Abstract
Sediments and surface water contamination by the industrial effluents containing heavy metals is the most detrimental environmental impact. Therefore, the present work attempts to determine the status of eight heavy metal distribution in sediments and water samples, and their ecological risks’ assessment in the studied area. The distribution pattern of heavy metals in the water and sediment follows the sequences: Zn > Cu > Pb > Cr > Mn > Ni > As > Cd and Mn > Zn > Cr > Pb > Cu > Ni > As > Cd, respectively. Gross water pollution is observed at different sampling points of Dhalai Beel and Bangshi River. The comparison of sedimentary mean metal concentrations with several environmental contamination monitoring parameters, viz, threshold effect level (TEL), probable effect level (PEL), and severe effect lever (SEL) indicates that the metal levels are less than PEL except Cr. Moreover, the level of contamination degree (C d) and modified degree of contamination (mC d) indicates ‘low’ and ‘nil to low’ degree of contamination, respectively. Pollution load indices (PLI) of the studied area are lower than unity, indicates no pollution. Furthermore, a toxic-response factor is applied to assess the potential ecological risk of these heavy metals into the water body. The results of this study exhibit a low potential ecological risk of heavy metals. The Pearson’s correlation and cluster analysis are also performed to assess the heavy metal interactions in water and sediment samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pollution of natural environment by heavy metals is recently a topic of much discussion; and the issue of heavy metal pollution in water and sediment of lakes and rivers has received much more attention from many environmental researchers over the past few decades. Due to industrial development as well as increasing usage of industrial products, everyday a huge amount of industrial wastages discharge into low lands and water bodies without proper treatment. As a consequence, it is assumed that the equilibrium balance between the metals in sediment/soils and ground water or surface water is disturbed (Klavinš et al. 2000; Yu et al. 2001) that might be a reason to increase water contamination.
Sediments usually provide useful information for environmental and geochemical pollution status (Uluturhan et al. 2011). Depending on hydrodynamics and environmental conditions, heavy metals tend to adsorb from water column onto surfaces of fine particles and usually move thereafter with the sediments, and can affect the benthic organisms and food chain if toxic levels are reached, resulting in health risk (Louriño-Cabana et al. 2011; Saha and Zaman 2013). Throughout the hydrological cycle, far less than 1 % of pollutants remain dissolved in water whereas over 99 % are stored in sediment (Filgueiras et al. 2004). Therefore, sediment represents one of the ultimate sinks for heavy metals discharged into the aquatic environment (Luoma and Bryan 1981; Joksimovic et al. 2011). Contaminants are not necessarily fixed permanently by the sediments, and under changing environmental conditions, they may be released to the water column by various processes of remobilization. Thus, in aquatic systems, sediments may be both a carrier and possible source of pollutants (Förstner 1989).
River water has been used as drinking water and irrigation water for agriculture and for fish culture. Rivers are also important in soil fertility maintenance, transportation, forest resources development, and wildlife conservations. However, most of the rivers in the urban areas of the developing world are at the end point of industrial effluents (Phiri et al. 2005). It is therefore, necessary to conduct a comprehensive river water quality monitoring program to safeguard public health and to protect the valuable fresh water resources (Kannel et al. 2007).
In order to create productive employment opportunity, poverty eradication, gender equality and rapid economic growth, the Government of Bangladesh decided to set up the Dhaka Export Processing Zone (DEPZ) at Ganakbari in Savar of Dhaka district in 1993. Another DEPZ was also established at the opposite side of the first DEPZ. Due to very cheap local work force and somewhat relaxed environmental and tariff regulations, foreign and local investors are being attracted heavily to set up manufacturing units for exportable commodities. It has been reported that DEPZ accommodates about 300 industries including textile, dyeing, plastic, metal fabrications, semiconductor goods, lather tanning and so on. Everyday a huge amount of heavy metals containing effluents produced by these industries of DEPZ are being released without proper treatment into the Dhalai Beel (a natural low land cum lake) and then carry away into the Bangshi River, which brings big concern for the aquatic environment. Moreover, local farmers are frequently using water from Dhalai Beel and Bangshi River for irrigation purposes and for fishing. However, no detailed study has so far been conducted in this area especially on ecological risk assessment for heavy metal contamination. Therefore, the present investigation is aimed to study the physicochemical properties (i.e., pH, EC, TSS, DO, BOD, COD, and Chloride) of water sample, and the levels of metals (Pb, Cd, Ni, Cr, Cu, Zn, Mn, and As) in water and sediment samples of Dhalai Beel and Bangshi River, primarily to generate data on seasonal variations and secondly to evaluate the degree of contamination and potential ecological risk in the study area.
Materials and methods
Description of site
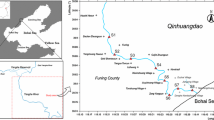
The present study area, Dhalai Beel (a natural low land cum lake) and Bangshi River, under Savar police station (23°5′ N and 90°15′ E) is located 30 km north of Dhaka City, capital of Bangladesh (Fig. 1a). The area is rich in industrial establishments like textile, dyeing, and apparel industries. Two export processing zones (EPZ) were established in this area on 533 acres with 586 plots (DEPZ 2000). The effluents from DEPZ are discharged into the adjacent Dhalai Beel on the western side of DEPZ. During the dry season, the effluents of the industries are deposited on the Dhalai Beel while in the rainy season, the land is overflowed and the toxic constituents of the effluents are carried away to the nearby villages and other water bodies including Bangshi River which is 2.5 km far away from Dhalai Beel.
Sampling
Water samples from Dhalai Beel were collected from four pre-determined sampling spots (Fig. 1b) during the pre-monsoon and post-monsoon. The Bangshi River water samples were collected from the five sampling spots; those were 500 m away from each other. Water samples were collected in pre-cleaned plastic bottles following filtering through Whatman No. 541 filter paper and kept in a refrigerator at 4 °C with addition of 2 mg/L HNO3 before laboratory analysis (Mastoi et al. 1997).
Sediment samples from the surface bed of Dhalai Beel and Bangshi River were collected with a stainless steel Ekman grab sampler, which allows free water through the sampler during descent penetration. The sampler was inspected for possible cross contamination (i.e., sediment from the previous sampling spot) and cleaned with ambient water for individual sample collection. The sediment samples were collected from the same sampling spots, which were previously indicated for water sampling. The sediment samples were firstly air dried for several days over Pyrex petri Dishes and then samples were dried in an oven at 105 °C in analytical chemistry laboratory, NRCD, Institute of Nuclear Science and Technology, Bangladesh. The dried samples were ground and screened through a 2-mm sieve to obtain a homogenous powder. The powder samples were stored in airtight plastic vials inside a desiccator for further physical and chemical analysis.
Sample coding and numbering
DB1 to DB4 = four sampling spots in Dhalai Beel for both water and sediment samples, ~500, ~700, ~900, and ~1,000 m, respectively, away from the effluent discharging point of DEPZ. The Bangshi River water and sediment samples were collected from the five sampling points (BR1 to BR5), which were 500 m away from one sampling point to another point.
Analytical procedure and analysis
Temperature, pH, conductivity, and total dissolved solid (TDS) in water samples of Dhalai Beel and Bangshi River were measured on the sites using portable mercury thermometer, pH meter (Hanna Instruments, HI 98106, Portugal), conductivity meter (Lutron-Model CD 4301, England), and TDS meter (Hanna Model HI 9635), respectively. Dissolved oxygen (DO) was measured iodometrically on site using the azide modification of Winkler’s method as recommended by American Public Health Association (APHA 1999). Biochemical oxygen demand (BOD5) by Winkler’s method, chemical oxygen demand (COD), total suspended solid (TSS), and chloride concentration were determined in the laboratory using standard water analysis methods (APHA 1999).
Accurately weighed amount (0.25 g) of each dried sediment sample was taken into a clean Pyrex test tube (12 × 2 cm) and digested with 1.0 mL 70 % HClO4, 4 mL concentration HNO3 at 150 °C in an oil bath. The solution was diluted with deionized water and filtered quantitatively (Whatman No. 541) in a 50-mL volumetric flask. One blank and one standard reference material (SRM) were digested in a similar manner for quality control and accuracy check. Determination of heavy metals (Pb, Cd, Ni, Cr, Cu, Zn, Mn, and As) in all samples (water and sediments) were carried out by atomic absorption spectrophotometer (Model AA-6800, Shimadzu Corporation, Japan) using air-acetylene flame with digital read out system in the Nuclear Analytical Chemistry Laboratory, NRCD, Institute of Nuclear Science and Technology, Gonakbari, Savar.
All samples were collected and analyzed in triplicate and the average results were used to represent the data. Statistical software, IBM SPSS 15.0 for Windows (IBM, USA), was used for hierarchical cluster analysis, and for correlation matrix of heavy metals in water and sediments. Other calculations were performed by Microsoft Excel 2010.
The accuracy of the analysis was verified by analyzing blanks and certified reference materials by the same procedure used for the samples. For the analysis of water, certified SLEW-2 estuarine water from the National Research Council of Canada was tested. For sediments, SRM, SL-1 (Lake Sediment) from International Atomic Energy Agency (IAEA) was used. All the samples were measured at least two times to assess the reproducibility of the measurement. Samples were reanalyzed if the relative standard deviation of the measurement exceeded 10 %.
Results and discussion
The results presented in this study comprise an attempt to report the seasonal variations and degree of heavy metal contamination (Pb, Cd, Ni, Cr, Cu, Zn, Mn, and As) in water and sediment samples collected from Dhalai Beel and Bangshi River, Savar, Bangladesh.
Heavy metals in water
The concentration variation of different heavy metals in the surface water of Dhalai Beel and Bangshi River in the pre-monsoon and post-monsoon is summarized in Table 1; and the examined metal contents are compared with recent past findings from other water bodies around the world (Table 2). Almost in all sampling spots of Dhalai Beel and Bangshi River, the higher lead (Pb) concentration was found in the post-monsoon and lower value was found during the pre-monsoon. This may be due to the flushing of the metal from rather immobilized deposits like domestic and industrial sludge in rainy season. Pb concentration (ranging from 0.173 to 0.680 mg/L) of the present observation was much higher than that of the recommended value (0.01 mg/L), set by WHO (1993). It was apprehended from the observation that high level of Pb in the river water was indicative of the fact that the source of higher Pb content was not only the industrial effluents but also some other outlets. Long-term accumulation of Pb from motor vehicles emission might be one of the major sources of higher Pb content in the water. From the observation, it would mean that the water in Bangshi River was not safe for any use by the humans as well for the safe growth of aquatic organisms in terms of its Pb content. The maximum cadmium (Cd) concentration was found in Bangshi River in post-monsoon. It might be happened due to flushing of the metal from immobilized deposits. The main source of Cd in wastewaters is the discharging of waste streams from various industrial processes, such as metallurgical alloys, ceramics, metal plating, photograph, pigment works, and textile printing industries (Rahman and Islam 2010). The operation of some tannery industries located by the bank of Bangshi River may also contribute to increase Cd concentration in the river water. Cd concentration in Dhalai Beel and Bangshi River water was 0.005–0.008 and 0.006–0.010 mg/L in the pre-monsoon and post-monsoon, respectively. The maximum permitted concentration of Cd in drinking water is 0.003 mg/L, set by WHO (1993). Cd concentration in water samples of Dhalai Beel and Bangshi River was higher than the above-recommended value. The maximum nickel (Ni) concentration was found in Dhalai Beel water in post-monsoon. Ni concentrations (ranging from 0.058 to 0.130 mg/L) in all sampling points were above the WHO recommended value (0.02 mg/L) for drinking water (WHO 1993). However, the Bangshi River water showed the maximum chromium (Cr) concentration in post-monsoon (0.157 mg/L) and the minimum was found in Dhalai Beel water in pre-monsoon (0.043 mg/L). EPC (1980) reported that most of the tannery industries used “Chrome tanning method” and the waste discharged by this method exerts toxicity to the fish and aquatic life. From the literature survey, it is noticed that some tannery industries are established along the bank of Bangshi River. Therefore, the data finding from this study has been suggested that the tannery wastes and dying effluents might be the principal source of Cr contamination in the study area. The recommended maximum concentration of Cr for drinking water is 0.05 mg/L set by WHO (1993). It was apparent (Table 1) that Cr concentration of the present investigation was higher than the recommended value. The concentration of Copper (Cu) in water samples of Dhalai Beel and Bangshi River ranged from 0.65 to 1.24 mg/L in pre-monsoon and 0.78 to 1.46 mg/L in post-monsoon, respectively. Regarding seasonal variation of Cu concentration in the water way from Dhalai Beel to Bangshi River, it was observed that in all sampling spots the maximum concentration of Cu was found in post-monsoon and minimum was found in pre-monsoon. Cu content of Dhalai Beel water samples was higher than the Bangshi River water. This is an indication of the fact that the presence of Cu in studied water system originates mostly from the DEPZ effluents. Cu enters in aquatic environment through industrial effluents and also from river run off, domestic waters, etc. (Rahman and Islam 2009). The observed values of Cu were lower than the permissible concentration of 2 mg/L, prescribed by WHO (1993). Zinc (Zn) concentration was found from 2.19 to 4.17 mg/L in pre-monsoon and 2.13 to 4.02 mg/L in post-monsoon. WHO (1993) recommended guideline value of Zn in drinking water is 3 mg/L. Zn concentration in almost all sampling stations were higher than the WHO’s recommended level. Manganese (Mn) concentration varied from 0.063 to 0.124 and 0.059 to 0.117 mg/L in pre-monsoon and post-monsoon accordingly. The concentration of Mn in Dhalai Beel water was slightly higher than the water samples of Bangshi River due to discharge of the DEPZ’s effluents. The maximum permissible limit of Mn concentration in drinking water is 0.5 mg/L, set by WHO (1993). Mn concentration of potable water quality for Bangladesh is 0.1 mg/L, set by BCAS (1998). In the present study, Mn level was in line of the BCAS standard and lower than the WHO prescribed value. In nine sampling spots, As concentration ranged from 0.009 to 0.039 mg/L. The maximum arsenic concentration for potable water quality standard is 0.05 mg/L, set by BCAS (1998); and the drinking water standard is 0.01 mg/L, set by both WHO (1993) and USEPA (1996). The arsenic concentration for this study was higher than the value set by WHO and USEPA, except the sampling point of W-BR5 (post-monsoon) and lowers than the BCAS recommended value.
Heavy metals in sediment
The variation of different heavy metals in sediment from Dhalai Beel and Bangshi River at Savar, Dhaka, Bangladesh is represented in Table 3. The analysis of the data depicted an order of heavy metal accumulation in sediment that was Mn > Zn > Cr > Pb > Cu > Ni > As > Cd. The data indicated that Mn was maximally accumulated in the sediments whereas Cd got least concentrated. The heavy metal concentrations were compared with threshold effect level (TEL) that represents the concentration below which adverse effects are expected to occur only rarely, probable effect level (PEL) representing the concentration above which adverse effects are expected to occur frequently and severe effect level (SEL) that signifies chronic, long-term impacts of contamination to benthic organisms (Table 4). The mean metals concentration obtained from the sediment samples did not exceed the PEL except Cr which exceeded severe effect level (SEL) in some sampling points. Comparisons of heavy metal concentration with the observed values by different authors around the world are listed in Table 4.
Water quality parameters
The physicochemical parameters such as temperature, pH, EC, TSS, DO, BOD, COD, and chloride at various sampling points of Dhalai Beel and Bangshi River water during the pre-monsoon and post-monsoon seasons are shown in Table 1. Water temperature showed higher values during pre-monsoon (28 to 29 °C) and lower values in the post-monsoon season (22 to 26 °C). In pre-monsoon the average pH value in Dhalai Beel water was rather higher than Bangshi River water due to stagnant of water in Dhalai Beel. High pH of waters during pre-monsoon could be due to the increased photosynthetic assimilation of dissolved inorganic carbon by plankton. A similar effect could also be produced by water evaporation through the loss of half bound CO2 and precipitation of mono-carbonate (Khan and Chowdhury 1994). The maximum permissible value for pH in industrial water, fishing water, and drinking water were 6.0–9.5, 6.5–8.5, and 6.5–8.5, respectively, set by EQS (1991) for Bangladesh. From our observation, it was seen that the average pH values of Bangshi River water were in line with the permissible limit of EQS. The electrical conductivity (EC) values ranged between 0.93 and 2.51 mS/cm. The high level of EC was due to significant amount of dissolved salt in the water under study. High conductivity in the pre-monsoon represents water with high electrolyte concentration due to evaporation of water. The average EC value (1.59 mS/cm) was in line of the WHO (2004) guidelines of 1.5 mS/cm for drinking water. Textile and dyeing industries release a lot of suspended solid waste. TSS measurements ranged between 51.55 and 115.24 mg/L. In Bangladesh there is no reliable data on permissible limit of TSS for fishing water, and no health-based guideline value is proposed. However, Hossain et al. (1988) reported that the concentration of suspended solids found to be well above the recommended levels in all rivers of Bangladesh. Therefore, the presence of high levels of TSS in water may be objectionable for aquatic environment. TDS comprise of inorganic salts and small amounts of organic matter that are dissolved in water. TDS values at various sampling points ranged between 880 and 1,340 mg/L. From the present observation, it was shown that the TDS values in Dhalai Beel water were much higher than that of Bangshi River water. However, all the TDS values were above the fresh water standard and the drinking water standard, set by WHO (1993). The dissolution of low molecular weight organic bases originated from dye industries may results in the higher TDS value of the samples. The measurement of DO gives a ready assessment of purity of water. Low DO values in the pre-monsoon might to due to higher microbial activities. Decomposition of organic matter might be an important factor in consumption of DO, as more vigorous deposition could be likely during warm weather. Dry period also decrease flow which reduces the amount of oxygen churned into the water. The higher concentration of DO in the post-monsoon might be occurring due to circulation and mixing of oxygen by inflow of water after monsoon rains. According to EQS (1991), the maximum admissible level of DO for drinking water, fishing water, industrial water, and irrigation water were 6, 4.6, 5 and 5, respectively. DO values (2.71–4.43 mg/L) at all locations in Dhalai Beel and Bangshi River were less than that of good quality water. It was reported that reduced DO below 3 mg/L delays hatching of fish eggs, interferes with growth rate, and also decreases tolerance to certain toxicants (Cairns and Scheier 1957). The BOD and COD are widely used for determining waste concentration, and applied primarily to pollutant mixture such as domestic sewage, agricultural and industrial waste. The BOD and COD measurements were 42.77–103.97 and 17–215 mg/L, respectively. The recommended BOD and COD values for drinking water are 6 and 10 mg/L, respectively (WHO 2004). The high BOD and COD values at Dhalai Beel and Bangshi River water may be accounted for the effluents from the DEPZ as well as effluents from domestic sewage. From this investigation, the highest concentration (446 mg/L) of chloride was recorded for the pre-monsoon in Dhalai Beel while the Bangshi River had the lowest concentration (147 mg/L) in post-monsoon. Chloride concentration in good quality water is less than 250 mg/L (WHO 1993). Therefore, it is hypothesized that Dhalai Beel water considered to be a risk for aquatic organism and human health, and might be caused unpleasant taste of water.
Contamination degree of heavy metals
The contamination factor is obtained from a ratio between the measured concentration of the heavy metals in sediment of the water body and the pre-industrial reference value for the same metal (Hakanson 1980). The degree of contamination is defined as the sum of all contamination factors.
The computing equation for contamination factor \(\left( {C_{\text{f}}^{i} } \right)\) and the degree of contamination \(\left( {C_{\text{d}} } \right)\) are as follows:
where \(C^{i}\) is the measured concentration of the heavy metals in sediment and \(C_{n}^{i}\) is the standard pre-industrial reference level (in mg/kg): 70 for Pb, 1.0 for Cd, 90 for Cr, 50 for Cu, 175 for Zn, 15.0 for As, 68 for Ni, 850 for Mn (Hakanson 1980; Turekin and Wedepohl 1961). In the present study, values of contamination factor \(\left( {C_{\text{f}}^{i} } \right)\) existed in the order of Cr > Pb > Zn > Cu > Cd > Mn > Ni > As in the Dhalai Beel and Bangshi River sediment (Table 5). The contamination factor and degree of contamination were classified into four groups by Hakanson (1980). Values of the contamination factor are characterized as follows: \(C_{\text{f}}^{i} \, < 1,{ 1 } \le \,C_{\text{f}}^{i} \, < { 3},{ 3} \le \,C_{\text{f}}^{i} \, < 6,{\text{ and}}\,C_{\text{f}}^{i} \, \ge 6\) indicate low, moderate, considerable, and very high contamination factor, respectively. The degree of contamination defines the quality of the environment in the following way: \(C_{\text{d}} \, < 8,{ 8} \le \,C_{\text{d}} \, < 1 6,{ 16} \le \,C_{\text{d}} \, < 3 2,{\text{ and}}\,C_{\text{d}} \, \ge 3 2\) indicate low, moderate, considerable, and very high degree of contamination accordingly. The \(\left( {C_{\text{f}}^{i} } \right)\) of Cr were viewed to be moderately contaminated in some sampling sites followed by Pb. But values of other metals were of low level. However, low degree of contamination (\(C_{\text{d}}\) < 8) was indicated in all sampling points (Table 5).
Modified degree of contamination \(\left( {{\text{m}}C_{d} } \right)\) and pollution load index (PLI)
The modified degree of contamination is defined as the sum of all contamination factors \(\left( {C_{\text{f}}^{i} } \right)\) derived from a set of contaminants divided by the number (n) of analyzed contaminants. The generalized form of \({\text{m}}C_{\text{d}}\) can be expressed as follows (Abrahim and Parker 2008):
Terminology used to describe the \({\text{m}}C_{\text{d}}\) as suggested by Abrahim and Parker (2008): \({\text{m}}C_{\text{d}} < 1. 5,{ 1}. 5\le \,{\text{m}}C_{\text{d}} \, < 2,{ 2} \le \,{\text{m}}C_{\text{d}} < 4,{ 4} \le \,{\text{m}}C_{\text{d}} \, < 8,{ 8} \le {\text{m}}C_{\text{d}} \, < 1 6,{ 16} \le {\text{m}}C_{\text{d}} \, < 3 2,{\text{ and m}}C_{\text{d}} \, \ge 3 2\) indicate nil to very low, low, moderate, high, very high, extremely high, and ultra high degree of contamination, respectively. This study demonstrated ‘nil to very low’ degree of sediment contamination with respect to the analyzed eight heavy metals (Table 5).
The extents of pollution by the heavy metals were assessed by employing the method based on pollution load index (PLI) developed by Tomlinson et al. (1980) and the expression is shown below:
where, n is the number of metals (n = 8 in this study) and \(C_{\text{f}}\) is the contamination factor. PLI provides a simple, comparative means for assessing a site or estuarine quality: a value of zero indicates perfection, a value of one indicates only baseline levels of pollutants present and values higher than one would indicate progressive deterioration of the site and estuarine quality (Tomlinson et al. 1980). In our study, all the stations indicated PLI values lower than one, means no pollution (Table 5).
Assessment of potential ecological risk
The assessment of the potential ecological risk of the heavy metal contamination was proposed as a diagnostic tool for water pollution control purposes as a result of the increasing content of heavy metals in sediments and their subsequent release into the water, which could threaten ecological health. Hakanson (1980) developed a method to assess the potential ecological risk index for aquatic pollution control purposes, i.e., to sort out which lakes or rivers and substances should be given special attention. According to this method, the potential ecological risk factor (\(\mathop {\text{E}}\nolimits_{\text{r}}^{i}\)) of single element and the potential ecological risk index (RI) of multi-element can be computed by the following equations:
where \(C_{\text{f}}^{i}\) is the contamination factor for the element of “i”; \(T_{\text{r}}^{i}\) is the toxic-response factor for the given element of “i”, which accounts for the toxic requirement and the sensitivity requirement. The toxic-response factors for Pb, Cd, Cr, Cu, Zn, As, Ni, and Mn were 5, 30, 2, 5, 1, 10, 5, and 1, respectively (Hakanson 1980; Xu et al. 2008). Using Eqs. 1, 2, 5, and 6, and indices and grades of potential ecological risk assessment suggested by Hakanson (1980), the results of evaluation on potential ecological risk factor \(\left( {{\text{E}}_{\text{r}}^{i} } \right)\) and the potential ecological risk index (RI) are summarized in Table 6. The order of potential ecological risk factor of heavy metal in sediments of Dhalai Beel and Bangshi River was Cd > Pb > Cu > Cr > Ni > As > Zn > Mn (Table 6). The potential ecological risk factors \(\left( {{\text{E}}_{\text{r}}^{i} } \right)\) of Pb, Cd, Ni, Cr, Cu, Zn, Mn, and As were all lower than 40, which belong to low ecological risk (Hakanson 1980). All the sampling sites were at low risk level where the RI values were much lower than 150 (Hakanson 1980). The results indicated that there was low potential ecological risk for the water body and the risk posed by the heavy metals at different sampling sites went down in the order of S-BR2 > S-DB1 > S-BR3 > S-DB2 > S-BR4 > S-DB3 > S-BR1 > S-BR5 > S-DB4, based on the value of RI.
Statistical analysis
The Pearson’s correlation coefficient analysis amongst the chemical parameters of both water and sediment samples was performed and presented in Tables 7 and 8. In water samples (Table 7), Pb showed significant positive correlations with all metals except Cd, Cr, and Mn. Similarly, a close relationship was noted between Ni and Cu (r = 0.86, p < 0.01), Ni and Zn (r = 0.48, p < 0.05), and Ni and As (r = 0.53, p < 0.05), suggesting a common source of these metals. A good correlation between Cu and Zn, and Cu and As were also observed in water samples. However, water temperature had relatively weak correlations with other parameters. The correlation coefficients between temperature and other parameters were less than or equal to 0.496. pH is positively correlated with all other parameters except DO (r = −0.70, p < 0.01). Strong positive correlation of EC with TDS (r = 0.79, p < 0.01), COD (r = 0.83, p < 0.01), Chloride (r = 0.82, p < 0.01), and strong negative correlation (r = 0.87, p < 0.01) with DO was observed. The matrix data for this study revealed that TDS was strongly positively correlated with other parameters (ranging from r = 0.72 to 0.97), whereas a strong negative correlation (r = −0.93, p < 0.01) was observed between TDS and DO. A strong negative correlation (r = −0.71 to −0.95) was also found between DO and other parameters. Correlation coefficients of BOD and other parameters ranged from 0.49 to 0.94. It was clear that COD was strongly correlated with other parameters which were significant at 0.01 levels. A strong correlation was also observed in terms of chloride and other examined parameters at significant level 0.01. Correlation coefficients (r) between the physicochemical properties and different heavy metal concentrations in water samples (Table 7) suggested that the concentrations of Pb, Ni, Cu, Zn, and As were significantly and positively correlated with pH, EC, TSS, TDS, BOD, COD, and Chloride. The concentrations of Pb, Ni, Cu, Zn, and As were significantly and negatively correlated with DO. No statistically significant correlations were observed between temp and heavy metal concentrations. The concentration of Cd, Cr, and Mn in water samples were statistically not correlated with any physicochemical properties of waters. In sediment samples (Table 8), significant positive correlations (p < 0.05) between Ni and Cu (r = 0.48), Ni and Zn (r = 0.54), Cu and Zn (r = 0.49) were observed.
A cluster analysis was performed on heavy metals of two data sets (sediment and water), using between-groups linkage method with Pearson correlation (Maitre 1982). The results are shown in Fig. 2. The distance axis represents the degree of association between groups of variables, i.e., the lower the value on the axis, the more significant the association. For sediment, Ni and Zn were very well correlated with each other (coefficient 0.54) and form another cluster with Cu. Secondly the association of Mn and As was shown, which was at a later stage associated with Ni-Zn cluster. Thereafter, another cluster was formed by Cd and Cr with positive coefficient of 0.08 (Fig. 2). For water, Ni and Cu were associated with each other (coefficient 0.86) and form another cluster with Pb. Whereas at 3rd, 4th and 5th stage Pb was correlated with Zn, As and Mn. Cd–Cr cluster was joined to Pb only very late.
Conclusion
This investigation reveals that the concentration of six heavy metals (Pb, Cd, Ni, Cr, Zn, and As) out of eight in water samples, are higher than the WHO (1993) recommended guideline for drinking water. Discharges of industrial, agricultural wastes and of municipal sewage water appear as the major sources for water quality deterioration in this study area. The elevated levels of these heavy metals could ultimately contaminate the cultivated crops, fish and thus making them toxic for human consumption. The average heavy metal concentrations in the sediments are lower than the PEL with exception of Cr. The sediment samples show a low degree of contamination by heavy metals and low potential ecological risk level. In conclusion it might be stated that, although, the present study area is an industrial zone but most of the factories do not have scientific effluent treatment systems. Consequently, Dhalai Beel and Bangshi River receive the considerably high quantity of industrial inputs from DEPZ and these contaminants threaten the ecological balance. Due legislative measures should be made to reduce the anthropogenic discharges in the lake and river; otherwise, high levels of pollution will greatly influence the population and will invite socio-economic disaster. Moreover, the regular monitoring program of the heavy metals is recommended to protect these water bodies and also to reduce environmental risk.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland. New Zealand. Environ Monit Assess 136(1–3):227–238
Ahmad MK, Islam S, Rahman S, Haque MR, Islam MM (2010) Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int J Environ Res 4(2):321–332
Ahmed G, Miah MA, Anawar HM, Chowdhury DA, Ahmad JU (2012) Influence of multi-industrial activities on trace metal contamination: an approach towards surface water body in the vicinity of Dhaka Export Processing Zone (DEPZ). Environ Monit Assess 184(7):4181–4190
Akbulut NE, Tuncer AM (2011) Accumulation of heavy metals with water quality parameters in KIzIlIrmak River Basin (Delice River) in Turkey. Environ Monit Assess 173(1–4):387–395
Akcay H, Oguz A, Karapire C (2003) Study of heavy metal pollution and speciation in Buyak Menderes and Gediz River sediments. Water Res 37(4):813–822
APHA (1999) Standard methods for the examination of water and waste water, 20th edn. Washington, DC
Bai J, Cui B, Chen B, Zhang K, Deng W, Gao H, Xiao R (2011) Spatial distribution and ecological risk assessment of heavy metals in surface sediments from a typical plateau lake wetland, China. Ecol Model 222(2):301–306
BCAS (1998) Guide to the Environmental Conservation Act 1995 and rules 1997. Bangladesh
Belhadj M, Ghezzar MR, Abdelmalek F, Benahmed AA, Ouddane B, Addou A (2006) Assessment of the sediment contamination by heavy metals of the Chelif River, Algeria. Fresen Environ Bull 15(3):186–192
Cairns JJ, Scheier A (1957) The effects of periodic low oxygen upon the toxicity of various chemicals to aquatic organisms. In: Proceedings 12th Industrial Waste Conference, Purdue University, USA
DEPZ (2000) Brochure Published by Bangladesh Export Processing Zone Authority
EPC (1980) A resume of environment pollution control in Bangladesh
EQS (1991) Environmental quality standards for Bangladesh. Department of Environment, Government of the Peoples Republic of Bangladesh
Filgueiras AV, Lavilla I, Bendicho C (2004) Evaluation of distribution, mobility and binding behaviour of heavy metals in surficial sediments of Louro River (Galicia, Spain) using chemo metric analysis: a case study. Sci Total Environ 330(1–3):115–129
Förstner U (1989) Contaminated sediments: lectures on environmental aspects of particle-associated chemicals in aquatic systems, vol 21. Springer
Gupta A, Rai DK, Pandey RS, Sharma B (2009) Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environ Monit Assess 157(1–4):449–458
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(8):975–1001
Hossain MH, Bhuiya MRH, Rahman MA (1988) A preliminary study on river-water pollution due to industrial wastes at some selected sites in Bangladesh. Bangladesh J Fish 11:59–65
Huang X, Hu J, Li C, Deng J, Long J, Qin F (2009) Heavy-metal pollution and potential ecological risk assessment of sediments from Baihua Lake, Guizhou, P.R. China. Int J Environ Health Res 19(6):405–419
Ikem A, Egiebor NO, Nyavor K (2003) Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut 149(1–4):51–75
Joksimovic D, Tomic I, Stankovic AR, Jovic M, Stankovic S (2011) Trace metal concentrations in Mediterranean blue mussel and surface sediments and evaluation of the mussels quality and possible risks of high human consumption. Food Chem 127(2):632–637
Kannel PR, Lee S, Lee YS, Kanel SR, Khan SP (2007) Application of water quality indices and dissolved oxygen as indicators for river water classification and urban impact assessment. Environ Monit Assess 132(1–3):93–110
Karadede-Akin H, Ünlü E (2007) Heavy metal concentrations in water, sediment, fish and some benthic organisms from Tigris River, Turkey. Environ Monit Assess 131(1–3):323–337
Karbassi AR, Monavari SM, Nabi Bidhendi GR, Nouri J, Nematpour K (2008) Metal pollution assessment of sediment and water in the Shur River. Environ Monit Assess 147(1–3):107–116
Khan MAG, Chowdhury SH (1994) Physical and chemical limnology of Lake Kaptai, Bangladesh. Trop Ecol 35(1):35–51
Klavinš M, Briede A, Rodinov V, Kokorite I, Parele E, Klavina I (2000) Heavy metals in rivers of Latvia. Sci Total Environ 262(1–2):175–184
Li C, Kang S, Zhang Q, Gao S, Sharma CM (2011) Heavy metals in sediments of the Yarlung Tsangpo and its connection with the arsenic problem in the Ganges-Brahmaputra Basin. Environ Geochem Health 33(1):23–32
Liu C, Xu J, Zhang P, Dai M (2009) Heavy metals in the surface sediments in Lanzhou Reach of Yellow River, China. Bull Environ Contam Toxicol 82(1):26–30
Louriño-Cabana B, Lesven L, Charriau A, Billon G, Ouddane B, Boughriet A (2011) Potential risks of metal toxicity in contaminated sediments of Deûle River in Northern France. J Hazard Mater 186(2–3):2129–2137
Luoma SN, Bryan GW (1981) A statistical assessment of the form of trace metals in oxidized estuarine sediments employing chemical extractants. Sci Total Environ 17(2):165–196
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39(1):20–31
Maitre RWL (1982) Numerical Petrology. Elsevier Scientific Publishing Company, Amsterdam
Mastoi GM, Khuhawar MY, Bozdar RB (1997) Physico-chemical analysis of industrial effluents from Kotri site area. In: Proc. NSMTCC’97 on Environmental Pollution, Islamabad Pakistan
Phiri O, Mumba P, Moyo BHZ, Kadewa W (2005) Assessment of the impact of industrial effluents on water quality of receiving rivers in urban areas of Malawi. Int Enviorn Sci Technol 2(3):237–244
Rahman MS, Islam MR (2009) Effects of pH on isotherms modeling for Cu(II) ions adsorption using maple wood sawdust. Chem Eng J 149(1–3):273–280
Rahman MS, Islam MR (2010) Adsorption of Cd(II) ions from synthetic waste water using maple sawdust. Energy Sour Part A: Recovery Util Environ Eff 32(3):222–231
Raphael EC, Augustina OC, Frank EO (2011) Trace metals distribution in fish tissues, bottom sediments and water from Okumeshi River in delta state, Nigeria. Environ Res J 5:6–10
Saha N, Zaman MR (2013) Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City. Bangladesh. Environ Monit Assess 185(5):3867–3878
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti River sediments: a tributary of the Ganges. India. J Hydrol 312(1–4):14–27
Suthar S, Nema AK, Chabukdhara M, Gupta SK (2009) Assessment of metals in water and sediments of Hindon River, India: impact of industrial and urban discharges. J Hazard Mater 171(1–3):1088–1095
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresuntersuchungen 33(1–4):566–575
Turekin KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192
Uluturhan E, Kontas A, Can E (2011) Sediment concentrations of heavy metals in the Homa Lagoon (Eastern Aegean Sea): assessment of contamination and ecological risks. Mar Pollut Bull 62(9):1989–1997
USEPA (1996) Drinking Water Regulations and Health Advisories. US Environmental Protection Agency, Washington
Wang CC, Niu ZG, Li Y, Sun J, Wang F (2011) Study on heavy metal concentrations in river sediments through the total amount evaluation method. J Zhejiang Univ: Sci A 12(5):399–404
WHO (1993) Guidelines for Drinking-Water Quality, 2nd edn. World Health Organization, Geneva
WHO (2004) Guidelines for Drinking-Water Quality, 3rd edn. World Health Organization, Geneva
Xu ZQ, Ni SJ, Tuo XG (2008) Calculation of heavy metals toxicity coefficient in the evaluation of potential ecological risk index. Environ Sci Technol 31:112–115
Yu KC, Tsai LJ, Chen SH, Ho ST (2001) Chemical binding of heavy metals in anoxic river sediments. Water Res 35(17):4086–4094
Acknowledgments
The authors thank the authority of Bangladesh Atomic Energy Commission for providing laboratory facilities to analyze effluent and water samples using conventional technique. The authors also grateful to the authorities of Dhaka Export Processing Zone (EPZ); especially to Md. Abdus Sobhan, Assistant Manager (Commercial Operation) for their kind cooperation to provide information and help to collect the samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rahman, M.S., Saha, N. & Molla, A.H. Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci 71, 2293–2308 (2014). https://doi.org/10.1007/s12665-013-2631-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-013-2631-5