Abstract
Groundwater is the major source of fresh water in regions where there is inadequate surface water resources. Forty-seven groundwater samples were collected from Lower Ponnaiyar basin, Cuddalore District, south India, during the premonsoon (PRM) and postmonsoon (POM) seasons of 2005. Out of 47 groundwater samples, 15 samples showing higher nitrate concentration were those collected during PRM 2005. Microbial analysis of these samples was carried out by employing 16S rRNA gene sequence tool. Detailed analysis was conducted to determine the hydrogeochemical processes and microbial contamination responsible for deterioration of quality. The abundance of the ions during PRM and POM are in the following order: Na > Ca > Mg > K = Cl > HCO3 > SO4 > CO3. The dominant water types in PRM are in the order of NaCl > CaMgCl > mixed CaNaHCO3, whereas during POM NaCl > CaMgCl > mixed CaNaHCO3, and CaHCO3. However, NaCl and CaMgCl are major water types in the study area. The quality of groundwater in the study area is mainly impaired by surface contamination sources, mineral dissolution, ion exchange and evaporation. Groundwater chemistry was used to assess quality to ensure its suitability for drinking and irrigation, based on BIS and WHO standards. Suitability for irrigation was determined on the basis of the diagram of US Salinity Laboratory (USSL), sodium absorption ratio (SAR), residual sodium carbonate (RSC), and Na%. According to SAR and USSL classification, 27.66% (PRM) and 40.43% (POM) of samples fall under C3S2 category, indicating high salinity and medium sodium hazard, which restrict its suitability for irrigation. Microbiological analysis and its effects on the water quality were also addressed. The 16S rRNA gene sequences of 11 bacterial contaminants exhibited five groups with 11 operational taxonomic units with aerobic and facultatively anaerobic organisms. The presence of aerobic organisms in the groundwater samples reflects the active conversion of ammonia to nitrite by Nitrosomonas sp. which is further converted to nitrates by other organisms. Further the presence of nitrate reducers could also play a role in the process of conversion of nitrate to ammonia and nitrate to molecular nitrogen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The quality of groundwater depends on a large number of individual hydrological, physical, chemical, and biological factors. In the recent years, the growth of industrial technologies, population, and water usage has increased the stress upon the land and water resources. Locally, the quality of groundwater has been degraded in many parts of the world. Groundwater quality is governed by lithology, groundwater flow, nature of geochemical reaction, residence time, solubility of salts, and human activities (e.g., Karanth 1987; Rajmohan et al. 2009; Nagarajan et al. 2010; Prasanna et al. 2010). The adverse effects on groundwater quality are the results of human activity (e.g., Almasri 2007), unintentionally due to agriculture, in the form of nitrate (e.g., Jordan and Smith 2005; Jeevanandam et al. 2006), domestic and industrial effluents, and unexpectedly by sub-surface or surface disposal of sewage and industrial wastes (e.g., Kelly et al. 1996). Atmospheric inputs also have an important influence in many shallow groundwater aquifers and majority of these solutes act as problematic sources (Van der Weijden and Pacheco 2006).
Agriculture also has direct (dissolution and transport of excess quantities of fertilizers, associated materials and hydrologic alterations related to irrigation and drainage) and indirect effects (changes in water–rock reactions in soils and aquifers caused by increased concentrations of dissolved oxidants, protons, and major ions) on the rates and composition of groundwater recharge and aquifer biogeochemistry. Agricultural contaminants loading into recharging groundwater resulted in societal problems related to drinking-water quality (Fan and Steinberg 1996) and ecological effects on groundwater discharge into water bodies (Howarth et al. 2000). Chemicals are not the only undesirables in groundwater supplies. The groundwater pollutants that mostly cause apprehension to human health also include microbiological agents which cause diseases and sometimes even death (e.g., Lerner and Harris 2009). Both the microbiological and chemical quality of water are important in determining its suitability for domestic supply and each can have significant and well-documented health impacts on users. Detection of bacterial indicators in drinking water indicates the presence of pathogenic organisms that are likely to be the source of water-borne fatal diseases (Macler and Merkel 2000). As millions of people world-wide continue to rely on shallow groundwater sources and on-site sanitation, it is important to develop and understand the causes of microbiological contamination of groundwater when considering the potential for improvement in water supplies and sanitation (van Ryneveld and Fourie 1997). In many developing countries there are no proper legislation and management plan to control groundwater pollution. In India, although both central and state governments are taking a lot of efforts to protect the groundwater quality and are regularly monitoring the status, it is still inadequate for addressing all the groundwater problems. In India, groundwater pollution is still a baffling problem. Cuddalore district is one of the areas prone to natural disasters like cyclones and floods. Low-lying areas and coastal areas have floods during the period of heavy rains particularly during NE monsoon. An early study in the Cuddalore district by DANIDA, a bilateral project between the Governments of India—Tamil Nadu and Denmark found that out of 491 water samples, 206 samples failed to meet the drinking-water quality standards. Majority of the sources failed on the basis of nitrate and fecal coliforms (FC). Due to floods, sudden outbreak of several water-borne diseases (malaria, cholera, typhoid), even death, also was witnessed in the past. Hence, the present study was carried out to evaluate the groundwater quality for domestic and agricultural activities, to identify the relevant contamination processes (chemical and microbiological) responsible for the chemistry of the groundwater, and also to understand the bacterial diversity of the groundwater in the study area. Also due to the high load of organic and inorganic nitrogen present in the study area, it was an essential prerequisite to conduct a study on the bacterial load and hence was carried out by enumerating the total coliforms (TCs) present in the groundwater samples. The diversity of the bacterial flora was also assessed by culture-based molecular approach by employing 16S rRNA gene sequence homology.
Study area and hydrogeological settings
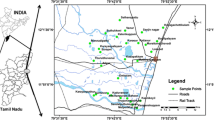
The study area forms the part of the Lower Ponnaiyar River basin, covering an area about 154 km2 and lies between latitudes 11°42′33″N and 11°51′45″N, and longitudes 79°33′21″E and 79° 47′34″E, and is located in Cuddalore district, north of Tamil Nadu, South India (Fig. 1). The northern and southern boundaries of the basin are defined by the Ponnaiyar and Gadilam Rivers (a distributary of Ponnaiyar River) which confluence into the Bay of Bengal sea. Geomorphologically, the area is more or less a plain terrain with slightly elevated tertiary upland hills and laterite hillocks occurring in the Cuddalore sandstone formations. The elevation varies between 20 and 160 m above mean sea level. Physiographically, Cuddalore district is broadly classified into: Ponnaiyar–Gadilam River Basins, Tertiary uplands, Vellar River, Veeranam Tank ayacuts, and hard-rock areas of Tittagudi taluk. Ponnaiyar River Basin is sandwiched between Cauvery River Basin in the west and Palar in the south and Tondiar basins in east and north, respectively. The total area of the basin is 11,257 km2. The total length of the river is 432 km, of which 112 km lie in Karnataka state. Thurinjalar and Musukundah rivers are the tributaries, while Mallatar is the distributary of the Ponnaiyar River. Near the tail end of the Ponnaiyar basin, Gadilam River flows, draining the streams in Kallakurichchi taluk of Villupuram district. Seshanadhi is a tributary for Gadilam River and Mallatar branching off from Ponnaiyar below Tirukovilur anicut and infalls into Gadilam River just above Panruti town. The Gadilam River flows almost parallel to Ponnaiyar and confluences with the sea south of Cuddalore. The study area experiences tropical climate with slight variation. The mean maximum temperature ranges from 30.16 to 40.34°C and the mean minimum temperature ranges from 20.37 to 26.68°C. Annual average precipitation ranges from 850 to 1,160 mm. In the monsoon season about 53.2% of the total precipitation occurs from October to December and 33.1% from June to September. In response to seasonal rainfall the groundwater table is generally the highest in the postmonsoon and lowest in the summer.
Geologically, the study area consists of sedimentary formations, which include sandstone, clay, alluvium, and small patches of laterite soils of Tertiary and Quaternary age. Charnockites and gneisses of Archaean age are found in the upper reaches of the Ponnaiyar River basin to the west of the study area. In the lower part of the basin, Ponnaiyar River has built up extensive alluvium consisting of mixtures of sand, silt, and clay in the delta portions in and around Cuddalore (Fig. 1). The thickness of this formation varies from 10 to 15 m. At some locations sandstones with intervening clay lenses underlie the alluvial sand up to a depth of 50 m below ground level. The Quaternary formations consist of sediments of fluvial, fluvio-marine and marine facies includes various types of soils, fine to coarse-grained sands, silts, clays, laterite, and lateritic gravels. In the coastal tract, except at the confluence point of river, wind-blown sands of 1.5 to 3 km width occur commonly in the form of low and flat-topped sand dunes. However, irregular mounts of 10 to 15 m altitude are a prominent feature due to wind action in the study area. The groundwater occurs in both unconfined and confined conditions in alluvial and sandstone formations, respectively. The groundwater level ranges from 1 to 7 m and 10 to 35 m below the ground level in the unconfined and confined aquifers, respectively (PWD 2000). The depth of bore well ranges from 50 to 180 m below ground level and deeper bore wells between 300 and 400 m were located near Panruti. The maximum discharge of 1,273 lpm is reported from a bore well located in Varakalpattu village. In general, the stratigraphy in the study area indicates the presence of alluvial sand and sandstones up to a depth of 50 m below ground level, underlain by sandstones continuing to considerable depth with intervening clay lenses. Based on pumping test data, the aquifer properties namely transmissivity is 98 m2 day-1 for alluvium formation; 46–134 m2 day-1 for Tertiary formation (Cuddalore sandstone) and hydraulic conductivity 19.7 m/day for alluvium formation and 16–33 m/day for Tertiary formation. The specific yield of the alluvium formation is 7.2%, while for the Tertiary formation is between 1.4 and 3.5% (CGWB 1997).
Materials and methods
Sampling and procedures
Groundwater samples from 47 borewells (Fig. 1) were collected during premonsoon (PRM) and postmonsoon (POM) seasons in 2005. In which 15 groundwater samples showing higher nitrate concentration were selected for microbial diversity study during premonsoon 2005. These wells are spatially distributed over quaternary alluvial formations. Groundwater samples were collected in 2-l high-density-polyethylene containers prewashed with 1:1 HCl followed by distilled water and then rinsed three to four times before sampling using sampling water. Water samples from borewells were collected at least after 10 min of pumping. Temperature, electrical conductivity (EC), hydrogen-ion activity (pH) were measured in situ using portable meters. Collected samples were transported to laboratory within the same day and stored at 4°C. Samples were filtered in the laboratory through 0.45-μm cellulose membranes prior to the analyses. Groundwater samples for cation analysis were acidified to pH < 2 with several drops of ultrapure HCl in the laboratory. Groundwater samples were analyzed based on standard methods (APHA 1995). Calcium (Ca), magnesium (Mg), carbonate (CO3), bicarbonate (HCO3), and chloride (Cl) were estimated by titrimetric method. Sodium (Na) and potassium (K) were analyzed by flame photometer, and sulphate (SO4), phosphate (PO4), nitrite (NO2), and nitrate (NO3) were analyzed using spectrophotometer. Measurement reproducibility and precision for each analysis were less than 2%. The analytical precision for the total measurements of ions was checked again by calculating the ionic balance errors and was generally within ±5%. The geochemical model PHREEQC (Parkhurst and Appelo 1999) was used to calculate the distribution of aqueous species and mineral saturation indices.
Microbiological analysis
Groundwater sampling and enumeration of total colony forming units (CFU)
Groundwater samples for microbial analysis were collected separately from 15 locations in 100-mL sterile plastic containers. Collected samples were brought to the laboratory in an ice pack and the laboratory analysis was conducted within 24 h. Serial dilution technique (Cappuccino and Sherman 1996) was employed for the assessment of total bacterial counts and TCs present in the groundwater samples. A set of seven sterile blanks, each containing 9 mL of distilled water were prepared. 1 mL of each groundwater samples was serially diluted to 10−7 dilutions aseptically. From the serially diluted samples, 0.1 mL from 10−5 and 10−6 dilutions were inoculated by pour-plate technique with nutrient agar for total CFU and with eosin–methylene blue agar for TCs. After 48 h of incubation at 37°C, the CFUs were enumerated.
Genomic DNA extraction and amplification, sequencing and phylogenetic analysis of 16S rDNA from the isolates
A total number of 11 morphologically distinguishable colonies were selected out of 110 isolates from high-nitrate-containing water samples. Subsequent identification of the bacterial isolates was carried out using the molecular tool based on the sequence homology of highly conserved 16S rRNA gene. Cell lysates were prepared by resuspending single colonies in 0.1 ml of TE buffer (0.01 mM pH 8.0) and boiling for 10 min to remove protein. After centrifugation, the supernatant was used for polymerase chain reaction (PCR). PCR amplifications for 16S rRNA genes were performed using Taq DNA polymerase (Invitrogen), primers 21F (5′ CCA GAG TTT GAT CMT GGC TCA G 3′) and 958R (5′ TTC TGC AGT CTA GAA GGA GGT GWT CCA GCC3′) from Brosius et al. (1978) using the following program: 94°C for 5 min, followed by 39 cycles of 94°C (90 s), 55°C (90 s) and 72°C (80 s) with a final 10 min extension step at 72°C. Amplified 16S rDNA was purified for sequence determination using the QIA-quick PCR purification kit (Qiagen), following the instructions of the manufacturer. The amplified 16S rRNA genes were sequenced by using the ABI PRISM Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer) and ABI PRISM 377 automated sequencer (Perkin-Elmer). Sequences have been deposited in GenBank under accession numbers EU849152-EU849162. Relevant sequences were obtained from GenBank (http://www.ncbi.nlm.nih.gov; http://blast.ncbi.nlm.nih.gov) using BLASTN. Operational taxonomic units (OTUs) were defined using DOTUR and furthest neighbour algorithm at 1% sequence distance (Schloss and Handelsman 2005). The most likely relationship between 16S rRNA sequences was identified using maximum likelihood reconstruction methods as implemented in PHYML under a TN93 model of sequence evolution + gamma distribution of rate heterogeneity among sites + fraction of invariant sites (Guindon and Gascuel 2003). Maximum parsimony trees were constructed using PAUP* (Swoford 2001). Supports for the best topologies were assessed using Bayesian inference (Rannala and Yang 1996) and non-parametric bootstrapping. Bayesian posterior probabilities were computed under the same maximum likelihood model using MrBayes 3.0b4 (Huelsenbeck and Ronquist 2001).
Results and discussion
Water chemistry and types
Maximum, minimum, and mean values for the analyzed variable in groundwater samples (n = 47), and their standard deviations for the PRM and POM are summarized in Table 1. The concentrations of dissolved major ions in the groundwater vary both spatially and seasonally. Abundance of the ions during both seasons is in the following order: Na > Ca > Mg > K = Cl > HCO3 > SO4 > CO3. Based on the land-use classification the upper and central part of the study area mostly consists of agricultural land, shrub land, and settlements, whereas the eastern part consists of settlements and coastal belts (Fig. 1). In the study area, the TDS (total dissolved solids) in the groundwater samples varies from 370 to 2,700 mg/l during PRM and 240 to 1,760 mg/l during POM (Table 1). Irrespective of seasons, high TDS values are recorded in the eastern part of the study area (Fig. 2a, b). The pH generally varies from 7.1 to 8.1 and 8.0 to 8.7 during PRM and POM, respectively, which indicates the groundwater is mostly alkaline in nature. HCO3 ranges from 49 to 412 mg/l during PRM and from 31 to 293 mg/l during POM. Maximum values of HCO3 are found in the western part and along the riverbanks (Fig. 2c, d), which might be due to the weathering of silicate rocks and dissolution of carbonate precipitates (Freeze and Cherry 1979). Bicarbonate and carbonate contribute about 24% (PRM) to 30% (POM) of the total anion concentration in the groundwater of the study area. Cl concentration varies from 34 to 951 mg/l in PRM and 44 to 780 mg/l in POM. The higher Cl content is recorded in the eastern part (Fig. 2e, f), which is classified as settlement area and coastal belt. SO4 concentration ranges from 54 to 288 mg/l during PRM and 15 to 324 mg/l during POM. Higher values of SO4 are observed as few pockets in the eastern part of the study area. The high concentration of SO4 may be attributed to the contamination by the untreated industrial and domestic waste effluents (Baruah et al. 2008). Na and K are released slowly upon dissolution of secondary minerals. Na is the dominant cation in the groundwater of the study area, and ranges from 51 to 744 mg/l in PRM and 28 to 425 mg/l in POM. Spatially, Na concentration is more in the eastern part of the study area during both seasons (Fig. 2g, h). K concentration is very low during both seasons and higher concentration is noted in the eastern part. Ca and Mg ions are added to the groundwater due to carbonate dissolution and from Ca- and Mg-bearing silicate rock types. Calcium is ranged between 16 and 98 mg/l during PRM and 4 and 92 mg/l during POM. As Mg is concerned, the concentration throughout the study area ranges from 9 to 48 mg/l in PRM and 2 to 44 mg/l in POM seasons, respectively. Nitrite concentration shows a minor variation, ranges from BDL to 4.10 mg/l during PRM and BDL to 4.9 mg/l during POM. Nitrate concentration of the groundwater of the study area ranges from 12 to 158 mg/l during PRM and BDL to 119 mg/l during POM. Ammonium is transformed to nitrates by the nitrification process in the presence of oxygen, which can be presented by the following reaction.
The presence of high nitrate content in groundwater may mainly be due to the leaching of NO3 from fertilizers and other agrochemicals used in agricultural and also by man made activities (other NO3 pollutions) (Almasri and Kaluarachchi 2004). Spatially, NO3 concentration is more in central and western parts (Fig. 2i, j), which reflects the land-use pattern (Fig. 1 i.e., agricultural-dominated area). Nitrate leaching is enhanced because soils in the study area have high infiltration rate and low runoff potential and high rates of nitrogen fertilizer application and extensive irrigation contributes to high NO3 concentration in the groundwater.
The Piper diagram (Piper 1944) is useful for evaluating the geochemical evolution of the groundwater in the study area. The dominant water types in PRM are in the order of NaCl > CaMgCl > mixed CaNaHCO3, whereas during POM the order of water types are slightly changed and shown as NaCl > CaMgCl > mixed CaNaHCO3 and CaHCO3 (Fig. 3). However, most of the samples are clustered in NaCl and CaMgCl segments for both seasons. Water types (NaCl and CaMgCl) suggest the mixing of high salinity water caused from surface contamination sources such as irrigation return flow, domestic wastewater, and septic tank effluents, with existing water followed by ion exchange reactions. According to Appelo and Postma (1996), some water types characterise saline groundwater: Na–Cl dominant type, Ca–Cl, indicating the seawater intrusion process, and Na–Mg-Cl-SO4 indicates a mixed water type. Samples have elevated Na concentrations coupled to low Ca2+ concentrations, suggesting that Ca2+–Na+ ion exchange is an important geochemical process for the Na–Cl type of groundwater. The Na–Cl type is the dominant groundwater type in PRM and POM contributing to 82.98 and 80.85%, respectively. The second facies is the mixed CaMgCl type and it contributes to 12.77 and 6.38% in PRM and POM, respectively. The CaNaHCO3 water type varies between PRM and POM seasons; it shows 2.13 and 12.77% respectively. During POM, more contribution of CaNaHCO3 and CaHCO3 water types express mineral dissolution and recharge of freshwater. In general, alkalis (Na + K) are significantly dominating over the alkali earth elements (Ca2++Mg2+) and strong acids (Cl, SO4) dominate over weak acid (CO3, HCO3) in most of the samples. Such water generally creates salinity problems both for drinking and irrigation use.
Geochemical processes
The plot Na + K versus Cl shows that most of the samples in PRM falls above the 1:1 line, whereas in POM, fall below the 1:1 line due to an enrichment of Cl (Fig. 4a). In general, Cl forms a compound with alkalis rather than alkali earth elements (Ca + Mg), it may be inferred that Cl has been consumed in the formation of alkali chlorides. It is evidenced by the higher alkali concentration observed in the samples than that required for the formation of chlorides. This can be further inferred from the high concentration of aqueous species such as alkali bicarbonate and alkali sulphate. Data points plotted near to equiline indicate that alkali content is balanced by chloride. The sequence is shown in POM by Cl enrichment in which surplus chlorides are balanced by the alkaline earth metal ions. Cl ion concentration in groundwater normally arises from the sources like ancient seawater-entrapped sediments, solution of halite, and related minerals in evaporate deposits, solution of dry fallout from the atmosphere especially in the arid region, and from anthropogenic sources (Walker et al. 1991). In the plot, Na + K vs Cl most of the PRM samples show enrichment in Na but POM samples fall below the 1:1 line due to Cl enrichment. The high concentration of Cl is caused by intrusion of saline water near the coastal area, which is a common phenomenon in SE coast of Tamil Nadu (e.g., Chidambaram et al. 2007). Stagnant backwater in the river percolates down, thereby increasing the Cl content and leaching of saline residues in the soil by the action of rainwater during POM and anthropogenic input. In particular, the variation in Cl concentration over the sampling locations indicate that evaporation may be the additional cause of enrichment in Cl concentration in POM due to water-level rise, which causes more salt dissolution from the soil. Na concentration is also being reduced by ion exchange. So Na and Cl do not increase simultaneously (Fig. 4b). Eastern part of the study area shows high concentration of Cl in both seasons, indicating more than one possibility such as seawater intrusion, dissolution of residues from soil and significant anthropogenic input from the settlement area. This was confirmed by lower Cl concentration obtained in agricultural area (central and western parts) than the settlement and coastal area of eastern part. Among the alkalis, K is apparently low, which might be due to the greater resistance of K to weathering and its fixation in the formation of clay minerals (Sarin et al. 1989). A strong positive correlation between Na and Cl suggests mixing of groundwater with different end member compositions (fresh and saline) and high concentration of SO4 and Cl may be related to long history of evaporations (Datta and Tyagi 1996). SO4 versus Cl may be good indicators of SO3 leaching to groundwater from surface sources. The variation in SO4/Cl ratios indicates the net changes to SO4, due to biogeochemical processes in the groundwater prior to recharge, or within the aquifer. The surface precipitation, irrigation or evapotranspiration lead to Cl enrichment in groundwater, which ultimately lowers the SO4/Cl ratios of groundwater. Very low SO4/Cl ratios (low concentration of SO4) (Fig. 4c) suggest that sulphate is being depleted, possibly by sulphate reduction (e.g., Lavitt et al. 1997). At the same time the enrichment of Cl in POM than in PRM, may be due to leaching from the soil and surface precipitation. Alkali ions are overwhelmingly abundant compared to Ca and Mg in the groundwater during PRM, whereas in POM, Ca + Mg are overwhelmingly abundant than Na + K (Fig. 4d). The deficiency of Ca in groundwater is due to the ion exchange process, which generally indicates the excess of alkalis over alkali earth elements. The plot of Ca + Mg versus HCO3 in Fig. 4e shows that most of the data during POM and significant number of samples during PRM fall below the equiline, although few points approach the line in PRM. This indicates that the excess of alkalinity in water has been balanced by alkalis (Na + K). The excess of alkali earth elements (Ca + Mg) over HCO3 in many samples during PRM and some samples during POM reflects an additional source of Ca and Mg ions. It might have been balanced by Cl and SO4 and/or supplied by silicate weathering (Zhang et al. 1995). High concentration of HCO3 in groundwater may be due to natural processes like dissolution of carbonate minerals and dissolution of atmospheric and soil CO2 gas (Jeong 2001). High base-exchange reaction in which alkali earth elements exchanged for Na ion (HCO3 > Ca + Mg), may be referred to as base-exchange softened water, whereas Na ion exchanged for the alkali earths (Ca + Mg > HCO3), can be referred to as base-exchange hard water. In present study, most of the samples are base-exchange hard water in nature during PRM, but in POM significant number of samples is converted to base-exchange softened water due to dilution (exchange of ions) after a monsoon except 15 samples, remain as hard water. The plot of Ca + Mg versus HCO3 + SO4 (Fig. 4f) is expected to fall close to the 1:1 equiline if the dissolution reactions of calcite, dolomite, and gypsum are dominant in the system. Ion exchange tends to shift the points to the right and left of the equiline due to an excess of SO4 + HCO3 and Ca + Mg, resepctively (Fisher and Mullican 1997). The samples approaching the equiline during PRM indicate dissolution of calcite and dolomite. But some samples show an enrichment of SO4 + HCO3, indicating ion exchange process, whereas during POM reverse ion exchange process contributed more Ca + Mg than SO4 + HCO3 (Fig. 4f). HCO3 versus Cl plot (Fig. 4g) shows that most of the samples during both seasons fall below 1:1 line due to domination of Cl. Generally, Gibbs plots (Gibbs 1970) are used to gain better insight into hydrochemical processes, such as precipitation, rock-water interaction, and evaporation on groundwater chemistry in the study area. Data points are observed (Fig. 5) mostly in the rock–water interaction zone and few samples in the evaporation zone. This phenomenon suggests that dissolution of carbonate and silicate minerals are the dominant controlling factor of the groundwater chemistry of the study area. Further surface contamination source such as irrigation return flow seems to affect the quality of the groundwater. Anthropogenic activities may amplify the role of evaporation and may lead to increase Na and Cl and thus rise in TDS (Karanth 1991).
Ion exchange processes and mineral dissolution
The evolution of groundwater towards a Na-rich type generally occurs by the precipitation of calcite and/or cation exchange, whereas Ca–Cl type water is commonly produced by reverse ion exchange reaction (Na + Ca-clay = Na-clay + Ca). These cation exchange and reverse ion exchange are encouraged by aquifer materials, particularly montmorillonite. This leads to the release of Na or Ca into groundwater and adsorption of Ca or Na, respectively (Alison et al. 1992). The ion exchange between the groundwater and their host environment during travel or residence can be calculated by using the Schoeller (1965, 1967) chloro-alkaline indices (i.e., CA I [((Cl – Na+ + K+)/Cl−)] and CA II [((Cl – Na+ + K+)/(SO4 + HCO3 − + CO3 + NO3 −))]. If the indices value is positive, this indicates base-exchange reaction where Na+ and K+ ions in groundwater exchanges with Ca and Mg cations. If the value is negative, this indicates chloro-alkaline disequilibrium (cation–anion exchange reaction), where host rocks are the primary source of dissolved solids in groundwater. The indices values of the groundwater samples of the study area during PRM reveal that cation–anion exchange reaction exists in most of the samples (64%) except at 17 locations (36%) showing base-exchange reaction. The indices value in POM show that 95.74% of samples except two samples have positive values (Cl > Na + K), which indicates base-exchange reaction (chloro-alkaline equilibrium). During POM the water table is generally shallow and near the ground surface, which increases the contact surface area of the water, so there is an exchange of Na in groundwater with Ca and Mg in the alluvium soils.
The equilibrium state of the water with respect to a mineral phase is determined by calculating a saturation index (SI) using analytical data. The potential for mineral precipitation or dissolution is assessed using the SI, which is based on the relation between analytical activity ion activity product (IAP) and thermodynamic calculations of the solubility product (K sp). Saturation index can be used to determine as to whether a solution is undersaturated (SI < 0), oversaturated (SI > 0), or in equilibrium (SI = 0) with a solid. If the solution is oversaturated, precipitation may be expected and if it is under-saturated dissolution might be possible. The SI of a mineral is determined using the following equation (Parkhurst and Appelo 1999).
The disequilibrium indices of carbonate minerals and sulphate minerals were presented in Table 1 from the data bank of PHREEQC (Parkhurst and Appelo 1999). The groundwater during PRM show equilibrium to oversaturated with calcite, dolomite, magnesite, and aragonite with minor indication of under saturation state (Table 1). The groundwater in POM fall between under-saturated to saturated state indicates the dissolution of carbonate minerals (Table 1). The status of groundwater in both seasons indicates that all the sulphate minerals are in the state of under saturation indicating the effect of dilution.
Statistical analysis
Twelve variables, such as Na, K, Ca, Mg, HCO3, CO3, Cl, SO4, NO3, NO2, PO4, and pH were used for factor analysis. The varimax rotation method with Kaiser Normalization was applied to extract the factors. The results of factor analysis based on four significant factors explain about 68.83% of the total variance. The communalities of variables, which can explain the variance degree of variable by the four factors are higher than 0.51 for PRM except NO2, and CO3 and POM except pH and Ca.
The factor 1 (F1) (PRM) has high loading of Na, K, Cl, PO4, and HCO3 (Fig. 6a) and accounts 26.81% of the total variance. During POM F1 is strongly loaded by Na, Cl and moderately loaded by Ca and Mg (Fig. 6b). These hydrochemical ions are the dominant solutes in seawater, especially Cl and Na (r = 0.948) and dominations of Na and Cl in F1 describes the salinization factor. The Na–Cl relationship can be used to acquire the mechanism for attaining salinity and saline intrusion in semi-arid regions (Dixon and Chiswell 1992). A parallel enrichment of Na and Cl indicates dissolution of chloride salts or concentration processes by evaporation, but the present study samples show significant enrichment of Na rather than Cl (in PRM) indicating seawater intrusion or solute diffusion from the marine clay aquitard. The distribution of the scores of F1 shows high values in the eastern part of the study area towards sea. This suggests that seawater ingression was predominantly related to water recharge from this tide-influenced Gadilam River in lower part of the study area (Prasanna et al. 2010). This area is the most densely populated and consequently witnesses higher groundwater abstraction. This probably establishes local freshwater depression cones, which induces seawater intrusion. (e.g., Gallardo and Marui 2007). Precipitation of minerals takes place during the cation exchange process, which was the later effect from seawater intrusion into aquifer. The lower concentration of Ca compared to Na was the result of cation exchange processes that occurs naturally during seawater intrusion.
Factor 2 (F2) explains 19.92% of the total variance with strong loadings of Ca, Mg, and SO4 (Fig. 6a). The high positive loadings in Ca and Mg are defined as hardness. This high loading of these ions (F2) is due to dissolution of carbonate and sulphate minerals. Low SO4 coupled to elevated hardness concentrations suggests that sulphate reduction is also important process for this loading in F2 (Cloutier et al. 2008). F2 in POM shows 14.60% of the total variance with strong loadings of CO3, HCO3 and moderate loading by pH (Fig. 6b). This factor describes dilution of groundwater by water recharge and/or mineralization by water soil/rock interaction. The average pH value is increased to 8.3 in POM from 7.6 in PRM, which is evident. The pH 8–8.7 (avg. 8.3 ± 0.19) indicates that the dissolved carbonates are predominantly in the HCO3 form (Adams et al. 2001). Hence the F1 and F2 are assumed to be indicative of natural processes which are dominating to control these variances.
Factor 3 (F3): NO2 and pH is positively loaded, whereas CO3 is negatively loaded in F3 during PRM (Fig. 6a) and accounts 12.40% of the total variance, whereas during POM, NO2 and K is positively loaded in F3 (Fig. 6b) and accounts 12.37%. The positive loading of nitrite in the groundwater may be due to the primary stage of nitrification, the oxidation of ammonia (NH3), performed by bacteria such as the Nitrosomonas sp., which converts NH3 to NO2 and subsequently is converted into NO3 by other bacteria. Presence of Nitrosomonas bacteria in the groundwater has been confirmed by microbiological analysis. SO4 also shows significant loading in F3, which provides evidence for the use of fertilizers with NH4 and SO4. Strong loadings of NO2 in F3 in POM combined with significant contribution from K, indicating that these ions derived from the agricultural area having high consumptions of fertilizers.
Factor 4 (F4): During POM the F4 is highly loaded with NO3, PO4, and K (Fig. 6b) and accounts the variance about 12.18%. It is very clear that these variables are related to agricultural source. The distribution of the factor scores for PRM shows of high NO3 concentration. Different N-fertilizers, potash and urea are widely used in this area apart from the organic manure to cultivate the major crops (GOI-TN-District profile 2001). These highly soluble NO3 are easily leachable on irrigation and percolated N move through the alluvium. In well-drained soils, the rate of nitrification is high but denitrification may be insignificant (Almasri 2007). The nitrification processes by microbial oxidation; NH3 is transformed to NO3 in the unsaturated zone (Stumm and Morgan 1996). In some locations NO3 concentration is low, where clay-rich sandy soils protect the aquifer from NO3 contamination. The role of human activities such as influence of liquid wastes, widespread forming and open dumping of manure has been contributed high concentration of NO3, NO2, and PO4 into the groundwater of the study area. In particular, nitrate is also derived from non-agriculture sources (point sources), such as waste disposal network, open-dumped animal wastes, including dung and urine (total livestock population in the district is comparatively significant i.e., 1.03 million), human excreta (common due to lack of sanitation facilities in the rural areas), and house building, etc. In high-density residential areas with no sewer systems, septic tanks also produce substantial amounts of nitrogen to sub-surface in the form of ammonium and organic nitrogen (MacQuarrie et al. 2001). So, it is clearly concluded that intensive consumption of fertilizer and high coarse soil are the important factors responsible for NO3 −, PO4 and K leaching to groundwater, which is well supported by the distribution of factor score recorded in the centre part of the study area.
Evaluation of groundwater quality
Drinking usage
The suitability of groundwater as a source of drinking water for the study area, was assessed on the basis of standards and water quality criteria specified by the World Health Organization (WHO 1993) and Indian Standards (BIS 1991), Table 2. The average values of individual parameters of groundwater are within the permissible limit except the parameter Na and NO3 during PRM, compared to the standards, whereas individual samples show higher concentrations which are presented in the Table 2. Based on TDS classification (Freeze and Cherry 1979), six samples each during PRM and POM were unsuitable for drinking purposes. According to Sawyer and Mc Carthy (1967), 14 samples during PRM are considered as very hard water, whereas in POM all the samples changed to soft water except one sample considered as very hard water. Ca and Mg concentrations are within the permissible limit for both seasons. Many samples show higher content of Na during both seasons than the guideline values, which may cause heart problem (Kumar et al. 2007; Mor et al. 2009). SO4 and Cl concentrations are within the permissible limit except two samples each in PRM and POM are exceeded the limit of WHO. SO4 content >200 mg/l is objectionable for domestic purposes; beyond this limit, SO4 causes gastro-intestinal irritation. People who are not habituated to high chlorine in drinking water are subjected to laxative effects and Cl is injurious to people who are suffering from heart and kidney diseases (WHO 1997). Elevated nitrate concentrations in drinking water can cause methemoglobinemia in infants resulting in decreased oxygen carrying capacity of haemoglobin in babies and stomach cancer in adults (Wolfe and Patz 2002). Usually nitrite is not present in significant concentration except in a reducing condition, since nitrate is the most stable oxidation state which can be formed by the microbial reduction of nitrate. Nitrite can also be formed chemically by Nitrosomonas bacteria in distribution pipes during stagnation of nitrate containing and oxygen poor drinking water in galvanized steel pipes or if chloramination is used to provide a residual disinfectant (WHO 2008). Hence, depletion of DO in water can encourage the microbial reduction of nitrate to nitrite and sulphate to sulphide. Twenty-two samples in PRM and 36 samples in POM are qualified for drinking according to WHO (1993), whereas 19 samples in PRM and 25 samples in POM are suitable for drinking as per BIS (1991). Based on NO2, two samples in PRM and 1 sample in POM do not meet the drinking water criteria. Due to the simultaneous occurrence of nitrate and nitrite in drinking water, the sum of the ratios of concentration of each to its guideline value should not exceed 1 (WHO 2008). i.e., C nitrate/GVnitrate + C nitrite/GVnitrite ≤ 1, where C-concentration (NO3 or NO2) in the sample and GV—guideline value by WHO. According to this ratio, 31 samples in PRM and 11 samples in POM were not qualified for drinking. As per drinking water quality standard, no sample should contain fecal coliform or E. coli, and ideally there should be no TC; however, a single sample may contain up to 10 total coliform CFU/100 ml. Thirty-six samples during PRM and 27 samples during POM show the presence of F.Coli, which are not suitable for drinking.
Irrigational suitability
Irrigational suitability of groundwater in the study area was evaluated by EC, SAR, RSC, USSL classification, Na%, and Wilcox diagram. Excessive Na content in water makes it unsuitable for soils that contain exchangeable Ca and Mg ions. The total content of soluble salts such as Na to Ca and Mg and its relative proportion affect the suitability of groundwater for irrigation. If the percentage of Na+ to [Ca2++ (Mg2+ + Na+)] is above 50% in irrigation waters, Ca and Mg exchange with Na, thus causing deflocculation and impairment of the tilth and permeability of soils (Karanth 1987). The irrigation water containing a high proportion of Na will increase the exchange of Na content of the soil, affecting the soil permeability, and the texture makes the soil hard to plough and unsuitable for seedling emergence (Trivedy and Goel 1984). The sodium or alkali hazard in the use of water for irrigation is expressed by determining the SAR, and it was estimated by the equation:
Units are expressed in milliequivalent per liter.
The calculated SAR values in the study area vary between PRM and POM as 1.98–16.47 and 1.82–12.53, respectively. According to SAR values 89.36% (n = 42) of samples in PRM and 91.49% (n = 43) of samples in POM are suitable for most types of crops and soils. 10.64% of groundwater in PRM and 8.51% in POM can be used if the organic content in the soil is high or it has a coarse texture with good permeability reference. A more detailed irrigation suitability analysis was made by plotting the data on the USSL diagram (USSL 1954). According to USSL, the groundwater of the study area falls into good to moderate category (Fig. 7). Overall 59.58% (PRM) and 48.93% (POM) of samples fall in C2S1 and C3S1 fields indicating of medium to high-salinity and low-alkalinity water, which can be used for irrigation, where moderate amount of leaching occurs and moderate permeability with leaching soil. Moreover 27.66% (PRM) and 40.43% (POM) of samples fall with in C3S2 field indicating high salinity and medium sodium hazard, which restrict its suitability for irrigation. Based on salinity hazard (EC) and SAR classification, 6.38% (PRM) and 8.51% (POM) of samples are considered to have very high salinity and the water class is poor (Table 3). High salinity and medium hazard type of water in fine-textured soil of high cation exchange capacity, under low-leaching conditions (unless gypsum is present in the soil), produces appreciable sodium hazard. But this type of water may be used in coarse textured or organic soils which have good permeability. In all natural waters, Na% is a common parameter to assess its suitability for irrigation (Wilcox 1948). Sodium combined with carbonate lead to form alkaline soils, whereas with chloride forms saline soils. Both these soils do not support plant growth. Na% was calculated using the following equation.
Units are expressed in mg/l. The values for the Na % in the study area range from 44 to 90% and 45 to 88% in PRM and POM seasons, respectively. About 78.72% (PRM) and 59.57% (POM) of the samples are higher than 60% of Na % and are considered unsafe for irrigation (Subrahmanyam and Yadaiah 2001). Calculated Na % for the groundwater of present study is plotted against specific conductance in Wilcox diagram (Fig. 8). According to this plot, three samples (PRM and POM) are excellent to good; three (PRM) and 12 (POM) samples are good to permissible; 34 samples during PRM and 25 during POM are permissible to doubtful; and seven samples during PRM and POM are doubtful to unsuitable. The effect of bicarbonate ion concentration on the water quality was assessed based on the residual sodium carbonate (RSC) and was estimated by the equation (Eaton 1950)
where, ionic concentrations are considered in milliequivalents per litre. Based on this criterion, 45 samples during PRM and 29 samples during POM are safe and two samples (1, 8) in PRM and 15 samples in POM are in marginal (Table 3). Two samples during POM are unsuitable for irrigation due to high RSC. Suitability of groundwater for irrigation purposes was also assessed using the criteria shown in Table 4. A comparison of TDS values with irrigation standards shows that 72.34% during PRM and 65.96% during POM is considered class II, which are suitable for permeable soil (Kumar et al. 2007). Only 23.40% in PRM and 34.04% in POM is considered as class I type, which is excellent to good category. Based on SO4, 87.23% in PRM and 89.36% in POM are considered as class I and 12.77% in PRM and 10.64% in POM are belongs to class II. The Cl content of water samples suggests that 57.45% in PRM and 40.43% in POM are of class II which may be injurious to crops. As per Cl and EC comparison 6.38% of samples during PRM and 8.51% of samples during POM are unfit for the irrigation purpose. The overall comparison between various suitability assessment five samples located in the eastern part of the study is highly not suitable for irrigation.
Microbiological quality
The microbiological quality of groundwater samples in 47 locations in the study area showed large temporal and spatial variation. The TC, in PRM ranges between 1.38 × 104 and 6.12 × 104 CFU/100 ml. The highest value was observed in the western part (sample No. 39). In POM, after heavy rainfall, the TC population ranged from 1.44 × 104 to 6.44 × 104 CFU/100 ml and sample No. 39 exhibited highest coliform counts. The heavy coliform load could be attributed to the rapid recharge of aquifer due to heavy rainfall. The magnitude of these values varied considerably but interestingly, contamination occurred at various depths throughout the profile. At two locations, highest coliform load was obtained at shallow levels (13 and 26 m), which reflect the increased influence of anthropogenic inputs. Elevated values in POM reflect that soils were fully saturated after exceptionally heavy rainfall. This allowed rapid flushing of sewage due to a reduced or absent vadose zone separating sewer leakage from underlying groundwater.
Out of 47 samples, 36 samples from PRM 27 samples in POM showed the presence of coliform. Powell et al. (2001) stated that the detection of fecally derived bacteria and viruses at such significant depths below the ground surface challenges current assumptions regarding microbial transport in consolidated sandstone aquifers. Indeed, the fact that the unsaturated-zone thickness is dependent on rainfall will produce seasonal variations in microbial loading. In addition, sewer-flow volumes are much greater at times of high rainfall. Microbial-contamination data indicate that sewage contamination can reach the aquifer system at depth and pose a serious threat to groundwater quality. This may be due to the contaminant-source characteristics whereby small amounts of sewer leakage dramatically affect microbial populations. The groundwater in consolidated sandstone aquifer is much more vulnerable to sewage-derived microbial contamination at depth than previously thought (Edworthy 1989). The seepage of E. coli is easier in sedimentary formation compared to hard rock terrain (Geldreich 1972), which supports the present study. Most of the groundwater samples with positive coliform bacteria had nitrate levels >45 mg NO3/l, which is due to various land-use activities occurring on the study area.
Bacterial diversity of groundwater samples
Results of the amplification and sequencing of 16S rRNA genes exhibited five groups of bacteria, each contain at least two different strains. The sequences of 11 isolates exhibited five groups with 11OTUs. The representative of the cultivated division of five groups was based on the least inclusive monophyly composed of the cultivated isolates of the groundwater samples and the nearest relatives of GenBank. The sequences were grouped within the phylogenetic divisions depending on the robustness of bootstrap values from the phylogenetic analysis in which, above 60% was considered to the good bootstrap values.
The first group of the phylogenetic tree (Fig. 9) represent Nitrosomonas group, which produced two phylogenetically different out groups such as Nitrosomonas VKMM 063, which is the closest relative of an ammonia-oxidizing strain Nitrosomonas sp. Nm84 isolated from marine systems (Purkhold et al. 2003) and the other strain Nitrosomonas VKMM060 which was also related to the above reference strain. Nitrosomonas are rod-shaped chemolithoautotrophs, genus of ammonia-oxidizing proteobacteria with an aerobic metabolism and these organisms play the role of oxidizing ammonia to nitrite, which is then converted to nitrate. It is presumed that the highest amount of nitrate in the groundwater samples could have been resulted from the conversion of ammonia initially by Nitrosonomonas sp. Even though the groundwater was sampled from the deeper wells (30 m), dissolved oxygen content was found to be normal (1.1 to 4.0 mg/l), which is assumed to support the metabolism of conversion of ammonia to nitrite and subsequently to nitrate.
The second group was Bacillus, which comprised of two isolates of Bacillus subtilis (VKMM 062 and VKMM 066). They were aligned very closely with B. subtilis strain PAB1C4 and MP-3. The closest relatedness of the isolates B. subtilis strain MP-3 (DQ462192) was revealed by the very high bootstrap values. The reference strain PAB1C4 had an origin from the rhizosphere of wheat that exhibited a good plant-growth-promoting ability. The other strain MP-3 isolated from agricultural soils having the ability of degrading methylparathion (unpublished data). The closest relatedness of the 16S rDNA sequences with bacillus strains of agricultural origin reflects that the present isolates (B. subtilis VKMM 062 and VKMM 066) could have been originated from agricultural soils. These organisms possess nitrate reduction capabilities (Graumann 2007), which perhaps convert the nitrate to ammonia as evidenced by a low quantity of ammonia in the samples (Table 5).
Group 3 exhibited two out groups, which are phylogenetically affiliated with Pseudomonas (Pseudomonas sp VKMM 068 and VKMM 070). The amplified 16S rRNA gene was the highest homology with Pseudomonas sp P12 DQ453821, a biocontrol agent producing the antifungal compound 2,4-diacetylphloroglucinol, isolated from Switzerland (Frapolli et al. 2007) and with an Antartican isolate Pseudomonas sp. P12 (AM491463) (Unpublished data). In nature, the genus demonstrates a great deal of metabolic diversity, and consequently is able to colonize a wide range of niches (Madigan 2005). Pseudomonas is major denitrifying bacteria (Miller et al. 2002) and previous pure-batch-culturing experiments have shown that Pseudomonas can utilize Fe(III) as terminal electron acceptors to grow in the absence of molecular oxygen. Typical Fe(III) reducing bacteria include both strictly anaerobic (such as Geobacter) and facultative microbes (such as Pseudomonas) that survive in the presence of minor amount of oxygen (Ehrlich 2002). The Pseudomonas sp have been employed for the remedial process of reducing nitrate toxicity (Ayyasamy et al. 2007), whereas the presence of Pseudomonas sp in the samples indicates the active bioremediation process.
Three OTUs of E. coli were exhibited from total 16S rRNA sequences obtained from the groundwater samples. The sequences identity of these isolates as E. coli strains VKMM 069, VKMM067 and VKMM 065 were revealed by the closest alignment with the reference strains E. coli strain 246 EF560775 and an ATCC strain E. coli 25922. Very high bootstrap values indicate the closest relatedness among the isolates. The presence of E. coli in the groundwater samples is attributed by the washed runoff of the domestic sewages and mixing up through water table and thus migrated to the wells. Only two Alcaligenes strains (VKMM061 and VKMM064) were obtained from the groundwater samples which showed a very little difference in their 16S rRNA gene sequences even though they exhibited a variation in colony morphology that phylogenetically affiliated with Alcaligenes xylosoxidense (AJ491840) and were Alcaligenes sp SBW1 (EU195847). NO3 reducing capacity was endowed with Alcaligenes sp. The presence of Alcaligenes sp was also reported previously from the nitrate rich groundwater (Ayyasamy et al. 2007). The cultivable isolates were found to be aerobic organisms that can survive at microaerophilic conditions by utilizing the dissolved oxygen. The presence of these organisms reflects the active conversion of ammonia brought to the groundwater to nitrite by Nitrosomonas sp and further converted to nitrates by other organism. Further, the presence of nitrate reducers could also play a role in the process of conversion of nitrate to ammonia and nitrate to molecular nitrogen. The cultivation independent approach of assessing the microbial diversity would reveal more about the exact microbial process of nitrogen cycling in the groundwater.
Conclusion
In the present study, 47 groundwater samples were collected during the PRM and POM seasons of 2005 and 15 groundwater samples were collected during PRM 2005 for microbial analysis from Lower Ponnaiyar Basin, India. Detailed analyses were carried to determine the geochemical and microbiological processes responsible for quality deterioration. NaCl and CaMgCl are major water types in the study area. The quality of groundwater in the study area is impaired by surface contamination sources, mineral dissolution, ion exchange, and evaporation. Apart from these processes agricultural activity and heavy groundwater withdrawal has led to seawater intrusion along the coastal part of the region are also source for groundwater quality deterioration. Influences of mineral dissolution was evaluated by PHREEQC and Gibbs plots and suggest that mineral dissolution, especially carbonate minerals regulate water chemistry. Chloro-alkaline indices indicate that cation–anion exchange reaction is a dominant process during PRM, whereas base-exchange reaction is a dominant process during POM. Groundwater suitability for drinking usage was evaluated by WHO and BIS standards and proposes 53.19% of samples during PRM and 25.53% during POM are not suitable for drinking. The total bacterial count showed higher values during POM of 2005 with significant amount of enteric pathogens like E. coli, contributing typhoid to the consumers. Suitability for irrigation was evaluated using USSL, SAR, residual sodium carbonate (RSC), Na% and Wilcox diagram. As per these comparison studies 6.38–27.66% of samples in PRM and 8.51–40.43% of samples during POM are unfit for the irrigation purpose. The overall comparison between various suitability assessments, five samples located in the eastern part of the study are highly unfit for irrigation. The cultivable isolates were found to be aerobic organisms that can survive at microaerophilic conditions by utilizing the dissolved oxygen. The presence of these organisms reflects the active conversion of ammonia brought to the groundwater to nitrite by Nitrosomonas sp and further converted to nitrates by other organism. Further, the presence of nitrate reducers could also play a role in the process of conversion of nitrate to ammonia and nitrate to molecular nitrogen. The cultivation-independent approach of assessing the microbial diversity would reveal more about the exact microbial process of nitrogen cycling in the groundwater.
References
Adams S, Titus R, Pietersen K, Tredoux G, Harris C (2001) Hydrochemical characteristics of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Alison EC, Janet SH, Blair FJ (1992) The chemical influence of clay minerals on groundwater composition in a lithologically heterogeneous carbonate aquifer. In: Kharaka YK, Maest AS (eds) Proceedings of 7th international symposium on Water–Rock Interaction (WRI7, Vol. 2). Balkema, Utah, pp 779–782
Almasri MN (2007) Nitrate contamination of groundwater: a conceptual management framework. Environ Impact Assess Rev 27:220–242. doi:10.1016/j.eiar.2006.11.002
Almasri MN, Kaluarachchi JJ (2004) Assessment and management of long-term nitrate pollution of groundwater in agriculture dominated watersheds. J Hydrol 295:225–245
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC, pp 1–467
Appelo C, Postma D (1996) Geochemistry, groundwater and pollution. Balkema, Rotterdam
Ayyasamy PM, Shanthi K, Lakshmanaperumalsamy P, Lee SJ, Choi NC, Kim DJ (2007) Two stage removal of Nitrate from groundwater using biological and chemical treatments. J Biosci and Bioeng 104(2):129–134
Baruah M, Bhattacharyya KG, Patgiri AD (2008) Water quality of shallow groundwater of core city area of Guwahati. In: Proceedings of sixteenth national symposium on environment, Haryana, India. pp 101–106
BIS (1991) Indian standard specification for drinking water. Bureau of Indian Standard No. 10500. Govt. of India
Brosius J, Palmer ML, Kennedy PJ, Nolther HF (1978) Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Nat Acad Sci (Wash) 75:4801–4805
Cappuccino JG, Sherman N (1996) Microbiology: a laboratory manual. Benjamin Cummings, California
CGWB (1997) Groundwater resources and development prospects in South-Arcot-Vallalar District, Tamil Nadu, Southeastern coastal region, Chennai
Chidambaram S, Prasanna MV, Vasu K, Shahul Hameed A, Unnikrishna Warrier C, Srinivasamoorthy K, John Peter A, Anandhan P (2007) Study on the stable isotope signatures in groundwater of Gadilam River Basin, Tamil Nadu. Ind J Geochem 22(2):209–221
Cloutier V, Lefebvre R, Therrie R, Savard MM (2008) Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J Hydrol 353:294–313
Datta PS, Tyagi SK (1996) Major ion chemistry of groundwater in Delhi area: chemical weathering processes and groundwater flow regime. J Geol Soc India 47:179–188
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland, Australia. J Hydrol 135:259–274
Eaton FM (1950) Significance of carbonates in irrigation water. Soil Sci 69:123–133. doi:10.1097/00010694-195002000-00004
Edworthy KJ (1989) Waste disposal and groundwater management. J IWEM 3:109–114
Ehrlich HL (2002) Geomicrobiology, 4th edn. Marcel Dekker, New York
Fan AM, Steinberg VE (1996) Health implications of nitrate and nitrite in drinking water: an update on methemoglobinemia occurrence and reproductive and developmental toxicity. Regul Toxicol Pharmacol 23:35–43
Fisher SR, Mullican WF (1997) Hydrogeochemical evolution of sodium-sulfate and sodium-chloride groundwater beneath the northern Chihuahua desert, Trans-Pecos, Texas, USA. Hydrogeol J 5:4–16. doi:10.1007/s100400050102
Frapolli M, Defago G, Moenne-Loccoz Y (2007) Multilocus sequence analysis of biocontrol fluorescent Pseudomonas spp. producing the antifungal compound 2,4-diacetylphloroglucinol. Environ Microbiol 9:1939–1955
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall, Englewood Cliffs, p 604
Gallardo AH, Marui A (2007) Modeling the dynamics of the freshwater-saltwater interface in response to construction activities at a coastal site. Int J Environ Sci Tech 4:285–294
Geldreich EE (1972) Waterborne pathogens. In: Mitchel R (ed) Water pollution microbiology. Wiley, New York, pp 207–241
GenBank. http://www.ncbi.nlm.nih.gov; http://blast.ncbi.nlm.nih.gov.
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
GOI-TN-District profile (2001) Government of India, Tamil Nadu, Cuddalore district profile. http://www.cuddalore.tn.nic.in/profile.htm
Graumann P (2007) Bacillus: cellular and molecular biology. Caister Academic Press, ISBN 978-1-904455-12-7
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate phylogenies by maximum likelihood. Syst Biol 52:696–704
Howarth R, Anderson D, Cloern J, Elfring C, Hopkinson C, Lapointe B, Malone T, Marcus N, McGlathery K, Sharpley A, Walker D (2000) Nutrient pollution of coastal rivers, bays, and seas. Ecol Soc Am Issues Ecol 7:1–16
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Jeevanandam M, Kannan R, Srinivasalu S, Rammohan V (2006) Hydrogeochemistry and groundwater quality assessment of lower part of the Ponnaiyar River Basin, Cuddalore District, South India. Environ Monit Assess 132:263–274
Jeong CH (2001) Effect of land use and urbanization on hydrochemistry and contamination of groundwater from Taejon area, Korea. J Hydrol 253:194–210
Jordan C, Smith RV (2005) Methods to predict the agricultural contribution to catchment nitrate loads: designation of nitrate vulnerable zones in Northern Ireland. J Hydrol 304:316–329
Karanth KR (1987) Groundwater assessment, development and management. Tata-McGraw-Hill, New Delhi
Karanth KR (1991) Impact of human activities on hydrogeological environment. J Geol Soc India 38:195–206
Kelly J, Thornton I, Simpson PR (1996) Urban geochemistry: a study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and nonindustrial areas of Britain. Appl Geochem 11:363–370
Kumar M, Kumari K, Ramanathan AL, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two agriculture dominated districts of Punjab, India. Environ Geol 53:553–574. doi:10.1007/s00254-007-0672-3
Lavitt N, Acworth RI, Jankowski J (1997) Vertical hydrogeochemical zonation in a coastal section of the Botany Sands aquifer, Sydney, Australia. Hydrogeol J 5:64–74
Lerner DN, Harris H (2009) The relationship between land use and groundwater resources and quality. Land Use Policy 26S:265–273. doi:10.1016/j.landusepol.2009.09.005
Macler AB, Merkel CJ (2000) Current knowledge on groundwater microbial pathogens and their control. Hydrogeol J 8:29–40
MacQuarrie KTB, Sudicky E, Robertson WD (2001) Numerical simulation of a fine-grained denitrification layer for removing septic system nitrate from shallow groundwater. J Hydrol 52:29–55
Madigan MT (2005) Brock biology of microorganisms, 11th edn. Prentice Hall, USA, ISBN 0131443291
Miller SCM, LiPuma JJ, Parke JL (2002) Culture-based and nongrowth-dependent detection of the Burkholderia cepacia complex in soil environments. Appl Environ Microbiol 68:3750–3758
Mor S, Singh S, Yadav P, Rani V, Rani P, Sheoran M, Singh G, Ravindra K (2009) Appraisal of salinity and fluoride in a semiarid region of India using statistical and multivariate techniques. Environ Geochem Health 31:643–655. doi:10.1007/s10653-008-9222-5
Nagarajan R, Rajmohan N, Mahendran U, Senthamilkumar S (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess 171:289–308. doi:10.1007/s10661-009-1279-9
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. USGS Water-Resources Investigations Report, pp 99–4259
Piper AM (1944) A graphical interpretation of water analysis. Trans AGU 25:914–928
Powell KL, Cronin AA, Barrett MH, Sellwood J, Pedley S (2001) Distribution of microbiological contaminants in Triassic sandstone urban aquifers. EA R&D Tech Rep P2-255/TR, July. Final Report to the Environ Agency, Bristol, p 26
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam Basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90
Purkhold U, Wagner M, Timmermann G, Pommerening-Röser A, Koops HP (2003) 16S rRNA and amoA-based phylogeny of 12 novel betaproteobacterial ammonia-oxidizing isolates: extension of the dataset and proposal of a new lineage within the nitrosomonads. Int J Syst Evol Microbiol 53:1485–1494
PWD (2000) Groundwater perspectives. a profile of Cuddalore district, Tamil Nadu. Public Works Department, Tamil Nadu, p 220
Rajmohan N, Al-Futaisi A, Al-Touqi S (2009) Geochemical process regulating groundwater quality in a coastal region with complex contamination sources: Barka, Sultanate of Oman. Environ Earth Sci 59:385–398
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Sarin MM, Krishnaswamy S, Dilli K, Somayajulu BLK, Moore WS (1989) Major ion chemistry of the Ganga-Brahmaputra river system: weathering process and fluxes to the Bay of Bengal. Geochim Cosmochim Acta 53:997–1009. doi:10.1016/0016-7037(89)90205-6
Sawyer CN, Mc Carthy PL (1967) Chemistry for sanitary engineers. McGraw-Hill, New York p 518
Schloss PD, Handelsman J (2005) Introducing species richness DOTUR, a computer program for defining operational taxonomic units and estimating. Appl Environ Microbiol 71:1501–1506
Schoeller H (1965) Qualitative evaluation of groundwater resources. In: Methods and techniques of groundwater investigations and development. UNESCO, pp 54–83
Schoeller H (1967) Geochemistry of groundwater. An international guide for research and practice UNESCO, chap 15, pp 1–18
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural waters, 3rd edn. Wiley, New York, p 1022
Subrahmanyam K, Yadaiah P (2001) Assessment of the impact of industrial effluents on water quality in Patancheru and environs, Medak district, Andhra Pradesh. India, Hydrogeol J 9:297–312. doi:10.1007/s100400000120
Swoford DL (2001) PAUP, Version 4.0b10 (Computer Software and Manual). Sinauer Associates, Sunderland, MA
Trivedy RK, Goel PK (1984) Chemical and biological methods for water pollution studies. Environ Publication, Karad
U.S. Salinity Laboratory Staff (1954) Diagnosis and improvement of saline and alkaline soils (60:147). U.S. Department of Agriculture, handbook
Van Ryneveld MB, Fourie AB (1997) Strategy for evaluating the environmental impact of on-site sanitation systems. Water SA 23:279–291
Vander Weijden CH, Pacheco FAL (2006) Hydrogeochemistry in the Vouga River basin (central Portugal): pollution and chemical weathering. App Geochem 21:580–613
Walker GR, Jolly ID, Cook PG (1991) A new chloride leaching approach to the estimation of diffuse recharge following a change in land use. J Hydrol 128:49–67
Wilcox LV (1948) The quality of water for irrigation use. US Department of Agriculture, Tech Bull 962, Washington, DC, pp 1–40
Wolfe AH, Patz JA (2002) Reactive nitrogen and human health: acute and long-term implications. Ambio 31:120–125
World Health Organization (1993) Guidelines for drinking water quality. Revision of the 1984 guidelines. Final Task Group Meeting, 21–25 Sept 1992, WHO, Geneva
World Health Organization (1997) Guideline for drinking water quality. 2nd edn. vol. 2, WHO, Geneva. Health criteria and other supporting information, pp 940–949
World Health Organization (2008) Guidelines for drinking-water quality: incorporating 1st and 2nd addenda, vol. 1, Recommendations., 3rd edn. Geneva, p 668, ISBN 978 92 4 154761 1(electronic resource)
Zhang J, Huang WW, Letolle R, Jusserand C (1995) Major element chemistry of the Huanghe (Yeloow River), China: weathering processes and chemical fluxes. J Hydrol 168:173–203
Acknowledgments
The first author is indebted to Prof. S. P. Mohan, Head of the Geology Department, and Prof.V. Rammohan, University of Madras, Chennai, for their constant support during his Doctoral degree program. The authors are grateful to anonymous reviewers and the editor for their constructive comments which improved the manuscript. This article is the 64th contribution from Earth System Science Group (ESSG), Chennai, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jeevanandam, M., Nagarajan, R., Manikandan, M. et al. Hydrogeochemistry and microbial contamination of groundwater from Lower Ponnaiyar Basin, Cuddalore District, Tamil Nadu, India. Environ Earth Sci 67, 867–887 (2012). https://doi.org/10.1007/s12665-012-1534-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1534-1