Abstract
Rapid urbanization and industrialization have aggravated heavy metal contamination in river sediments of the riverine ecosystem in developing countries like Bangladesh owing to their toxicity and persistence. Sediments are dynamic components and useful indicators to understand the level of contamination and their associated ecological risks in the aquatic environment. The study was conducted to investigate the heavy metal contamination in sediments for assessing the ecological risks of an urban river of Bangladesh using principal component analysis (PCA), Pearson’s correlation matrix, geo-accumulation index (Igeo), contamination factor (CF), contamination degree (CD), pollution load index (PLI), enrichment factors (EF), and potential ecological risk factor (RI). The ranges of Zn, Cr, Cu, Pb, and Cd in sediments were 42.22–99.55, 11.12–57.83, 7.98–53.31, 6.76–22.41, and 0.38–0.87 mg/kg, respectively. In the present study, heavy metal concentration in sediments followed the descending order of Zn > Cr > Cu > Pb > Cd, while the concentrations of Cu, Cr, and Cd were higher and the concentrations of Pb and Zn were lower than the toxicity reference value (TRV). Geoaccumulation index (Igeo) demonstrated that most of the sediment samples were unpolluted to moderately polluted. The PLI ranged from 0.334 to 1.209 that stated that sediments were moderately polluted by studied metals. The multivariate statistical analysis revealed that heavy metal contamination was influenced by multiple pollution sources. The extent of heavy metal pollution in the Shitalakhya River implies that the condition is much frightening to both the aquatic biota and inhabitants in the vicinity of the river.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, metal contamination in the aquatic environment has attracted global attention and reached an anxious level owing to its abundance, persistence, and toxicity to the environment (Islam et al. 2015a, 2018, 2020; Ahmed et al. 2012; Proshad et al. 2020). Accumulation of heavy metals can be transmitted to the trophic level of the food chain resulting in the breakdown of the natural balance of the ecosystems (Alam et al. 2007). Apart from destabilizing the ecosystem, their potential long-term impact on ecosystem decontamination cannot be disregarded due to its toxic effects, exorbitance and persistence in the environment, and successive development in the environment (Varol 2011; Ali et al. 2019). Moreover, heavy metal residues from the contaminated habitat may lead to bioaccumulation as well as biomagnification in microorganisms, aquatic vegetation, and fauna, which consequently may enter into the human food chain and create many problems related to human health (Haque et al. 2005; Ahmed et al. 2015; Kormoker et al. 2020; Islam et al. 2017a). Typically, sources of heavy metals are varied from region to globally depending on various contexts. Major anthropogenic sources of heavy metals include fossil fuel and coal combustion, industrial effluents, solid waste disposal, domestic and municipal wastewater, fertilizers, pesticides, paint and dyes, mining, and metal processing (Malik et al. 2010; Islam et al. 2018) whereas natural sources include weathering of mineral deposits, brush burning, and windblown dust (Zhang and Liu 2002), and these metals are distributed between the aqueous phase and bed sediment during their conduction (Sakan et al. 2009; Ali et al. 2018). Due to the adsorption, hydrolysis, and co-precipitation, only an inconsiderable amount of free metal ions remain dissolved in water and an extensive quantity of them get deposited in the sediment which eventually creates ecological risk (Gaur et al. 2005; Islam et al. 2017b, 2018; Tusher et al. 2020).
Sediments are important sinks for diverse pollutants especially for heavy metals and play a weighty role in the remobilization of contaminants in aquatic systems under inclinable environments and interactions between water and sediment (Mohiuddin et al. 2011). Sediments are also significant in fostering the trophic level of any water body as they have extended residence time (Rahman et al. 2014). In developing countries, the river plays an effective role in assimilating or carrying off industrial and municipal wastewater, manure discharges, and agricultural runoff, etc. which are accountable for river pollution (Reza and Singh 2010). Thus, the river sediments are the weighty source for the assessment of heavy metal contaminations in any aquatic environment (Ahmad et al. 2010). In Bangladesh, about 1176 industries (situated beside the studied river) are continuously discharging 0.4 million m3 of untreated wastewater into the rivers per day (Islam et al. 2015a) and these metals also gain access to ecosystem and get distributed in the water body, total suspended solids, and sediments during their mobility (Banu et al. 2013; Islam et al. 2015 b,c). On one hand, sediments act as a bearer of contaminants from the sources and also acting as the characteristic of secondary pollutants. In surface sediments, heavy metal concentrations are tremendously high than the water column; as a consequence, metals tend to accumulate in lowermost deposits reported by numerous studies globally (Nobi et al. 2010; Islam et al. 2015a; Bhuyan et al. 2017). Therefore, the assessment of river sediments is an effective tool to study the environmental toxicity caused by heavy metals (Moore et al. 2009). Recently, the aquatic environment of Bangladesh is being contaminated by heavy metals in many ways (Kabir et al. 2020). The waste discharged from various industries is responsible for elevated metal levels in the exposed sediment of the surrounding river of Dhaka city (Mohiuddin et al. 2011). Potential ecological risk analysis in surface sediments of the Rupsa River indicates a moderate to serious toxicity of heavy metals in sediment (Proshad et al. 2019). Sediments of the Old Brahmaputra river have been contaminated by heavy metals as a result of the anthropogenic sources that include industrial, domestic, and irrigation discharges (Bhuyan et al. 2019). Moreover, numerous studies conducted by Banu et al. (2013), Ahmed et al. (2012), Rahman et al. (2014), and Mamun et al. (2013) revealed that rivers of Bangladesh are being polluted in different extents with time. Worldwide various studies also showed that owing to the effect from the point and nonpoint sources such as metropolitan runoffs, atmospheric deposition, leaded gasoline, chemical manufacturing, and steelworks in city areas have straight influences on heavy metal contamination in aquatic environments (Shikazono et al. 2012; Gaur et al. 2005; Barakat et al. 2012; Abrahim and Parker 2008).
The Shitalakhya river flows through the Narsingdi district town, one of the most vital industrial zones of the country, and various types of industrial units have been established on the bank of the river. Most of these industries directly or indirectly discharge a huge quantity of wastewater and effluents into the river without any treatment, and also, municipal and domestic wastewater from Narsingdi urban area finds their way untreated into this river (Islam et al. 2014). Plenty of raw materials for industrial production and processed industrial products are also transported through this river regularly by ships and cargo; therefore, the risks of pollution impact are rising upwards sequentially (Islam et al. 2008). The Shitalakhya River in Bangladesh recently has raised attention to public concern due to its extreme pollution caused by pollutants from two large cities that include Narayanganj and Narsingdi (Islam et al. 2014; Mottalib et al. 2016). The two big cities are established on the bank of the Shitalakhya River, and the aquatic environment of this river system is dominated by these cities (Kabir et al. 2020). Previously, two studies were conducted for the assessment of heavy metal pollution in sediments of the Shitalakhya River at Narayanganj area by Islam et al. (2014) and Islam et al. (2016). On the other hand, assessment of physicochemical water quality and heavy metal concentrations in water and fishes of the Shitalakhya River at Narsingdi area were documented by Mottalib et al. (2016) and Kabir et al. (2020). To date, no scientific research regarding heavy metal issues with relation to sediment contamination in the study area has been conducted so far. Therefore, the present study was an attempt to investigate the present status of heavy metal pollution in sediments of the Shitalakhya River using different pollution indices.
Materials and methods
Study area
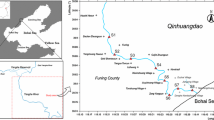
The study area was located in the Shitalakhya river of Narsingdi district, Bangladesh, which was approximately within latitude between 23°55′36″ and 23°91′92″ N and longitude between 90°43′54″ and 90°71′76″E (Fig. 1). The Shitalakhya river is one of the most prominent distributary of the Old Brahmaputra river in the flood plain region of Bangladesh and flows through Gazipur district forming its border with Narsingdi for some distance and then through Narayanganj District. The Shitalakhya river is a silt-carrying and flooding river and very much alive during the monsoon and carries the major discharge of the Old Brahmaputra river on its way down. The river is about 110 km long and widest 0.3 km across and remains navigable year-round, whereas maximum depth is 21 m and the average depth is 10 m (Ahmed et al. 2010). For the collection of surface sediment samples, three sampling stations namely as Saorait Bazar (St-1), Jamalpur Bazar (St-2), and Fuleshawri Bazar (St-3), along with the Shitalakhya river of Bangladesh. Stations were selected depending upon the presumed sediment quality and extent of pollution by prior visiting the study area. St-1 (upstream) located at the 1 km upstream of the Ghorashal Fertilizer Company Limited, where agricultural runoff, animal manure, and wastewater from municipal drains were the main sources of pollution. St-2 (upstream), was dominated by cement, textile, pesticide, and fertilizer factory and food beverage industrial park. The heavy metal-containing wastewater from plant discharge and wastewater drain that collect mixed domestic, municipal, and industrial wastewater was the major pollution source in St-2. St-3 (downstream) was severely dominated by large-scale industries such as paper and pulp industry, plastic industrial park, cement factory, pesticide, and the fertilizer factory and textile industry. Ghoshal Railway Bridge and Ferry Ghat were 1 km upstream near the St-3. The wastewater drains that collect plant discharge, municipal, industrial, and agricultural runoff were the most significant sources of pollution in St-3.
Sample collection
Sediments samples were collected randomly from the St-1, St-2, and St-3 (Fig. 1) during the period from April to December 2015, approximately 1.0 m below the water surface at 4-month interval, i.e., in April, August, and December 2015, and these 3 months were considered as pre-monsoon, monsoon, and post-monsoon season, respectively. From each sampling station, three sediment samples were collected during each season and then mixed thoroughly to get a composite sample, and therefore, a total of 27 sediment samples were collected for analysis. Approximately 1000-g samples from each sampling station were collected with the help of Grab sampler and kept in polyethylene bags and transported immediately to the laboratory for further analysis. Samples were labeled properly and preserved in a refrigerator at 4 °C temperature (Varol 2011). Before sampling, the polyethylene was washed with detergents and 10% HNO3 solutions and rinsed with distilled water for avoiding external contamination (Ogbeibu et al. 2014).
Sample analysis
After collection, sediment samples were air-dried in a dust-free place at room temperature, ground into powder form using pestle and mortar whereas stone, plant fragment, or unwanted materials were removed by passing the dried sample through a 2-mm sieve (Hassan et al. 2015). The sieved samples were then finally passed through a 500-μm sieve and stored in acid-washed and deionized water rinsed glass bottles. Sediment samples then digested in Teflon vessels with a 3:1 mixture of nitric acid (HNO3) and hydrochloric acid (HCl) in a microwave oven (USEPA 2007). After microwave digestion, the sample solutions were filtered and adjusted to a compatible volume with double deionized water. Sediment extracts were then analyzed for Cu, Cr, and Zn by a flame atomic absorption spectrometry (FAAS) equipped with deuterium background correction, whereas Pb and Cd extracts were measured by graphite furnace atomic absorption spectrometry (GFAAS) with Zeeman background correction (Varol 2011) in the Laboratory of the Soil Science, Bangladesh Institute of Nuclear Agriculture (BINA), Mymensingh-2201, Bangladesh.
Sediment quality assessment
Background or pre-industrial values play a significant role for the interpretation of geochemical data and numerous authors used the average shale standard values, average crustal abundance values, world surface rock average values, and uncontaminated sediment values (Hassan et al. 2015; Sakan et al. 2009; Banu et al. 2013; Varol 2011). The present study followed average shale standard values by Turekian and Wedepohl (1961) and world surface rock average values by Martin and Meybeck (1979) for calculations. In this study, five different indices were used to assess the degree of heavy metal contamination in sediments of the Shitalakhya river.
Geoaccumulation index
The Igeo enables the assessment of metal contamination in sediment by comparing the presently measured concentrations with pre-industrial background contents and Igeo can be calculated using the following formula proposed by Muller (1969):
where Cn is the measured concentration of the metal (n) and Bn is the geochemical background of that metal (n). The average shale standard for different metals recorded by Turekian and Wedepohl (1961) was used as a background concentration for this study. Factor 1.5 was applied for the possible variations of the background values due to the lithological effect. The Igeo scale composed of seven grades or classes ranging from unpolluted to extremely polluted, i.e., grade 0 (Igeo ≤ 0): unpolluted; grade 1 (Igeo = 0–1): unpolluted to moderately polluted; grade 2 (Igeo = 1–2): moderately polluted; grade 3 (Igeo = 2–3): moderately to strongly polluted; grade 4 (Igeo = 3–4): strongly polluted; grade 5 (Igeo = 4–5): strongly to extremely polluted; and grade 6 (Igeo ≥ 6): extremely polluted reported by Müller (1981). Due to the nature of the Igeo calculation which entangles a log function and a background multiplication of 1.5, the Igeo index is not deliberately analogous to other indices of metal affluence (Abrahim and Parker 2008).
Contamination factor and contamination degree
The Contamination factor (CF) and contamination degree (CD) were used to investigate the heavy metal pollution load status of the sediments in the Shitalakhya River. The CF is the ratio obtained by dividing the concentration of each metal in the sediment by the background value reported by Turekian and Wedepohl (1961) and the CF for each metal was calculated by the following formula proposed by Tomlinson et al. (1980).
The CD for each station was computed as the sum of all contamination factors (Hassan et al. 2015). Hakanson (1980) classified four grade ratings of sediments concerning CF values such as CF < 1: low, 1 ≤ CF < 3: moderate, 3 ≤ CF < 6: considerable, and CF ≥ 6: very high; on the other hand, Tomlinson et al. (1980) reported four classes of sediment based on the CD levels, i.e., CD < 6: low, 6 ≤ CD < 12: moderate, 12 ≤ CD < 24: considerable, and CD ≥ 24: very high.
Pollution load index
The pollution load index (PLI) is used to explore the mutual pollution effect at diverse stations by the diverse metals in soils and sediments and gave an appraisement of the overall toxicity status of each sampling station (El-Sammak and Aboul-Kassim 1999). For the entire sampling stations, PLI was computed as the nth root of the product of the multiplications of the contents by the following equation proposed by the Tomlinson et al. (1980):
This pragmatical index provides a simple, comparative means for assessing the level of heavy metal pollution, whereas PLI > 1 indicates that pollution exists; on the other hand, if PLI < 1, it indicates that there are absence metal pollution (Varol 2011).
Enrichment factor
Enrichment factor (EF) is a significant tool in assessing the degree of anthropogenic heavy metal pollution (Sakan et al. 2009). The EF was calculated using the formula:
For geochemical normalization, iron (Fe) is used as the reference element in this study due to the fact that its geochemistry is uniform to that of numerous heavy metals, combined with the thin solid base, and its native concentration tends to be identical (Bhuiyan et al. 2010). The EF values were interpreted as EF < 1 (no enrichment), EF < 3 (minor enrichment), EF = 3–5 (moderate enrichment), EF = 5–10 (moderately severe enrichment), EF = 10–25 (severe enrichment), EF = 25–50 (very severe enrichment), and EF > 50 (extremely severe enrichment) reported by Sakan et al. (2009).
Potential ecological risk index
The potential ecological risk index (RI) method developed by Hakanson (1980) was applied in this study which describes the number of times by which the heavy metal contents in the sediment exceed the geochemical background concentrations and provide a multitude expression of the level of heavy metal toxicity in a particular specimen (Barakat et al. 2012). Based on this method, the potential ecological risk coefficient \( {E}_{\mathrm{r}}^{\mathrm{i}} \) of a single metal and the potential ecological risk index Ri of multi-metal can be computed via the following equations:
In these equations, \( {C}_{\mathrm{f}}^{\mathrm{i}} \) is the accumulating coefficient of metal (i) and \( {T}_{\mathrm{f}}^{\mathrm{i}} \) is the toxic response factor of metal (i), which reveals its levels of toxicity and the sensitivity of bio-organism to it. The toxic response factors for Pb, Cu, Cr, Zn, and Cd were considered 5, 5, 2, 1, and 30, respectively, reported by Banu et al. (2013). Accumulating coefficients of heavy metals in sediments from the three different stations were computed and applied to depict the accumulation status of heavy metal in the sediments from each sampling station. The computing equation for accumulating coefficient \( {C}_{\mathrm{f}}^{\mathrm{i}} \) is as follows:
where \( {C}_{\mathrm{m}}^{\mathrm{i}} \) is the value of heavy metal concentration in the sediment samples and \( {C}_{\mathrm{n}}^{\mathrm{i}} \) is the pre-industrial background values in sediments. In this study, the pre-industrial background values for Pb, Cu, Cr, Zn, and Cd are considered 20, 32, 97, 129, and 0.2 mg/kg, respectively, proposed by Martin and Meybeck (1979). Chen and Zhou (1992) stated four ratings of ecological pollution degree of heavy metal in sediment like as \( {E}_{\mathrm{r}}^{\mathrm{i}} \) < 40 or RI < 150: low, 40 ≤ \( {E}_{\mathrm{r}}^{\mathrm{i}} \) < 80 or 150 ≤ RI < 300: moderate, 80 ≤ \( {E}_{\mathrm{r}}^{\mathrm{i}} \) < 600 or 300 ≤ RI < 600: considerable, and 160 ≤ \( {E}_{\mathrm{r}}^{\mathrm{i}} \) < 320 or 600 ≤ RI: very high ecological risks for the water body.
Statistical analysis
The collected data were compiled and tabulated in proper form and were subjected to statistical analysis, whereas MS Office Excel 2010 and IBM Statistics 20.0 software were used. Pearson’s correlation analysis and principal component analysis (PCA) were employed to reveal the interrelationship among the studied heavy metals in the sediment samples in order to identify the possible sources of contamination (Benson et al. 2016). Kaiser-Meyer-Olkin (KMO) and Bartlett’s test of sphericity were used to confirm the adequacy of data used for PCA, while two components were extracted. The mapping of spatial distribution of heavy metals during different sampling seasons was done by using ArcGIS software.
Results and discussion
Heavy metal concentrations in sediments
The concentrations of heavy metals in sediments of the Shitalakhya River in three different seasons and their comparison with TRV are given in Table 1. Moreover, the spatial distributions of the heavy metals during different seasons are presented in Fig. 2, indicating higher concentrations of heavy metals in the downstream areas, regardless of the seasons. The wide range of metal concentrations was witnessed among the sampling stations and seasons. Various influences such as geomorphological outfits, land runoff, and industrial discharge might have played a dynamic role in the variation of metal concentrations. The concentrations of Pb, Cu, Cr, Zn, and Cd in sediments were ranged from 6.76 to 22.41, 7.98 to 53.31, 11.12 to 57.83, 42.22 to99.55, and 0.38 to 0.87 mg/kg, respectively, while the mean concentrations followed the order of Zn (75.48 mg/kg) > Cr (38.39 mg/kg) > Cu (24.60 mg/kg) > Pb (13.16 mg/kg) > Cd (0.64 mg/kg). The results of the present study revealed that concentrations of Cu, Cr, and Cd were higher than the TRV value given by the USEPA (1999), whereas the concentrations of Pb and Zn were lower than the TRV. The maximum concentration of Zn (99.55 mg/kg) was found at St-3 in the post-monsoon season, while the minimum concentration of Cd (0.38 mg/kg) was found at S-1 in monsoon season. Variations in heavy metal concentrations of the stations’ overall seasons followed the order of St-3 > St-2 > St-1. Moreover, the result also depicted that St-3 was more contaminated than others due to receiving a large volume of industrial, domestic, municipal wastewater, and agricultural runoff. The variations of heavy metal concentrations from stations to stations may be correlated with the flow of the rivers and location of industries and their wastewater discharge system (Alam et al. 2004; Pandey et al. 2019). However, the study areas were dominated by paint and dyes, pesticides, textile, plastic, paper and pulp, fertilizer, and cement factory, and most of the industry uses the chlor-alkali process and releases a large amount of wastewater into the river. The high concentration of heavy metals was discharged from various industries in the form of base materials, abrasive blasting materials, surface coating, and welding materials (Kura et al. 2006; Monirul et al. 2014). Mean concentrations of Pb, Cu, Cr, Zn, and Cd were found 11.64 ± 0.87, 17.37 ± 4.05, 45.20 ± 7.36, 87.25 ± 3.16, and 0.67 ± 0.03 mg/kg, respectively, in pre-monsoon; 7.85 ± 1.27, 10.04 ± 2.42, 15.00 ± 3.60, 45.21 ± 2.59, and 0.44 ± 0.07 mg/kg, respectively, in monsoon; and 19.98 ± 2.92, 46.38 ± 6.87, 54.98 ± 3.42, 93.99 ± 4.82, and 0.81 ± 0.05 mg/kg, respectively, in post-monsoon season. The study showed that higher concentrations of heavy metal were found in post-monsoon followed by pre-monsoon and monsoon season might be due to the discharge of a huge volume of industrial, domestic, and municipal wastewater and absence of less rainfall water for mixing are the main reasons for this result (Islam et al. 2015a). The concentrations of Cr, Pb, Cd, Zn, and Cu were ranged from 105 to 2017, 56 to 1592, 3.5 to 7.8, 129 to 2163, and 30 to 743 mg/kg, respectively, in summer, whereas 105 to 4249, 110 to 1584, 4.1 to 9.5, 166 to 3002, and 55 to 459 mg/kg, respectively, in winter season in the sediment of Buriganga River, Bangladesh (Mohiuddin et al. 2011), and these results are almost similar to the present study. The concentrations of Pb, Cu, Cr, and Cd in Ganges and Gomti river sediments of India were 4.3–8.4, 0.98–4.4, 1.8–6.4, and 0.14–1.4 mg/kg (Gupta et al. 2009), respectively, and 40, 5.0, 8.15, and 2.4 mg/kg, respectively (Singh et al. 2005). High Pb and Cd concentrations in Gomti river sediments were observed than the present study which might be attributed to municipal runoffs, industrial doings, atmospheric giving off, leaded gasoline, chemical manufacturing, steelworks, and Cd plating in the urban area (Shikazono et al. 2012; Proshad et al. 2019). Concentrations of Pb (26–78 mg/kg), Cu (30–102 mg/kg), and Cr (41–128 mg/kg) in Yellow river of China were much higher than the present measured concentration of the Shitalakhya river (Liu et al. 2009) that could be relating to the waste discharged from municipal and industries (Mohiuddin et al. 2011). Moreover, lower levels of Pb (0.45 mg/kg), Cr (0.87 mg/kg), and Cd (1.32 mg/kg) concentrations were perceived in the Okumeshi River sediments of Nigeria (Raphael et al. 2011) than present study that might be due to the fact that the higher water flow could not support to accumulate heavy metals in sediment (Kabir et al. 2020; Mohiuddin et al. 2011; Ali et al. 2016). As a whole, concentrations of Cu, Cr, and Cd exceeded some well-recognized standard values and in agreement with some previous studies (Table 3).
Pearson’s correlation analysis was performed to determine the relationships among the heavy metals in the sediment samples. The calculated values of Pearson correlation coefficients showed strong significant correlation between Pb-Cu during pre-monsoon (r = 1.000) and monsoon seasons (r = 1.000) at p < 0.01 level, whereas Pb was significantly correlated with Cd (r = 1.000) at p < 0.01 level during monsoon and with Cr (r = 1.000) at p < 0.05 level during post-monsoon seasons (Table 2). Moreover, strong significant positive correlation between Cu and Cd was observed during monsoon (r = 1.000) and post-monsoon (r = 0.999) seasons at p < 0.05 level. However, the rest of the elemental pairs showed a strong positive correlation with each other. The strong positive correlations between the studied heavy metals indicated perhaps the common sources of origins which may be anthropogenic and similar pathways into the aquatic environment (Banu et al. 2013; Hassan et al. 2015; Varol 2011). The principal component analysis (PCA) was also performed in the rotated space to assess the interrelationships among the studied heavy metals and to explore the possible and the probable metal distribution patterns (Fig. 3). The PCA of heavy metals explained about 99.55% cumulative variance of the data. In this study, two components were extracted, while the component 1 accounted for about 63.36% of the total variance and the component 2 explained about 36.19% of the total variance. The component 1 showed a positive correlation with Cr, Zn, and Cd, and the component 2 exhibited a positive loading to the Cu and Pb, indicating that the heavy metals in the study area originated from various pollution sources, predominantly from anthropogenic inputs.
The concentrations of heavy metal in sediments of the Shitalakhya River were compared with other major rivers of Bangladesh (Table 3). The concentrations of Pb, Cu, Cr, and Cd in the Shitalakhya River at Narayanganj (Islam et al. 2014) were higher than the present study whereas Zn content was found almost similar in both studies. Moreover, Cu, Cr, and Zn concentrations quantified by Islam et al. (2016) were several times higher as compared with the values observed by Islam et al. (2014) and in the present study. Precisely, the study revealed that the heavy metal concentrations of the Shitalakhya River sediments in Narayanganj areas were comparatively more contaminated than the Narsingdi city area of Bangladesh. The concentrations of five studied heavy metals by Saha and Hossain (2011) in the Buriganga river were found several times higher compared with the present investigation. Conversely, all measured metal concentrations of the Old Brahmaputra (Bhuyan et al. 2019) and Louhajang (Kormoker et al. 2019a) river sediments were quite a few times lower than the Shitalakhyariverin present examinations. According to Banu et al. (2013) except Cd, all of the studied heavy metal concentrations in Turagriver were found higher than this study, whereas the Meghna river (Hassan et al. 2015) showed the lower level of Pb, Cr, and Cd apart from Zn. Mean concentrations of Pb, Cu, and Cd were found higher and Cr was found lower in the Dhaleshwari River reported by Ahmed et al. (2012) than the present measured concentration of the Shitalakhya river. However, Cd concentrations of the Jamuna River (Datta and Subramanian 1998) were almost three times higher when compared with the Shitalakhya River (current study). Rahman et al. (2014) reported higher levels of Pb, Cr, and Zn and a lower level of Cd in the Bangshi River than the present investigation (Table 3). The Cu, Zn, and Cd concentrations of the Khiru River (Rashid et al. 2012) were a few times higher but Pb concentration was almost three times lower than the Shitalakhya River (present study). The concentrations of Pb, Cu, and Cd of the Rupsa River described by Proshad et al. (2019) were many times higher and Cr concentration was much lower when compared with the present investigation in the Shitalakhya River. The Pb, Cu, and Zn concentrations were found higher, while Cr concentration was found almost similar to the Padma River (Jolly et al. 2013) in comparison with the present explored concentration. Moreover, mean concentrations of Pb, Cr, and Cd in the Karnafuli River (Mamun et al. 2013) were found several times higher than the present study.
Assessment of heavy metal contamination using pollution indices
The universal criterion to evaluate the metal pollution in sediments is the Igeo that has been widely used since the late 1960s. According to Müller (1981), the calculated geoaccumulation index (Igeo) values showed that the sediments of Shitalakhya river were unpolluted (Igeo ≤ 0) for all metals over the pre-monsoon, monsoon, and post-monsoon season except for Cd which showed unpolluted to moderately polluted (0 ≤ Igeo ≥ 1) quality during the period in pre-monsoon and post-monsoon seasons (Table 4). The mean Igeo values of the studied five metals followed the descending order of Cd (0.4715) > Zn (− 0.9893) > Pb (− 1.3036) > Cu (− 1.7711) > Cr (− 2.0300). The highest Igeo value in the sediment was found for Cd (0.9510) at St-3 in the post-monsoon season, whereas the lowest was observed for Cr (− 3.6029) at St-1 during monsoon season (Table 4). The Igeo values for the studied heavy metals in the sediment revealed that geoaccumulation of the heavy metals was higher in the post-monsoon season followed by pre-monsoon and monsoon season.
The highest CF value was found 2.90 for Cd at St-3 among all studied heavy metals during the post-monsoon season which might be due to the presence of large-scale industrial and wastewater drainage in the St-3 area. In pre-monsoon and monsoon season, CF values for Cd were > 1 which indicated the moderate level of contaminations, whereas CF values for Pb, Cu, Cr, and Zn were < 1 that indicated the lower level of contaminations at all stations (Table 5). The CF values for Pb and Cu at St-2 and St-3, for Zn at St-3, and for Cd at all stations in post monsoon season were > 1 which showed a moderate level of contamination due to the acceptance of large volume of municipal, domestic, and industrial wastewater and St-1 indicated lower level of contamination for all studied metals. The mean CF values of all metals were found in the following order: Cd (2.15: moderate) > Zn (0.79: low) > Pb (0.66: low) > Cu (0.55: low) > Cr (0.43: low) and the study stated that sediments of the Shitalakhya river were improvised with heavy metals (Table 5).
The maximum CD value was found 6.896 at St-3 during the post-monsoon season; on the other hand, the minimum CD (2.349) was found at St-1 in monsoon season. The CD values for all metals in all the stations over all the seasons were found less than 6 which indicated a lower level of contamination apart from the St-2 and St-3; the CD was higher than 6 that reported moderate level of contaminations which might be due to the receiving of extensive amount of municipal, agricultural, domestic, and industrial wastewater at these stations. Based on the CD values of all studied metals, post-monsoon season was higher contamination level followed by pre-monsoon and monsoon season (Table 5). The PLI describes the number of times by which the heavy metal contents in the sediment exceed the geochemical background concentrations and provide a multitude expression of the level of metal toxicity in a particular specimen (Barakat et al. 2012; Kormoker et al. 2019b). The PLI for the studied heavy metals was ranged from 0.33 to 1.21, with the mean PLI value 0.75 stating that the Shitalakhya River was moderately polluted. The highest PLI (1.21) was found at St-3 in the post-monsoon season that indicated that the sediments of the St-3 were moderately polluted by the studied heavy metals. In this study, the PLI values of all sampling stations in pre-monsoon and monsoon seasons were < 1 which indicated no pollution, whereas PLI values in post-monsoon season in all stations were > 1 revealing the moderate level of pollution (Table 5). The PLI value is significant for understanding the quality of a component of their environment and represents the trend over time and area (Hassan et al. 2015; Islam et al. 2015d). From the investigation, the PLI of the sediments at all seasons followed the order of post-monsoon > pre-monsoon > monsoon.
The average EF for Pb, Cu, and Zn was found minor enrichment category (1 < EF <3), Cd was found moderate enrichment category (EF = 3–5), on the other hand, Cr found no enrichment category (EF < 1). Among the five studied metals, Cd had the most enrichment factor value to a large extent in all sampling station overall seasons which revealed that EF value for Cd in the sediments was moderate to moderately severe enrichment. The mean EF values for all metals were in the descending order of Cd > Zn > Pb > Cu > Cr. The EF values for Cd were found highest in monsoon, whereas Zn found highest in pre-monsoon. Moreover, Pb, Cu, and Cr were found the highest in the post-monsoon season, so the study stated that the rate of EF was high in the post-monsoon season (Table 6). The EF values greater than 1.5 indicate that the sources of metal enrichment are thoroughly from anthropogenic activities, whereas EF values less than 1.5 report that sources are more likely to be natural origins (Zhang and Liu 2002). In the present study, EF values for Zn and Cd in all seasons and Pb and Cu in the post-monsoon season were found > 1.5 in the sediments of the Shitalakhya river that stated anthropogenic effects on the metal extents in the river (Varol 2011; Ustaoğlu and Islam 2020).
The result of the study showed that the RI of heavy metals in sediments of the Shitalakhya river was in the order of Cd (96.83) > Cu (3.84) > Pb (3.29) > Cr (0.79) > Zn (0.58). The richest and significant metal was Cd (57.00–130.50) in all stations over the three seasons and the risk factors of Cd that was considerable for the water body, whereas rest of the metals such as Cu, Pb, Cr, and Zn was found the low risk for the water body. The RI was ranged from 60.50 to 146.39, and the maximum RI was found at St-3 during the post-monsoon while the minimum was found at St-1 during the monsoon season. The mean RI of the Shitalakhya River was found 105.34 which depicted that the river poses low potential ecological risks concerning heavy metal contaminations (Table 7). The highest RI value was recorded in the post-monsoon season which might be due to the lack of heavy rainfall for mixing followed by pre-monsoon and monsoon season (Islam et al. 2015a).
Conclusion
The study found that the concentrations of Cu, Cr, and Cd were higher than the TRV, whereas Pb and Zn were found lower and the highest concentrations were found in the post-monsoon season. Strong significant positive correlations among the studied metals were also reported. The highest Igeo, CF, CD, PLI, and RI values were found in the post-monsoon season that might be owing to a lack of massive amount of water for mixing of received waste and wastewater from various sources, whereas the lowest was found during the monsoon season. The spatial distribution of heavy metals showed that the downstream station was heavily contaminated with heavy metals, regardless of the seasons. On the other hand, the highest EF value was found in the monsoon season that could be due to the input of an enormous amount of silt. Multivariate statistical analysis revealed that the heavy metal contamination was influenced by multiple pollution sources, mainly of anthropogenic origins. The study concluded that the quality of the Shitalakhya river sediments was lower to moderately contaminated with heavy metals as well as low ecological risk for the aquatic environment, especially for sediment-dwelling biota. However, further study is suggested considering more sampling points based on land-use patterns along the river bank to appropriately identify the sources of heavy metals in order to take necessary initiatives to safeguard the riverine environment. This study suggested that chemical fractionation of sediments should be considered to provide more accurate appraisal of the risk of heavy metals in aquatic ecosystems. Moreover, to investigate the comprehensive risks in this riverine environment, both the biological and toxicological or bioaccumulation data in the benthic environment should be considered in future studies.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess 136:227–238. https://doi.org/10.1007/s10661-007-9678-2
Ahmad MK, Islam S, Rahman S, Haque MR, Islam MM (2010) Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int J Environ Res 4(2):321–332
Ahmed MK, Bhowmik AC, Rahman S, Haque MR (2010) Heavy metal concentration in water, sediments, freshwater mussels and fishes of the river Shitalakhya, Bangladesh. Asian J Water Environ Pollut 7(1):77–90
Ahmed ATA, Mandal S, Chowdhury DA, Tareq ARM, Rahman MM (2012) Bioaccumulation of some heavy metals in Ayre fish (SperataAor Hamilton 1822), sediment and water of Dhaleshwari River in dry season. Bangladesh J Zool 40(1):147–153. https://doi.org/10.3329/bjz.v40i1.12904
Ahmed MK, Shaheen N, Islam MS, Al-Mamun MH, Islam S, Mohiduzzaman M, Bhattacharjee L (2015) Dietary intake of trace elements from highly consumed cultured fish (Labeo rohita, Pangasius pangasius and Oreochromis mossambicus) and human health risk implications in Bangladesh. Chemosphere 128:284–292
Alam AMS, Islam MA, Rahman MA, Ahmed A, Islam S, Sultana KS, Siddique MN (2004) Transport of toxic metal through the major river system of Bangladesh. J Chem Soc Pak 26(3):328–332
Alam MJB, Muyen Z, Islam MR, Islam S, Mamun M (2007) Water quality parameters along the rivers. Int J Environ Sci Technol 4(1):159–167. https://doi.org/10.1007/BF03325974
Ali MM, Ali ML, Islam MS, Rahman MZ (2016) Preliminary assessment of heavy metals in water and sediment of Karnaphuli River. Bangladesh. Environ Nanotechnol Monit Manag 5:27–35. https://doi.org/10.1016/j.enmm.2016.01.002
Ali MM, Ali ML, Islam MS, Rahman MZ (2018) Assessment of toxic metals in water and sediment of Pasur River in Bangladesh. Water Sci Technol 77:1418–1430. https://doi.org/10.2166/wst.2018.016
Ali MM, Proshad R, Islam MS, Kormoker T, Rahman M, Tusher TR, Mamun M (2019) Heavy metal concentrations in commercially valuable fishes with health hazard inference from Karnaphuli River. Bangladesh Hum Ecol Risk Assess:1–17. https://doi.org/10.1080/10807039.2019.1676635
Banu Z, Chowdhury MSA, Hossain MD, Nakagami K (2013) Contamination and ecological risk assessment of heavy metal in the sediment of Turag river, Bangladesh: an index analysis approach. J Water Resource Prot 5:239–248. https://doi.org/10.4236/jwarp.2013.52024
Barakat A, Baghdadi ME, Rais J, Nadem S (2012) Assessment of heavy metal in surface sediments of Day river at Beni-Mellal Region, Morocco. Res J Environ Earth Sci 4:797–806
Benson NU, Asuquo FE, Williams AB, Essien JP, Ekong CI, Akpabio O, Olajire AA (2016) Source evaluation and trace metal contamination in benthic sediments from equatorial ecosystems using multivariate statistical techniques. PLoS One 11(6):e0156485. https://doi.org/10.1371/journal.pone.0156485
Bhuiyan MAH, Parvez L, Islam MAS, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392. https://doi.org/10.1016/j.jhazmat.2009.08.085
Bhuyan MS, Bakar MA, Akhtar A, Hossain MB, Ali MM, Islam MS (2017) Heavy metal contamination in surface water and sediment of the Meghna River, Bangladesh. Environ Nanotechnol Monit Manag 8:273–279. https://doi.org/10.1016/j.enmm.2017.10.003
Bhuyan MS, Bakar MA, Rashed-Un-Nabi M, Senapathi V, Chung SY, Islam MS (2019) Monitoring and assessment of heavy metal contamination in surface water and sediment of the Old Brahmaputra River, Bangladesh. Appl Water Sci 9:125. https://doi.org/10.1007/s13201-019-1004-y
Chen JS, Zhou JY (1992) Study of heavy metals in the water environment of China. China Environmental Science Press, Beijing
Datta DK, Subramanian V (1998) Distribution and fractionation of heavy metals in the surface sediments of the Ganges–Brahmaputra–Meghna River system in the Bengal Basin. Environ Geol 36(1–2):93–101
El-Sammak A, Aboul-Kassim T (1999) Metal pollution in the sediments of Alexandria Region, Southern Mediterranean, Egypt. Bull Environ Contam Toxicol 63(2):263–270. https://doi.org/10.1007/s001289900975
Gaur VK, Gupta SK, Pandey SD, Gopal K, Mishra V (2005) Distribution of heavy metals in sediment and water of river Gomti. Environ Monit Assess 102(1–3):419–433. https://doi.org/10.1007/s10661-005-6395-6
Gupta A, Rai DK, Pandey RS, Sharma B (2009) Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environ Monit Assess 157(1–4):449–458. https://doi.org/10.1007/s10661-008-0547-4
Hakanson L (1980) Ecological risk index for aquatic pollution control. A Sedimentological approach. Water Res 14(8):975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Haque MR, Ahmad JU, Chowdhury MDA, Ahmed MK, Rahman MS (2005) Seasonal variation of heavy metals concentration in surface water of the rivers and estuaries of Sundarbans mangrove forest. Pollut Res 24(2):463–472
Hassan M, Mirza ATM, Rahman T, Saha B, Kamal AKI (2015) Status of heavy metals in water and sediment of the Meghna river, Bangladesh. Am J Environ Sci 11(6):427–439. https://doi.org/10.3844/ajessp.2015.427.439
Islam MZ, Azim MA, Islam R, Quraisi SB (2008) Seasonal variation of heavy metals in water samples from Shitalakhya river. J Bangladesh Acad Sci 32(1):13–22. https://doi.org/10.3329/jbas.v32i1.2437
Islam MM, Rahman SL, Ahmed SU, Haque MKI (2014) Biochemical characteristics and accumulation of heavy metals in fishes, water and sediments of the river Buriganga and Shitalakhya of Bangladesh. J Asian Sci Res 4(6):270–279
Islam MS, Ahmed MK, Raknuzzaman M, Mamun MHA, Islam MK (2015a) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Indic 48:282–291. https://doi.org/10.1016/j.ecolind.2014.08.016
Islam MS, Ahmed MK, Al-Mamun MH (2015b) Geochemical speciation and risk assessment of heavy metals in sediments of a river in Bangladesh. Soil Sediment Contam Int J 24:639–655
Islam MS, Ahmed MK, Raknuzzaman M, Al-Mamun MH, Masunaga S (2015c) Metal speciation in sediment and their bioaccumulation in fish species of three urban rivers in Bangladesh. Arch Environ Contam Toxicol 68:92–106
Islam MS, Ahmed MK, Al-Mamun MH, Hoque MF (2015d) Preliminary assessment of heavy metals contamination in surface sediments from a river in Bangladesh. Environ Earth Sci 73:1837–1848
Islam SMD, Bhuiyan MAH, Rume T, Mohinuzzaman M (2016) Assessing heavy metal contamination in the bottom sediments of Shitalakhya River, Bangladesh; using pollution evaluation indices and geo-spatial analysis. Pollut 2(3):299–312
Islam MS, Ahmed MK, Proshad R, Ahmed S (2017a) Assessment of toxic metals in vegetables with the health implications in Bangladesh. Adv Environ Res Int J 6(4):241–254
Islam MS, Al-Mamun MH, Ye F, Tokumura M, Masunaga S (2017b) Chemical speciation of trace metals in the industrial sludge of Dhaka City, Bangladesh. Water Sci Technol 76:256–267
Islam MS, Proshad R, Ahmed S (2018) Ecological risk of heavy metals in sediment of an urban river in Bangladesh. Hum Ecol Risk Assess Int J 24(3):699–720
Islam MS, Ahmed MK, Al-Mamun MH, Eaton DW (2020) Human and ecological risks of metals in soils under different land use in an urban environment of Bangladesh. Pedosphere 30:1–12
Jolly YN, Akter S, Kabir J, Islam A, Akbar S (2013) Trace elements contamination in the river Padma. Bangladesh J Phys 13:95–102
Kabir MH, Islam MS, Tusher TR, Hoq ME, Muliadi, Mamun SA (2020) Changes of heavy metal concentrations in Shitalakhyariver water of Bangladesh with seasons. Indonesian J SciTechnol 5(3):395–409. https://doi.org/10.17509/ijost.v5i3.25007
Kormoker T, Proshad R, Islam MS (2019a) Ecological risk assessment of heavy metals in sediment of the Louhajang River, Bangladesh. SF J Environ Earth Sci 2(2):1030
Kormoker T, Proshad R, Islam MS, Ahmed S, Chandra K, Uddin M, Rahman M (2019b) Toxic metals in agricultural soils near the industrial areas of Bangladesh: ecological and human health risk assessment. Toxin Rev:1–20. https://doi.org/10.1080/15569543.2019.1650777
Kormoker T, Proshad R, Islam MS, Shamsuzzoha M, Akter A, Tusher TR (2020) Concentrations, source apportionment and potential health risk of toxic metals in foodstuffs of Bangladesh. Toxin Rev:1–14. https://doi.org/10.1080/15569543.2020.1731551
Kura B, Kambham K, Sangameswaran S, Potana S (2006) Atmospheric particulate emissions from dry abrasive blasting using coal slag. J Air Waste Manage Assoc 56:1206–1215. https://doi.org/10.1080/10473289.2006.10464533
Liu C, Xu J, Liu C, Zhang P, Dai M (2009) Heavy metals in the surface sediments in Lanzhou Reach of Yellow River, China. Bull Environ Contam Toxicol 82(1):6–30. https://doi.org/10.1007/s00128-008-9563-x
Malik N, Biswas AK, Qureshi TA, Borana K, Virha R (2010) Bioaccumulation of heavy metals in fish tissues of a freshwater lake of Bhopal. Environ Monit Assess 160:267–276. https://doi.org/10.1007/s10661-008-0693-8
Mamun A, Sumon KA, Sukhan ZP, Hoq E, Alam MW, Haq MS, Rashid F, Rashid H (2013) Heavy metal contamination in water and sediments of the river Karnafuli from south-east coast of Bangladesh. In: Conference paper, 4th the International Conference on Environmental Aspects of Bangladesh, at Fukuoka, Japan, pp 113–116
Martin JM, Meybeck M (1979) Elemental mass balance of materials carried by major world rivers. Mar Chem 7(3):173–206. https://doi.org/10.1016/0304-4203(79)90039-2
Mohiuddin KM, Ogawa Y, Zakir HM, Otomo K, Shikazono N (2011) Heavy metals contamination in water and sediments of an urban river in a developing country. Int J Environ Sci Technol 8(4):723–736. https://doi.org/10.1007/BF03326257
Monirul IM, Rahman SL, Ahmed SU, Haque MKI (2014) Biochemical characteristics and accumulation of heavy metals in fishes, water and sediments of the river Buriganga and Shitalakhya of Bangladesh. J Asian Sci Res 4(6):270–279
Moore F, Forghani G, Qishlaqi A (2009) Assessment of heavy metal contamination in water and surface sediments of the Maharlu Saline Lake, SW Iran. Iranian J. Sci Technol 33(1):43–55. https://doi.org/10.22099/ijsts.2009.2201
Mottalib MA, Al-Razee ANM, Abser MN, Aman EUM (2016) Assessment of physicochemical properties of surface water of Shitalakhya river near Polash, Narsingdi, Bangladesh. Int J Adv Res 4(8):915–924. https://doi.org/10.21474/IJAR01/1288
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geochem J 2:108–118
Müller G (1981) Die Schwermetallbelastung der sedimente des Neckars und seiner Nebenflusse: EineBestandsaufnahme. Chemiker-Zeitung 105:157–164
Nobi EP, Dilipan E, Thangaradjou T, Sivakumar K, Kannan L (2010) Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuar Coast Shelf Sci 87(2):253–264. https://doi.org/10.1016/j.ecss.2009.12.019
Ogbeibu AE, Omoigberale MO, Ezenwa MI, Eziza JO, Igwe JO (2014) Using pollution load index and geoaccumulation index for the assessment of heavy metal pollution and sediment quality of the Benin river, Nigeria. Nat Environ 2(1):1–9. https://doi.org/10.12966/NE.05.01.2014
Pandey LK, Park J, Son DH, Kim W, Islam MS, Choi S, Lee H, Han T (2019) Assessment of metal contamination in water and sediments from major rivers in South Korea from 2008 to 2015. Sci Total Environ 651:323–333
Persuad D, Jaagumagi R, Hayton A (1993) Guidelines for the protection and management of aquatic sediment quality in Ontario. Ontario Ministry of the Environment, Canada
Proshad R, Kormoker T, Islam S (2019) Distribution, source identification, ecological and health risks of heavy metals in surface sediments of the Rupsa River. Bangladesh Toxin Rev:1–25. https://doi.org/10.1080/15569543.2018.1564143
Proshad R, Islam MS, Tusher TR, Zhang D, Khadka S, Gao J, Kundu S (2020) Appraisal of heavy metal toxicity in surface water with human health risk by a novel approach: a study on an urban river in vicinity to industrial areas of Bangladesh. Toxin Rev:1–17. https://doi.org/10.1080/15569543.2020.1780615
Rahman MS, Saha N, Molla AH (2014) Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci 71(5):2293–2308. https://doi.org/10.1007/s12665-013-2631-5
Raphael EC, Augustina OC, Frank EO (2011) Trace metals distribution in fish tissues, bottom sediments and water from Okumeshi River in delta state, Nigeria. Environ Res J 5:6–10
Rashid H, Hasan MN, Tanu MB, Parveen R, Sukhan ZP, Rahman MS, Mahmud Y (2012) Heavy metal pollution and chemical profile of Khiru River, Bangladesh. Int J Environ 2:57–63
Reza R, Singh G (2010) Heavy metal contamination and its indexing approach for river water. Int J Environ Sci Technol 7(4):785–792. https://doi.org/10.1007/BF03326187
Saha PK, Hossain MD (2011) Assessment of heavy metal contamination and sediment quality in the Buriganga river, Bangladesh. Proceedings of the 2nd International Conference on Environmental Science and Technology, (EST’ 11), IACSIT Press, Singapore, pp 384–388
Sakan SM, Djordjevic DS, Manojlovic DD, Polic PS (2009) Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J Environ Manag 90(11):3382–3390. https://doi.org/10.1016/j.jenvman.2009.05.013
Shikazono N, Tatewaki K, Mohiuddin KM, Nakano T, Zakir HM (2012) Sources, spatial variation, and speciation of heavy metals in sediments of the Tamagawa River in Central Japan. Environ Geochem Health 34(1):13–26. https://doi.org/10.1007/s10653-011-9409-z
Singh KP, Malik A, Sinha S, Singh VK, Murthy RC (2005) Estimation of source of heavy metal contamination in sediments of Gomti River (India) using principal component analysis. Water Air Soil Pollut 166(1-4):321–341. https://doi.org/10.1007/s11270-005-5268-5
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresun 33(1–4):566–575. https://doi.org/10.1007/BF02414780
Turekian KK, Wedepohl KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192. https://doi.org/10.1130/0016-7606(1961)72[175:DOTEIS]2.0.CO;2
Tusher TR, Sarker ME, Nasrin S, Kormoker T, Proshad R, Islam MS, Al-Mamun S, Tareq ARM (2020) Contamination of toxic metals and polycyclic aromatic hydrocarbons (PAHs) in rooftop vegetables and human health risks in Bangladesh. Toxin Rev:1–16. https://doi.org/10.1080/15569543.2020.1767650
USEPA (United States Environment Protection Agency) (2007) Method 3051a: microwave assisted acid dissolution of sediments, sludges, soils, and oils, revision 1, Washington DC, USA
USEPA (United States Environmental Protection Agency) (1999) Screening level ecological risk assessment protocol for hazardous waste combustion facilities, Appendix E: Toxicity reference values. Washington DC, USA
Ustaoğlu F, Islam MS (2020) Potential toxic elements in sediment of some rivers at Giresun, NortheastTurkey: a preliminary assessment for ecotoxicological status and health risk. Ecol Indic 113:106237–106251
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364. https://doi.org/10.1016/j.jhazmat.2011.08.051
Zhang J, Liu CL (2002) Riverine composition and estuarine geochemistry of particulate metals in China-weathering features, anthropogenic impact and chemical fluxes. Estuar Coast Shelf Sci 54(6):1051–1070. https://doi.org/10.1006/ecss.2001.0879
Acknowledgments
Sincere gratitude to Ms. Nowara Tamanna Meghla (Assistant Professor) and Dr. Iqbal Bahar (Assistant Registrar, Lab), Department of Environmental Science and Resource Management, Mawlana Bhashani Science and Technology University, Bangladesh, for their cooperation during the study period.
Funding
This project is funded by Mawlana Bhashani Science and Technology University, Bangladesh, for the synthesis of materials, characterization, and all chemical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Kabir, M.H., Islam, M.S., Hoq, M.E. et al. Appraisal of heavy metal contamination in sediments of the Shitalakhya River in Bangladesh using pollution indices, geo-spatial, and multivariate statistical analysis. Arab J Geosci 13, 1135 (2020). https://doi.org/10.1007/s12517-020-06072-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-06072-5