Abstract
The Houjing River flows through Kaohsiung, the most industrialized city in southern Taiwan. In this study, heavy metal concentrations in water and sediments from samples along the river were investigated to illustrate metal contamination levels and call for the awareness of industrial pollution prevention. The heavy metal concentrations in the water samples were low and appear to pose little direct risk to aquatic life and irrigation, but heavy metal concentrations in the sediments are locally very high and present an environmental risk. Cadmium, Cu, and Zn were found in higher concentrations in the river sediments than those recommended in some sediment quality guidelines and findings of river sediments in similar studies worldwide. Hence, the ecological risk of heavy metal contamination in sediments was assessed using the pollution load index (PLI) and potential ecological risk index (RI). Three of the eleven sites sampled were found to have PLI values higher than 1 and 8 of them had ‘considerable’ to ‘very high’ RI values, suggesting a considerable ecological risk. These findings provide an insight into elemental metal contamination of the Houjing River and present a baseline data set, which will be critical for future development and environmental protection plans devised for the region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although heavy metals naturally occur in the environment, they are considered environmental contaminants when they are introduced into an environment where they are not common or are artificially concentrated. Their persistence, non-biodegradability, bio-accumulation, and toxicity in the ecosystem have attracted wide attention in both public and scientific communities (Tessier and Campbell 1987; Tomlinson et al. 1980). Heavy metals can enter ecosystems through various processes, including dissolution, precipitation, sorption, and complexation (Dhanakumar et al. 2015; Pejman et al. 2015). At elevated levels, they are harmful to organisms (e.g., some of benthic invertebrates and fish species) and pose different negative effects to human and ecosystem health (Chapman et al. 1998; Eeva and Lehikoinen 2000; Kiffney and Clements 1993; Vu et al. 2017b). Although heavy metals can occur naturally, industrial manufacturing, transportation, and agricultural irrigation often lead to elevated or excess heavy metal concentrations (El Nemr et al. 2016a; Venkatramanan et al. 2015; Yan et al. 2016).
Industrialization has led to numerous sites contaminated by heavy metal through the release of chemical products used in different industrial processes (Dhanakumar et al. 2015; Pejman et al. 2015). Metals entering aquatic ecosystem are rapidly deposited, connected to organic and inorganic compounds, including complexation, and eventually settled out in sediments. Since the mobility and bio-availability of heavy metals in aquatic environment can be altered, heavy metals can be accumulated in the bodies of aquatic organisms and enter the food chain (Tessier and Campbell 1987). Heavy metals are difficult to degrade and/or excrete and excessive amounts of them may cause negative effects on the growth and physiological functions of organisms, including humans (Deniseger et al. 1990). Therefore, understanding the distribution and ecological risk of heavy metals is important for the management of the environment.
The rapid industrial transformation from an agricultural society in Taiwan has created high environment pressure (Huang et al. 2016; Vu et al. 2017a). One of the most noticeable environmental problems on the island is the widespread heavy metal pollution of water bodies (Huang 2003; Lin et al. 2009, 2010; Williams and Chang 2008). Kaohsiung City, the most industrialized area in Taiwan, is where heavy industries concentrated over the last 40 years. Located in the northern part of Kaohsiung City, the Houjing River has a long pollution history due to the industrial plants situated along its banks (Lin et al. 2017; Vu et al. 2017b). Many widely known incidents of illegal discharge from metal-utilizing companies into the river over the years have drawn wide attention from the public and resulted in a demand for risk assessment and frequent monitoring of heavy metal pollution (Chen 2016; Tsai 2014). Therefore, we investigated the heavy metal contamination at 11 sampling sites along the Houjing River and assessed environmental and ecological risks. The results of the current contamination status of the Houjing River are essential for guiding city development plans and restoration studies or plans for land-use changes, and are, thus, paramount for the city’s future economic and environmental development.
Materials and methods
Location
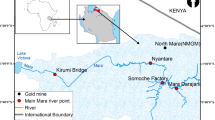
The Houjing River, situated in north–west Kaohsiung City in southern Taiwan (Latitude 22°69′21.63′′N–22°73′17.74′′N and Longitude 120°25′74.36′′E–120°33′74.70′′E), has two upstream origins: one from the north-east in Dashe District and one from the south-east in Renwu District (Fig. 1). The waters meet near the Si-Chingpu Landfill and flow from there through the city and into the Taiwan Strait. The river is about 13 km long. Southern Taiwan has long winters and summers, and short springs and autumns. The basins main water supply is a composite of rainfall and effluent discharges from industrial wastewater treatment plants along the river. In the past, the river water was the main source for agricultural activities in the area, but recent urbanization and industrialization have impacted water and sediments (Lin et al. 2010). The previous studies showed that the river is polluted with the elevated levels of organic compounds including di-(2-ethylhexyl) phthalate (DEHP) in sediments (Lin et al. 2009), benzene, toluene, ethylbenzene, xylene (BTEX) in water (Lin et al. 2007), and emerging contaminants in water (Jiang et al. 2015).
Sampling
Figure 1 shows a map of the 11 sites which we sampled from September 2014 to September 2015. The sites Sannaitan (H1), Xinggong (H2), and Jingjian (H3) were chosen to investigate the potential heavy metal influx and contamination caused by different anthropogenic activities in the upstream Dashe District that includes a large petrochemical manufacturing complex and some metal-surface-coating processes. Sites H1 and H2 are upstream from the industrial facilities of Dashe Industrial Park with wastewater discharged between sites H2 and H3. Sites Bakong (H4), Renwu (H5), and Huifeng (H6) were selected to investigate pollution caused by industrial activities upstream in Renwu District, where plastic resin and traditional metal-surface-processing industries are located. Site H4 is upstream of the Renwu Industrial Park with wastewater discharged between sites H4 and H5, while Zhuhou Industrial Park’s wastewater is discharged between sites H5 and H6. Sites Demin (H7), CingPu (H8), and Dehuei (H9) focused on industrial activities in the midstream Nanzih Export Processing Zone (NEPZ). The NEPZ has various heavy metal-processing plants, including computer chip, packaging, electroplating, and surface-coating manufacturing. Youchangda (H10) and Xingzhong (H11) are downstream sites before the water masses join the Taiwan Strait. The heavy industrial activities are putting the environmental health of the river and ocean systems under a significant pressure as wastewater discharge of plants is poorly managed. Kaohsiung’s Environmental Protection Bureau only monitors and mandates the discharge of NEPZ, Dashe, and Renwu Industrial Parks. These Industrial Parks have two discharge options, either directly into the Houjing River (limit 20,000 m3/day) or into the ocean through an ocean outflow pipe constructed in 1989 (5 km away from the coastal line, limit 74,800 m3/day). More importantly, the discharge management only focuses on the level of total chemical oxygen demand (COD), i.e., limit of 100 mg/L for the Houjing River and 300 mg/L for the ocean outflow (Lin et al. 2010) and not the level of individual contaminants. Sadly, illegal discharge activities through deep-water pipeline or wastewater trucks also take place (Lin et al. 2009, 2007). Hence, a study of heavy metal concentrations and contaminations of water and sediments of the Houjing River is long overdue.
In this study, we followed the standard US Environmental Protection Agency methods of water (USEPA 2013) and sediment sampling (USEPA 2014). A total of 88 sediment and 88 water samples were collected. An Ekman Dredge was used to collect sediment samples, which were then stored in polyethylene bags. Water samples were collected with a bucket and transferred into plastic bottles. Samples were collected in the middle of the river, approximately 5 m from the river banks. The samples were stored at 4 °C until they were analyzed.
Instrumental analysis
Temperature, salinity, dissolved oxygen (DO), pH, turbidity, and conductivity in water samples were assessed on-site using a Multi-Parameter Water Quality Sonde (YSI 6600 V2-4). Biochemical oxygen demand (BOD5) (Winkler’s method) and chemical oxygen demand (COD) were measured using the standard methods (APHA 1999)
For heavy metal analysis, water samples were first acidized with HNO3 (Zhang et al. 2016a) and aliquots of 20 mL were then filtered through Whatman® filter paper. Sediment samples were homogenously mixed, and rock fragments were removed manually before 0.2 g of the samples were taken and lyophilized in a vacuum freeze dryer (Eyela FDU-1200) at − 50 °C and 10 Pa for 24 h. Acids (3 mL HCl and 1 mL HNO3) were added to both water and sediment samples before microwave digestion (Topex, Preekem) according to the instruction of the manufacturer. After the digestion, the samples were filtered and analyzed using an Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES Optima 2100 DV, PerkinElmer) and a Mercury Analyzer (NIC MA-2, Systematic). ICP-OES was used to quantify As, Cd, Cr, Cu, Pb, Ni, and Zn concentrations.
The plastic sample containers and glassware used for ICP analysis were treated with 5% HNO3 prior to sampling and analysis. Similarly, ship-shaped ceramic sample containers used for mercury analysis were soaked in 5% HNO3 and baked in an oven at 500 °C for 18 h before the analysis. Standard ICP and mercury solutions were purchased from High-Purity Standards (Charleston, USA) and J.T.Baker (Avantor Performance Materials, USA), respectively. For all analyses, analytical grade reagents and deionized water were used.
Minimum detection limits (MDL) were set based on triple standard deviations of the analysis results of seven samples at the same concentration. A calibration curve was constructed using the seven levels of standard concentrations. Relative standard deviation (RSD) was less than 10%. The acceptable correlation coefficient (r value) for each element was greater than 0.995. A set of quality control (QC) samples was required for every batch of ten samples analyzed. Average deviation of continuing calibration recovery was assured to be less than 10%. Blanks (laboratory blank, field blank, and trip blank) were less than MDL. Spike sample recovery fell within the 80–120% range. Relative percent differences (RPD) of all duplicate samples were below 20%.
Risk assessment methods
Pollution load index
Pollution load index (PLI) is an important index that is often used to describe the severity of the heavy metal contamination. PLI is calculated as follows:
\(\text{where}{ C}_{\text{f}1}\)is the contamination factor; n the number of heavy metals, which is 8 in this study,\({C}^{i}\) the heavy metal concentration in sediment and water samples; \({C}_{\text{ref}}^{i}\) the reference value of the element (Hakanson 1980; Yan et al. 2016), and \({C}_{\text{f}}^{i}\) the contamination factor of each element for the local site. If PLI value equals 0, the site is not polluted. If it is less than 1, the site is considered ‘unpolluted’. If the value equals or is greater than 1, the site is considered ‘polluted’ (Tomlinson et al. 1980). The greater the value, the more polluted the site.
Potential ecological risk index
Potential ecological risk index (RI), proposed by (Hakanson 1980), is a commonly used index to evaluate the potential risk of one or multiple heavy metals to the ecological system:
where \({C}_{\text{f}}^{i}\) is the contamination factor; \({T}_{\text{f}}^{i}\) is the toxicity response coefficient of each element (As = 10, Cd = 30, Cr = 2, Cu = Pb = Ni = 5, Zn = 1 and Hg = 40) (Yan et al. 2016), and \({E}_{\text{f}}^{i}\) is the potential ecological risk factor of each element.
The values of Cd and RI were simplified using the scale displayed in Table S1.
Results and discussion
Ecological risk of heavy metal concentrations in water samples
Table 1 shows the results of heavy metal concentrations of the water samples and the water quality parameters. At the time of sampling, heavy metal concentrations in the waters were low. Copper was found at slightly elevated concentrations at the sites H3 (0.0655 mg/L), H9 (0.0700 mg/L), H6 (0.0903 mg/L), and H10 (0.0523 mg/L), while Zn had marginally high concentrations at the sites H5 (0.0710 mg/L), H7 (0.0640 mg/L), and H11 (0.0503 mg/L). Other metals, including As, are found in negligible concentrations at all sites.
Unlike heavy metal ecological risk assessment methods for sediments which have been well established (El Nemr et al. 2016b), heavy metal ecological risk assessment for water has rarely been addressed. However, the contamination factor and potential ecological risk factor for heavy metals in water can be calculated following the approach of Sharifi et al. (2016). Similar to the calculation of contamination factor and potential ecological risk factor for heavy metals in sediments, Sharifi et al. (2016) used the reference values (\({C}_{\text{ref}}^{i}\)) of different water quality guidelines to calculate contamination and potential ecological risk factors for heavy metals in water. In the current study, we used the values of \({C}_{\text{ref}}^{i}\) taken from heavy metal water quality indices issued by the Canadian Council of Ministers of the Environment (CCME 2007), the National Resource Management Ministerial Council, the Commonwealth of Australia (NRMMCA 2011), and the Australian and New Zealand Environment and Conservation Council & Agriculture and Resource Management Council of Australia and New Zealand (ANZECC and ARMCANZ 2000).
The risk factors of the metal concentrations in the water are several orders of magnitude lower than the reference values issued by the above organizations (Table S2). This implies that the ecological risk of the Houjing River is relatively small, but more detailed monitoring or more frequent sampling of the water masses is suggested to confirm the findings.
Ecological risk heavy metal contamination in sediment
Heavy metal content in sediment
The analytical results of the heavy metal concentrations in sediments of the Houjing River are provided in Table 2. Sites H1 and H2 are upstream of the Dashe Industrial park and provide some background measurements, although the area is fully developed. In comparison to sites H1 and H2, site H3 near Dashe Industrial Park (petrochemical resin production) had 4–5 times the concentrations of Pb (110.94 ± 103.78 mg/kg), 3 times the concentration of Zn (642.75 ± 424.67 mg/kg), and 6 times the concentrations of Hg (0.63 ± 0.61 mg/kg). Site H8, situated on Dashe upstream reach near Si-Cingpu Landfill and after sites H1 to H3, showed lower metal concentrations compared to site H3. For example, concentrations of Pb, Zn, and Hg at site H8 were 18.95 ± 6.55, 345.05 ± 186.30, and 0.16 ± 0.16 mg/kg, which are much lower than the above-mentioned concentrations of these metals at site H3. Probably, the dilution of the flow caused the heavy metal concentrations to decrease from site H3 to site H8. Site H4, the upstream background site for Renwu Park, had highly elevated levels of As, Cr, Ni, and Zn compared to sites H1 and H2, probably due to the recently rapid growth of the Renwu urban district. Ever since the establishment of the industrial parks in Renwu district, the commercial and residential area here has grown rapidly and become increasingly crowded, making the management of solid-waste disposal and wastewater collection and treatment a difficult task for the local government. Illegal discharge is sometimes observed, although the local authority has made series of propaganda campaigns to raise the awareness of the local inhabitants about the negative impacts of that action. Probably, stricter regulations and higher penalties are needed to address this problem. Site H5, situated near Renwu Industrial Park (including Formosa Plastics and other companies mostly involved with metal, chemical and plastic production), had high concentrations of Pb (63.40 ± 82.50 mg/kg). Site H6 showed high concentrations of Zn (376.70 ± 74.85 mg/kg). From site H5 to site H6, there is no clear increasing or decreasing pattern of metal concentrations. High concentrations of Cd (43.75 ± 72.92 mg/kg), Pb (60.81 ± 70.56 mg/kg), Ni (110.48 ± 82.11 mg/kg), Cu (677.83 ± 924.75 mg/kg), and Cr (97.88 ± 86.55 mg/kg) occurred at the site H7. High concentrations of Ni (153.54 ± 188.45 mg/kg) appeared at the site H9. The sites H7 and H9 are situated next to the discharges of NEPZ-Kaoshiung’s semiconductor manufacturing center. The related inputs from NEPZ also supported by the concentrations increase from H6 to H7; thus, intensive monitoring and regulatory enforcement are warranted. The downstream site H10 showed high concentrations of Cr (67.84 ± 36.50 mg/kg), Cu (384.48 ± 319.08 mg/kg), Ni (101.15 ± 58.84 mg/kg), and Zn (571.75 ± 330.90 mg/kg). The heavy metal concentrations at the next site (site H11) are mostly lower heavy metal concentrations than those at site H10. Site H11 only showed high concentrations of Zn (187.73 ± 81.03 mg/kg) and Cu (65.04 ± 22.52 mg/kg).
Next, average concentrations of heavy metals, which are calculated by the total concentration of each metal divided by the number of sediment samples collected throughout the whole river basin during the entire sampling period, are compared with average metal concentrations of other water bodies in south-east Asia in order to have an overview over the seriousness of sediment contamination in the Houjing River (Table 3). Although the heavy metal concentrations vary between sampling sites, the average concentrations of Cd, Cu, Zn, and Hg in sediment samples of the Houjing River are higher than those of other coastal or river sediments in the comparison. Noticeably, the concentration of Ni in this study was considerably higher than those of the Bangshi River, the Ganga River, the Shuangtaizi River, the Bortala River, and rivers in Eastern China.
The data can also be shown in comparison with limits determined by various environmental management organizations (Table 4), such as the Taiwan EPA, the New Zealand, and Australian environmental conservation council or NOAA. The comparison shows that the metal concentrations in the sediment of the Houjing River are very high, and often fall between the lower and upper values of the selected guidelines. The ecological effects of the heavy metals are deemed “rarely observed” concerning the lower limit value, but “frequently observed” concerning the upper limit. It would succeed that, when concentrations of the metals are either equal to or greater than the lower limit but below the upper limit, their effects would be realized as “occasionally observed” (Duodu et al. 2017; Vu et al. 2017a). Although As concentrations were only high at sites H4 and H7, our average As value fell between the low and high levels of Taiwan EPA, ANZECC and ARMCANZ and NOAA, suggesting a need for consideration by future monitoring and pollution control plans, especially at sites H4 and H7. Most alarming were our findings for average concentrations of Cd (10.72 mg/kg), Cu (200.96 mg/kg), and Zn (413.17 mg/kg), which exceeded the upper concentrations of most guidelines. Hence, the sites with such high metal concentrations along the Houjing River are clearly at high ecological risk and further or future metal contributions at these sites or remediation action have to be very carefully considered. Average concentrations of Cr (60.28 mg/kg) and Pb (40.16 mg/kg) are within the guidelines and only slightly above the lower limit of CCME ISQG. For Ni, although our average concentration exceeded the upper levels established by ANZECC and ARMCANZ and NOAA, it only fell between the upper and lower limits of Taiwan EPA. Therefore, Ni needs to be included in future monitoring plans and if possible, remediation projects.
Ecological risk assessment of heavy metals in sediments
Table 5 shows the results of the calculation of contamination factor (\({C}_{\text{f}}^{i}\)) and pollution load index (PLI) for heavy metals in sediments. From the sites H1 and H2 to H3, all metals, except Cd, showed around 1.5–6 times increase in the values of\({C}_{\text{f}}^{i}\). The increasing trend implies the pollutants discharge and accumulation potentials while the water body entering Dashe Industrial Park. Although the values of \({C}_{\text{f}}^{i}\) fluctuate mostly between 0.2 and 3.0 from site H4 to site H6, there are two noticeably high values of \({C}_{\text{f}}^{i}\), which are 12.24 for As at site H4 and 17.61 for Cd at site H5. At the site H7, Cd and Cu showed very high values of \({C}_{\text{f}}^{i}\), being 43.75 and 13.56 respectively. From site H8 to site H11, the values of \({C}_{\text{f}}^{i}\) vary strongly and no clear patterns could be observed.
Regarding PLI, the values at sites H2 (PLI = 0.99), H3 (PLI = 1.43), and H5 (PLI = 0.99) were high, probably because they were affected by the industrial discharges from Dashe and Renwu Industrial Parks. The site H4 showed a ‘very high’ value of PLI (1.83), which is due to the high value \({C}_{\text{f}}^{i}\) of As (12.25) at this site. Although the site H4, which is located at the upstream Renwu origin, should be a background site, high values of contamination factors at this site imply that it could be affected by the rapid urbanization and the rapid development of small and medium businesses. As discussed above, due to the occurrence of Renwu and Zhuhou Industrial Parks, the area around site H4 has become a busy residential and commercial area with numbers of condominiums and shopping streets. Probably, the illegal discharge of wastewater and solid waste from the residential and busy commercial activities has contaminated site H4. The site H7, located at the discharge of NEPZ, had the highest PLI (2.64). The downstream sites H10 and H11 showed high PLI values (1.11 and 0.77, respectively). According to Tomlinson et al. (1980), if the PLI value equals or is greater than 1, the site is considered polluted. As shown on Table 5, H3, H4, H7, H9, and H10 are considered ‘polluted’ and are geographically associated with industrial parks along sides of the Houjing River. It also suggests that the Houjing River in its present state is a threat to aquatic life in the ocean, and further investigations and monitoring are required.
Table 6 shows the potential ecological risk index of the sites and highlights that 8 of 11 sites posed “considerable”-to-“very high” risk. These findings suggest that metal pollution needs to be curbed and reduced to reduce the ecological risks in the area. From site H1 to site H3, many metals showed increasing trends in the values of\({E}_{\text{f}}^{i}\). From site H4 to site H6, the values of \({E}_{\text{f}}^{i}\) varies greatly. At site H7, a very high value of \({E}_{\text{f}}^{i}\) (1312.50) occurs. From site H7 onward, the values of \({E}_{\text{f}}^{i}\) fluctuate strongly and no clear pattern is observed. The RI values of the sampling sites depend mostly on Cd levels with sites H2, H5, and H7 having “very high” RI values of 524, 574, and 1443, respectively, implying that the ecological system is at its highest threat at these sites. These sites are also located near the major industrial plants of Dashe Industrial Park, Renwu Industrial Park, and NEPZ. The sites H1 (204.24), H4 (265.90), H8 (310.33), H9 (259.31), and H11 (232.92) showed “considerable high” RI values, indicating a need for continued monitoring and future ecological risk assessment. Regarding to site H4 that has the highest \({E}_{\text{f}}^{i}\)for As (\({E}_{\text{f}}^{i}\hspace{0.17em}\)= 122.54) implies that a special forensic investigation on arsenic is warranted. It should be noticed that As is actually very toxic for certain species, i.e., fish, invertebrates, and humans (Eisler 1988).
As a summary for the Houjing River basin, Cd is obviously the most critical element, follows by Cu, Hg, and As, that need to be curbed and reduces. Therefore, strict regulations on these heavy metals discharge into the water body should be strictly established and enforced.
The rapid development of heavy industries has created wealth and improved economic status of Kaohsiung City and its citizens (Huang et al. 2016; Vu et al. 2017a; Williams and Chang 2008). However, those industries have also seriously destroyed the environment of the city (Vu et al. 2017a, b). The Houjing River is one of the most endured “victims” of those environmentally degrading industries. The main reason for those industries to continue damaging the environment is the lenient regulation of the government, who has entire authority to prevent the adverse impacts resulted from those environmentally damaging industrial activities. Kaohsiung City is a “typical” rapidly industrially developed city in Asia, where hundreds of other industrial cities reside, i.e., those in China, India, Bangladesh, and Vietnam. The lesson learned here from this study of the case of the Houjing River is that economic growth from rapid industrial development should not compromise the inherently natural environment; otherwise, the damage would be too immense and too costly to recover. Therefore, hopefully, the Kaohsiung City’s government and Taiwan EPA would recognize that and impose strict regulations on the environmentally damaging industrial activities. In addition, despite the possibly incurred great cost, regular monitoring plans and remediation projects are needed to prevent environmentally degrading activities and to reclaim the degraded environment of Kaohsiung City.
Conclusions
The concentrations of heavy metals (As, Cd, Cr, Cu, Pb, Ni, Zn, and Hg) in water and sediments of the Houjing River were between ND–0.09 mg/L and 0.05–677.83 mg/kg, respectively. Concentrations, contamination factors (\({C}_{\text{f}}^{i}\)), and potential ecological risk factors (\({E}_{\text{f}}^{i}\)) of heavy metals in collected water samples were low, and thus, a limited ecological risk is identified. In contrast, metal concentrations in sediments were elevated to high and occasionally above EPA guidelines depending on site and element. The study determined factors and indices to evaluate which element at which sites may pose “considerable” or “very high” potential ecological risks (as expressed by high values of \({C}_{\text{f}}^{i}\) and \({E}_{\text{f}}^{i}\)). The comparisons of our findings with other similar studies and sediment quality guidelines indicate that the heavy metal concentrations in sediments of the Houjing River exceeds recommended limits and thus place some site at high ecological risks which are recommended to be considered for pollution control and remediation plans of the city’s government. The values of PLI and RI at sites H4 (Bakong) and H7 (Demin) were very high, which implies that pollution prevention needs to be carefully considered and planned while in the urbanization processes and that of the economic investment in the emerging semiconductor manufacturing industry. Since almost all sites show some sort of elevated metal concentrations or factors, the sediments of the Houjing River are clearly contaminated and require monitoring and pollution control programs if its negative impacts on the ecological system along the riverside, estuary, and the ocean are to be limited. The heavy metal contamination of the river is clearly a result of decades of poor environmental and wastewater management policies and guidelines and other rapid industrially developing countries should take notice of the lessons learned in Kaohsiung City as metal contamination could be limited, reduced, or even prevented. However, decades of negligence now result in very high risk and remediation costs.
References
Ali MM, Ali ML, Islam MS, Rahman MZ (2016) Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ Nanotechnol Monit Manag 5:27–35. https://doi.org/10.1016/j.enmm.2016.01.002
ANZECC, ARMCANZ (2000) Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Australia. https://www.environment.gov.au/system/files/resources/e080174c-b267-455e-a8db-d3f79e3b2142/files/nwqms-guidelines-4-vol3.pdf. Accessed 26 Mar 2017
APHA (1999) Standard Methods for the Examination of Water and Wastewater. American Public Health Association, USA. https://www.mwa.co.th/download/file_upload/SMWW_1000-3000.pdf. Accessed 27 Mar 2017
CCME (1999) Canadian sediment quality guidelines for the protection of aquatic life. Canadian Council of Ministers of the Environment, Winnipeg, Canada. http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/. Accessed 26 Mar 2017
CCME (2007) Canadian Water Quality Guidelines for the Protection of Aquatic Life: Summary table. Canadian Council of Ministers of the Environment, Canada. http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/. Accessed 26 Mar 2017
Chapman PM, Wang F, Janssen C, Persoone G, Allen HE (1998) Ecotoxicology of metals in aquatic sediments: binding and release, bioavailability, risk assessment, and remediation. Can J Fish Aquat Sci 55(10):2221–2243. https://doi.org/10.1139/f98-145
Chen WH (2016) Court revokes ASE pollution fine, Taipei Times. Taiwan. http://www.taipeitimes.com/News/taiwan/print/2016/03/24/2003642322
Deniseger J, Erickson LJ, Austin A, Roch M, Clark MJR (1990) The effects of decreasing heavy metal concentrations on the biota of Buttle Lake, Vancouver Island, British Columbia. Water Res 24(4):403–416. https://doi.org/10.1016/0043-1354(90)90222-R
Dhanakumar S, Solaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Saf 113:145–151. https://doi.org/10.1016/j.ecoenv.2014.11.032
Duodu GO, Ogogo KN, Mummullage S, Harden F, Goonetilleke A, Ayoko GA (2017) Source apportionment and risk assessment of PAHs in Brisbane River sediment, Australia. Ecol Ind 73:784–799. https://doi.org/10.1016/j.ecolind.2016.10.038
Eeva T, Lehikoinen E (2000) Pollution—recovery of breeding success in wild birds. Nature 403(6772):851–852. https://doi.org/10.1038/35002672
Eisler R (1988) Arsenic Hazards to Fish, Wildlife, and Invertebrates: a Synoptic Review Contaminant Hazard Reviews. Laurel, MD
El Nemr A, El-Said GF, Khaled A, Ragab S (2016a) Distribution and ecological risk assessment of some heavy metals in coastal surface sediments along the Red Sea, Egypt. Int J Sedim Res 31(2):164–172. https://doi.org/10.1016/j.ijsrc.2014.10.001
El Nemr A, El-Said GF, Ragab S, Khaled A, El-Sikaily A (2016b) The distribution, contamination and risk assessment of heavy metals in sediment and shellfish from the Red Sea coast, Egypt. Chemosphere 165:369–380. https://doi.org/10.1016/j.chemosphere.2016.09.048
Hakanson L (1980) An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Huang WB (2003) Heavy metal concentrations in the common benthic fishes caught from the coastal waters of eastern Taiwan. J Food Drug Anal 11(4):324–330
Huang WY, Hung WT, Vu CT, Chen WT, Lai JW, Lin CT (2016) Green and sustainable remediation (GSR) evaluation: framework, standards, and tool. A case study in Taiwan. Environ Sci Pollut Res 23(21):21712–21725
Islam MS, Ahmed MK, Raknuzzaman M, Habibullah-Al-Mamun M, Islam MK (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Ind 48:282–291. https://doi.org/10.1016/j.ecolind.2014.08.016
Jiang JJ, Lee CL, Fang MD, Boyd KG, Gibb SW (2015) Source apportionment and risk assessment of emerging contaminants: an approach of pharmaco-signature in water systems. Plos One 10(4):e0122813
Kiffney PM, Clements WH (1993) Bioaccumulation of heavy metals by benthic invertebrates at the arkansas river, colorado. Environ Toxicol Chem 12(8):1507–1517. https://doi.org/10.1002/etc.5620120818
Li C, Song C, Yin Y, Sun M, Tao P, Shao M (2015) Spatial distribution and risk assessment of heavy metals in sediments of Shuangtaizi estuary, China. Mar Pollut Bull 98(1–2):358–364. https://doi.org/10.1016/j.marpolbul.2015.05.051
Lin C, Mao WM, Nadim F (2007) Forensic Investigation of BTEX Contamination in the Houjing River in Southern Taiwan. J Environ Eng Manag 17(6):395–402
Lin C, Lee CJ, Mao WM, Nadim F (2009) Identifying the potential sources of di-(2-ethylhexyl) phthalate contamination in the sediment of the Houjing River in southern Taiwan. J Hazard Mater 161(1):270–275
Lin CE, Kao CM, Jou CJ, Lai YC, Wu CY, Liang SH (2010) Preliminary identification of watershed management strategies for the Houjing river in Taiwan. Water Sci Technol 62(7):1667–1675
Lin C, Nguyen KA, Vu CT, Senoro D, Villanueva MC (2017) Contamination levels and potential sources of organic pollution in an Asian river. Water Sci Technol. https://doi.org/10.2166/wst.2017.419
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19(1):81–97. https://doi.org/10.1007/BF02472006
NRMMCA (2011) Australian Drinking Water Guidelines Paper 6 National Water Quality Management Strategy. National Resource Management Ministerial Council, Commonwealth of Australia, Canberra, Australia. https://www.nhmrc.gov.au/guidelines-publications/eh52. Accessed 26 Mar 2017
Pandey M, Tripathi S, Pandey AK, Tripathi BD (2014) Risk assessment of metal species in sediments of the river Ganga. CATENA 122:140–149. https://doi.org/10.1016/j.catena.2014.06.012
Pejman A, Bidhendi GN, Ardestani M, Saeedi M, Baghvand A (2015) A new index for assessing heavy metals contamination in sediments: a case study. Ecol Ind 58:365–373. https://doi.org/10.1016/j.ecolind.2015.06.012
Rahman MS, Saha N, Molla AH (2014) Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci 71(5):2293–2308. https://doi.org/10.1007/s12665-013-2631-5
Sharifi Z, Hossaini SMT, Renella G (2016) Risk assessment for sediment and stream water polluted by heavy metals released by a municipal solid waste composting plant. J Geochem Explor 169:202–210. https://doi.org/10.1016/j.gexplo.2016.08.001
Tang W, Shan B, Zhang H, Zhang W, Zhao Y, Ding Y, Zhu X (2014) Heavy metal contamination in the surface sediments of representative limnetic ecosystems in Eastern China. Sci Rep 4:7152. https://doi.org/10.1038/srep07152
TEPA (2010) Soil and groundwater pollution remediation Act. Taiwan Environmental Protection Administration, Taipei, Taiwan. https://sgw.epa.gov.tw/. Accessed 26 Mar 2017
Tessier A, Campbell PGC (1987) Partitioning of trace metals in sediments: relationships with bioavailability. Hydrobiologia 149(1):43–52. https://doi.org/10.1007/BF00048645
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol Meeresunters 33(1):566–575. https://doi.org/10.1007/BF02414780
Tsai HS (2014) Environment health check needed, Taipei Times. Taipei, Taiwan. http://www.taipeitimes.com/News/editorials/archives/2014/10/31/2003603303. Accessed 26 Mar 2017
USEPA (2013) Surface water sampling. United States Environmental Protection Agency, Washington, DC, USA. https://www.epa.gov/sites/production/files/2015-06/documents/Surfacewater-Sampling.pdf. Accessed 26 Mar 2017
USEPA (2014) Sediment sampling. United States Environmental Protection Agency, Washington, DC, USA. https://www.epa.gov/sites/production/files/2015-06/documents/Sediment-Sampling.pdf. Accessed 26 Mar 2017
Venkatramanan S, Chung SY, Ramkumar T, Selvam S (2015) Environmental monitoring and assessment of heavy metals in surface sediments at Coleroon River Estuary in Tamil Nadu, India. Environ Monit Assess 187(8):1–16. https://doi.org/10.1007/s10661-015-4709-x
Vu CT, Lin C, Shern C-C, Yeh G, Le VG, Tran HT (2017a) Contamination, ecological risk and source apportionment of heavy metals in sediments and water of a contaminated river in Taiwan. Ecol Ind 82:32–42. https://doi.org/10.1016/j.ecolind.2017.06.008
Vu CT, Lin C, Yeh G, Villanueva MC (2017b) Bioaccumulation and potential sources of heavy metal contamination in fish species in Taiwan: assessment and possible human health implications. Environ Sci Pollut Res 24(23):19422–19434. https://doi.org/10.1007/s11356-017-9590-4
Williams JF, Chang C-YD (2008) Taiwan’s environmental struggle: toward a green silicon island. Routledge and Taylor & Francis, London
Yan N, Liu WB, Xie HT, Gao LR, Han Y, Wang MJ, Li HF (2016) Distribution and assessment of heavy metals in the surface sediment of Yellow River, China. J Environ Sci 39:45–51
Zhang ZQ, Wang JJ, Ali A, DeLaune RD (2016a) Heavy metals and metalloid contamination in Louisiana Lake Pontchartrain Estuary along I-10 Bridge. Transp Res Part D Transp Environ 44:66–77
Zhang ZY, Li JY, Mamat Z, Ye QF (2016b) Sources identification and pollution evaluation of heavy metals in the surface sediments of Bortala River, Northwest China. Ecotoxicol Environ Saf 126:94–101
Acknowledgements
The authors acknowledge the enthusiastic assistance of the sampling and analysis staff at the Center of Environmental Analysis Services (CEAS), National Kaohsiung Marine University. The authors appreciate the reviewers whose knowledgeable comments and suggestions have largely contributed to the improvement of the quality of our work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vu, C.T., Lin, C., Nguyen, K.A. et al. Ecological risk assessment of heavy metals sampled in sediments and water of the Houjing River, Taiwan. Environ Earth Sci 77, 388 (2018). https://doi.org/10.1007/s12665-018-7573-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7573-5