Abstract
Industrial wastewater discharged into aquatic ecosystems either directly or because of inadequate treatment of process water can increase the concentrations of pollutants such as toxic metals and others, and subsequently deteriorate water quality, environmental ecology and human health in the Dhaka Export Processing Zone (DEPZ), the largest industrial belt of 6-EPZ in Bangladesh. Therefore, in order to monitor the contamination levels, this study collected water samples from composite effluent points inside DEPZ and the surrounding surface water body connected to effluent disposal sites and determined the environmental hazards by chemical analysis and statistical approach. The water samples were analysed by inductively coupled plasma mass spectrometry to determine 12 trace metals such as As, Ag, Cr, Co, Cu, Li, Ni, Pb, Se, Sr, V and Zn in order to assess the influence of multi-industrial activities on metal concentrations. The composite effluents and surface waters from lagoons were characterized by a strong colour and high concentrations of biochemical oxygen demand, chemical oxygen demand, electrical conductivity, pH, total alkalinity, total hardness, total organic carbon, Turb., Cl−, total suspended solids and total dissolved solids, which were above the limit of Bangladesh industrial effluent standards, but dissolved oxygen concentration was lower than the standard value. The measurement of skewness and kurtosis values showed asymmetric and abnormal distribution of the elements in the respective phases. The mean trend of variation was found in a decreasing order: Zn > Cu > Sr > Pb > Ni > Cr > Li > Co > V > Se > As > Ag in composite industrial effluents and Zn > Cu > Sr > Pb > Ni > Cr > Li > V > As > Ag > Co > Se in surface waters near the DEPZ. The strong correlations between effluent and surface water metal contents indicate that industrial wastewaters discharged from DEPZ have a strong influence on the contamination of the surrounding water bodies by toxic metals. The average contamination factors were reported to be 0.70–96.57 and 2.85–1,462 for industrial effluents and surface waters, respectively. The results reveal that the surface water in the area is highly contaminated with very high concentrations of some heavy/toxic metals like Zn, Pb, Cu, Ni and Cr; their average contamination factors are 1,460, 860, 136, 74.71 and 4.9, respectively. The concentrations of the metals in effluent and surface water were much higher than the permissible limits for drinking water and the world average concentrations in surface water. Therefore, the discharged effluent and surface water may create health hazards especially for people working and living inside and in the surrounding area of DEPZ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many different chemicals are used for the benefit of daily life, medicine, food production and industrial purposes, and a good proportion of these compounds lack natural counterparts. The majority of these compounds have a rather poor biodegradability. Hence, freshwater resources, a valuable asset for human and other living beings, become more and more contaminated with these man-made chemical pollutants. Global modernization and growth of industrial activities have discharged industrial wastewater into aquatic ecosystems either directly or because of inadequate treatment of process water that can lower water quality by increasing concentrations of pollutants such as heavy metals, organic matter, micropollutants and nutrients (phosphorus and nitrogen), resulting in adverse effects on human health, aquatic biota, fish and macrophytes. It becomes increasingly clear that societal attitude towards water pollution is associated with rising economic costs because of the ensuing depletion of water resources for specific uses. The reduction of pollution in wastewater will depend on what a given community or an industrial area allows into the effluent stream and on the efficiency and effectiveness with which these effluents are treated (Schröder et al. 2007). Barzilay et al. (1999) have very clearly depicted the relation between water pollution and public health problems, and nowadays, it is well known to the general public also.
Metals, especially ‘toxic trace metals’, are among the most common environmental pollutants, and their occurrence in waters and biota results from natural or anthropogenic sources (Hussain and Ahmed 1997). Nowadays, the major sources of metal pollution in freshwater systems are industrial activities. Influx of metal ions into the aquatic environment results in the elevation of water electrical conductivity (EC) and dissolved solid (DS) content at low pH conditions, but at high pH, most metal ions undergo precipitation or adsorption onto the sediment surface. Elevated EC and DS affect the osmoregulation of the freshwater organisms, reduce the solubility of gases (O2) and affect the utility of water for domestic, industrial and agricultural usages (Mowka 1988). Trace and heavy metals in environment cannot be decomposed, but rather they accumulate in the food chain of the ecosystem through a biomagnification process (Mortula and Rahman 2002; More et al. 2001; Lee et al. 2001).
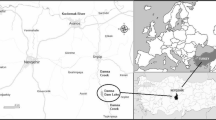
Bangladesh is a developing country with a low land-to-man ratio and a low resource base. In order to cope with increasing unemployment and to improve economic growth, the government of Bangladesh started allocating special industrial installations called “export processing zones” (EPZs) in the early 1990s to private entrepreneurs, and so far, 6-EPZ has been established in the country. Because of the very cheap local work force and the somewhat relaxed environmental and tariff regulations, foreign and local investors are being attracted heavily to set up manufacturing units for exportable commodities. The Dhaka Export Processing Zone (DEPZ) is the largest industrial belt of 6-EPZ established in 1993 with 141 acres of land and later extended in 1997 to 355 acres divided into 383 industrial plots. It is 40 km away from Dhaka City and located in densely populated villages, locality separated by a water body (Fig. 1) named Dhalai Beel and of low-lying farming lands. DEPZ accommodates 300 industries whose activities range from textile and dyeing to plastics, metal fabrications, semiconductor goods, lather tanning and so on. These industries release their effluents either on open land or in surrounding surface water bodies, contaminating the soil, surface water and, ultimately, groundwater. Very recently, it was reported that the surface water body connected to DEPZ effluent disposal sites has been steadily contaminated with a huge number of trace and toxic metals (Ahmed et al. 2009; Mortula and Rahman 2002; Mahfuz et al. 2004). Therefore, it is a worrying need to figure out the present status of toxic trace metals in this water body, redress the affected subsequent environmental problems and adopt future mitigation strategy. The government of Bangladesh has recently passed a strict environmental legislation to control environmental contamination. Remediation of these contaminated areas will involve very high costs, and therefore it is essential that the contamination of water bodies is controlled rather than remediated (Gowd and Govil 2008).
Therefore, the main objective of the present work was to measure the physical, chemical and biological properties, and the concentration of 12 trace metal ions (Li, V, Cr, Co, Ni, Cu, Zn, As, Se, Sr, Ag and Pb) in water samples collected from selected locations of the composite effluents flow and the natural water flow near DEPZ. Then, the results were projected by descriptive statistics together with the evolution of simple and multiple correlations that would provide the correlation patterns of the metals in effluents and surface water. This approach would, in turn, help adopt an effluent management strategy towards control over enhanced metal levels with recycling of effluents for toxic metal separation (Tariq et al. 2005; Hardle and Simar 2003; Hussain et al. 2000) and, thus, to avert the current gross environmental pollutions around the DEPZ area.
Methods and materials
Site description and geology
The study area, Savar, is situated at the southern tip of a Pleistocene terrace, the Madhupur Tract. Two characteristic geological units cover the area, viz. Madhupur Clay of the Pleistocene age and alluvial deposits of recent age. The major geomorphic units of the area are: the high land, the low lands or floodplains, depressions and abandoned channels. Low-lying swamps and marshes located in and around the area are other major topographic features. The incised channels and depressions within the area are floored by recent alluvial floodplain deposits and are further subdivided into lowland alluvium and highland alluvium. The subsurface sedimentary sequence, up to the explored depth of 300 m, shows three distinct entities: one is the Madhupur Clay of the Pleistocene age, characterized by reddish plastic clay with silt and very fine sand particles. This Madhupur Clay uncomfortably overlies the Dupi Tila Formation of the Plio-Pleistocene age, composed of medium to coarse yellowish brown sand and occasional gravel. The Dupi Tila sands aquifer is the main source of water in the area. Madhupur Clay overlies the aquifer with a thickness of 8–45 m (averages 10 m). The aquifer varies in thickness from 100 to 200 m (averages 140 m).
Sample collection
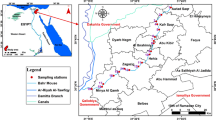
Twelve sampling points were selected inside DEPZ to represent the whole composite effluent points and assess the selective trace metal loads in various composite effluents, combined drains and discharge points in both the old and new DEPZ. The same number (12) of sampling points was selected randomly in the water body to evaluate the influence of the composite effluent sources on the receiving surface water body. In addition, five groundwater (GW) samples of different sites that supply pure water to DEPZ were collected to know the background or reference level before its use in the industrial operation. In January and March 2005, the samples were collected by a proper sampling procedure and stored in 2.5-L cleaned plastic containers (Robinson et al. 2001; More et al. 2001; Mastoi et al. 1997) and then transferred to the laboratory. A portion of each sample was filtered through a Millipore membrane filter (pore size, 0.45 μm) and HNO3 (65%) added as a preservative to make the pH < 2, stored in 100-mL, cleaned acid-washed plastic bottles, and kept in a refrigerator at 4°C (HACH 1997; FALP 2006) until analysis. The sampling points in this study were selected in such a way that these may be representative to evaluate the environmental impact on the receiving water body of the effluent discharge points (Olajire and Imeokparia 2000).
Sample analysis
The physical, chemical and biological characteristics of the industrial composite effluents and surface water of lagoons were determined through extensive laboratory tests. The detailed standard experimental methods to determine the colour, odour, temperature, pH, EC, total dissolved solids (TDS), turbidity (Turb), total suspended solids (TSS), total alkalinity (TA), total hardness (TH), Cl−, CO2, dissolved oxygen (DO), biochemical oxygen demand (BOD), chemical oxygen demand (COD) and total organic carbon (TOC) are described in HACH (1997) and FALP (2006).
Inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7500 cc, ICP-MS, Octopole Reaction System, USA) was used to carry out the quantitative analysis of trace metals at the International Environmental Research Center, Gwangju Institute of Science and Technology (GIST), Republic of Korea. The samples were diluted with ultrapure water mixed with 2% (v/v) HNO3. To calibrate the instrument, a commercial multi-element standard solution (Merck) with a concentration of 100 mg/L was used as a stock solution. The calibration standard solutions, prepared using ultrapure water in 2% (v/v) HNO3, have concentrations of 0, 10, 25, 50 and 100 μg/L. For ion formation, temperature was maintained at 7,000–8,000 K. Three replicates for each sample were analysed, and the percentages of RSD of the elements were within the range 0.25–12%. The accuracy of the procedure was determined by triplicate analysis of the Standard Reference Material NIST-1643d. The reported results are the mean values of triplicate determinations multiplied by the dilution factor and are expressed as micrograms per litre. Statistical software SPSS (Hannan 2007) was used to compute the relevant statistical analysis of the data.
Results and discussion
Physicochemical characteristics
The composite effluents from multi-industrial activities and surface water samples are characterized by a strong colour (reddish dull brown) and high concentrations of BOD, COD, EC, pH, TA, TH, TOC, Turb., Cl−, TSS and TDS (Tables 1 and 2), similar to those of tannery waste effluent and contaminated water reported by Gowd and Govil (2008). The concentrations of TSS in surface water and effluent were within the range of 20–336 mg/L, with a mean value of 126 mg/L, and 24.9–422 mg/L, with a mean value of 281 mg/L, respectively, which were found to be above the limit of Bangladesh industrial effluent standards of 100 mg/L. Similarly, the concentrations of TDS and BOD5 in surface water and effluent samples exceeded Bangladesh industrial effluent standards of 2,100 mg/L for dissolved solids and 50 mg/L for BOD5; however, DO concentration was lower than the standard value (4.5–8 mg/L).
Heavy metal concentrations
The descriptive statistical measures reflecting the maximum, minimum, mean, standard deviation (SD), standard error (SE), skewness, kurtosis and average contamination factor (ACF) of the metals in industrial composite effluents and surface water samples are presented in Tables 3 and 4, respectively. The mean concentrations of the different metals were found in the order: Zn > Cu > Sr > Pb > Ni > Cr > Li > Co > V > Se > As > Ag in effluent and Zn > Cu > Sr > Pb > Ni > Cr > Li > V > As > Ag > Co > Se in surface water (Tables 3 and 4).
Zinc
Out of 12 trace metals, Zn exhibited the highest mean concentration in effluent (1,488 μg/L) and surface water samples (788 μg/L). These results reflect the use of large amounts of Zn in DEPZ, viz. construction materials as Zn alloys, protective and coating for iron and steel. Furthermore, zinc is used as follows: dust used as a pigment and reducing agent; ZnCl2 in dry cell batteries, cotton processing, soldering and welding flux; ZnCrO4 as corrosion inhibitor in paints; and ZnO as pigment in the rubber industry, glass, enamels, plastics, lubricants, cosmetics, pharmaceutical, agent for burns and ointments. Zinc sulphide is used as white pigments, semiconductor, in manufacturing soap, lubricants, and waterproofing of textiles, paper and concrete.
Lead and other metals
Metal spraying, printing, cables, fuses, bearing metals, brass making and dye casting are used in manufacturing automobile carburetors, pumps, hubcaps and drills (Ayres and Hellier 1998; Cobelo and Ricardo 2004). Out of all the elements, the concentrations of Cu (353 μg/L), Sr (189 μg/L), Pb (109 μg/L), Ni (66.15 μg/L), Cr (44.03 μg/L), Li (38.92 μg/L) and Co (27.04 μg/L) in effluent and those of Cu (201 μg/L), Sr (171 μg/L), Pb (68.76 μg/L), Ni (59.77 μg/L), Cr (34.37 μg/L) and Li (13.65 μg/L) in surface water are alarmingly high due to the wide use of these metal compounds, metal complexes and metal alloys in the manufacturing of machine, catalyst, pigment, preservative, electrolytes, protective coatings, decorative finishing agent, mordent in dyeing and printing, corrosion resistance, medicine, pyrotechnics, manufacture of special glass and colour picture tubes, etc. (Nriagu 1988; Appelo and Postma 1996; Tariq et al. 2006; Gowd and Govil 2008) in DEPZ multi-industrial activities. During the process of leather manufacturing, chemical like Cr(SO4)3 is extensively used (Gowd and Govil 2008).
Lead, a potentially toxic metal, had very high concentrations in both industrial composite effluents and surface water samples. The concentrations of lead in industrial composite effluents and surface water samples ranged 56.50–235 μg/L, with an average value of 109 μg/L, and 36.10–142 μg/L, with an average value of 68.76 μg/L, respectively. These values are greater than the WHO permissible limit of 0.01 μg/L (Obiri et al. 2010). There is high variability in the concentration of lead in both industrial composite effluents and surface water samples. The high concentrations of Pb in surface waters pose significant health hazards to residents consuming it. Hence, regular consumption of water from these sources by resident children could pose a serious neurological health problem from a long-term Pb exposure in the vicinity of the DEPZ, which would eventually affect their academic performance. The resident children may suffer multiple exposures to lead via drinking water, air and soil intake in the study area. According to the US Center for Disease Control, very low concentrations of lead pose a serious public health hazard to sensitive populations such as infants, children and pregnant women (as surrogates for foetuses). For the concentrations of Li, Co, Ni, Cu, Zn and Sr, there is also a very high variability in the industrial composite effluents, but very low in surface water samples (Tables 3 and 4).
Arsenic
Arsenic, a priority toxic element, can cause arsenicosis-related disease and internal cancers, even though a trace amount of As is present in drinking water. The As concentrations in industrial composite effluents and surface water samples in this study ranged 2.0–8.0 μg/L, with an average value of 4.92 μg/L, and 4.30–11.60 μg/L, with an average value of 7.63 μg/L, respectively. The As concentrations in some of the surface water samples exceeded the maximum permissible limit of WHO (10 μg/L) in drinking water.
Average contamination factor
The average contamination factor (ACF) is determined from trace metal concentrations in the respective effluent and water phases divided by the background level (groundwater concentrations) for the effluent and world average concentrations (WAC) for surface water (Table 8), respectively. The average contamination factors of Pb, As, Se, Cu, Zn, Li and Co in composite effluents were higher by 13.17–28.83 times, whilst Cr, Ni, Ag, Sr and V had lower to moderate contamination factors ranging from 0.7 to 5.0 (Table 3). In surface water samples, the average contamination factors of Pb, Se, Cu, Zn, Ni and Cr were higher by 30.57–1,462 times, whilst Ag, Sr, V, As, Li and Co had lower to moderate average contamination factors of 2.85–24.67 (Table 4). Comparatively higher ACF in surface waters than in effluents was found (Tables 3 and 4), which is supposed to be due to a continuous discharge of the untreated effluent and metal accumulation in surface water near the effluent discharge points of DEPZ (Ahmed et al. 2009; Roychoudury and Starke 2006).
Statistical analysis
Significant variations between the reliant statistical measures, viz. maximum–minimum and mean–median, and the elevated SD of the physicochemical characteristics and trace metals in effluent and surface water (Tables 1 and 4) reflect the heterogeneous discharge and accumulation of the pollutants. The skewness and kurtosis values evidence the abnormal distributions of trace metals in effluents and surface waters. The variations of metal distribution in effluents and surface water samples connected to effluent disposal points of DEPZ are in agreement with the discharge of effluent and in sequence of the industrial operations conducted in different batches (Tariq et al. 2005).
Tables 5 and 6 show the statistical analysis of metal-to-metal correlation matrix in terms of linear correlation coefficient (r) values (significant at 0.05) in composite effluents and surface water, respectively. The listed r values reveal the high degree of positive correlations and significant linear regression relation between various pairs of metals, reflecting their simultaneous release and identical source from the DEPZ zone, transport and accumulation in surface water. The inter-metallic correlation coefficients in composite effluents with p < 0.05 were: Co–Li (r = 0.99), Cu–Cr (r = 0.73), Zn–Ni (r = 0.92), As–Cr (r = 0.74), As–Se (r = 0.54), Se–V (r = 0.52), Sr–Ni (r = 0.76), Sr–Zn (r = 0.89), Ag–Cr (r = 0.74) and Pb–Sr (r = 0.51). There are well-defined relationships between these various trace metals.
The trace metal concentrations in effluents exhibited a positive correlation with those in surface water (Table 7), with p < 0.05; the following pairs of trace elements confirmed the significant positive correlations: V–Se, V–V, Cr–Co, Cr–Cu, Co–Cu, Co–Se, Ni–Cr, Ni–Zn, Ni–Ni, Cu–Pb, Zn–Cr, Zn–Ag, As–Sr, As–Ni, Se–Cu, Ag–Li, Ag–Co and Pb–Co. A non-significant correlation exists between the other metals, and significant negative values of a few pairs of the metals represent their natural control.
The average concentrations of Zn, Pb, Cu, Sr and Ni possessed elevated levels when compared with other trace metals (Tables 3 and 4 and Fig. 2), and their levels are comparatively higher in effluents than in surface water (Fig. 2). The metal concentrations in groundwater, used in industrial activities of DEPZ, and the world average concentrations are summarized in Table 8.
Based on the correlation matrix, the true natural trace elements can be separated from possible trace metals of anthropogenic (e.g. industrial) origin. The results of this study reveal a good relationship between the different trace metals in the effluents and surface water. Both statistical analysis and the geographical distribution of the concentrations are being used to assess the possible contamination compared with natural background values (groundwater concentrations) and the WAC values. The statistical analysis reveals that there is considerable contamination of Pb, Zn, Cu, Ni and Cr occurring in the DEPZ industrial area causing surface water systems toxic for human health, agricultural use and ecology. It was observed that the people in the area are seriously affected and suffering from occupational diseases such as asthma, ulcers, skin diseases, etc. Cr, Ni and Zn from surface water can be adsorbed on and contaminate the soils (Gowd and Govil 2008), and infiltrate or percolate downwards into groundwater. However, the trace metals can be remobilized again from soils/sediments to water systems depending on redox and pH conditions of water systems and sediments.
Conclusions
The present work demonstrates the application of descriptive statistics in identifying the pattern of distribution and levels of trace metal contamination in surface water connected to effluent disposal points of the DEPZ industrial area. In comparison with the world average concentrations and maximum contaminant level, the results show that the surface water is heavily contaminated by toxic trace metals like Zn, Pb, Cr, Cu and Ni, which may give rise to various health problems or diseases. The random dumping of hazardous waste and liquid waste from industries is the main cause of the contaminant spreading by rainwater and wind. The natural occurrence of metals is not a source of contamination in the whole area. The elevated levels of these metals could ultimately affect the soil sediment and cultivated crops and fish, thus making them toxic for human consumption. The extensive and severe contamination of Zn, Pb, Cr, Cu and Ni is a matter of concern due to the fact that many residential areas are located near the industrial area. To mitigate and stop the contamination, due legislative measures must be made legally binding on the individual industries towards controlling the discharge of untreated effluents. The regular monitoring of toxic metals in the surface water is needed to check the water quality. All existing waste should be cleaned up and future waste should be dumped in a permanent landfill site. The present surface wastes can be treated by remedial technology including excavation and replanting, stabilization of the soil, phytoremediation and bioremediation.
References
Ahmed, G., Uddin, M. K., Khan, G. M., Rahman, M. S., & Chowdhury, D. A. (2009). Distribution of trace metal pollutants in surface water system connected to effluent disposal points of Dhaka Export Processing Zone (DEPZ), Bangladesh: A statistical approach. Journal of Nature Science and Sustainable Technology, 3(4), 293–304.
Appelo, C. A. J., & Postma, D. (1996). Geochemistry, groundwaters and pollution. Rotterdam: Balkema (536 pp).
Ayres, D. C., & Hellier, D. G. (1998). Dictionary of environmentally important chemicals. London: Blackie A and P.
Barzilay, J. I., Weinberg, W. G., & Eley, J. W. (1999). The water we drink: Water quality and its effects on health. New Brunswick: Rutgers University Press (192 pp).
Cobelo, G. A., & Ricardo, P. (2004). Influence of point sources on trace metal contamination and distribution in a semi-enclosed industrial embayment: The Ferrol Ria (NW Spain). Estuarine, Coastal and Shelf Science, 60, 695–703.
Field and Laboratory Protocol (FALP). (2006). Bangladesh environmental technology verification support to arsenic mitigation (BETV-SAM). Dhaka: OCETA, DPHE.
Gowd, S. S., & Govil, P. K. (2008). Distribution of heavy metals in surface water of ranipet industrial area in Tamil Nadu, India. Environmental Monitoring and Assessment, 136, 197–207.
HACH. (1997). Measuring of conductivity/TDS/salinity: Water analysis handbook (3rd ed.). Loveland: HACH Co.
Hannan, J. M. A. (2007). Medical and pharmaceutical statistics. Fakirapool, Dhaka: Sangbed Printing Publication.
Hardle, W., & Simar, L. (2003). Applied multivariate statistical analysis. Berlin: Springer.
Hussain, T., & Ahmed, A. H. (1997). Environmental and economic aspects of wastewater reuse in Saudi Arabia. Water International, 22, 108–112.
Hussain, S. T., Hajira, T., Saleem, M., & Afzal, M. (2000). Trace metals pollution assessment of sediments and liquid wastes from textile industries of Pakistan. Journal of Trace and Microprobe Techniques, 18(1), 99–109.
Lee, G. C., Chon, T. H., & Jung, C. M. (2001). Heavy metal contamination in the vicinity of the Daduk Au–Ag–Pb–Zn mine in Korea. Applied Geochemistry, 16, 1377–1386.
Mahfuz, M. A., Ahmad, J. U., Sultana, M. S., Rahman, M. M., Goni, M. A., & Rahman, M. S. (2004). Status of physico-chemical properties of wastewater in Bangladesh: A cash studies in Dhalai Beel of DEPZ. Bangladesh Journal of Environmental Research, 2, 9–15.
Mastoi, M. G., Khuwar, Y. M., & Bosdar, B. R. (1997). Physicochemical analysis of industrial effluents from Kotri site area. Protocol NSMTCC-97 on Environ. Pollution, February 24–26, Islamabad, Pakistan.
More, V. S., John, B., Rao, S. S., Nair, U. B., & Laxman, S. R. (2001). Chromium removal and reduction in COD of tannery effluents by actinomycetes. Indian Journal of Environmental Health, 43, 108–113.
Mortula, M. M., & Rahman, M. S. (2002). Study on waste disposal at DEPZ. Bangladesh Environment (BAPA), 2, 807–817.
Mowka, E. (1988). Understanding factors that affect pH, and guide to alkalinity and pH control. Sea Scope Aquarium Systems, Vol. 5, Fall, 1988.
Nriagu, J. O. (1988). Production and uses of chromium. In J. Nriagu & E. Niebner (Eds.), Chromium in the natural and human environments (pp. 81–104). New York: Wiley.
Obiri, S., Dodoo, D. K., Armah, F. A., Essumang, D. K., & Cobbina, S. J. (2010). Evaluation of lead and mercury neurotoxic health risk by resident children in the Obuasi municipality, Ghana. Environmental Toxicology and Pharmacology, 29, 209–212.
Olajire, A. A., & Imeokparia, E. F. (2000). Water quality assessment of Osun River: Studies on inorganic nutrients. Environmental Monitoring and Assessment, 69, 17–28.
Robinson, T., McMullan, G., Marchant, P., & Nigam, R. (2001). Remediation of dyes in textile effluents: A critical review on current treatment technologies with a proposed alternative. Journal of Bioresource Technology, 77, 247–255.
Roychoudury, N. A., & Starke, F. M. (2006). Partitioning and mobility of trace metals in the Blesbokspruit: Impact assessment of dewatering of mine waters in the East Rand, South Africa. Applied Geochemistry, 21, 1044–1063.
Schröder, P., Navarro-Aviñó, J., Azaizeh, H., Goldhirsh, A. G., DiGregorio, S., Komives, T., et al. (2007). Using phytoremediation technologies to upgrade waste water treatment in Europe. Environmental Science and Pollution Research, 14(7), 490–497.
Tariq, R. S., Munir, H., Shah, N., Shaheen, A., Khalique, S., & Manzoor, M. J. (2005). Multivariate analysis of selected metals in tannery effluents and related soil. Journal of Hazardous Materials, 122, 17–22.
Tariq, S. R., Shah, M. H., Shaheen, N., Khalique, A., Manzoor, S., & Jaffar, M. (2006). Multivariate analysis of trace metal levels in tannery effluents in relation to soil and water: A case study from Peshawar, Pakistan. Journal of Environmental Management, 79(1), 20–29.
Acknowledgement
We are grateful to Dr. Sharrif E. Kabir, Vice Chancellor of Jahangirnagar University, professor of the Department of Chemistry, and Dr. Rabiul Islam, Dean of Faculty of Mathematical and Physical Sciences and professor of the Department of Chemistry, Jahangirnagar University, Bangladesh, for their help in the collection of samples and their digestion and preservation. We also thank the staffs of UNU-GIST Joint Research Programme in Korea for analysing trace metals using the ICP-MS method.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, G., Miah, M.A., Anawar, H.M. et al. Influence of multi-industrial activities on trace metal contamination: an approach towards surface water body in the vicinity of Dhaka Export Processing Zone (DEPZ). Environ Monit Assess 184, 4181–4190 (2012). https://doi.org/10.1007/s10661-011-2254-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2254-9