Abstract
Hydrochemical and stable isotope (18O and 2H) analyses of groundwater samples were used to establish the hydrochemistry of groundwater in the Ankobra Basin. The groundwater was generally mildly acidic, low in conductivity and undersaturated with respect to carbonate phases. Major ions except bicarbonate were low and dissolved silica was moderately high. Silicate minerals weathering is probably the main process through which major ions enter the groundwater. Groundwater samples clustered tightly along the Global Meteoric Water Line suggesting integrative, smooth and rapid recharge from meteoric origin. The majority of the boreholes and a few hand dug wells cluster towards the Ca–Mg–HCO3 dominant section of the phase diagram, in conformity with the active recharge and short residence time shown by the isotope data. Aluminium, arsenic, manganese, iron and mercury were the only trace metals analysed with concentrations significantly above their respective detection limits. Approximately 20%, 5%, 40% and 25% respectively of boreholes had aluminium, arsenic, iron and manganese concentrations exceeding the respective WHO maximum acceptable limits for drinking water. The relatively large percentage of boreholes with high concentration of aluminium reflects the acidic nature of the groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
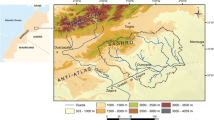
Ankobra Basin lies between latitudes 4.8° N and 6.5° N, and longitudes 1.75° W and 2.5° W respectively, and occupies approximately 8,400 km2. The location map for the Ankobra Basin is presented in Fig. 1. It is one of the most important river basins in Ghana, in that it has some of the richest gold and manganese mines in the country. Diamonds are also known to occur within the Ankobra Basin (Appiah et al. 1993). Minerals are not the only commodities produced. Large volumes of timber, palm-oil and coconut as well as rubber are also produced in this Basin.
Ghana government’s policy of trade liberalization and amendment of mining laws to attract foreign investments into the mining industry area have resulted in intensified and uncontrolled mining (particularly gold mining) activities in the Ankobra Basin with the subsequent degradation of the environment, putting water resources, particularly surface water, at high risk of pollution (Acquah 1993).
Realising that most of the surface water resources are polluted, the government of Ghana has shifted attention from developing surface water resources to groundwater resource development and supply to the communities in the Basin because of the anticipated high cost of treating polluted surface water resources. Consequently, groundwater has become the principal source of potable water supply for the communities within the Ankobra Basin.
Groundwater in hard rock aquifers, particularly in mining areas, are known to be vulnerable to quality problems as rocks are often carbonate-deficient and give rise to poorly buffered groundwater (Smedley et al. 1995). Gold ores in the Ankobra Basin are partly sulphidic ores (Acquah 1993), and exposure of the sulphides to the atmosphere either through natural processes of weathering, or anthropogenic processes such as mining, road cutting results in oxidation that often leads to generation of acid mine drainage. Acid mine drainage may eventually infiltrate into aquifers and lower the pH of the groundwater, depending on the buffering capacity of the aquifer. Additionally, acidic groundwater may encourage dissolution of minor and trace elements such as Al, As, Mn, Be and Fe from most minerals if they are present in the rock matrix and render the groundwater unsafe for drinking. Thus, there is a need to examine the hydrochemical composition and quality of groundwater in the Basin.
In spite of the importance of groundwater to this Basin, there is hardly any baseline information on hydrochemical composition of the groundwater or water quality data on which the present and future groundwater quality assessment could be based. The main objective of this paper is to determine the hydrochemistry and assess the groundwater quality in relation to drinking water.
2 Physiography and climate
Geomorphologically, the Ankobra Basin consists of a series of ridges separated by steep-sided, flat-bottomed valleys varying in altitude from 46 m in the south to 76 m in the north that are parallel to one another and to the strike of the rocks. The ridges at some places reach a height of 200 m. Due to the heavy rainfall and forest vegetation that prevent sheet erosion, the whole area is highly dissected and reduced to uniformly moderate relief with a gentle slope to the south (Service 1938; Dickson and Benneh 1980). Erosion is mainly restricted to river channels that cut up the plateau surfaces and the hills are usually capped with iron-pan (laterite) and bauxite (Dickson and Benneh 1980).
The climate of the Ankobra Basin falls partly under the wet semi-equatorial and partly under south-western equatorial climatic zones of Ghana (Dickson and Benneh 1980). It is thus characterised by double rainfall maxima. The first peak occurs in June whilst the second peak occurs in October. The mean annual rainfall values in the northern part of the Basin (wet semi-equatorial climatic zone) and in the southern part (equatorial climatic zone) are approximately 1600 mm and 2000 mm, respectively. The highest mean monthly temperature for the two climatic zones is 30°C and occurs between March and April and the lowest temperature is approximately 26°C and occurs in August (Dickson and Benneh 1980).
3 Hydrogeological setting
The geology of the Ankobra Basin consists mainly of lower Proterozoic rocks divided into the Birimian (Lower and Upper) system, unconformably overlain by the Tarkwaian system. Sills and dykes of igneous rocks ranging from felsite and quartz porphyry to meta-dolerite, gabbro and norite intrude into the Birimian and the Tarkwaian Systems at several places. The Lower Birimian rocks underlie approximately 60% of the Basin and are mainly pelitic in origin and consist of great thicknesses of alternating shales, phyllites, greywacke and argillaceous beds with tuffs and lavas. The Upper Birimian system covers approximately 8% of the Ankobra Basin and is dominated by rocks of volcanic and pyroclastic origin. The rocks consist of a bedded group of green lavas (greenstones), tuffs and sediments with minor bands of phyllite that include a zone of manganiferous phyllites containing manganese ore (Junner et al. 1942; Kesse 1985).
The Tarkwaian system that forms nearly 15% of the rock cover occupies an elongated and narrow geosyncline about 16 km wide that stretches in a north-eastern to south-western direction. It consists of an overall thick clastic sequence of arenaceous and argillaceous sediments with two well-defined zones of pebbly beds and conglomerate in the lower members of the system (Kesse 1985). The Granitoid occupies about 10% of the Ankobra Basin while the Eocene and Cretaceous Deposits occupy approximately 3% of the Basin. The Eocene and Cretaceous Deposits occur along the coast. A geological map of the Ankobra Basin is presented in Fig. 2.
Groundwater occurrence in the Ankobra Basin is associated with the development of secondary porosities as a result of fissuring and weathering. Tectonic lines of weakness created by the orogenic regimes have facilitated extensive and deep weathering and thick regolith development under the highly humid tropical climate with distinct wet and dry seasons. The weathering depth is greatest in the Birimian rock areas, where it has reached between 90 m and 120 m. The depth of the weathering in the granites, porphyries, felsites and other intrusive igneous rocks is between 20 m and 80 m. However, in the Tarkwaian system, the weathered depth rarely exceeds 20 m (Junner et al. 1942; Kesse 1985). Aquifers are mostly either semi-confined or confined due to the high argillite content of the regolith and occur mainly in the transition zone between weathered zones and the fresh rock or in the fractured or fissured zone normally at some depth below the weathered zone. Borehole yields are highly variable and in the range of 0.3–36.0 m3 h−1 with a mean 2.5 m3 h−1 while static water levels vary from 0.0 (flowing well) to 26.5 m below ground level (Nii Consult 1998).
4 Sampling and analytical techniques
Water samples were collected from 64 boreholes, 12 hand-dug, 3 major rivers and 2 streams that drain mining concession in January 2005 for physico-chemical analysis. Additionally 4 rainwater samples were also collected. At each sampling point, two samples were collected for major ions and trace elements analyses. Each sample was collected in acid-washed high-density linear polyethylene (HPDE) 100 ml bottle with strict adherence to the sampling protocol as described by Claasen 1982; Barcelona et al. 1985; Gale and Robins 1989. Samples were filtered using Sartorius polycarbonate filtering apparatus and a 0.45 μm cellulose acetate filter membrane. Sample for metal analyses were acidified to a pH < 2 after filtration using reagent grade nitric acid. Samples for anion analyses were without preservation. In this case, unfiltered sample was collected in 100 ml polyethylene bottles. The bottles were tightly capped to protect samples from atmospheric CO2 and stored in ice chest (with temperature variation between 4°C and 8°C). Precaution was also taken to avoid sample agitation during transfer to the laboratory. Furthermore, 39 water samples were collected from selected boreholes and hand dug wells, rivers and rainwater for deuterium and Oxygen-18 analyses. These consisted of 25 borehole, 4 hand dug well, 4 river and 6 rainwater samples. The isotope samples were collected unfiltered in 30 ml glass bottles with poly-sealed lids.
On-site analyses of temperature, redox potential (Eh), pH and electrical conductivity (EC) were conducted using a WTW-Multiline P4 Universal Meter in flow-through cell attached in line to the borehole pump outlet. Before taking readings, pumping was carried out until the meter readings were stable for each parameter. Since the boreholes were already being used, the pumping time before stable meter readings were achieved was, on average, 5 min. Hand-dug wells were not pumped, so measurements were carried out at the wellhead or water point as soon as the samples were taken. Alkalinity titrations were carried out at the wellhead or water point using a HACH Digital Titrator Model 16900. All major ions [sodium (Na+), potassium (K+), calcium (Ca2+), magnesium (Mg2+), bicarbonate (HCO\({_{3}^{-}}\)), chloride (Cl−), sulphate (SO\({_{4}^{2-}}\))] and minor elements such as nitrate (NO\({_{3}^{-}}\)), fluoride (F−) etc. were analysed using double column Dionex DX-120 ion chromatograph. Trace metals analyses were carried out using ICP-MS. ICP-MS and stable isotope analysis of deuterium and oxygen −18 analyses were performed at the Geological Institute of the University of Copenhagen, Denmark. The ionic balances for the analyses varied from −5.9% to 10.1%, though more than 85% of the samples had an ionic balance within ±5%. An ionic balance outside ±5% was largely associated with samples with very low total dissolved solids (TDS).
5 Results and discussions
5.1 Summary statistics
A statistical summary of chemical parameters measured in the groundwater samples is presented in Table 1 and actual results are put in appendices 1 and 2. Minimum, maximum, median are non-parametric statistics, which may be used to summarise non-normally distributed water quality data (Caritat et al. 1998; UNESCO/WHO/UNEP 1996).
The groundwater pH is typically low, in the range 3.89–6.78 with mean and median values of 5.70 and 5.72, respectively. However, the majority of the samples (>95%) falls within the pH range 4.5 to 6.9. Natural water pH values generally lie between 4.5 and 9.0 (Langmuir 1997). Electrical conductivity values are generally low. Maximum and minimum values are 780 μS cm−1 and 37 μS cm−1 respectively (Table 1).
Major cations (Ca2+, Mg2+, Na+ and K+) are generally low. In the majority of the cases (>60%) no particular cation shows a clear dominance but in a few cases, either Na+ or Ca2+ appears to be the dominant cation. Apart from bicarbonate (HCO −3 ), which is by far the dominant anion and forms approximately 40% of the TDS content, the concentrations of other major anions are generally low. Sulphate and nitrate occur only in trace concentrations in the groundwater. Dissolved silica concentration is moderately high and on average accounts for 15% of the TDS load.
5.2 Saturation indices
The saturation state of the groundwater was assessed with respect to the major carbonate minerals in order to investigate the thermodynamic controls on the composition of the groundwater and the approximate degree to which the groundwater has equilibrated with the various carbonate mineral phases since HCO −3 is the dominant anion. The saturation indices were determined using the hydrogeochemical equilibrium model, Phreeqc for Windows (Parkhurst and Appelo 1999). The saturation index (SI) of a given mineral is defined in Eq. 1
where IAP is the ion activity product and K sp is the solubility product at a given temperature (the thermodynamic equilibrium constant adjusted to the temperature of the given sample).
The thermodynamic data used in this computation were those contained in the default database of the ‘Phreeqc for Windows’. Supersaturation (SI > 0) indicates that precipitation is thermodynamically favourable in spite of the fact that slow rates of reaction can inhibit precipitation. On the other hand, undersaturation (SI < 0) signifies that dissolution is favoured. The summary statistics of saturated indices of some of the common mineral phases is presented in Table 2.
A plot of the computed saturation indices for calcite and dolomite (the commonest carbonate phases) based on the analytical results and measured field temperatures and pH for groundwater samples is shown in Fig. 3 (data for the plot is presented in appendix 3). In the diagram, calcite saturation is indicated on the ordinate and dolomite on the abscissa. A central band, of 0.4 units wide along each axis, represents essential equilibrium with respect to either mineral to account for the possible errors that may occur in the measurement pH, Mg2+ and Ca2+. The four quadrants of the plotting field, I–IV, outside the equilibrium area represents different kinds of equilibrium conditions with respect to calcite and dolomite. Quadrant I represents supersaturation with respect to both carbonates. This condition represents water brought to equilibrium with respect to these carbonates and subsequently transported into a different environment where a higher pH or an apparent condition caused by the failure of the measured pH to accurately represent the actual equilibrium pH of the water in the aquifer (Langmuir 1971). Quadrant II represents super-saturation with respect to calcite but undersaturation with respect to dolomite. Water undergoing incongruent dissolution of dolomite or precipitation of calcite would plot in this quadrant. Quadrant III represents undersaturation with respect to both calcite and dolomite. A sample plotting in this quadrant represents water that has come from an environment where carbonates are impoverished. Water that has not reached equilibrium with the carbonates due to the short residence time would also plot in this quadrant (Langmuir 1971). Quadrant IV represents supersaturation with respect to dolomite and undersaturation with respect to calcite.
All the groundwater samples plotted in quadrant III, is an indication that the groundwater is undersaturated with respect to the major carbonate species. Thus, either the groundwater originates from an environment where calcite and dolomite are impoverished or the residence time is short and the groundwater has inadequate time for the mineral phases to react to equilibrium. Petrographic evidence, however, indicates the presence of significant proportion of carbonates in some of the rock types that have underlain the Basin. Various researchers (Jacobson and Langmuir 1970; Back and Hanshaw 1970; Kenoyer and Bowser 1992) observed that vast majority of groundwater samples approach saturation with respect to calcite and dolomite even in fractured bedrock aquifers, if these carbonates are present to significant degree in an aquifer. Thus, the undersaturation of the groundwater with respect to calcite and dolomite can be attributed to short residence time of the groundwater in the aquifer or the carbonates may have occurred in some insoluble form. The groundwater is also undersaturated with respect to gypsum and anhydrite due to their absence from the rocks. The groundwater is uniformly supersaturated with respect to quartz but undersaturated with respect to amorphous silica (Table 2) suggesting that amorphous silica possibly controls the silica concentration in the groundwater.
5.3 Hydrochemical facies
The chemical composition of groundwater (borehole samples) from the Ankobra Basin is shown on the phase diagrams in Fig. 4. The majority of the boreholes and a few hand dug wells cluster towards the Ca–Mg–HCO3 dominant Section (A) of the diamond field, in conformity with the active recharge and short residence time shown by the isotope data. A few of the boreholes (Section B) also show Na–HCO3 characteristic reminiscent of aggressive recharging water reacting with silicate rocks mainly containing albite. Other processes that can lead to Na–HCO3 water type include cation exchange, anaerobic degradation of organic matter and proton exchange. However, as can be seen later, albite weathering is the main process. The Na–HCO3 water type does not appear to be associated with any particular geology or area. The remaining hand dug wells and few boreholes are Na–Cl in character (Section C). Less than 10% of the groundwater show Na–Cl water type. Hand dug wells in the Aboso area and low pH and conductivity boreholes in Tamso area (please refer to Fig. 1) show this character. This water type may, therefore, be due to the influence of the local rain and illustrates the importance of local recharge conditions in this Basin. The rivers and springs plot mainly in section D. This is mixed water type where there is neither dominant cation nor anion.
5.4 Sources of major ions
Figure 5 shows the relationship between Ca + Mg (meq l−1) vs. SO4 + HCO3 (meq l−1) for the groundwater. A 1:1 relationship would indicate gypsum, anhydrite, calcite and dolomite dissolution as the predominant processes controlling solution composition while groundwater falling below the 1:1 dissolution line signifies ion exchange, in which case Ca2+ + Mg2+ are being depleted with respect to SO\({_{4}^{2-}+\hbox{HCO}_{3}^{-}}\) (McLean and Jankowski 2000). Several samples fall on the 1:1 line. Gypsum and anhydrite have not been detected in the rocks within the Ankobra Basin, thus the 1:1 ratio probably implies the dissolution of calcite or dolomite or both. In spite of petrographic evidence that the rocks in the study area contain carbonates, the groundwater is largely undersaturated with respect to the common carbonates (calcite and dolomite) suggesting the carbonates dissolution is not enough to explain the 1:1 relationship. The HCO −3 /SiO2 ratio for about 80% of the water samples is relatively low, varying between 1 and 5, while the Mg2+/(Mg2+ + Ca2+) equivalent ratio for approximately 46% of the samples is greater than 0.5, suggesting silicate (mainly, ferromagnesian mineral) weathering is likely Hounslow (1995). Thus, silicate weathering may have contributed significantly to the concentrations of the Ca2+ and Mg2+. Approximately 50% of the water samples have Na+/(Na+ + Cl−) ratio within the range 0.5 ± 0.1 or plot along or close to 1:1 line in Na+ vs. Cl− graph (Fig. 6) suggesting that either sea aerosols or halite dissolution is partially responsible for Na+ in the groundwater. However, halite is not known to be associated with the rocks in this area implying sea aerosols is partially the likely source of sodium in the groundwater. A few (8%) of the samples plot below and away from the 1:1 line suggesting reverse ion exchange also occurs but is not a major process responsible for chemical evolution of groundwater in this Basin. Approximately 32% of the water samples, however, have Na+/(Na+ + Cl−) ratios significantly greater than 0.5 (samples falling above the 1:1line in Na vs. Cl graph indicating other sodium sources than sea spray; perhaps cation exchange or alumino-silicate (Na-plagioclase or albite) dissolution). Figure 7 is a plot of \({\hbox{Ca}^{2+}+\hbox{Mg}^{2+}-\hbox{SO}_{4}^{2-}-\hbox{HCO}_{3}^{-}}\) (meq l−1) vs. Na+–Cl− (meq l−1) for groundwater samples that show Na+/(Na+ + Cl) > 0.5. Waters undergoing ion exchange would plot along a line whose slope is −1 while waters plotting close to the zero value on the x-axis are not influenced by ion exchange (Jankowski et al. 1998). A closer look at Fig. 7 reveals that the samples plot away from the zero value of the x-axis with a slope of −2. This slope is twice as steep as the slope of −1 expected if cation exchange were the dominant process through which Na+ enters the groundwater system. Ion exchange can, therefore, not explain the observed excess of Na+ over Cl−. Therefore, if ion exchange is taking place, other processes like Na-plagioclase(albite) weathering may be masked.
5.5 Silicate weathering
The predominant mineralogy of the igneous rocks and the low-grade metamorphic rocks of the Ankobra Basin consists of plagioclase feldspars (mainly albite), hornblende and pyroxenes. The groundwater composition is influenced by the reaction between these mineral phases and the groundwater. The degree to which the mineral phases react with the groundwater depends on the availability of protons (H+), the contact time and the surface area per unit volume of water (Hem 1985). The forest vegetation and the humid climatic conditions of the Ankobra Basin provide avenues for the decay of leaves and other organic matter as well as root respiration that produce substantial amount of CO2 in the soil zone and hence the H+ availability for circulation in the groundwater. Dissolution of sodium feldspars (albite (NaAlSi3O8) or intermediate plagioclase (Na0.62Ca0.38Al1.38Si2.62O8)) is probably the source of Na–HCO3 water type and produces the secondary mineral products kaolinite and/or gibbsite. Meteoric water dissolving Na+ from Na-bearing silicates results in Na–HCO3 waters having Na+/Cl− ratios greater than one (Garrels and Mackenzie 1967). The alteration of albite and intermediate plagioclase to kaolinite is given by Eqs. 2 and 3, respectively.
Theoretically, the H4SiO4/Na+ molar ratio is 2.0 for the reactions in both Eqs. 2 and 3. Similarly the H4SiO4/HCO −3 ratio is 2 for albite Eq. 2 and 0.9 for intermediate plagioclase dissolution Eq. 3. Molar ratios of H\({_{4}\hbox{SiO}_{4}/\hbox{HCO}_{3}^{-}}\) and H\({_{4}\hbox{SiO}_{4}/\hbox{Na}^{+}}\) for the groundwater are in the ranges 0.01–2.80 and 0.02–2.30 with median values of 0.40 and 0.80 respectively (Table 2). These ratios are within the limits of the theoretical values, suggesting the plausibility of albite and intermediate plagioclase weathering to kaolinite and contributing Na+, HCO\({_{3}^{-}}\) and H4SiO4 to the groundwater and the possible source of excess of Na+ over Cl−. The evolution of Na–HCO3 water type in some of the wells can, therefore, be explained by dissolution of albite by CO2 charged meteoric recharging waters. The stability diagram of albite and its possible weathering products gibbsite, kaolinite and Na-montmorillonite (Tardy 1971) for the groundwaters in the Ankobra Basin is shown in Fig. 8. All the groundwater samples plotted in the kaolinite field as would be expected for a system controlled by reactions in Eqs. 2 and 3. Similar to the dissolution of albite and intermediate plagioclase, the dissolution hornblende (NaCa2(Mg,Fe,Al)5Si8O22(OH)2) and other ferromagnesian silicates probably resulted in the Ca–Mg–Na–HCO3 or Na–Ca–Mg–HCO3 water types.
The stability of albite and its possible weathering products gibbsite, kaolinite and Na-montmorillonite with respect to the groundwaters in the Ankobra Basin (modified after Tardy 1971)
5.6 Stable isotopes
The results of the oxygen-18 (\(\delta^{18}\hbox{O}\permille\)) and deuterium (\(\delta ^{2}\hbox{H}\permille\)) analyses are presented in Table 3. The variation in δ18O was from \(-2.65\permille\)to \(-0.99\permille\) with mean and median values of \(-2.12\permille\) and \(-2.21\permille\) respectively. Deuterium values varied between \(-11.7\permille\) and \(-2.2\permille\) with mean and median values of \(-6.99\permille\) and \(-7.3\permille\), respectively. The relationship between \(\delta ^{2}\hbox{H}\permille\) and \(\delta ^{18}\hbox{O}\permille\) of the samples is shown in Fig. 9. The groundwater samples were relatively tightly clustered along the Global Meteoric Water Line (δ2H = 8δ18O + 10 (Craig 1961)) suggesting an integrative, smooth and rapid recharge from meteoric origin. Consequently recharge to the aquifer system within the Ankobra Basin is mainly meteoric water with little or no isotopic modification. A few groundwater samples (e.g. the open hand-dug wells at Huni Valley and Aboso, with (δ18O, δD) values of (−1.16, −2.2) and (−0.99, −2.2), respectively), showed evidence of some degree of isotopic enrichment by evaporative processes. Since evaporation may have taken place in these open wells under the humid climatic conditions of the Ankobra Basin, the deviation from the Global Meteoric Water Line has not been as much pronounced as may have occurred under less humid climatic conditions (IAEA 1983). Two of the surface water samples with isotopic composition (δ18O, δD) of (\(-1.54\permille, 8\permille\)) and (\(-1.54\permille, 7.5\permille\)) respectively, plot relatively far below the Global Meteoric Water Line indicating a degree of isotopic enrichment as a result of surface evaporation. The samples were taken in the dry season when the rivers were at their lowest stage and flow was comparatively slow and sluggish and thus there was adequate time for the almost stagnant water to be subjected to intense evaporation. It is therefore not surprising that these two surface water bodies show isotopic enrichment typical of surface evaporation. Alternatively, the isotope enrichment could be reflecting the integration of the isotopic composition of the various tributaries that take their sources from a relatively drier part of Ghana than the Ankobra Basin.
5.7 Minor and trace ions
Contrary to expectation from a mining area, the analytical results did not indicate high levels of trace metal loading. Nonetheless, aluminium, arsenic, iron and manganese showed concentrations significantly above their detection levels in a considerable number of wells. The spatial distributions and the histograms of the concentrations of aluminium, arsenic, iron and manganese are shown in Figs. 10a–d, 11a–d, respectively.
5.7.1 Aluminium (Al3+)
The Al3+ ion concentration in the groundwater varies from <0.1 μg l−1 (below detection limit) to 2,510 μg l−1 with median value 0.10 μg l−1. Approximately 20% of the boreholes have Al3+ concentrations exceeding the WHO maximum acceptable limit of 200 μg l−1 for drinking water (WHO 1993) and thus pose the risk of significant aesthetic problems to groundwater usage. As expected, higher Al ion concentrations are associated with the boreholes with lower pH (3.89–4.2) water. The very low pH boreholes, nonetheless, constitute only 5% of the total number of boreholes. Dissolution of kaolinite or alunite, to provide extra acid neutralisation capacity to the groundwater, and appears to be the main source of Al3+ in the low pH waters. The typical kaolinite dissolution reaction in low pH water is given by Eq. 4
5.7.2 Arsenic (As)
The concentration of arsenic is generally low varying from <0.05 μg l−1 to 491 μg l−1 with a median value of 0.05 μg l−1. Relatively high As concentrations (>5 μg l−1) occur in the western and the northwestern part of the study area, where the Birimian rocks contains significant amount of pyrite and arsenopyrites (Fig. 2). However, only 5% of the wells and boreholes have arsenic concentrations slightly in excess of the WHO guideline limit of 10 μg l−1 (WHO 1993). Arsenic therefore does not potentially pose any significant physiological problem for groundwater usage in the Ankobra Basin. On the contrary, the surface waters (creeks) draining mining concessions have relatively high levels of arsenic concentrations (130–2,700 μg l−1) and high sulphate levels (190.0–1,840 mg l−1). This indicates pyrite and arsenopyrite oxidation. Low concentrations of arsenic in the shallow groundwater (boreholes) regardless of the high presence of arsenopyrite in association with the gold ore, suggest a level of co-precipitation of arsenic with ferric oxyhydroxide or adsorption onto the ferric oxyhydroxide masses in the creeks before possible infiltration into the aquifer. Nevertheless, arsenopyrite oxidation can take place to produce a measurable arsenic concentration, even with only a small quantity of arsenopyrite. The reaction involving arsenopyrite oxidation is given in Eq. 5.
If complete and congruent reactions were assumed, the highest measured arsenic concentration in the boreholes within the Ankobra Basin of 49 μg l−1 would require the oxidation of 10,749 μg l−1 of arsenopyrite. This would also produce corresponding concentrations of only 36 μg l−1 and 63 μg l−1 of Fe and SO\({_{4}^{2-}}\) respectively. Thus, arsenopyrite oxidation appears to be the main source of arsenic concentration in the groundwater in spite of the generally low sulphate concentrations.
5.7.3 Iron
The minimum and maximum concentrations of iron in the groundwater are <10 μg l−1 and 12,100 μg l−1 respectively with a mean and median values of 1,300 μg l−1 and 150 μg l−1 respectively. The WHO guideline maximum for iron in drinking water is 300 μg l−1 but an upper limit of 1,000 μg l−1 should suffice for most purposes (WHO 1993). Approximately 40% of boreholes in the Ankobra Basin have Fe2+ greater than 1,000 μg l−1. This poses major aesthetic quality problems to groundwater usage for domestic purposes in the Basin as it may lead to consumer complaints and indeed a significant number of boreholes has already been abandoned due to the iron problem. It has been mentioned earlier that iron minerals, particularly pyrites and arsenopyrite are common in the rocks that have underlain the Ankobra Basin. Oxidation and dissolution of pyrites and arsenopyrite should naturally be a major source of iron and sulphate (SO\({_{4}^{2-}}\)) in the groundwaters. However, SO\({_{4}^{2-}}\) concentration in the boreholes is generally low, 0.002–0.57 mmol l−1 with a median value of 0.06 mmol l−1 and only a few samples satisfy the iron to sulphate (Fe2+/SO 2−4 ) molar ratios of 0.5 and 1 for the stoichiometry of pyrite and arsenopyrite oxidation respectively. Median (Fe2+/SO\({_{4}^{2-}}\)) is 0.05 (Table 2). Additionally, boreholes with high iron concentration also have (Fe2+/SO\({_{4}^{2-} > 1}\)) suggesting that unless SO\({_{4}^{2-}}\) reduction or gypsum precipitation had taken place, which has not been evident, pyrite and/or arsenopyrite oxidation cannot exclusively explain the high iron concentration in some of the boreholes. The calculated saturation indices of the iron species indicate that the waters are highly supersaturated with respect to goethite, hematite and magnetite. The supersaturation of the groundwater with respect to these iron-bearing minerals suggests that the groundwater has reacted with these minerals for long enough time to reach equilibrium and should be thermodynamically incapable of dissolving more of these iron species unless equilibrium conditions are changed. In other words, the groundwater should be precipitating these iron minerals if it is oxygenated as in the case of phreatic aquifers and therefore, reducing the iron concentration in the boreholes. However, this does not seems to be the case. As indicated by White and Yee (1985), Fe3+ is reduced to Fe2+ at the mineral surface. Goethite, hematite and magnetite are the main iron species present in the rock matrix within the study area. Iron may, therefore, have been derived from the reductive dissolution of hematite and goethite and to a limited extent magnetite in the presence of organic matter. The reduction is likely microbially mediated. Hematite dissolution in the presence of protons (acid) is extremely slow. However, in the presence of organic ligands the reaction can be fast (Appelo and Postma 1999). The presence of organic matter in the soil zone of the study area is indicated by the brown colouration of rivers and streams throughout the year. An additional source of iron could be the leaching from ferromagnesian silicates, such as hornblende and pyroxenes.
5.7.4 Manganese
Manganese occurs in the rocks mainly as manganese and manganiferrous oxides, particularly in areas underlain by Birimian rocks (Kesse 1985). Reaction between these oxides and the mildly acidic groundwater results in the production of manganous ions (Mn2+). Mn2+ concentration varies from 1.0 μg l−1 to 1,300 μg l−1 and is greater than 500 μg l−1 (WHO permissible limit for potable water) in approximately 25% of the boreholes and wells within the Ankobra Basin. Similar to iron, the manganese concentration in the groundwater also poses major quality problems in the Ankobra Basin.
5.7.5 Mercury
The concentration of mercury (Hg) in the groundwaters varies from <0.05 μg l−1to 36.8 μg l−1. The maximum acceptable limit for mercury in drinking water is 1.0 μg l−1. The occurrence of mercury in water is mainly due to leaching from rocks and sometimes from release into the water in the form of wastewater effluents from industrial plants that use mercury (Holden 1972). Mercury has not been detected in any of the rocks within the Ankobra Basin. Neither are there industries in the area, apart from mining, that can release mercury in significant quantities to the environment. Mercury amalgamation used to be the main method for the processing of gold ores in the Ankobra Basin. Currently, the major gold-mining companies use the more efficient cyanide leaching method. However, because mercury amalgamation requires little capital investment in equipment, it is still popular and widely used by small-scale miners and the galamsey (illegal gold miners) operators in the Ankobra Basin. The occurrence of mercury in the groundwater bodies is, therefore, suspected to be largely due to contamination from surface sources, perhaps resulting from indiscriminate use of mercury for gold processing. The concentration of mercury exceeds the WHO permissible limit for drinking water in approximately 10% of the boreholes in the Ankobra Basin. Because mercury toxicity can lead to kidney damage, brain and nervous system disorder (WHO 1980; Carlos et al. 1997) therefore, it potentially poses the greatest physiological problem for using the groundwater for drinking purposes in the Ankobra Basin.
5.8 Groundwater acidity
Acidity in groundwater could be due to natural biogeochemical or anthropogenic processes. Natural acidification is obvious in areas with weathering-resistant soils and rocks, where the climate is humid and the dominating water movement as well as the transport of chemical components is downward, resulting in run off of base cations as in the Ankobra Basin (Knutsson 1994). Figure 12 illustrates the relationship between total hardness and alkalinity of groundwater in the Ankobra Basin. The non-marine total hardness-alkalinity plot is usually used to evaluate the status of ground and surface waters (Von Brömssen 1989; Caritat 1995). The total hardness values are assumed to be non-marine because chlorine which is conservative and originates exclusively from marine source is very low in concentration and also there is no petrographic evidence to suggest the existence of marine carbonate in most part of the Ankobra Basin, particularly areas where relatively low pH values were obtained. Figure 12 shows that most of the groundwater samples plot along the 1:1 line suggesting that acidification is principally due to natural biogeochemical processes (CO2 generation in the soil zone through root respiration and decay of organic matter). The presence of organic matter in the soil zone is supported by the brown colouration of surface waters through out the year. The few samples that plot along or above the 2:1 line probably resulted from sulphide (pyrite or arsenopyrite) oxidation enhanced by mining.
Groundwater acidification (Aci.) can also be defined as loss of alkalinity and computed using Eq. 6.
where all concentrations are expressed in meq l−1 (Henriksen and Kirkhusmo 1986; Caritat et al. 1998). Similarly, the acid neutralising capacity of the groundwater can be computed using Eq. 7.
where all concentrations are expressed in meq l−1 (Stumm 1992; Caritat 1998)
The relationships between ANC, NANC (net acid neutralising capacity) and Aci are presented in Fig. 13. The groundwaters are low in acid neutralising capacity with ANC varying from −1.7 meq l−1 to 4.26 meq l−1 with a mean value of 1.15 meq l−1 confirming buffering agents other than carbonates. Nonetheless, the Aci of the groundwaters is also very low varying between −15.1 meq l−1 and −10.7 meq l−1 with a mean value of −14.1 meq l−1. It is obvious from Fig. 13 that though the ANC is low it remains positive for most samples while Aci remains negative. The net acid neutralising capacity is thus positive for all the groundwater samples. This gives an indication that, notwithstanding the moderately low pH, the groundwaters still have the potential to neutralise acids probably due to the presence of alumino-silicates and some mafic rocks.
6 Summary and conclusions
Analysis of the hydrochemical survey data from the Ankobra Basin has revealed that the groundwater is generally mildly acidic (pH 4.5–6.9) with a few of the boreholes showing strong acidic character (pH < 4.5). Major cations are generally low and in about 60% of the wells no clear-cut dominant cation emerged. Bicarbonate is by far the most dominant anion and in a large number of wells forms approximately 40% of the TDS.
Three major water types have been delineated using the phase diagrams. These are Ca–Mg–HCO3, Na–HCO3 and Na–Cl waters. The Ca-Mg-HCO3 water type represents over 60% of the groundwater in the Ankobra Basin and it is not associated with any particular rock type. The Na–Cl water-type comprises less than 10% of the groundwater and is mainly associated with boreholes with very low pH. The groundwater is generally undersaturated with respect to major carbonate species namely calcite, dolomite and ankerite. Dissolution of alumino-silicate minerals particularly plagioclase, amphiboles and pyroxenes by mildly acidic groundwater is responsible for the major ions in the groundwater.
The trace metal loading of the groundwater is not high. Only aluminium, arsenic, manganese, iron and mercury show significant concentrations in the boreholes. Approximately 20% of the boreholes have Al3+ concentration exceeding the WHO maximum acceptable limit of 0.2 mg l−1 for drinking water. The elevated concentration of aluminium in the groundwater reflects the acidic nature of the groundwater and aluminium probably entered the groundwater as result of dissolution of kaolinite and alunite to provide extra acid neutralisation. Approximately 5% of the boreholes have arsenic concentrations slightly in excess of the WHO (1993) guideline limit of 10 μg l−1. Relatively high arsenic concentrations occur in the western and the north-western part of the study area where the Birimian rocks contain significant amounts of pyrite and arsenopyrites. Nearly 40% of boreholes in the Ankobra Basin have Fe2+ greater than 1.0 mg l−1. Unless iron is removed through the use of aerators or iron removal plants, it poses significant water quality problems for domestic purposes in the Ankobra Basin. Sources of iron in the groundwater include oxidation of pyrite and arsenopyrite, the reductive dissolution of hematite and goethite and, to a limited extent, magnetite in the presence of organic matter and leaching from ferromagnesian silicates, such as hornblende and pyroxenes. All these iron minerals are common in the Ankobra Basin. Manganese occurs in greater concentrations than 0.5 mg l−1 (WHO permissible limit for potable water) in approximately 25% of the boreholes within the Ankobra Basin and thus also poses the risk of major aesthetic quality problems.
Mercury potentially poses the greatest physiological problem for using the groundwater for drinking purposes in the Ankobra Basin. Its concentration exceeded the WHO (1993) permissible limit for drinking water in 10% of the boreholes. It originates from anthropogenic sources since it has not been detected in any of the rocks in the Ankobra Basin. Mercury amalgamation, which is widely used by small-scale miners for gold extraction is suspected to be the source.
References
Acquah PC (1993) Emerging trends in gold ore processing and some related environmental issues in Ghana. Regional Trends in African Geology. Proc. of the 9th Int. Geol. Conf. (Accra, 2nd to 7th November 1992). Geological Society of Africa
Appiah H, Norman Di, Kuma JS, Nartey RS, Dankwa JBK (1993) Sources of diamonds in the Bonsa field. Regional Trends in African Geology. Proc. 9th Int. Geol. Conf. (Accra, 2nd to 7th November 1992). Geological Society of Africa
Appelo CAJ, Postma D (1999) Chemical analysis of groundwater. Geochemistry, Groundwater and Pollution. Fourth corrected print. AA Balkema, Rotterdam, Brookfield
Back W, Hanshaw B (1970) Comparison of chemical hydrology of the carbonate peninsulas of Florida and Yucatan. J Hydrol 10:330–368
Barcelona M, Gibb JP, Helfrich JA, Garske EE (1985) Practical Guide for Groundwater Sampling. Illinois State Water Survey ISWS Contract Report 374
Claasen HC (1982) Guidelines and techniques for obtaining water samples that accurately represent the water quality for an aquifer. U.S. Geological Survey Open File Report 82-1024, 49 pp
Caritat PDE (1995) Intensifying groundwater acidification at Birkenes, southern Norway. J Hydrol 170:47–67
Caritat PDE, Danilova S, Jaeger Ø, Reimann C, Storrø G (1998) Groundwater composition near the nickel-copper smelting industry on the Kola Peninsula, central Barents Region (NW Russia and NE Norway). J Hydrol 208:92–107
Carlos D, da Rosa JD, Lyon JS, Udall SL, Hocker PM (1997) Golden dreams, poisoned streams. Mineral Policy Center, Washington, DC
Craig H (1961) Isotopic variations in meteoric water. Science 133:1702–1703
Dickson KB, Benneh G (1980) A new geography of Ghana. Longmans Group Limited, London
Gale IN, Robins NS (1989) The Sampling and Monitoring of Groundwater Quality. British Geological Survey. Hydrogeology Report, No. 89/37
Garrels RM, Mackenzie FT (1967) Origin of the chemical compositions of some springs and lakes. In: Gound RF (ed) Equilibrium concepts in natural water systems. American Chemical Society Publications, Washington, DC
Hem JD (1985) Study and interpretation of chemical characteristics of natural water Geological Survey Water Supply Paper 2254. Washington, United States Government Printing Office, 264 p
Henriksen A, Kirkhusmo LA (1986) Water chemistry of acidified aquifers in southern Norway. Water Qual Bull 11(1):34–38, 61
Holden AV (1972) Present levels of mercury in man and his environment. In: Mercury contamination in man and his environment. Vienna, Internatioinal Atomic Energy Agency. Technical Report Series No. 137
Hounslow AW (1995) Water quality data analysis and interpretation. Lewis Publishers, Boca Raton, New York
Iaea (1983) Guidebook on nuclear techniques in hydrology (1983 edn). Technical reports series No. 91 IAEA, Vienna, 1983
Jacobson RL, Langmuir D (1970) The chemical history of some spring waters in carbonate rocks. Groundwater 8:5–9
Jankowski J, Acworth RI, Shekarforoush S (1998) Reverse ion-exchange in deeply weathered porphyritic dacite fractured aquifer system, Yass, New South Wales, Australia. In: Arehart GB, Hulston JR (eds) Proc. 9th Int. Symp. Water–Rock interaction. Taupo, New Zealand, 30 March–April 1998, 243–246. Balkema, Rotterdam
Junner NR, Hirst T, Service H (1942) Tarkwa Goldfield. Memoir, No. 6 Gold Coast Geological Survey
Kenoyer GJ, Bowser CJ (1992) Groundwater chemical evolution in a sandy silicate aquifer in Northern Wisconsin, 1. Patterns and Rates of Change. Water Resour Res 28(2):579–589
Kesse GO (1985) The mineral and rock resources of Ghana. AA Balkema, Rotterdam, Boston
Knutsson G (1994) Acidification effects on groundwater – prognosis of the risks for the future. In: Future groundwater resources at risk. Soveri J, Suokko T (eds) Proc. Helsinki Conf., June 1994. IAHS Publ. No. 222
Langmuir D (1971) The geochemistry of some carbonate groundwaters in central Pennsylvania. Geochim Cosmochim Acta 35:1023–1045
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River, New Jersey 07458
McLean W, Jankowski J (2000) Groundwater quality and sustainability in an alluvial aquifer, Australia. In: Sililo et al (eds) Proc. XXX IAH congress on Groundwater: Past Achievements and Future Challenges. Cape Town South Africa 26th November–1st December 2000. AA Balkema, Rotterdam, Brookfield
Nii Consult (1998) Information Building Block. Ghana Water management Study. Unpublished Consultancy Report for the Ministry of Works and Housing, Ghana, DANIDA, World Bank
Parkhurst DL and Appelo CAJ (1999) PHREEQC for windows version 1.4.07. A hydrogeochemical transport model. U.S. Geological Survey Software
Service (1938) Annual Report of the Gold-Coast Geological Survey
Smedley PL, Edmunds WM, West JM, Gardner SJ, Pelig-ba KB (1995) Vulnerability of Shallow Groundwater Quality due to Natural Geochemical Environment. Health Problems related to Groundwater in the Obuasi and Bolgatanga Areas, Ghana. Report prepared for ODA under the ODA/BGS Technology Development and Research Programme, Project 92/5
Stumm W (1992) Chemistry of the solid-water interface. John Wiley, New York
Tardy Y (1971) Characterization of the principal weathering types by the geochemistry of waters from some European and African crystalline massifs. Chem Geol 7:253–271
UNESCO/WHO/UNEP (1996) Water quality assessments. In: Chapman D (ed) A guide to the use of biota, sediments and water in environmental monitoring. E & FN SPON, London and New York
Von Brömssen U (1989) Acidification trends in Swedish groundwaters. Review of time series 1950–85. National Swedish Environmental Protection Board, Report 3547, 67 pp
White AF, Yee A (1985) Aqueous oxidation–reduction kinetics associated with coupled electron-cation transfer from iron-containing silicates at 25°C. Geochim Cosmochim Acta 49:1263–1275
World Health Organisation (WHO) (1980) Recommended health-based limits in occupational exposure to trace metals. Technical Report Series No. 647, Vienna
World Health Organisation (WHO) (1993) Guidelines for Drinking Water Quality. Revision of the 1984 Guidelines. Final Task Group Meeting. Geneva 21–25 September 1992
Acknowledgements
I wish also to express my gratitude to DANIDA, The Danish International Development Authority that partially funded the project through Grant No. Dau. 8l/300. I wish also to thank the Water Research Institute for providing the remainder of the funds for this project.
Author information
Authors and Affiliations
Corresponding author
Appendices
Rights and permissions
About this article
Cite this article
Kortatsi, B.K. Hydrochemical framework of groundwater in the Ankobra Basin, Ghana. Aquat Geochem 13, 41–74 (2007). https://doi.org/10.1007/s10498-006-9006-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-006-9006-4