Abstract
Mangrove forests are one of the most productive and biodiverse wetlands on earth. Yet, these unique coastal tropical forests are among the most threatened habitats in the world. Muthupet mangroves situated in the southeastern coast of India, has a reverse “L” shaped structure. Four cores were collected in 2008, sliced and subsampled at 2.5 cm length. The heavy metals (Mn, Cu, Zn, Ni, Pb, Cr, Cd) and other associated geochemical parameters were evaluated to determine pollution history of Muthupet. An evaluation of the status of heavy metal pollution through the index analysis approach was attempted by computing geoaccumulation index, anthropogenic factor, enrichment factor, contamination factor and degree of contamination, pollution load index and metal pollution index. To compensate for the natural variability of heavy metals in the core sediments, normalization using Al was carried out so that, any anthropogenic metal contributions may be detected and quantified. Results of the study reveal that significant metal contamination exists, and all the metals are found to be higher than continental crustal values. The fine sediments of Muthupet vary between uncontaminated and moderately contaminated with almost no enrichment (EF < 1) to severe enrichment (EF > 10). On comparison, the core collected close to aquafarms and dense mangrove forest (C3) is the most polluted core and the core retrieved where minor rivers drain (C2) is the least polluted.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sediments in the coastal zone are normally dominated by terrigenous particles due to the supply of continental material. With growing industrial activity, contaminants are released into the coastal environment causing undesirable effects on the marine ecosystem. Among these innumerable contaminants, pollution by heavy metals in coastal environment has become a global phenomenon because of its toxicity, persistence for several decades in the environment, bioaccumulation and biomagnification in the food chain (Burger and Gochfeld 2003). Sediment heavy metal data can be used to uncover the pollution history of an aquatic system, because they are more widely available, and more reliable than dissolved metal concentrations in a water body (Zwolsman et al. 1996).
Mangroves are interface ecosystems that occur at the confluence of rivers and seas in tropical and subtropical areas (Lugo and Snedaker 1974). Mangrove forests are considered to be highly productive ecosystems (Clough 1992). With growing industrial activity, worldwide mangroves can also contribute to the cycling of certain pollutants in the local environment (Murray 1985), either as sinks or conveyors of pollutants to marine food chains (Harbison 1981; Lacerda 1998). The value of mangrove communities, and particularly of mangrove forest sediments, as a buffer between potential sources of metalliferous pollutants and marine ecosystems has been noted previously (Harbison 1981, 1986; Saenger 2002; Ong Che 1999; Kuitwagen et al. 2008). Due to their inherent physical and chemical properties, mangrove sediments have an extraordinary capacity to accumulate materials discharged to the near shore marine environment (Harbison 1986). Mangrove sediments are anaerobic and reduced and their richness in sulphide and organic matter favor the retention of water-borne heavy metals (Silva et al. 1990; Tam and Wong 2000), and the subsequent oxidation of sulphides between tides allows metal mobilization and bioavailability (Clark et al. 1998).
In India, mangroves occur on the west coast, on the east coast and on Andaman and Nicobar Islands, but in many places they are highly degraded. According to Government of India statistics (1987), India has lost 40 % of its mangrove area in the last century. The National Remote Sensing Agency recorded a decline of 7,000 ha of mangroves in India within a 6-year period from 1975 to 1981. In Andaman and Nicobar Islands, about 22,400 ha of mangroves was lost between 1987 and 1997. Mangroves in Tamil Nadu exist on the Cauvery deltaic areas viz., Vedaranyam, Kodiakarai (Point Calimere), Muthupet, Chatram, and Tuticorin. Geochemical studies on the mangrove ecosystem are vital as pollution level and the source can be identified from the sediments. The spatial and temporal variability observed in sediment contaminant concentrations reflects the complexity of estuarine biogeochemical cycles and the various sources of contamination. Further by studying the dynamic nature of ecosystem both spatially and geochemically it is possible to understand the present and future trend in degradation. The aim of the study was to determine historic levels of seven commonly polluting metals: manganese (Mn), copper (Cu), zinc (Zn), nickel (Ni), lead (Pb), chromium (Cr), and cadmium (Cd); and to assess the status of Muthupet mangrove through computation of various pollution indices.
Regional setting
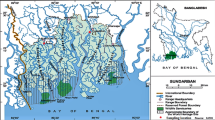
The study area lies along the southeastern coast of India, also known as Coromandel Coast, having a reverse “L” shaped structure (Fig. 1). It lies on the Cauvery river delta passing through Nagapatinam and Thiruvarur districts. The study area extends from 79°19′41″ to 79°38′24″ along East and 10°16′23″ to 10°24′43″ along North. This area is drained by distributaries of Cauvery, i.e., Agniar, Nasuvunniyar, Kattar, Paminiyar, Korayar, Kilaithangiyar, Marakkakoryar and Varavanar. According to international classification, study area falls in the category of medium tropical transitional bio-climate, characterized by monthly average temperatures above 27 °C. The total annual rainfall varies from 1,000 to 1,500 mm. The average humidity of the area is 75 %. The maximum elevation of the study ranges between 0 and 6 m.
Mangroves are present near Atirampattinam and Mullippallam creek. The whole area is marshy up to 1,000 m from the coast. From Point Calimere to Atirampattinam, the coast stretches for almost 100 km and it known as the “Great Vedaranniyam Salt Swamp”. The width generally varies between 8 and 10 km from the coast and the uniqueness of the swamp is the occurrence of two periodical high tides, i.e., the Chittrai parvam and Visakhavellam, happening around the full moon day in May and June, respectively. Presently, wetland is occupied by six species of mangrove vegetation, namely, Avicennia marina, Acanthus ilicifolius, Aegiceras corniculatum, Excoecaria agallocha, Rhizophora mucronata and Lumnitzera racemosa. In Muthupet wetland, A. marina represents 95 % of mangrove species. The major industry in the vicinity of the study area is bromine extracting. Saltpans have been present for a long time and recently aquaculture farms have come into existence, affecting the adjacent mangrove ecosystem. People belonging to 26 hamlets of 16 revenue villages with a total population of about 35,900 depend on the fishery and forestry resources of Muthupet. Fishing in mangrove waters is only seasonal. A preliminary estimate indicates that about 106 tons of fish and shellfish are harvested every year from the Muthupet mangroves. Pandian (1985) recorded 73 species of finfish in Muthupet mangrove, but according to the local fishermen only 30 species are commonly occurring. Apart from fish, the lagoon is also found to be rich in prawns and crabs (ICMAM 2005). For the benefit of the local fishermen, a fishing harbor is constructed under the Sethusamudram Ship Channel Project. Furthermore, fertilizers from the agricultural areas drain into the sea. The other sources of pollution include transport of pollutants from Gulf of Mannar and Tuticorin.
Materials and methods
Four core samples were collected with the help of a fiber boat across the study area during June 2008 (Table 1). C1 was collected from Muthupet Lagoon to understand the influence of tides and the input from the coastal environment. C2 was retrieved from the adjoining areas that are drained by minor rivers, which flow during monsoon periods and small scale aquafarms are found about 500 m away from C2. C3 is located very close to aquafarm and adjacent to dense mangroves. C4 is close to the fishing harbor. The cores were collected by driving an acid washed PVC pipe of 2.5 m length and 2.5 in. diameter. The geochemical data presented in this study has not been corrected for compaction, as it is likely to be uniform down the length of the core (Clark et al. 1998). All the cores were frozen prior to subsampling (slicing) at 2.5 cm intervals. To avoid contamination from the PVC pipe, a thin film of sediment adjacent to the pipe was left in the core itself. The subsamples were stored in clean polythene covers.
A representative portion of each sample was used for the determination of sediment composition (Krumbein and Pettijohn 1938). A portion of the sample was finely powdered in an agate mortar and used for chemical analysis. Organic carbon and calcium carbonate was determined following the procedure of Loring and Rantala (1992) and Gaudette et al. (1974), respectively. Analyses of metals (Mn, Cu, Zn, Ni, Pb, Cr and Cd) were carried out by flame atomic absorption spectroscopy (Varian Spectrometer AA 200), after total digestion following the procedure of Agemian and Chau (1976) and Taliadouri (1995). International standard reference materials were used to calibrate the instrument viz., BEST and BCSS1 and the metal concentrations were within the reported values except for Zn and Mn, which were consistently higher by about 10 % and lower by about 5 % than the standard values, respectively. The indices used in the present study are geoaccumulation index (Muller 1979), anthropogenic factor (Szefer et al. 1998), and enrichment factors were interpreted as suggested by Bloundi et al. (2009), contamination factor and contamination degree (Hakanson 1980), pollution load index (Tomlinson et al. 1980), and metal pollution index (Usero et al. 1997).
Results and discussion
Sediment composition
In all the cores, mud (silt + clay) dominates, reflecting calm conditions at the time of deposition (Fig. 2). However, there are local variations in sediment composition from core to core, and also at different depths within the core. The sand content ranges from 0.73 to 23.21 %. The minimum percentage of sand is recorded in C2 at 52.50 cm (last subsample) and the maximum percentage is recorded in C4 at 2.5 cm depth (first subsample). The mud (silt + clay) ranges from 76.79 to 99.27 % with the lowest and the highest values occurring in C4 at 2.5 cm depth and C2 at 52.50 cm, respectively. Only few samples contain mud content <80 %. Most of the samples fall in the field of either sandy silt or clayey silt indicating domination by silt. Hydrodynamic processes induce the accumulation of fine sediments associated with organic matter in zones characterized by lower hydrodynamic energy.
Significantly lower values for sand in the top layers of C1 along with corresponding increase in silt predicts an increased inflow of fine sediments to the coastal area. An extreme event is evident beyond 60 cm which is most likely due to a highly turbulent water column, which may be attributed to the tsunami which occurred in southeast India during 26th June 1941 (Madan Kumar 2006). Considering the sedimentation rate of 0.95 cm/year (Madan Kumar 2006), the sediments at 57.5–60 cm depth might have been deposited in 1941 and higher content of sand at this depth correlated with the prevalence of high-energy environment. Hence the significant change in concentration is related to a major paleo-washover event, tsunami. In C2, an increased sand and silt content between 0 and 5 cm interval with corresponding decrease in clay points to the presence of resuspension of clay due to tidal and wave action. C3 records low clay, moderate sand, and higher levels of silt again implying relatively calm environment of deposition due to the presence of dense mangroves for a long time. Highest sand is present in C4 owing to the apparent closeness to the harbor, wherein turbulence is quite common. While the eastern coastline of India runs in the north–south direction, the part of the coastline where this core was collected runs in the east–west direction facilitating the movement of sediment through wave action.
Calcium carbonate and organic carbon
The calcium carbonate in the core sediments of the Muthupet is found to be low (Fig. 3). The estimated values of carbonate in the surface sediments range from 0.32 to 6.61 %, with an average content of 2.65 %. In C1, CaCO3 in the upper half, i.e., from 2.5 to 22.5 cm reveals a downward sloping trend, with values ranging from 1 to 1.86 % which is well supported by the low sand contents (8.63–10.68 %) at these depths. Increase of CaCO3 is observed from 25 to 60 cm, with CaCO3 content of 1.92–3.18 %. The steady increase in CaCO3 is attributed to the dissolution of CaCO3 at the top levels. Moreover, this is due to reprecipitation of carbonates in the reduced sediment layers because of increase in alkalinity, which may be generated by sulphite reduction (Oenema et al. 1988; Gaillard et al. 1989). The sudden decrease in sand and CaCO3 content at 60.0 cm indicates that it could also be due to a sudden change in environment in the mangrove region or it could be related to a paleo-washover event, may be a tsunami, as discussed above in the grain size analysis (Shumilin et al. 2002). In C2, low values encountered at intermediate depths indicate the dissolution of CaCO3 at these depths due to change in pH conditions. Average CaCO3 concentration in C3 is 3.55 % which is higher than C1 and C2. Surface enrichment of CaCO3 is observed in C3 (6.61 %). C4 has the highest CaCO3 when compared to the other cores. Surface enrichment occurs till a depth of 7.5 cm with values ranging from 5.72 to 6.22 %. The high values in the surface sediment of C3 and C4 are due to the location of the core sample on the western side of the salt marshes and also due to high sand content at these depths. The CaCO3 content is high where higher sand content is observed indicating that carbonates are attached to coarse sediments.
OC concentration varies from 0.86 to 6.70 % (C1), 2.57 to 6.09 % (C2), 2.1 to 7.97 % (C3), and 0.83 to 4.76 % (C4) as shown in Fig. 3. Average organic carbon content is 3.66 % in C1, 4.55 % in C2, 4.28 % in C3, and 2.37 % in C4. OC contents are high where CaCO3 content is low and mud content is high signifying that they are attached to finer particles (Jonathan et al. 2004). The clayey silt and sandy silt sediments in these regions also favor the deposition of organic debris. Surface enrichment of OC content is seen at 5.0–10.0 cm depth (5.59–6.70 %). The high concentration observed in the upper layer is due to the adsorption and incorporation of organic materials from the overlying water column with rapid accumulation of fine-grained terrigenous inorganic material and reducing environment prevailing down the core. The organic carbon values reveal a steady increase from 4.40 to 6.09 % at 35.0–52.5 cm in the bottom part of C2, which is due to the erosion of mangrove sediments by heavy rainfall and the degradation of mangrove leaves (Dittmar 1999). Low values (2.85–2.94 %) at upper layers of C3 illustrate that it could be due to the changes in salinity and a much higher tidal exchange and increase in water flow. Overall, the low organic carbon in C4 can be attributed to the sandy nature of the core sample.
Down core variations in trace metal concentrations
Due to their stability within the sedimentary column, most of the contaminants can leave their fingerprint in sediments, i.e., when there is no or insignificant post-depositional mobility. Metals enter the environment and oceans by two means: natural processes (including erosion of ore-bearing rocks, wind-blown dust, volcanic activity and forest fires); and processes derived from human activities by means of atmospheric deposition, rivers, and direct discharges or dumping (Clark 2001). The spatial and temporal geochemical records of sediments are deemed to be vital to evaluate recent contamination levels and pollution histories within estuarine, coastal and shelf regions worldwide (Szefer et al. 1995; Zwolsman et al. 1996; Nolting et al. 1999; Selvaraj et al. 2004).
In an attempt to compensate for the natural variability of trace metals in the core sediments, normalization was carried out so that, any anthropogenic metal contributions may be detected and quantified. Normalization is done commonly by using geochemical reference elements such as Sc, Mn, Ti, Al and Fe (Pacyna and Winchester 1990; Quevauviller et al. 1989; Reimann and de Carital 2000; Schiff and Weisberg 1999; Sutherland 2000; Sugirtha Kumar and Patterson Edward 2009). In the present study, Al was used as the reference element. The metal concentrations in average shale (Turekian and Wedepohl 1961) or Continent Crust Values (CCV) after Taylor (1964) listed in Table 2 were taken to represent the uncontaminated levels of the trace metals (Salomons and Forstner 1984). The down core variations in heavy metals are illustrated in Fig. 4 and Table 3.
Overall observation of Mn/Al ratios in the study area (Fig. 4) exhibit concentrations lower than CCV and only in certain depths of cores, in C2 and C4 it is above the crustal average. According to Calvert and Pedersen (1993) the coarse-grained ferromagnesian minerals in the shallow coastal zone, do not contain high Mn concentration and the degree of enrichment in surficial sediments is controlled by the depth of penetration of dissolved oxygen from overlaying water, which depends upon the flux of organic matter to the sediments. In addition, high Mn/Al ratios in C1 (7.5–10.0 cm) and C2 (0–2.5 and 5.0–20.0 cm) is due to the redox potential affecting upward diffusion, which together with reductive solubilization processes in the lower sediment layers cause a permanent deposition of the mobile Mn compounds in the lower most horizon of the oxidized sediment (Froelich et al. 1979). The Cu/Al ratios indicate that external input of Cu to the study area. Important anthropogenic inputs of copper in estuarine and coastal waters include sewage sludge dumpsites, municipal waste discharge, and antifouling paints (Kennish 1997). The down core profiles of Cu/Al ratios in C1 reveal a twofold increase over the CCV at 0–35.0 cm (11.57–16.01 × 10−4) except at 5.0–7.5 cm. In the lower half of the core, the Cu/Al ratio differs from 8.31 to 10.80 × 10−4 again higher than the crustal average. In C3, the Cu/Al ratio is well above the CCV from surface to 80.0 cm depth interval (7.23–12.64 × 10−4) except for low concentration (6.02 × 10−4) at 60–62.5 cm depth. Below 80 cm depth the values are well below the crustal average.
The average Zn/Al ratio in the core samples are 3.75 × 10−3, 7.19 × 10−3, 7.24 × 10−3 and 10.66 × 10−3 in C1, C2, C3 and C4 respectively. C1 reveals a low average than the other core samples, but with almost threefold increase over the reference value. Core 2 depicts a seven-time increase (7.19 × 10−3) over the crustal average (0.85 × 10−3). The Zn/Al ratio in C3 show 8–10 times increase over crustal average at the central part 20.0–60.0 cm depth (8.31–10.67 × 10−3), whereas the lower part shows a sixfold increase at 62.5 cm till the bottom (5.87–7.01 × 10−3). Zn/Al ratio in C4 exhibits a ten fold increase than the crustal average at the depth of 17.5–70.0 cm (10.20–13.41 × 10−3) except for low value of 6.52 × 10−3 at 60.0–62.5 cm depth. Higher concentration in the central part than the lower part can be attributed to the maritime activities and the anti corrosive paints used for various purposes, which could have accumulated in the region. Ni/Al shows a double fold increase in concentration compared to CCV (more than 18 × 10−4) except at 57.5–60.0 cm, 70.0–75.0 cm in C1; 27.5–32.5 cm in C2; 0–12.5 cm, 60.0–62.5 cm, 75.0–77.5 cm in C3 and 10.0–12.5 cm, 57.5–70.0 cm in C4, which exhibit concentrations above the crustal average. According to Zwolsman et al. (1996), the increase of Ni content at subsurface layers is due to Ni sorption onto manganese oxyhydroxides.
Pb/Al ratios for the core samples vary from 4.99 to 10.87 × 10−4, 7.37 to 17.13 × 10−4, 4.14 to 11.51 × 10−4 and 11.47 to 24.70 × 10−4 in C1, C2, C3 and C4 respectively. In C1, Pb/Al ratio reveals high concentration at the surface (10.87 × 10−4) and 47.5–57.5 cm depth (10.08–10.52 × 10−4) which is greater than the average crustal value (1.52 × 10−4). At other depths, the enrichment is 7–9 times greater than the crustal values. In C3, higher values in the upper half (7.37–11.57 × 10−4) and lower in bottom half (7.08–8.71 × 10−4) with least value (4.14 × 10−4) occurring at 60.0–62.5 cm depth is observed. In C4, the average Pb/Al ratio is 19.33 × 10−4 (maximum concentration). The down core profile shows a gradual increase with depth till 37.5 cm (14.99–24.20 × 10−4). In the lower half from 37.5 cm till the last subsample, the values are marginally lower ranging between 16.33 and 21.35 × 10−4. Abu-Hilal (1987) and Laxen and Harrison (1983) attributed high Pb concentrations to several sources such as boat exhaust systems, spillage of oil, and other petroleum products from mechanized boats employed for fishing, and the discharge of sewage effluents into water.
Cr/Al ratios in the study area range from 1.60 to 9.24 × 10−3, 2.86 to 4.59 × 10−3, 1.79 to 4.08 × 10−3 and 1.80 to 4.38 × 10−3 with an overall average of 3.89 × 10−3, 3.41 × 10−3, 2.81 × 10−3 and 3.29 × 10−3 for C1, C2, C3 and C4 respectively. Cr/Al ratios in all the cores show higher values than the crustal average with very high values at the surface layer. The Cr/Al ratios values in the upper half and central part of the core indicates that Cr contamination is taking place continuously. Cd/Al ratio from the surface to 30 cm depth in C1 varies from 13.52 to 19.94 × 10−6 and from thereon till the bottom there is a twofold reduction (6.84–9.92 × 10−6) except at 57.5–60.0 cm depth. In C2, high Cd/Al ratio is observed at 5.0–10.0 cm, 32.5–35.0 cm (20.17–21.21 × 10−6), whereas at the other depths, it varies from 10.95 to 18.77 × 10−6 indicating a four fold increase over the reference value (2.43 × 10−6). C3 illustrates that the bottom subsamples records a low ratio than the upper half. Similar to C2, C4 also records a high Cd/Al ratio (12.85 × 10−6) with greater values occurring in 0–40 cm depth.
Pollution status inferred from indices
Various authors (Salomons and Forstner 1984; Muller 1979; Hakanson 1980) have proposed pollution impact scales or ranges to convert the calculated numerical results into broad descriptive bands of pollution, ranging from low to high intensity. Turekian and Wedepohl’s (1961) average shale value was used as the background concentration for computation of the indices.
Geoaccumulation index
The Geoaccumulation index (I geo) introduced by Muller (1979) was used to assess pollution status in the mangrove sediments. I geo is expressed as:
where, C n, measured concentration of heavy metal in the sediment; B n , geochemical background value in average shale (Turekian and Wedepohl 1961) of element n, 1.5 is the background matrix correction in factor due to lithogenic effects.
Geoaccumalation index classes to assess sediment quality are as follows:
-
<0 Uncontaminated
-
1 Uncontaminated to moderately contaminated
-
2 Moderately contaminated
-
3 Moderately to highly contaminated
-
4 Highly contaminated
-
5 Highly to very highly contaminated
-
6 Very highly contaminated
Sediments of Muthupet mangroves fall under class 0 or class 1. C1 is uncontaminated for all the metals except for Cr in the surface sediment (0–5 cm) which falls under the category uncontaminated to moderately contaminated. Ni and Pb are found to lie in class 1 in C2. C3 and C4 show uncontamination to moderate contamination for Zn and Pb. On comparison, Pb is lying in class 1 for all the cores except C1 indicating the extent to which anthropogenic activities have an impact on the mangrove ecosystem.
Anthropogenic factor
The anthropogenic factors (AF) of elements in the cores were calculated as suggested by Szefer et al. (1998)
where C s and C d refer to the concentrations of the elements in the surface sediments and at depth in sediment column. According to Ruiz-Fernández et al. (2001),
-
AF is >1 for a particular metal, it means contamination exists.
-
AF is ≤1, there is no metal enrichment of anthropogenic origin.
The AFs of Muthupet mangroves are tabulated in Table 4. Mn in C1 and C3, Cu in C4, Zn in C3 and C4, Pb in C4 are <1 indicating the absence of metal enrichment from anthropogenic origin. The highest recorded AF value is 16.32 for Mn in C2 followed by 3.12 for Cr in C1. The calculated AF’s are found in the following sequences: Mn > Cd > Cr > Cu > Ni > Pb > Zn.
Enrichment factor
The enrichment factor (EF) (Szefer et al. 1998) permits to calculate the heavy metal contamination by distinguishing natural changes from those induced by anthropogenic activity. Al has been chosen as normalization element because of its origin being exclusively lithospheric (Bloundi et al. 2009). In the present study, Al was used since Al is closely associated with alumino-silicate fraction that is dominant metal-bearing phase of the sediment. EF is calculated according to the following formula
where (Me/Al)Sample, ratio of the metal (Me) to Al in samples of interest and (Me/Al)Reference, natural reference value of the metal to Al.
EF values were interpreted according to Bloundi et al. (2009) where EF < 1 indicates no enrichment; 1–3 is minor; 3–5 is moderate; 5–10 is moderately severe; 10–25 is severe; 25–50 is very severe; and >50 is extremely severe. EF values for Muthupet mangroves are tabulated in Table 5. EF ranges between no enrichment to severe enrichment for the mangrove sediments. Mn shows no enrichment in C1 and C3, whereas in C2 and C4, a few subsamples fall under the category of minor enrichment. C1 comparatively shows higher EF values for Cu showing moderately severe enrichment. The maximum (7.63) is recorded in the surface sediment indicating recent activity. Minor enrichment of Cd is seen in C2, C3 and C4 with few samples showing moderate enrichment. Zn and Pb record the highest EF values and found to vary between moderately severe and severe enrichment. Minor to moderate enrichment is observed for Ni and Cr whereas for Cd it reaches up to moderately severe enrichment. The enrichment factor of the metals in the entire study area is found to be of the order: Zn > Pb > Cd > Cr > Ni > Cu > Mn.
Contamination factor and degree of contamination
Hakanson (1980) suggested a contamination factor (C if ) and the degree of contamination (C d) to describe the contamination of given toxic substance as follows:
where, \(C_{0 - 1}^{\text{i}}\), mean content of the substance; C in , reference value for the substance.
Table 6 gives the details about contamination factor and degree of contamination.
Mn, Cu and Ni (C4) show a low degree of contamination (Table 7) and all the other metals record a moderate degree of contamination. The CF yielded the following ranking Zn > Pb > Cd > Cr > Ni > Cu > Mn similar to EF. The core wise ranking for contamination degree is C3 > C4 > C1 and C2. The high metal concentration C3 and C4 can be attributed to apparent closeness of core collection to aquafarms and fishing harbor.
Pollution load index
Pollution load index (PLI) was computed according to Tomlinson et al. (1980) from the following equation
where, CF, contamination factor, C metal, concentration of pollutant in sediment, C background, background value for the metal, n, the number of metals.
PLI value of >1 is polluted whereas <1 indicates no pollution.
The results of PLI calculation for Muthupet mangroves are tabulated in Table 7 and for all the cores the value is greater than unity indicating pollution with the order as C3 > C4 > C1 > C2.
Metal load index
The metal concentration was evaluated using the metal pollution index (MPI) calculated according to Usero et al. (1997) with the formula:
where, M n, metal concentration, n, number of metals considered for the study.
MPI also yielded a sequence similar to that of PLI viz. C3 > C4 > C1 > C2 (Table 7), since these two indices are mathematically proportional to each other and the ratios MPI/PLI does not significantly change (about 39.8). Although computation of this index is simple it does not compare the contaminant concentration with neither baseline/guidelines nor with a threshold classification from unpolluted to high pollution (Caeiro et al. 2005).
The difference in these trend sequence patterns may be attributed to the different methods of calculation. In the case of the EF, the enrichment of the elements is normalized relative to Al in the surficial sediments whereas in the case of the anthropogenic factor, the enrichment of the elements is normalized relative to depth in the sediment cores (Szefer et al. 1998). CF is calculated based on the mean concentration of the metal whereas PLI is calculated based on CF (Seshan et al. 2011).
Statistical analysis
The study of the inter-element relationship has gained attention worldwide to know the different parameters that correlate with one another in the environment. Especially, to know the various behavior and diagenetic changes in the mangrove environment it is very important to have knowledge of how they behave in different environmental conditions. Pearson correlation matrix was generated for the present study using SPSS (Table 8).
Coarse grained fraction sand indicates positive correlation with CaCO3 except C2 suggests that the distribution of CaCO3 is mainly due to the shell materials in the sandy sediments and it is controlled independently (Rao 1978). The positive relationship of CaCO3 versus Cu and Cd in C3 and C4 indicates that these trace metals have also been adsorbed by the calcareous materials present in the study area. In addition, the association of acid leachable trace metals shows that substantial portions of it occur in the calcareous materials indicating some anthropogenic input in the study area (Abu-Hilal 1987; Jonathan and Ram-Mohan 2003). Correlation analysis indicates that OM in the study area does not play a major role in the study area except for C1, which is located in the mangrove region. The relationship of sand with Cu, Cd, Cr (except C1) reflects that these trace metals are held in the coarse-grained fractions. The inter-relationships among metals viz., Cu with Zn and Cd, Zn with Ni and Cd, Ni with Cr and Cd, Pb with Cr, etc., imply that the source of these elements could be from the river or localized contamination (Willey and Fitzgerald 1980; Turner 2000). The differences in correlation among the above elements reflect differential behavior and environmentally specific, sources and geochemical behaviors, which require further examination.
Conclusions
Muthupet mangroves situated on the Cauvery delta away from industrial hubs were assessed for determining the historic levels of commonly polluting metals. The prominent observation made during the study is a paleo-washover event, i.e., a tsunami dating back to 1941. The study area is dominated by mud (silt + clay), which in turn favors accumulation of heavy metals and organic carbon. An index analysis approach was attempted to evaluate the status of heavy metal pollution in Muthupet. The geoaccumulation indices suggest that fine sediments in the study area range from unpolluted to moderately polluted with respect to the analyzed metals. The calculated AF’s are found in the following sequences: Mn > Cd > Cr > Cu > Ni > Pb > Zn. Zn and Pb record the highest EF values, which vary between moderately severe and severe enrichment. C3 and C2 are the most polluted and the least polluted cores, respectively. The results indicate the influence of anthropogenic activities on the mangrove sediments. Further mangrove sediments have been trapping the heavy metals for a long time thereby affecting the sensitive ecosystem.
References
Abu-Hilal AH (1987) Distribution of trace elements in nearshore surface sediments from the Jordan Gulf of Aqaba (Red Sea). Mar Poll Bull 18(4):190–193
Agemian H, Chau ASY (1976) Evaluation of extraction technique for the determination of metals in aquatic sediments. Analyst 101:761–767
Bloundi MK, Duplay J, Quaranta G (2009) Heavy metal contamination of coastal lagoon sediments by anthropogenic activities: the case of Nador (East Morocco). Environ Geol 56:833–843
Burger J, Gochfeld M (2003) Spatial and temporal patterns in metal levels in eggs of common terns (Sterna hirundo) in New Jersey. Sci Total Environ 311:91–100
Caeiro S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Medeiros G, Painho M (2005) Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol Ind 5:151–169
Calvert SE, Pedersen TF (1993) Geochemistry of recent oxic and anoxic marine sediments: implications for the geological record. Mar Geol 113:67–88
Clark RB (2001) Marine pollution. Oxford University Press, Oxford
Clark MW, McConchie DM, Lewis DW, Saenger P (1998) Redox stratification and heavy metal partitioning in Avicennia dominated mangrove sediments: a geochemical model. Chem Geol 149:147–171
Clough BF (1992) Primary productivity and growth of mangrove forests. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystem. American Geophysical Union, Washington, DC, pp 249–255
Dittmar T (1999) Outwelling of organic matter and nutrients from a mangrove in north Brazil: evidence from organic tracers and flux measurements. ZMT-contribution 5, Centre for Tropical Marine Ecology, Breman, 229
Froelich PN, Klinkhammer GP, Bender ML, Luedtke NA, Heath GR, Cullen D, Dauphin P, Hammond D, Hartman B, Maynard V (1979) Early oxidation of organic-matter in pelagic sediments of the Eastern Equatorial Atlantic-suboxic diagenesis. Geochim Cosmochim Acta 43:1075–1090
Gaillard JF, Pauwels H, Michard G (1989) Chemical diagenesis in coastal marine sediments. Oceanologia Acta 12:175–187
Gaudette HE, Flight WR, Toner L, Folger DW (1974) An inexpensive titration method for the determination of organic carbon in recent sediments. J Sediment Petrol 44:249–253
Government of India (1987) Mangroves in India—status report. Ministry of Environment and Forests, New Delhi, pp 52–55
Hakanson L (1980) Ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14(5):975–1001
Harbison P (1981) The case for the protection of mangrove swamps: geochemical considerations. Search 12:273–276
Harbison P (1986) Mangrove muds—a sink and a source for trace metals. Mar Poll Bull 17:246–250
ICMAM (2005) Ecosystems modelling for Muthupet Lagoon along Vedernayam coast (Tamil Nadu), 9
Jonathan MP, Ram-Mohan V (2003) Heavy metals in sediments of the inner shelf off the Gulf of Mannar, southeast coast of India. Mar Poll Bull 46:258–268
Jonathan MP, Ram-Mohan V, Srinivasalu S (2004) Geochemical variations of major and trace elements in recent sediments, off the Gulf of Mannar, the southeast coast of India. Env Geol 45:466–480
Kennish MJ (1997) Heavy metals. In: Practical handbook of estuarine and marine pollution. CRC Press Marine Science Series, Boca Raton, pp 253–327
Krumbein WC, Pettijohn FJ (1938) Manual of sedimentary petrography. D. Appleton Century Co., Inc., New York, pp 549–551
Kuitwagen G, Pratap HB, Covaci A, Wendelaar Bonga SE (2008) Status of pollution in mangrove ecosystems along the coast of Tanzania. Mar Pollut Bull 56:1022–1042
Lacerda LD (1998) Biogeochemistry of trace metals and diffuse pollution in Mangrove ecosystems. International Society for Mangrove Ecosystems, Okinawa
Laxen DPH, Harrison RM (1983) The physio-chemical speciation of selected metals in the treated effluent of a lead-acid battery manufacturer and its effect on metal speciation in the receiving water. Water Res 17:71–80
Loring DH, Rantala RTT (1992) Manual for geochemical analyses of marine sediments and suspended particulate matter. Earth Sci Rev 32:235–283
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annu Rev Ecol Syst 5:39–64
Madan Kumar M (2006) Chemical diagenesis of wetland sediments near Muthupet mangroves, southeast coast of India. Ph.D. thesis, Anna University
Muller G (1979) Schwermetalle in den Sedimenten des Rheins Veranderungen seit. Umschau 79(24):778–783
Murray F (1985) Cycling of fluoride in a mangrove community near a fluoride emission source. J Appl Ecol 22:277–285
Nolting RF, Ramkema A, Everaats JM (1999) The geochemistry of Cu, Cd, Zn, Ni and Pb in sediment cores from the continental slope of the Banc d’Arguin (Mauritania). Cont Shelf Res 19:665–691
Oenema O, Steneker R, Reynders J (1988) The soil environment of the intertidal area in the Westerschelde. Hydrobiol Bull 22:21–30
Ong Che RG (1999) Concentration of 7 heavy metals in sediments and mangrove root sediments from Mai Po, Hong Kong. Mar Pollut Bull 39:269–279
Pacyna JM, Winchester JW (1990) Contamination of the global environment as observed in the Arctic. Palaeogeogr Palacoclimatol Palaeoecol 82:149–157
Pandian C (1985) Icthyofauna of Muthupet estuary with special reference to pearl spot, Etroplus suratensis Bloch. Ph.D. thesis
Quevauviller P, Lavigne R, Cirtex I (1989) Impact of industrial and mine drainage wastes on the heavy metal distribution in the drainage basin and estuary of the Sado River (Portugal). Environ Pollut 59:267–286
Rao CHM (1978) Distribution of CaCO3, Cu2+ and Mg2+ in sediments of the northern half of western continental shelf of India. Indian J Mar Sci 7:151–154
Reimann C, de Carital P (2000) Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ Sci Technol 34:5084–5091
Ruiz-Fernández AC, Páez-Osuna F, Hillaire-Marcel C, Soto-Jime′nez M, Ghaleb B (2001) Principal component analysis applied to assessment of metal pollution from urban wastes in the Culiacán river estuary. Bull Environ Contam Toxicol 67:741–748
Saenger P (2002) Mangrove ecology, silviculture and conservation. Kluwer Academic Publishers, Dordrecht, p 360
Salomons W, Forstner U (1984) Metals in the hydrocycle. Springer Publishing Co., Berlin 349
Schiff KC, Weisberg SB (1999) Iron as a reference element for determining trace metal enrichment in Southern California coastal shelf sediments. Mar Environ Res 48:161–176
Selvaraj KV, Mohan Ram, Szefer Piotr (2004) Evaluation of metal contamination in coastal sediments of the Bay of Bengal, India: geochemical and statistical approaches. Mar Pollut Bull 49:174–185
Seshan BRR, Natesan Usha, Deepthi K (2011) Geochemical evidence of terrigenous influence in sediments of Buckingham canal. Environ Earth Sci, Ennore. doi:10.1007/s12665-011-1258-7
Shumilin EV, Carriquiry JD, Camacho-Ibar VF, Sapozhnikov D, Kalmykov S, Sánchez A, Aguiňiga-Garcia S, Sapozhinikov YA (2002) Spatial and vertical distributions of elements in sediments of the Colorado river delta and upper Gulf of California. Mar Chem 79:113–131
Silva CAR, Lacerda LD, Rezende CE (1990) Heavy metal reservoirs in a red mangrove forest. Biotropica 22:339–345
Sugirtha Kumar P, Patterson Edward JK (2009) Assessment of metal concentration in the sediment cores of Manakudy estuary, southwest coast of India. Indian J Mar Sci 38(2):235–248
Sutherland RA (2000) Bed sediment—associated trace metals in an urban stream Oahu, Hawaii. Environ Geo 39:611–627
Szefer P, Glasby GP, Pempkowiak J, Kaliszan R (1995) Extraction studies of heavy-metal pollutants in surficial sediments from the southern Baltic Sea off Poland. Chem Geol 120:111–126
Szefer P, Geldon J, Ahmed Ali A, Paez Osuna F, Ruiz-Fernandes AC, Guerro Gaivan SR (1998) Distribution and association of trace metals in soft tissue and bussus of Myeella strigata and other benthal organisms from Mazatlan harbour, mangrove lagoon of the northwest coast of Mexico. Environ Int 24(3):359–374
Taliadouri FV (1995) A weak acid extraction method as a tool for the metal pollution assessment in surface sediments. Mikrochim Acta 119:243–249
Tam NFY, Wong YS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamp. Environ Pollut 110:195–205
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28:1273–1285
Tomlinson DC, Wilson JG, Harris CR, Jeffery DW (1980) Problems in the assessment of heavy metals levels in estuaries and the formation of a pollution index. Helgol Wiss Meeresunters 33(1–4):566–575
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192
Turner A (2000) Trace metal contamination in sediments from UK Estuaries: an empirical evaluation of the role of hydrous iron and manganese oxides. Estuar Coast Shelf Sci 50:355–371
Usero J, Gonzalez-Regalado E, Gracia I (1997) Trace metals in the bivalve mollusks Ruditapes descussatus and Ruditapes philippinarum from the Atlantic Coast of Southern Spain. Environ Int 23:291–298
Willey JD, Fitzgerald RA (1980) Trace metal geochemistry in sediments from the Miramichi estuary, New Brunswick. Can J Earth Sci 17:254–265
Zwolsman J, Van Eck G, Burger G (1996) Spatial and temporal distribution of trace metals in sediments from the Scheldt estuary, south-west Netherlands. Estuar Coast Shelf Sci 43:55–79
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Natesan, U., Madan Kumar, M. & Deepthi, K. Mangrove sediments a sink for heavy metals? An assessment of Muthupet mangroves of Tamil Nadu, southeast coast of India. Environ Earth Sci 72, 1255–1270 (2014). https://doi.org/10.1007/s12665-014-3043-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-014-3043-x