heavy metals in soil
The Hg content in the soil of Xinjian Dyke ranged from 2.14 to 2.23 μg/kg, with HgYD2 (the Hg content of strip YD2) exhibiting the maximum value and HgYD3 exhibiting the minimum (Table 1, Fig. 3); the As content ranged from 27.32 to 35.33 μg/kg, AsYD1 was the maximum and AsYD3 was the minimum; the Cd content ranged from 1.38 to 2.4 μg/kg, CdYD2 was the maximum and CdYD4 was the minimum; the Cr content ranged from 115.83 to 134.32 μg/kg, CrYD4 was the maximum and CrYD2 was the minimum; the Pb content ranged from 54.33 to 69.44 μg/kg, PbYD4 was the maximum and PbYD3 was the minimum; the Cu content ranged from 50.11 to 86.89 μg/kg, CuYD4 was the maximum and CuYD1 was the minimum; the Zn content ranged from 205.76 to 223.42 μg/kg, ZnYD3 was the maximum and ZnYD4 was the minimum.
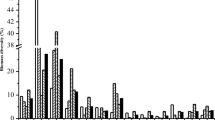
The PMF model was originally used in the analysis of air pollution sources and has been applied for the assessment of water and soil pollution in recent years (Salim, et al. 2019; Meng, et al. 2021). The average contents of the seven heavy metals were all higher than the CK values, and the contents of Hg, Cd, Cu, and Zn were higher than the maximum allowable values established by the GB15618-2018 (Table 1). This demonstrated that although the distribution difference of soil heavy metals in the study was not obvious, it was affected by human activities to a certain extent. Therefore, PMF sources were analyzed for seven heavy metals. After 20 iterations, the minimum q (robustness value = 128.5) was obtained. When the number of factors was 5, the model had the smallest difference between the objective function and objective function true value, meaning that the calculation scheme was the most stable. Therefore, the source of heavy metals was locked into 5 factors. Fitting results obtained by source analysis are shown in Fig. 4. The Hg element fitting curve R2 was 0.73, and those of the other elements were greater than 0.9, which indicated that the overall accuracy of the PMF model was relatively good. Therefore, this model could fully explain the information contained in the original data, thereby meeting the needs of source analysis. The output results of the model operation are shown in Fig. 5. The source component spectrum overlap was high, indicating the high similarity of the results. Previous studies have shown that heavy metals originate from a variety of industrial sources such as mining and fossil fuel burning, nonferrous metal smelting, machinery manufacturing and electroplating, instrument fabrication, organic synthesis, and manufacturing (Chaturvedi et al. 2018; Kartal et al. 2006; Loska and Wiechuła, 2003; Men et al. 2018). These industrial sources were excluded after field investigation in this study. Additionally, coal heating emission sources were not considered and no film has been used in agricultural land in recent years.
Spectrum of heavy metal sources in four belt strips. F1 is the traffic source; F2 is the agricultural non-point source of organic fertilizer emissions; F3 is the mixed source of soil parent material and organic fertilizer; F4 is a mixture of vehicle exhaust and organic fertilizer; F5 is the soil parent material source
As indicated in Table 2, the main contributing elements of F1 were Cd and Pb, with a contribution rate of 83.9%, whereas the contribution of Hg was 0. The external sources of Cd include industrial sources, fossil fuel combustion, and pesticides, among others. Pb mainly originates from industrial raw material production, metal mining, and transportation emissions (He et al. 2013; Li and Jia 2018). After the investigation, industrial sources such as mining, fossil fuel combustion, nonferrous metal smelting, machinery manufacturing, electroplating instruments, and organic synthesis were excluded. There were no coal heating emission sources and no film had been used in farmlands in recent years. Moreover, the roads were mainly internal roads with less traffic pollution. Therefore, F1 was the pesticide source.
The main contributing elements of F2 were Cu and Hg, which contributed to 73.7%, whereas Cd made an extremely low contribution. Cu mainly comes from mining, smelting processes, fossil fuel combustion, and organic fertilizers. As is mainly related to fossil fuel combustion, soil-forming matrix, pesticides, and thin film use (Cai et al. 2015). Therefore, F2 was a mixed source of soil-forming matrix and organic fertilizer.
The main contributing elements of F3 were Zn and Pb, which contributed to 64.2%, and Cd had an extremely low contribution. The main sources of Zn are zinc mining, smelting processing, and industrial emissions, as well as the use of livestock manure (Xu et al. 2014; Yuan et al. 2019). The production of Pb is related to mineral mining, smelting and processing, industrial emissions, and transportation emissions (Cloquet et al. 2006). Therefore, F3 was an agricultural source of livestock manure.
The main contributing elements of F4 were Cu and Pb, which contributed to 66.5%. Therefore, F4 was an organic fertilizer pollution source.
The main contributing elements of F5 were Hg and Cr, which contributed to 75.7%, whereas the contributions of Cu and Pb were 0. Hg mainly comes from industrial sources, sewage irrigation, household garbage, and coal burning for heating (Wu and Zhang 2010; Talukder et al. 2021). Cr is a typical product of the plating process and is also derived from diagenesis (Du et al. 2008), which was also shown as such in previous studies (Xu et al 2015). Therefore, F5 is a mixed source of sewage irrigation, domestic waste, and soil-forming matrix.
In summary, the main source of Cd was pesticide; Cu and As originated from a mixed source of the soil matrix and organic fertilizer; Zn from agricultural livestock manure; Pb mainly from pesticide, organic fertilizer, and livestock manure; Hg and Cr from sewage irrigation, domestic waste, and the soil matrix.
Analysis of the community species composition
The structure of plant communities at different taxon levels (family, genus, and species) can serve as an indicator of the responses of these communities to various environmental factors. There were 25 species of wild plants belonging to 22 genera and 13 families in the Xinjian Dyke wetland, among which Gramineae and Compositae accounted for 24% and 16% of the total species, respectively (Table 3). The most common species were Zizania latifolia (Griseb.) Stapf, Cynodon dactylon (L.) Pers., Eccoilopus cotulifer (Thunb.) A.Camus, Monochoria vaginalis (Burm.f.) C.Presl, Artemisia annua L., Erigeron annuus (L.) Pers., Pterocypsela elata (Hemsl.) C.Shih, Ranunculus polii Franch. ex Hemsl., Polygonum criopolitanum Hance, Polygonum perfoliatum (L.) L., Broussonetia papyrifera (L.) Vent., Morus alba L., Calystegia sepium (L.) R.Br., Cerastium glomeratum Thuill., and Pyrus betulifolia Bunge.
The community was divided into eight types based on the IV values: Z. latifolia community (Two), E. cotulifer community (Two), C. dactylon/Setaria viridis (L.) P.Beauv. community, Kalimeris indica (L.) Sch.Bip./C. dactylon community, Astragalus sinicus L. community, R. polii community, B. papyrifera community (Two), and P. betulifolia community (Two). With the highest IV value as the dominant species and an IV of 15 as the companion species, the shrub and herb community similarities were very high. The plots showed a single species as the absolute dominant species. The dominant species with the highest IV value was the shrub P. betulifolia (IV = 33.6%), and the highest IV in the herb communities belonged to Z. latifolia (29.45%). The communities were mostly consociations, the six communities with the dominant species of Z. latifolia, B. papyrifera, and P. betulifolia contained 3–4 companion species, the A. sinicus community had 2, and the K. indica community had 1. In contrast, the R. polii community, E. cotulifer community, and C. dactylon/S. viridis communities had no companion species. The important value (IV) is a characteristic indicator of community building species and dominant species, reflecting the functional status of species in the community. The B. papyrifera community and Z. latifolia communities had 3–4 companion species and higher IV, with no more than 7 community species, two E. cotulifer communities, the K. indica/C. dactylon communities, and the K. indica/S. viridis communities had lower IV and only had 0–1 companion species. However, the number of species exceeded nine. This was because higher importance values or more dominant species will occupy more niche temporal or spatial dimensions, and the inhibition of non-companion species growth reduced species diversity.
Analysis of the community diversity index
The diversity index reflects the community species diversity. The species diversity of the four strip zones was compared using different indices.
Shannon–Wiener index
The H values of the four belt strips were the highest in the E. cotulifer-YD4 community and lowest in the B. papyrifera community-YD2 (Fig. 6). The species diversity of the community exhibited the following descending order according to the H value: E. cotulifer community-10/YD4 > E. cotulifer community-3/YD1 > K. indica/C. dactylon community-7/YD3 > A. sinicus community-12/YD4 > C. dactylon/S. viridis community-8/YD3 > R. polii community-11/YD4 > B. papyrifera community-9/YD3 > P. betulifolia community-6/YD2 > P. betulifolia community -4/YD2 > Z. latifolia community-1/YD1 > Z. latifolia community-5/YD2 > B. papyrifera community-2/YD1.
Simpson index
The DS values of the four strip zones were all the highest in the E. cotulifer community-YD4 and the lowest in the B. papyrifera-YD2 community. The community species diversity exhibited the following descending order based on the DS values: E. cotulifer community-10/YD4 > E. cotulifer community-3/YD1 > K. indica/C. dactylon community-7/YD3≈ A. sinicus community-12/YD4 > C. dactylon/ S. viridis community-8/YD3≈ R. polii community-11/YD4 > B. papyrifera community-9/YD3 > P. betulifolia community-6/YD2≈ Z. latifolia community-1/YD1 > P. betulifolia community-4/YD2 > Z. latifolia community-5/YD2 > B. papyrifera community-2/YD1 (Fig. 6). The above-described analyses indicated that the change trend of the DS value of the four strip communities is basically the same as the H value. Correlation analysis showed that the H and DS values were significantly correlated (p < 0.01, r = 0.969) (Table 4).
Margalef index
The highest DM value of the four belt strips was that of the E. cotulifer community-YD4 community, whereas the lowest DM value was that of the Z. latifolia community-YD1. According to the DM value, the community species diversity exhibited the following descending order: E. cotulifer community-10/YD4 > K. indica/C. dactylon community-7/YD3 > E. cotulifer community-3/YD1 > C. dactylon/S. viridis community-8/YD3 > R. polii community-11/YD4 > B. papyrifera community-9/YD3 > A. sinicus community-12/YD4 > P. betulifolia community-6/YD2 > B. papyrifera community-2/YD1 > P. betulifolia community-4/YD2 > Z. latifolia community-5/YD2 > Z. latifolia community-1/YD1. However, the change trend was inconsistent with that of the Shannon–Wiener and Simpson indices. Correlation analysis showed that the DM value was not correlated with the H and DS values (Table 4).
Discussion
Relationship between index change and species diversity
The Shannon–Wiener index includes richness and evenness and can therefore comprehensively reflect the diversity and evenness of the community (Wilsey and Stirling 2007). A larger Shannon–Wiener index means that there are more plant species in the community, and therefore, there is a higher species richness. At the same time, the uniformity of the individual distribution among species increases the community diversity. The Margalef index reflects the species richness, whereas the Simpson index is more closely related to frequency and species uniformity. Zhang et al. (2015) reported that species richness takes the number of species as an indicator, implying that all species contribute equally to the community. However, plants do not have equivalent functions in a community (Zhang et al. 2015; Adrián et al. 2021), and therefore, species richness alone cannot explain the functional aspects of plant communities. Our findings suggested that the Simpson and Shannon–Wiener indices of the plant community in the study area remained consistent. The developing wetland community has a more uniform succession trend, and therefore, the uniformity of the individual distribution among species was an important determinant of community diversity. The Margalef index was strongly complementary to the Simpson and Shannon–Wiener indices. After comparing the community diversity index in the same zone (Fig. 6), the H values and DS values of the three clusters in the YD1 strip change trends were consistent. The highest value was observed in the E. cotulifer community, the lowest value was observed in the B. papyrifera community, with both showing significant differences (p < 0.05). The highest DM value was observed in the E. cotulifer community, whereas the lowest value was observed in the Z. latifolia community, all communities showed significant differences (p < 0.05). Among the H and DM trends of the three clusters in the YD2 strip, the highest value was observed in the P. betulifolia community, whereas the lowest value was observed in the Z. latifolia community, and all of these values showed significant differences (p > 0.05). The lowest DS value was observed in the Z. latifolia community; however, there was no significant difference between the two P. betulifolia communities (p > 0.05). Among the H and DM trends of the three clusters in the YD3 strip, the highest value was observed in the K. indica/C. dactylon community, whereas the lowest value was observed in the B. papyrifera community, all of these values showed significant differences (p > 0.05). The highest DS value was observed in the K. indica/C. dactylon community. However, no significant difference was observed between the R. polii community and the B. papyrifera community (p > 0.05). The changing trend of the H values and DS values of the E. cotulifer community in the YD4 strip was as follows. The highest value was observed in the E. cotulifer community. There were no significant differences between the A. sinicus community and the C. dactylon/S. viridis community. The highest DM value was observed in the E. cotulifer community, whereas the lowest value was observed in the A. sinicus community, both with significant differences (p > 0.05).
The trend of the H and DS values within the YD1 and YD4 strips was the same, showing that the Shannon–Wiener index was closely related to the enriched species. The H and DM values exhibited identical trends in the clusters within the YD2 and YD3 strips, indicating that species richness contributes significantly to the Shannon–Wiener index. From the same zone of species diversity index comparison and the overall comparison of the diversity of four strips, the convergence and specificity of the three indices have some conflict. This uncertainty indicated that the diversity indices were not only related to biological factors such as abundance, richness, dominant species, and individual growth, but also to abiotic environmental factors (Fu et al. 2020; Jin et al. 2011; Zhang et al. 2016).
Effect of heavy metal stress on plant diversity
The survey in February 2021 indicated that the high degree of anthropogenic disturbance was evidenced by the high degree of community similarity and small patch aggregation and distribution in the study area. The abiotic factors that affect the diversity of terrestrial plants in the study area include water level, water quality, grazing, mowing, the Yangtze-to-Huaihe Water Diversion Project, and soil quality. The four belt strips were all located in the alternate areas of land and water, and therefore, the effects of the difference in water level and water quality can be excluded. There was no difference in grazing and mowing activity intensity between the four strips. The research area was carrying out the Yangtze-to-Huaihe Water Diversion Project, which had little interference in plant diversity, and sampling in this study was conducted away from the engineering area. Therefore, the above factors did not contribute to the differential variation of the community species diversity. The factors affecting soil quality included artificial factors such as pH, salinity, nutrients, soil texture, and structure. The pH value of the four strips was 5.37–6.63, the soil was weakly acidic, the soil organic matter (SOM) was 1.09–1.88%, with a variation coefficient of 0.112 (previous investigation of this study, unpublished) and weak dispersion. Moreover, there was a small spatial difference in soil texture and structure, with pollutants likely being the main influencing factor. Therefore, this study explored the impact of heavy metal pollution on species diversity.
Table 4 summarizes the results of the correlation analysis of heavy metal content and plant diversity indices. There was no significant correlation between Hg and the three diversity indices. Cr was positively correlated with the Shannon–Wiener and Simpson indices (P < 0.01), with correlation coefficients of 0.741 and 0.762, respectively. As was positively correlated with the Margalef index (P < 0.05, r = 0.652). These findings indicated that as the content of Cr and As in the strip increased, the corresponding diversity index also increased. The positive effect of these metals on plant diversity could potentially be attributed to a combination of factors including SOM and other soil physical and chemical parameters, as well as the biological characteristics of plant species, microorganisms, and other neglected environmental factors (Ma et al. 2022). Alternatively, the presence of heavy metals may reduce the advantages of a single plant, increase competition among plants, and promote plant coexistence (Garbisu et al. 2020; Lalor et al. 2004; Li et al. 2019a, b). The positive effect of heavy metals on species diversity might be related to their complementary effect or selection effect on plant species. The complexity and specificity of regional plant diversity increased with increasing soil heavy metal content, which may be due to the use of supplementary resources by some species to promote inter-species diversity. It may also be that increasing species numbers increases the likelihood of the random introduction of high-yielding species (Zhang et al. 2015). Additionally, the existence of some species with heavy metal enrichment ability alleviates the toxic stress of heavy metals on other species, thereby improving community species diversity.
In contrast, Cd and Zn were negatively correlated with the diversity index. The correlation coefficient between Cd and the Shannon–Wiener index was − 0.666 (P < 0.05), whereas that between Cd, Zn, and the Simpson index was − 0.636 and − 0.594 (P < 0.05), respectively, indicating that as Cd and Zn content increased, the corresponding diversity index decreased. The content of Cd and Zn in this study exceeded the GB 15,618–2018 guidelines and exhibited different degrees of toxicity and ecological risk to individual plants and their populations. Therefore, high concentrations of these heavy metals had negative effects on community diversity indices. The content of Hg exceeded GB 15,618–2018 but was not correlated with the community species diversity index. The toxicity of Hg on individuals and populations may be inhibited by other environmental factors, thus indirectly reducing the negative effect of Hg on species diversity. This hypothesis was mentioned in Connell’s theory of intermediate interference (Connell 1978). Fantaw et al. (2006) demonstrated that the distribution characteristics of vegetation were greatly influenced by the soil and other environmental factors, in addition to their mutual influence.
Four strips had a high Cu background value (66.40 mg/kg) and were above the standard value (50 mg/kg). The Cu contents of the YD1 and YD2 strips were lower than the background values, whereas the Cu contents of the YD3 and YD4 strips were higher than the background values. Cu occurred mainly as a mixed source of the soil matrix and organic fertilizer, which made the Cu distribution uneven. Deng et al. (2021) found that the Cu concentrations under the plant tolerable conditions were significantly positively correlated with plant height, stem thickness, crown diameter, and plot coverage, which promotes the species diversity of the community. Therefore, the plant species in the study area developed a certain tolerance to Cu, and Cu showed a very significant positive correlation with the Shannon–Wiener and Simpson indices (p < 0.01; the correlation coefficient r was 0.983 and 0.970, respectively). After analysis, the Cu content of the four strips exceeded the Chinese GB 15,618–2018 standard value (Table 1). Although Cu is a trace element needed for plant growth, the growth and distribution of plants can be limited if the Cu content exceeds the upper limit of the plant’s nutrient tolerance. This might partly explain the negative correlation between Cu and the Margalef index, which indicated that Cu had a certain inhibitory effect on the community species composition and limited the community richness.
Conclusion
Plant communities often exhibit a single absolute dominant species, with dominant and associated species with high IV occupying key ecological niches. However, this inhibits the growth of non-associated species and thus reduces the community species diversity. Source analyses of seven heavy metals by PMF in the wetland indicated that the seven heavy metals originated from pesticides, fertilizers, livestock manure, sewage irrigation, agricultural sources, and soil matrix sources. Heavy metals affect community species diversity by influencing the enriched species evenness and richness of plant communities.
In conclusion, we must reasonably increase the number of tolerant species and maximize species niche differentiation to improve the differences and functional complementarity between coexisting individuals. Native pioneer plants should be given priority in artificial planting, whereas functional redundant species should be avoided. Additionally, more studies are needed to confirm the changes in soil heavy metal distribution characteristics and heavy metal accumulation process before, during, and after ecological restoration.
Data availability
Not applicable.
References
Adrián E, Matesanz S, Pescador DS, Cruz MDL, Valladares F, Cavieres LA (2021) Every little helps: the functional role of individuals in assembling any plant community, from the richest to monospecific ones. J Veg Sci. https://doi.org/10.1111/jvs.13059
An L, Liao K, Zhu L, Zhou B (2021) Influence of river-lake isolation on the water level variations of the Caizi Lake, lower reach of the Yangtze River. J Geog Sci 31(4):551–564
Cai L, Xu Z, Bao P, He M, Dou L, Chen L, Zhou Y, Zhu Y (2015) Multivariate and geostatistical analyses of the spatial distribution and source of arsenic and heavy metals in the agricultural soils in Shunde, Southeast China. J Geochem Explor 148:189–195
Chaturvedi A, Bhattacharjee S, Singh AK, Kumar V (2018) A new approach for indexing groundwater heavy metal pollu- tion. J Ecol Ind 87:323–331
Cloquet C, Carignan J, Libourel G, Sterckeman T, Perdrix E (2006) Tracing source pollution in soils using cadmium and lead isotopes. J Environ Sci Technol 40(8):2525–2530
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science (new York, NY) 199(4335):1302–1310
Cyriac M, Gireeshkumar TR, Furtado CM, Fathin KF, Shameem K, Shaik A, Vignesh ER, Nair M, Kocherla M, Balachandran KK (2021) Distribution, contamination status and bioavailability of trace metals in surface sediments along the southwest coast of India. Mar Pollut Bull 164:112042
Deng J, Li B, Zhang S, Li Z, Zu Y, He Y, Chen J, Li T (2021) Plant species diversity of plant communities and heavy metal accumulation in buffer zone of momianhe stream along a long-term mine wastes area, china. B Environ Contam Tox 107(6):1136–1142
Du P, Xue N, Liu L, Li F (2008) Distribution of Cd, Pb, Zn and Cu and their chemical speciations in soils from a peri-smelter area in northeast China. Environ Geol 55(1):205–213
Ekengele LN, Blaise A, Jung MC (2017) Accumulation of heavy metals in surface sediments of lere lake. Chad Geosci J 21:305–315
Fantaw Y, Ledin S, Abdelkadir A (2006) Soil property variations in relation to topographic aspect and vegetation community in the south-eastern highlands of Ethiopia. For Ecol Manage 232(1–3):90–99
Fu H, Yuan G, Ge D, Li W, Jeppesen E (2020) Cascading effects of elevation, soil moisture and soil nutrients on plant traits and ecosystem multi-functioning in poyang lake wetland, China. Aquat Sci 82:1–10
Gao P, Zhou Z, Shuyong MA, Sun QA, Renxin XU (2011) Vegetation distribution pattern and community succession in the trans ition from macro- phyte-to phytoplank ton-dom inated state in shallow lakes, a case study of the Caizi Lakein Anhui Province. Lake Sci (China) 23(1):13–20
Garbisu C, Alkorta I, Kidd P, Epelde L, Mench M (2020) Keep and promote biodiversity at polluted sites under phytomanagement. Environ Sci Pollut Res 27(36):44820–44834
GB 15618-2018 (n.d.) Soil environmental quality. Soil pollution risk control standard for agricultural land (Trial) (in Chinese)
GB/T 30363-2013 (n.d.) Technical Specification for Forest Vegetation Monitoring (in Chinese)
He B, Yun Z, Shi J, Jiang G (2013) Research progress of heavy metal pollution in China: Sources, analytical methods, status, and toxicity. J Chin Sci Bull 58(2):134–140
HJ 710.1.11-2014 (n.d.) Technical guidelines for biodiversity monitoring—terrestrial vascular plants (in Chinese)
HJ 803-2016 (n.d.) Determination of 12 metal elements in soil and sediment by royal water extraction-inductively coupled plasma mass spectrometry (in Chinese)
HJ/T 166-2004 (n.d.) The technical specification for soil environmental monitoring (in Chinese)
Jia T, Wang R, Chai B (2019) Effects of heavy metal pollution on soil physicochemical properties and microbial diversity over different reclamation years in a copper tailings dam. J Soil Water Conserv 74(5):439–448
Jin JS, Wang RS, Li F, Huang JL, Chuan BZ, Zhang HT, Yang WR (2011) Conjugate ecological restoration approach with a case study in Mentougou district, Beijing. Ecol Complex 8(2):161–170
Kartal Ş, Aydın Z, Tokalıoğlu Ş (2006) Fractionation of metals in street sediment samples by using the BCR sequential extraction procedure and multivariate statistical elucidation of the data. J Hazard Mater 132(1):80–89
Lalor GC, Rattray R, Williams N, Wright P (2004) Cadmium levels in kidney and liver of Jamaicans at autopsy. West Indian Med J 53(2):76–80
Li S, Jia Z (2018) Heavy metals in soils from a representative rapidly developing megacity (SW China): Levels, source identification and apportionment. CATENA 63:414–423
Li C, Li H, Zhang Y, Zha D, Zhao B, Yang S, Zhang B, Boer WFD (2019) Predicting hydrological impacts of the Yangtze-to-Huaihe Water Diversion Project on habitat availability for wintering waterbirds at Caizi Lake. J Environ Manag 249:1–10
Li Z, Colinet G, Zu Y, Wang J, An L, Li Q, Niu X (2019) Species diversity of Arabis alpina l. communities in two pb/zn mining areas with different smelting history in Yunnan province, China. Chemosphere. 233(OCT):603–614
Loska K, Wiechuła D (2003) Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. J Chemosphere 51(8):723–733
Ma J, Hao Z, Sun Y, Liu B, Jing W, Du J, Li J (2022) Heavy metal concentrations differ along wetland-to-grassland soils: a case study in an ecological transition zone in Hulunbuir, Inner Mongolia. J Soils Sediments. https://doi.org/10.1007/s11368-021-03132-5
Matayoshi CL, Pena LB, Arbona V, Gómez-Cadenas A, Gallego SM (2021) Biochemical and hormonal changes associated with root growth restriction under cadmium stress during maize (Zea mays L.) pre-emergence. Plant Growth Regul 96:269–281
Men C, Liu R, Xu F, Wang Q, Guo L, Shen Z (2018) Pollution characteristics, risk assessment, and source apportionment of heavy metals in road dust in Beijing, China. J Sci Total Environ 612:138–147
Meng M, Yang LS, Yu JP, Wei BG, Li HR, Cao ZQ, Chen Q, Zhang GY (2021) Identification of spatial patterns and sources of heavy metals in greenhouse soils using geostatistical and positive matrix factorization (PMF) methods. Land Degrad Dev 32(18):5412–5426
Mulya H, Santosa Y, Hilwan I (2021) Comparison of four species diversity indices in mangrove community. Biodiversitas 22:3648–3655
Salim I, Sajjad RU, Paule-Mercado MC, Memon SA, Lee BY, Sukhbaatar C, Lee CH (2019) Comparison of two receptor models PCA-MLR and PMF for source identification and apportionment of pollution carried by runoff from catchment and sub-watershed areas with mixed land cover in South Korea. Sci Total Environ 66:764–775
Sofowote UM, Mccarry BE, Marvin CH (2008) Source apportionment of PAH in hamilton harbour suspended sediments: comparison of two factor analysis methods. J Environ Sci Technol 42(16):6007–6014
Sun YQ, Xiao K, Wang XD, Lv ZH, Mao M (2021) Evaluating the distribution and potential ecological risks of heavy metal in coal gangue. Environ Sci Pollut Res 28(15):18604–18615
Talukder R, Rabbi MH, Baharim NB, Carnicelli S (2021) Source identification and ecological risk assessment of heavy metal pollution in sediments of Setiu wetland, Malaysia. Environ Forensics 23(1–2):241–254
Timoney K (2008) Factors influencing wetland plant communities during a flood-drawdown cycle in the Peace-Athabasca Delta, Northern, Alberta Canada. J Wetlands 28(2):450–463
Tóth G, Hermann T, Da Silva MR, Montanarella L (2016) Heavy metals in agricultural soils of the European Union with implications for food safety. Environ Int 88:299–309
Wang XY, Jiang B, Tian ZF, Cai J, Lin G (2018) Impact of water level changes in Lake Caizi (Anhui Province) on main wetland types and wintering bird habitat during wintering period. Lake Sci (China) 30(6):1636–1645
Wilsey B, Stirling G (2007) Species richness and evenness respond in a different manner to propagule density in developing prairie microcosm communities. J Plant Ecol 190:259–273
Wu C, Zhang L (2010) Heavy metal concentrations and their possible sources in paddy soils of a modern agricultural zone, southeastern China. Environ Earth Sci 60(1):45–56
Xie Z, Sun Z, Zhang H, Zhai J (2014) Contamination assessment of arsenic and heavy metals in a typical abandoned estuary wetland-a case study of the Yellow River Delta Natural Reserve. Environ Monit Assess 186(11):7211–7232
Xu X, Zhao Y, Zhao X, Wang Y, Deng W (2014) Sources of heavy metal pollution in agricultural soils of a rapidly industrializing area in the Yangtze Delta of China. Ecotoxicol Environ Saf 108:161–167
Xu ML, Fang FM, Lin YS (2015) Contents and pollution evaluation of heavy metals in soils of wetlands after returning farmland to lake in Caizi Lake in Anqing City. Wetland Sci (China) 4:437–443
Yang HJ, Bong KM, Kang TW, Hwang SH, Na EH (2021) Assessing heavy metals in surface sediments of the Seomjin River Basin, South Korea, by statistical and geochemical analysis. Chemosphere 284:131400
Yang Y, Ding J, Chi Y, Yuan J (2020) Characterization of bacterial communities associated with the exotic and heavy metal tolerant wetland plant spartina alterniflora. Sci Rep 10(1):17985
Yuan Z, Luo T, Liu X, Hua H, Zhuang Y, Zhang X, Zhang L, Zhang Y, Xu W, Ren J (2019) Tracing anthropogenic cadmium emissions: from sources to pollution. J Sci Total Environ 676:87–96
Zhang Y, Wang R, Kaplan D, Liu J (2015) Which components of plant diversity are most correlated with ecosystem properties? A case study in a restored wetland in northern China. Ecol Ind 49:228–236
Zhang XN, Yang XD, Lü GH (2016) Diversity patterns and response mechanisms of desert plants to the soil environment along soil water and salinity gradients. Acta Ecol Sin 36(11):3206–3215
Zheng X, Fu J, Ramamonjisoa N, Zhu W, He C, Lu C (2019) Relationship between wetland plant communities and environmental factors in the Tumen river basin in northeast China. Sustainability 11(6):1559–1582
Zuran Li, Colinet G, Zu Y, Wang J, An L, Li Q, Niu X (2019) Species diversity of Arabis alpina L. communities in two Pb/Zn mining areas with different smelting history in Yunnan Province, China. Chemosphere 233:603–614
Acknowledgements
The authors would also like to thank reviewers for commenting on this paper.
Funding
This research was supported by a grant from the Natural Science Research Project of Anhui Education Department (No. KJ2019A0552& No. KJ2019A0582), Natural Science Foundation of Anhui Province (No. 1908085MD103& No. 2008085QE243), and Anqing Yangtze River Ecological Environmental Protection and Restoration stagnation Point Tracking Research Project (III) (No. 20190911).
Author information
Authors and Affiliations
Contributions
Huiqun Sun: Data curation, writing-original draft preparation. Zhangying Zheng: Conceptualization, methodology, software. Shuqin Chen: Supervision. Jingjing Cao: Software, validation. Mengxin Guo: Supervision. Yi Han: Writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, H., Zheng, Z., Chen, S. et al. Source apportionment of heavy metals and their effects on the species diversity of plant communities in the Caizi Lake wetland, China. Environ Sci Pollut Res 30, 60854–60867 (2023). https://doi.org/10.1007/s11356-023-26815-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26815-7