Abstract
The intensification of economic development, population growth, and energy consumption have led to the pollution of the groundwater in Gaobeidian City. This may exert great influence on human health. In the study, the chemical characteristics of the groundwater have been analyzed by Piper diagram and the possible health risks posed to the human health in Gaobeidian City has been evaluated using the model recommended by the Ministry of Environmental Protection of China. Furthermore, metal-species-weighted human health risk assessment is adopted for Cr6+, which is the major contributor to the carcinogenic health risk. The results show that groundwater is weakly alkaline, and the hydrochemical types is mainly Ca-HCO3. Non-carcinogenic pollutants mainly include arsenic (As), F−, and Fe. Carcinogenic pollutants mainly include Cr6+ and As. The carcinogenic risks of Cr6+ and As in the groundwater of all sampling sites greatly exceed their maximum acceptable limits, and contribution rate of Cr6+ is higher than that of As. The most important chemical species of Cr6+ is CrO42−, followed by CaCrO4(aq) and HCrO4−. The carcinogenic risk of above species is greater than the allowable limit. Among them, CrO4− exhibits the highest carcinogenic risk, and the maximum carcinogenic risk through ingestion to children and adults is 1.27E−03 and 5.98E−04, respectively. The economy in this area has developed rapidly because of its superior geographical location, but the groundwater pollution may have a great impact on the health of local residents, which must be paid attention to by local decision makers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater resources are indispensable for domestic drinking, irrigation, and industrial activities worldwide, especially for arid and semiarid regions (Li et al. 2014a, b; Hu et al. 2015; Su et al. 2020; Zhai et al. 2017; Wang et al. 2020a). Numerous problems related to groundwater, such as water shortage, inadequate supply, deterioration of water quality and severe water pollution have caused human health problems and threatened human life (Adimalla et al. 2019; He et al. 2020a). For example, heavy metal pollution and nitrate pollution will increase the incidence rate of human body (such as gastric cancer, esophageal cancer and skin disease, etc. (Adimalla et al. 2019; Adimalla and Qian 2019; He et al. 2020a). At the same time, they have also affected the sustainable development of the ecology, environment and the entire society (Li et al. 2018a, b). In particular, due to the toxicity, persistence, and bioaccumulation of some contaminants such as arsenic (As), chromium (Cr), manganese (Mn), fluoride, nitrate or aniline (Li et al. 2019a, b; Wu et al. 2019, 2020; Zhou et al. 2020b; Wang et al. 2020b), the health risks caused by such groundwater pollution has been paid significant attention worldwide (Cai et al. 2015; Chanpiwat et al. 2014; Chen et al. 2016; Olujimi et al. 2015; Yang et al. 2015; Adimalla and Qian 2019; Adimalla and Li 2019). Groundwater was the only source of domestic water in the study area. After the South-to-North Water Transfer Project (in 2016), some urban residents obtained water from the South-to-North Water Transfer Project. However, residents in suburbs, rural areas, and other areas still rely on groundwater for domestic water sources. If the groundwater is polluted, it significantly harms the human health.
Gaobeidian City has a superior geographical position in the economic circle around the Capital of Beijing, Municipality of Tianjin, and Province of Hebei, and is an important industrial city. A variety of metallurgy, machinery, automobile manufacturing, and other large manufacturing industries have developed rapidly in the study area. Moreover, there are 350 luggage and bag enterprises, more than 10,000 individual processing enterprises and over 4000 employees in this area. Gaobeidian is considered as the largest production and marketing base of luggage and bags. Furthermore, the rapid growth of urban population has intensified the exploitation of underground water in recent years. The rapid population growth and fast economic development not only increase fresh water demand but also bring problems associated with water pollution. The industrial and domestic wastewater in the area may induce a variety of pollutants, such as Cr, As, F−, Cd, Cu, and Fe, which may cause certain harm to the human health. Among them, heavy metal pollution is concerned by scholars. Heavy metals are rich and difficult to degrade in the environment (Li et al. 2015; He and Li 2020). Metals can interact strongly with proteins, making them inactive. If the human body comes in contact with heavy metals present in groundwater beyond the limit that human body can tolerate, it causes human poisoning, threatens the health of people in the area, and even causes great harm to human body (Li and Wu 2019a, b). For example, Cr6+ is an extremely inhalable poison. For humans, skin contact may induce allergic symptoms and might cause genetic defects in severe cases. Inhalation may increase the risk of cancer (Ghosh et al. 2012; Zhang et al. 2017). As for the environment, there might be a long-term latent danger. Therefore, exposure to high Cr6+ poses adverse effects on human health, causing a great public health and environmental concern on underground water safety.
Total concentrations of heavy metal contaminants in groundwater are often used for human health risk assessments. In recent years, some studies have found that total concentrations are not sufficient to assess the potential impacts of contaminated sites (Reis et al. 2014; Mashal et al. 2015; Yang et al 2015; Gu et al. 2016). The toxicity, mobility, and bioavailability of metals in the environment depend to a large extent on the metal species and their state of metal binding (Song and Ma 2017; Kelly et al. 2002; Ruby et al. 1996). Moreover, the human digestive system cannot fully absorb the pollutants present in the conjugate (Yang et al. 2015). Many researchers reported that the chemical species and bioavailability of heavy metals in the soil has become an important method for assessing the risks due to heavy metals in the soil (Liu et al. 2017; Lei et al. 2007; Guo et al. 2013; Dai et al. 2018). Therefore, this study evaluated the health risk due to contaminants in groundwater in the study area, and deeply analyzed the sources and distribution characteristics of pollution factors that bring great harm. In particular, the chemical species of the main pollutants were analyzed in order to provide scientific basis for groundwater pollution control.
The main objectives of this study are as follows: (1) to analyze the ions present in groundwater through water chemistry, and understand the current groundwater chemical characteristics of Gaobeidian City; (2) to assess the pollutants in groundwater through intake and skin contact according to the groundwater pollution health risk assessment study. Study of risks related to exposure to carcinogenic and non-carcinogenic materials, providing importance of chemical speciation assessment for the development of management or treatment and remediation programs for risks due to contaminated groundwater.
Study Area
Location and Climate
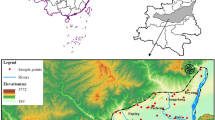
Gaobeidian City is located in the central part of Hebei Province, southwest of Beijing, China. The geographical coordinates of the city are: 115°47′–116°12′ E, 39°5′–39°23′ N. Gaobeidian is under the jurisdiction of Baoding City, with a total area of 672 square kilometers. The location of the study area is shown in Fig. 1. Gaobeidian City is a region with temperate continental monsoon climate with an average annual temperature of 12.4 °C, an average precipitation of 600 mm, and a frost-free period of 183 days. It is cold and dry in winter, dry and windy in spring, and hot and rainy in summer. Every year from June to August is the flood season for Gaobeidian City. The annual precipitation in summer is 332.0 mm, accounting for about 67% of the entire year. The average annual evaporation is 1315.9 mm (Shi 2016). The study area is located in river alluvial plain area to the east of the Taihang Mountains. The terrain gradually decreases from northwest to southeast. The terrain is flat, and the elevation is about 10–30 m, with a surface slope less than 1%. The upper part of the alluvial plain of the river consists of inclined land, alluvial lowland and highland, river floodplain (Fig. 1).
Hydrogeology
The study area belongs to the Daqing River system of the Haihe River Basin. Daqing River water system originates from the Taihang Mountains, with many tributaries and short-flowing sources. It is mainly divided into two tributaries corresponding to the northward and the southward flowing systems. The main tributary flowing through Gaobeidian City is the Baigou River, which is the main flood channel of Daqing River, and located in the middle reaches of Daqing River. Baigou River has water all year round. The river channel is a compound river channel and main channel is about 200 m wide, and river bottom slope is 1/4000. The annual runoff distribution of Baigou River is basically consistent with precipitation, and runoff mainly changes with the amount of precipitation.
The study area is located in the Daqing River basin and its aquifer can be divided into four layers according to the lithology and occurrence conditions. As shown in Fig. 2, the first and second aquifers are shallow groundwater, and they are closely related to each other; the third and fourth aquifers are deep groundwater. The lithology of the first and second aquifer is mainly fine sand and medium fine sand, with 20–40 m in thickness. The bottom boundary is about 150 m deep with 6–8 m in buried depth. The lithology of the third aquifer is medium sand and fine sand, 60–80 m in thickness. The bottom boundary is 300–350 m deep, and the buried depth is 8–10 m. The bottom boundary of the fourth aquifer is 500–600 m deep, the lithology is medium fine sand and fine sand, and the water-richness is poor.
The water sources in the study area are all from groundwater sources, mainly used for farmland irrigation, residential life, urban public water, and industrial water. Water consumption for farmland irrigation is the largest, accounting for 4/5.
Materials and Methods
Sample and Sample Description
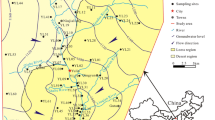
In this study, a total of 18 shallow water samples (80–120 m below the ground surface) and 8 deep groundwater samples (150–350 m below the ground surface) were obtained. The sampling sites are shown in Fig. 1. The water samples were mainly taken from monitoring wells and pumped wells during the 2019 monsoon season (July–September). Water samples were taken, sealed, and transported in strict accordance with the national technical regulations (Ministry of Environmental Protection of P.R. China, 2004). Detailed test methods, instrument specifications and detection limits of each indicator are shown in Table 1. The pH and electrical conductivity (EC) of the groundwater samples were measured at the site, while other indicators were measured in the laboratory. Before collection, all the sampling containers are required to be rinsed and washed according to the standard. Samples for dissolved oxygen need to be sampled with a special dissolved oxygen bottle, and the others were sampled with polyethylene bottles. Samples for K+, Na+, Fe, Mn2+, Pb, and Cd all need to add HNO3 (10 mL in 1 L of water), for As and NH4+-N need to add H2SO4 to make pH less than 2, and for Cr6 + needs to be added with NaOH to make pH between 8 and 9. After collection, all samples were labeled and transported immediately to the laboratory and analyzed in the laboratory of the Hebei GEO University, using standard methods recommended by technical specifications for environmental monitoring of groundwater (Ministry of Environmental Protection of P.R. China 2004). Precision and accuracy of the data have been examined by calculating the charge balance errors (within ± 5%) and the recovery ratio (within ± 10%) (Li et al. 2016b). The charge balance error percentage (%CBE) was calculated to determine the accuracy of each sample as per Eq. (1), and the results are shown in Fig. 3.
Health Risk Assessment
Various industries such as metallurgy, automobile manufacturing, leather and luggage manufacturing, and electroplating industry are present in Gaobeidian city. In recent years, the agricultural scale of Gaobeidian City has been increasing year by year, the demand for agricultural water has increased sharply, and the use of pesticides and fertilizers has changed the distribution of groundwater ions. Presence of F−, NO2−, Mn, Fe, Pb, Cr6+, Cd, NH4+, and As in groundwater was evaluated by using the model recommended by the Ministry of Environmental Protection of China for non-carcinogenic and carcinogenic health risks. Both children and adults may get exposed to the contaminated site for a long time. Owing to different factors such as body weight and daily water intake, the exposed population was divided into three groups: children, female adults, and male adults. The lifetime cancer risks of contaminants were assessed based on exposure during children and adults. Notably, the non-carcinogenic hazard effects of contaminants were generally assessed based on children exposure.
Health risks due to contaminants in groundwater were calculated and the risks of oral ingestion and skin exposure to groundwater were assessed. The models recommended by the Ministry of Environmental Protection of Environmental Protection of the P.R. China are based on United Stated Environmental Protection Agency (USEPA) models. However, the Chinese models assign unique parameters to reflect specific conditions in China (Wu and Sun 2016).
The average daily dose for oral and skin contact is as follows (Ji et al. 2020; Li et al. 2017):
where intakeoral is the average daily dose of oral intake route, (mg (kg d)−1) and C is the concentration of pollutants in groundwater (mg L−1), which depends on laboratory analysis. DA and SA are defined as the exposure dose (mg cm−2) and skin contact area (cm2) of each event, respectively. The values of three types of sensitive groups through the intake route and skin contact parameters are listed in Table 2.
Non-carcinogenic risk of oral intake:
HQoral and RfDoral are non-carcinogenic hazard quotients and reference doses through oral intake route. In this study, RfD value of F−, NO2−, Mn, Fe, Pb, Cr6+, Cd, ammonia nitrogen (in terms of N), and As were found to be 0.04, 0.1, 0.14, 0.3, 0.0014, 0.0003, 0.003, 0.97, and 0.0003 mg (kg d)−1, respectively (Ministry of Environmental Protection of the P.R. China 2014).
The non-cancer risk is expressed by skin contact with groundwater as follows:
where HQdermal and RfDdermal represent the risk quotient and reference dose (mg (kg d)−1) of non-carcinogenic risk through skin contact pathway, respectively. RfDdermal is derived from RfDoral, which is a gastrointestinal absorption factor, except for Cr6+ having an ABSgi value of 0.025 and an ABSgi value of 1.
Non-carcinogenic risk of oral intake and skin contact absorption is calculated as the total risk (Eqs. 9 and 10)
where HI is a risk index. The HI refers to the sum of more than one HQ for multiple substances and two exposure pathways. HQ and HI values less than 1 are considered safe for human health. In contrast, when these values exceed 1, residents may face non-carcinogenic risks (Ministry of Environmental Protection of the P.R. China 2014).
Carcinogenic effects on human health risks were measured by carcinogenic factors. The risk of carcinogenesis in the periphery of residents has a certain negative impact on the health of residents, which may cause common human tumors such as lung cancer and digestive tract cancer. The main carcinogenic factors found in the study area were Cr6+ and As. The carcinogenic risk of As and Cr6+ through drinking water intake and skin contact is calculated as follows:
where CR indicates the risk of cancer. According to the regulations of the Ministry of Environmental Protection of China, the acceptable limit is 10−6. SF is the slope factor of carcinogenic pollutants. The SForal values of As and Cr6+ were set to 1.5 and 0.5 (mg (kg d)−1), respectively (Ministry of Environmental Protection of the P.R. China 2014).
Metal-Species-Weighted Human Health Risk Assessment
Quantitative and qualitative assessment of human health risks caused by metals in groundwater was carried out using metal-species-weighted human health risk assessment (MSRA) (Zhang et al. 2017). MSRA was proposed to quantify and distinguish the contribution of metal species risk on human in site-specific groundwater. It could also compare risk effects of exposure concentrations for metal species with the level of total metal concentration (Ogunbanjo et al. 2016). Visual MINTEQ, a geochemical software code for speciation of metals, was used to understand the concentration and activity of metals species. The concentration and activity of each metal species were simulated by using the Visual MINTEQ tool. Chemical equilibrium model is an important tool to analyze metal morphology of groundwater. This model can simulate the effect of many factors on metal morphology in groundwater environment (Tipping 1994; Mosley et al. 2015; Stefansson et al. 2015). Health risks of Cr6+ morphologies in groundwater were assessed by modifying the average daily dose from exposure pathways.
Visual MINTEQ3.1 system was used to obtain the concentration and activity of metal species of 26 groundwater samples in the study area. Inputs to Visual MINTEQ included measured groundwater pH, temperature (25 °C), and cations of Na+, K+, Ca2+, Mg2+, and measured concentrations of target metals (mg L−1). Anions such as Cl−, HCO3−, CO32−, and SO42− and alkalinity need to be added to the model.
Exposure Assessment
The average daily dose was used for calculating the risk of human exposure. The main ways of human exposure are oral intake, skin contact, and exposure to the air environment (Ministry of Environmental Protection of the P.R. China 2014). For the region where metal is mainly present in groundwater, the source of exposure is mainly skin contact and oral intake. There is a protective layer on the surface of the skin, which has a small amount of water and a small conversion of inhalation. The risk of skin contact health is much less than the amount of oral intake (Nguyen et al. 2009) and the oral intake is calculated as follows (Li et al. 2016a; Zhang et al. 2018; He and Wu 2019; He et al. 2019):
where intakei,j is the modified average daily dose from ingestion of the j speciation in i heavy metal (mg kg−1 d−1), and intake`i,j is the average daily dose from ingestion of the j speciation in heavy metal (mg kg−1 d−1), CMi is the concentration of i heavy metal (mg L−1).
Organism intake degree of metal determines human health risk. The intake dose of heavy metals was inconsistent with the amount of pollutants actually absorbed, which could affect human health (Cai et al. 2015). A certain correlation exists between the total concentration of pollutants in groundwater and the extent to which these pollutants are absorbed by the body. Therefore, it is necessary to rectify the intake of substances by human. The average daily dose of orally ingested metal was modified to more accurately assess health risks and concentration correction based on metal weight.
where M′i,j is the concentration of j speciation (mg L−1), Ci,j is the concentration of j speciation in i heavy metal in groundwater (mol L−1), and Ai,j is the activity of j speciation in i heavy metal. Mi is the relative atomic mass of the metal (g mol−1), ni,j is the number of target metal from the j speciation in i heavy metal, wi,j is the weight value of the j speciation in i heavy metal, Mi,j is the modified concentration of j speciation in i heavy metal in groundwater (mg L−1), and ri,j is the weight assignment of the j speciation in i heavy metal. When the target metal is Cr6+, i takes a value of 1, and j represents a different species morphology of Cr6+, j = 1, 2, …, n.
Risk characterization
where CRi,j represents the modified cancer risk of the j species in i heavy metal, CR′i,j is the cancer risk of j speciation in i heavy metal, CRitotal is the total modified cancer risk of i heavy metal, CR′itotal is the total cancer risk of i heavy metal, and CR is the cancer risk of i heavy metal.
Non-carcinogenic calculations (He et al. 2020b):
where RfDi is reference dose of i heavy metal, and the value for Cr6+ is 0.003 (mg kg−1 d−1). HQi,j is modified hazard quotient of the j speciation in i heavy metal, HI the total modified non-cancer risk of i heavy metal, HQʹi,j is revised hazard quotient of the j speciation in heavy metal, HIʹi is the total revised non-cancer risk of i heavy metal, and HQi is hazard quotient of i heavy metal.
Results and Discussion
Hydrochemical Parameters
The range of pH of shallow groundwater is from 7.64 to 7.99, with an average value of 7.77, thus shallow groundwater is weakly alkaline in Gaobeidian City. The salinity ranges from 373.546 to 1427.84 mg L−1, with a mean value of 702.806 mg L−1, which is low salinity water. According to TDS the types of shallow groundwater are divided into fresh water and brackish water, of which fresh water and brackish water account for 6.25 and 93.75%, respectively. The total hardness of shallow groundwater varies from 160.0 to 749.8 mg L−1 with a mean value of 321.8 mg L−1. The contents of Ca and Mg ions in three samples are extremely high, and the total hardness exceeds 450 mg L−1. According to the standard for groundwater quality (Ministry of Health of the P.R. China 2006), the concentration of NO2− in 2 samples (0.074 and 0.065 mg L−1) exceeds the standard, the concentration of F− in 3 samples exceeds the standard (level III), and the concentration of Fe in 9 samples (0.347–10.68 mg L−1) exceed drinking water standard. Components of the shallow groundwater mainly include HCO3−, Na+, Ca2+, Mg2+, followed by SO42− and Cl−. The main anion is HCO3−, the range of variation is 247.7–1024.1 mg L−1, and the main cations are Ca2+ and Mg2+ followed by Na+.
The range of pH of deep groundwater is from 7.78 to 8.04, with an average value of 7.88, thus deep groundwater is weakly alkaline. The TDS ranges from 338.826 to 382.195 mg L−1, with a mean of 366.307 mg L−1. According to TDS, the type of deep groundwater in this area is fresh water. One sample of Fe ion in groundwater exceeds the groundwater standard, 0.445 mg L−1. The total hardness varies from 106.2 to 184.4 mg L−1, with a mean of 144.4 mg L−1. The components of deep groundwater are mainly HCO3−, Na+, Ca2+, and Mg2+. The main anion is HCO3−, and the main cations are Ca2+, Na+, followed by Mg2+.
The chemical composition of shallow groundwater in the Gaobeidian area is affected by human activities, producing and discharging more Cl− and SO42− into groundwater, which leads to the change in its chemical composition. More metallurgical industries in the region produce F−, Fe ions (Fe2+ and Fe3+), and Cr6+ and As into the groundwater and cause contamination, which exert a negative impact on the health status of residents in the area.
Types of Groundwater Based on Hydrochemical Characteristics
In general, the Piper diagram is a graphical method for analyzing the distribution characteristics of chemical ions present in water (He and Li 2019; Piper 1944). It can be used to visually reflect the general chemical characteristics and water chemistry types of water samples (Li et al. 2016b). Groundwater chemical type in this area has obvious horizontal zoning from west to east (Fig. 4). In the vicinity of the inclined land (Figs. 1 and 2), the aquifer particles are coarse. The groundwater in this area is abundant, and the type of water chemistry is relatively simple, which is mainly bicarbonate. Moreover, to the east of Gaobeidian, with the gradual changes in groundwater runoff conditions and the long-term effects of climate and hydrogeochemical effects and human activities, shallow groundwater hydrochemistry is changing. From west to east, the proportion of Ca2+ decreases and that of Na+ ions increase gradually, and the distribution of salinity has a certain regionality.
Hydrochemical type, indicated by Fig. 5, transits from Ca·Mg-HCO3 to Mg·Ca-HCO3, Na·Mg-HCO3 and then to Na-HCO3 type along the flow path, from northwest to southeast. The type of water chemistry that appears in the local part is of the bicarbonate-chloride type (Mg·Ca-HCO3·Cl), which may be due to the excessive exploitation and utilization of shallow groundwater by humans, the increase of pollutants emissions from industrial and agricultural wastewater discharges, and the unreasonable discharge of urban domestic sewage. Chloride ions and sulfate ions in groundwater increase the chemical characteristics of shallow groundwater (Li et al. 2016a). The impact of human activities on water sources close to the surface is more obvious. The surface water samples taken from the types are Na·Ca–Cl and Na·Ca -SO4·Cl in Pingjing Town. Deep groundwater, similar to shallow groundwater, has obvious water chemistry (Fig. 6). The deep groundwater in the study area is distributed from northwest to southeast: Ca·Mg–HCO3, Ca·Na–HCO3, Na·Ca–HCO3.
Health Risk Assessment
Children, female adults, and male adults in the study area are exposed to F−, NO2−, Mn, Fe, Pb, Cr6+, Cd, ammonia nitrogen (in N), and As in groundwater through skin contact or oral administration. Non-carcinogenic risks are listed in Tables 3 and 4. The results indicated that irrespective of the population being the children, adult female adults, or male adults, the contact quotient of NO2−, Mn, Pb, Cr6+, Cd, NH4+-N, and As in groundwater through two types of exposure routes is less than 1. Thus the impact on human health is small.
Calculation results revealed that there are 9 water samples containing F− with non-carcinogenic risk, and all from shallow groundwater. F− enters the soil via adsorption and migration, leaching into groundwater and causing its contamination, thus affecting water quality and causing harmful effects on human health. For groups affected by non-cancer risk, children showed the greatest exposure to F− through skin contact and intake pathways, hazard quotient of children ranged from 0.2423 to 1.984; followed by non-cancer risk to female adults, the range was from 0.182 to 1.49; and the non-cancer risks to male adults were all less than 1. The non-carcinogenic risk to male adults is relatively small.
The hazard quotient of Fe2+ ions in groundwater in S20 exceeds 1, which can lead to non-cancer risk. The concentration of Fe ions (including Fe2+, Fe3+) was 10.68 mg L−1, and the non-cancer risk to children, female adults, and male adults was 2.380, 1.378, 1.123, respectively. The concentration of Fe ions in shallow groundwater in S23 was 5.767 mg L−1. Children were affected by non-carcinogenesis, and the hazard quotient was 1.285. Moreover, in case of the non-carcinogenic risk caused by F−, Fe ions, As, and NO2− in groundwater, the risk of oral intake is much greater than the risk due to skin contact. The non-carcinogenic risk values for oral intake t children, female adults, and male adults were 378, 346, and 300 times of skin contact, respectively, accounting for 99.7% of non-carcinogenic risk.
The 26 water samples were considered for the total non-carcinogenic speciation based on all influencing factors (Tables 4, and 5, and Fig. 7). The non-carcinogenic risks to children, male adults, and female adults were from 0.83 to 4.44, 0.41 to 2.14, and 0.48 to 2.57, respectively. The HI of 20 samples for children, 13 for female adults, and 10 for male adults were more than 1, which indicates that HIchildren > HIfemale adults > HImale adults (Fig. 7). For the purposes of human health, there is a requirement for improved understanding of the main influencing factors and, where possible, the spatial distributions. The spatial distributions of the non-carcinogenic risks to children in shallow and deep groundwater are demonstrated in Fig. 8. The results show that samples with significant health risk in shallow groundwater are distributed in the west part of the study area which are along the Baigou River (Fig. 8a). However, the high risk in deep groundwater is distributed around Xiaoxinzhuang and Guangedian (Fig. 8b). Furthermore, exposure of residents to non-oncogenic pathways indicates that the non-carcinogenic risk by oral intake to children, female adults, and male adults is 1.21 × 102, 1.11 × 102, and 0.96 × 102 times that of non-carcinogenic skin contact, respectively. Furthermore, exposure of residents to non-oncogenic pathways indicates that the non-carcinogenic risk of oral intake by children, female adults, and male adults is 1.21 × 102, 1.11 × 102, and 0.96 × 102 times that of non-carcinogenic skin contact, respectively. They accounted for 98.5, 98.4, and 98.1% of the total non-cancer risk, respectively.
This indicates that the way in which residents of Gaobeidian City are exposed to non-carcinogenic risks is mainly drinking.
The total carcinogenic risk present in groundwater in study area is presented in Table 4. The statistics show that the total carcinogenic risk for children, female adults and male adults is from 2.108E−04 to 7.166E−04, 1.23E−04 to 4.17E−04 and 1.01E−04 to 3.44E−04, respectively, which in all the cases exceeds the allowable value of 102 times (Table 6). The carcinogenic risk is in the order of children > female adults > male adults (Fig. 9). Children are lighter and have higher exposures, thus it can be inferred that children in the same area are more likely to develop cancer than adults. The spatial distribution of the carcinogenic risk to children indicates that significant health risk of shallow groundwater is mainly distributed on both sides of the river (Fig. 10a). It may be concluded that the sources for high carcinogenic risk in shallow groundwater may be identified as discharge of the polluted surface water. What’s more, the areas with the highest shallow water risk are mainly distributed in the southwest of the study area, nearby the Baigou town. Baigou town is a luggage trading center in North China, with more than 300 luggage enterprises. Cr6+ produced by industries such as leather factories may cause some pollution to the shallow water in the study area, which has brought a great risk of carcinogenic to local residents, especially children. The carcinogenic risk in deep water is mainly distributed in the southern part of the study area, and the maximum risk is higher than that that in shallow water (Fig. 10b). The three samples S3, S8 and S9 have relatively high health risk values, which are caused by high concentration of Cr6+. Combined with the analysis of water chemistry types, the types at these three groundwater samples are all Na-HCO3, while the main water chemistry types of deep groundwater in the northern study area are Ca-HCO3 or Mg-HCO3. The regional water flow direction is from northwest to southeast, which shows that with the flow of water, the exchange between cations may occur, causing the increase of Na+ and the decrease of Ca2+ and Mg2+, which promotes the further dissolution of Cr6+. And the concentration of HCO3− increases, so the pH of the deep groundwater increases. The pH of S3 is the highest in the study area (8.04). High alkalinity environment of groundwater is conductive to the desorption of Cr6+, which may be one of the reasons for the higher concentration of Cr6+ in deep groundwater. In addition, the DO value in shallow water is lower than that in deep groundwater. This may be due to the higher pollution of organic matter in shallow water, which consumes some dissolved oxygen. The oxidizing environment is also conducive to the enrichment of Cr6 +, so this may be the other reason for the higher concentration of Cr6+ in the deep water.
Obviously, Cr6+ is the main factor for carcinogenic risk in both shallow and deep groundwater (Table 7). Cr6+ is an essential element for human health and also a significant health risk assessment index. As shown in Table 7, the health risks of Cr6+ to children, female adults and male adults range from 7.372E−05–6.266E−04, 4.31E−05–3.659E−04, 3.56E−05–3.027E−04, respectively. At the deep groundwater sampling S3, the Cr6+ concentration is the largest, which is 0.017 mg L−1. The presence of high levels of Cr6 + in groundwater is a significant problem in many parts of China (Liu et al. 2016; Li et al. 2018c, 2013) and many other studies surrounding the research area have also indicated the similar results (Zhou et al. 2020a). Many other studies on human health risk (Wongsasuluk et al. 2014) also indicate that the public health risks of non-carcinogenic pollutants to local residents are generally negligible, while the hazards by carcinogenic pollutants can usually be much higher and cannot be neglected. Effective way of decreasing the health risk is of great concern in the study area, particularly in the sampling sites where groundwater has high concentration of Cr6+ (Broadway et al. 2010).
Metal-Species-Weighted Human Health Risk Assessment
According to the above analysis, the Cr6+ present in groundwater in Gaobeidian City was found to be the most contributive to the carcinogenic risk. The carcinogenic risk values for children and adults were 7.372E−05 to 6.266E−04 and 3.56E−05 to 3.027E−04, respectively. The risk of carcinogenesis of Cr6+ greatly exceeded the maximum acceptable limit (10−6). The main pollution pathway of Cr6+ is oral intake. Children and adults are exposed to the toxic heavy metal Cr6+ through intake route.
Based on the above results, Cr6+ in groundwater was assessed by MSRA. Different forms of Cr6+ and their corresponding concentration and activity were calculated by using Visual MINEQ (VM) (Table 8). 10 species were found to simulate Cr6+: CaCrO4(aq), CrO42−, H2CrO4(aq), NaCrO4−, Cr2O72−, CrO3Cl−, CrO3SO42−, HCrO4−, KCr2O7−, and KCrO4−. Statistics and analysis of all forms of Cr6+ indicated CaCrO4(aq), CrO42−, and H2CrO4(aq) to be the dominant species.
The results of VM program indicate that CrO42− accounts for an average of 75% of all species, and accounts for the largest proportion of Cr6+ species. The variation range of CrO42− is 63.96–82.29%, which is privilege speciation of Cr6+ in groundwater. CaCrO4(aq) is a subspecies, accounting for 14.65–27.14%, with an average proportion of 21%. The speciation CrO4− and CaCrO4(aq) reach a total of 95% of Cr6+ speciation. The species HCrO4− has an average proportion of 3% and NaCrO4− accounts for about 1%. The concentration and activity values of Cr2O72−, CrO3Cl−, CrO3SO42−, HCrO4−, KCr2O7−, and KCrO4− are relatively small, and their aqueous components play a small role and are negligible. CaCrO4(aq), CrO4−, and HCrO4− are still the dominant species after modifying, and average daily dose of these species exposed to the human body are still high. Average daily dose of CaCrO4(aq) and HCrO4− were found to reduce. For children, reduction of average daily dose was greater than that of adults. Nonetheless, the modified average daily dose of children was still greater than that of adults. In contrast, the dominant speciation of CrO4− dose increased. Average daily dose of other species decreased, and modified average daily dose was very small, approaching zero.
For non-carcinogenic aspects (Table 9 and Fig. 10), the non-carcinogenic risks (adults and children) of different species of Cr6+ are in the following order: CrO42− > CaCrO4(aq) > HCrO4− > NaCrO4− > KCrO4− > Cr2O72− > H2CrO4(aq) > CrO3SO42− > KCr2O7− > CrO3Cl−. However, the non-carcinogenic risk value of all Cr6+ species is less than hazard quotient, indicating that different species of Cr6+ in groundwater do not cause large non-carcinogenic risks.
According to the Ministry of Environmental Protection of the P.R. of China, the acceptable limit for carcinogenic risk is 10−6. The carcinogenic risk results of different Cr6+ species related to groundwater are presented in Tables 10 and 11. The morphological carcinogenic risk value of Cr6+ in groundwater in study area is more than 10−6 for CrO42−, CaCrO4(aq), HCrO4−, and NaCrO4−. Children and adults in the study area exhibited the highest exposure to CrO42− and CaCrO4(aq) through oral intake. Carcinogenic risk is between 10−6 and 10−3. For the children and adults, the maximum carcinogenic risk of CrO42− is 09E−03 and 5.14E−05, respectively. The modified carcinogenic risk of CrO42− increases, and the maximum value for children and adults is 0.00127 and 0.000598, respectively. Carcinogenic risk of CaCrO4(aq) decreases, and HCrO4− is found to be a high-risk contaminant, with carcinogenic risk at 10−6 to 10−5. Moreover, when the modified risk carcinogenesis of HCrO4− was reduced (< 10−6), its harmful effect on human health is also reduced. In some areas of Gaobeidian City, modified carcinogenic risk for NaCrO4− is > 10−6, and the modified carcinogenic risk is lower than the allowable value (CRchildren = 3.85E−08, CR adult = 1.8147E−08). The datum indicates that different species of Cr6+ have higher carcinogenic hazards to children than to adults.
The modified carcinogenic risk of different species of Cr6+ was CrO42− (1.93E−04 for children, 9.02E−05 for adults) > CaCrO4(aq) (1.80E−05 for children, 8.34E−06 for adults) > HCrO4− (8.37E−07 for children, 3.89E−07 for adults) > NaCrO4− (1.66E−08 for children, 7.83E−09 for adults) > KCrO4− (4.5E−12 for children, 2.06E−12 for adults) > Cr2O72− (2.25E−19 for children, 7.15E−19 for adults) > H2CrO4(aq) (1.67E−21 for children, 7.74E−22 for adults) > CrO3SO42− (5.23E−24 for children, 2.44E−24 for adults) > CrO3Cl− (2.14E−26 for children, 1.00E−26 for adults) > KCr2O7− (3.82E−27 for children, 1.59E−27 for adults). The carcinogenic risk of Cr2O72−, CrO3Cl−, CrO3SO42−, KCr2O7−, HCrO4−, and KCrO4− was close to zero, thus they could be ignored (Fig. 6). This also indicates that the carcinogenic risk of Cr6+ is derived from the species HCrO4−, CaCrO4(aq), and CrO42−.
Although groundwater has been widely used for irrigation, drinking, and economic development, human health risks still exist, especially carcinogenic risks, which can be clearly seen from the results above. However, there might be uncertainties in the health risk assessment used in this report. Under the assumption that the individual indexes are average, such as AT, ED and IR, the calculation results are inevitably deviate. In addition, other toxic pollutants that may cause harm to the human body, which are not calculated in the health risk assessment, such as pesticides pesticide (Skevas 2020; Kiefer et al. 2019), will also cause deviations in the results. Nevertheless, the results of calculation can still lay a foundation to the decision makers to improve the current situation about groundwater.
Conclusions
Exposure to a contaminated environment can pose serious risk to human health based on considering the weight of the residents in the area, exposure time, and exposure route. A qualitative and quantitative evaluation of human health risks has been assessed in the study and the main conclusions are as follows:
The pH of groundwater is weakly alkaline. The TDS ranged from 338.826 to 1427.84 mg L−1, except for three water samples, and the values for others were less than 500 mg L−1. Total hardness was 144.6–749.8 mg L−1. Fluoride, iron ions, and nitrite of shallow groundwater in the study area exceeded the allowable values of groundwater quality standards, and the deep groundwater iron ions in one site exceeded the groundwater quality standards (III level). The shallow groundwater was polluted by iron ions, F−, Cr6+, and arsenic (As) to some extent.
Non-carcinogenicity is mainly caused by As, F−, and Fe ions (including Fe2+, Fe3+), and the health risks due to oral intake are higher than that due to skin contact. Oral intake exposure to risk can reach 98.5% of the total risk value. Cr6+ and As are the main pollutants causing cancer risk, and their presence in groundwater at all groundwater samples is carcinogenic, and the order is CRchildren > CRfemale > CRmale. The carcinogenic risk value of As and Cr6+ contaminating groundwater through the intake route far exceeded the allowable limit value, which may cause carcinogenic damage to human health.
The speciation of Cr6+ in groundwater was modified in contact with human body. This difference is small when the concentration of Cr6+ in groundwater was low, and strengthened when the content of Cr6+ is high. The dominant speciation of Cr6+ in groundwater was CrO42−, followed by CaCrO4(aq) and HCrO4−. The health risk distribution of different species of Cr6+ was in the following order: CrO42− > CaCrO4(aq) > HCrO4− > NaCrO4− > KCrO4− > Cr2O72− > H2CrO4(aq) > CrO3SO42− > KCr2O7− > CrO3Cl−. All of its non-carcinogenic hazards were less than 1, and the carcinogenic risk values of CrO42−, CaCrO4(aq), and HCrO4− were greater than the allowable value of 10−6.
References
Adimalla N, Li P (2019) Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Hum Ecol Risk Assess 25(1–2):81–103. https://doi.org/10.1080/10807039.2018.1480353
Adimalla N, Qian H (2019) Spatial distribution and health risk assessment of fluoride contamination in groundwater of Telangana: a state-of-the-art. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125548
Adimalla N, Qian H, Li P (2019) Entropy water quality index and probabilistic health risk assessment from geochemistry of groundwaters in hard rock terrain of Nanganur County, South India. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125544
Broadway A, Cave M, Wragg J, Fordyce F, Bewley R, Graham M (2010) Determination of the bioaccessibility of chromium in Glasgow soil and the implications for human health risk assessment. Sci Total Environ 409(2):267–277. https://doi.org/10.1016/j.scitotenv.2010.09.007
Cai L, Xu Z, Qi J, Feng Z, Xiang T (2015) Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 127:127–135. https://doi.org/10.1016/j.chemosphere.2015.01.027
Chanpiwat P, Lee B, Kim K, Sthiannopkao S (2014) Human health risk assessment for ingestion exposure to groundwater contaminated by naturally occurring mixtures of toxic heavy metals in the Lao PDR. Environ Monit Assess 186:4905–4923. https://doi.org/10.1007/s10661-014-3747-0
Chen H, Teng Y, Lu S, Wang Y, Wu J, Wang J (2016) Source apportionment and health risk assessment of trace metals in surface soils of Beijing metropolitan, China. Chemosphere 144:1002. https://doi.org/10.1016/j.chemosphere.2015.09.081
Dai S, Li H, Yang Z, Dai M, Shi L (2018) Effects of biochar amendments on speciation and bioavailability of heavy metals in coal-mine-contaminated soil. Hum Ecol Risk Assess 24(7):1887–1900. https://doi.org/10.1080/10807039.2018.1429250
Ghosh S, Basu A, Saha R, Ghosh A, Mukherjee K, Saha B (2012) Micellar catalysis on picolinic acid promoted hexavalent chromium oxidation of glycerol. J Coord Chem 65(7):1158–1177. https://doi.org/10.1080/00958972.2012.669035
Gu Y, Huang H, Lin Q (2016) Concentrations and human health implications of heavy metals in wild aquatic organisms captured from the core area of daya bay's fishery resource reserve, south china sea. Environ Toxicol Pharmacol 45:90–94. https://doi.org/10.1016/j.etap.2016.05.022
Guo X, Wei Z, Penn C, Xu T, Wu Q (2013) Effect of soil washing and liming on bioavailability of heavy metals in acid contaminated soil. Soil Sci Soc Am J 77(2):432. https://doi.org/10.2136/sssaj2011.0371
He S, Li P (2019) A MATLAB based graphical user interface (GUI) for quickly producing widely used hydrogeochemical diagrams. Geochemistry. https://doi.org/10.1016/j.Chemer.2019:125550
He X, Li P (2020) Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): occurrence, sources and health risks. Expo Health. https://doi.org/10.1007/s12403-020-00344-x
He S, Wu J (2019) Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, Northwest China. Expo Health 11(2):125–137. https://doi.org/10.1007/s12403-018-0289-7
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess 25(1–2):32–51. https://doi.org/10.1080/10807039.2018.1531693
He X, Li P, Ji Y, Wang Y, Su Z, Elumalai V (2020a) Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: occurrence, distribution and management. Expo Health. https://doi.org/10.1007/s12403-020-00347-8
He X, Li P, Wu J, Wei M, Ren X, Wang D (2020b) Poor groundwater quality and high potential health risks in the Datong Basin, northern China: research from published data. Environ Geochem Health. https://doi.org/10.1007/s10653-020-00520-7
Hu Y, Wang X, Dong Z, Liu G (2015) Determination of heavy metals in the groundwater of the Huaibei plain, China, to characterize potential effects on human health. Anal Lett 48:349–359. https://doi.org/10.1080/00032719.2014.940530
Ji Y, Wu J, Wang Y, Elumalai V, Subramani T (2020) Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain, China. Expo Health. https://doi.org/10.1007/s12403-020-00357-6
Kelley M, Brauning S, Schoof R, Ruby M (2002) Assessing oral bioavailability of metals in soil. Battelle Press, Columbus
Kiefer K, Muller A, Singer H, Hollender J (2019) New relevant pesticide transformation products in groundwater detected using target and suspect screening for agricultural and urban micropollutants with LC-HRMS. Water Res 165:114972. https://doi.org/10.1016/j.watres.2019.114972
Lei M, Liao B, Qin P (2007) Evaluation of bioavailability of chemical forms of heavy metals in soil. J Eco-Environ 16(5):1551–1556. https://doi.org/10.3969/j.issn.1674-5906.2007.05.043
Li P, Wu J (2019a) Sustainable living with risks: meeting the challenges. Hum Ecol Risk Assess 25(1–2):1–10. https://doi.org/10.1080/10807039.2019.1584030
Li P, Wu J (2019b) Drinking water quality and public health. Expo Health 11(2):73–79. https://doi.org/10.1007/s12403-019-00299-8
Li H, Qian X, Hu W, Wang Y, Gao H (2013) Chemical speciation and human health risk of trace metals in urban street dusts from a metropolitan city, Nanjing, SE China. Sci Total Environ 456–457(7):212–221. https://doi.org/10.1016/j.scitotenv.2013.03.094
Li P, Qian H, Howard KWF, Wu J, Lyu X (2014a) Anthropogenic pollution and variability of manganese in alluvial sediments of the Yellow River, Ningxia, northwest China. Environ Monit Assess 186:1385–1398. https://doi.org/10.1007/s10661-013-3461-3
Li P, Wu J, Qian H, Lyu X, Liu H (2014b) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36:693–712. https://doi.org/10.1007/s10653-013-9590-3
Li P, Qian H, Howard KWF, Wu J (2015) Heavy metal contamination of Yellow River alluvial sediments, northwest China. Environ Earth Sci 73(7):3403–3415. https://doi.org/10.1007/s12665-014-3628-4
Li P, Li X, Meng X, Li M, Zhang Y (2016a) Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Expo Health 8(3):361–379. https://doi.org/10.1007/s12403-016-0205-y
Li P, Wu J, Qian H (2016b) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9(1):15. https://doi.org/10.1007/s12517-015-2059-1
Li P, Feng W, Xue C, Tian R, Wang S (2017) Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake tourist area, northwest China. Expo Health 9(3):213–225. https://doi.org/10.1007/s12403-016-0237-3
Li H, Yu X, Zhang W (2018a) Risk assessment of groundwater organic pollution using hazard, intrinsic vulnerability, and groundwater value, Suzhou City in China. Expo Health 10:99–115. https://doi.org/10.1007/s12403-017-0248-8
Li P, He S, He X, Tian R (2018b) Seasonal hydrochemical characterization and groundwater quality delineation based on matter element extension analysis in a paper wastewater irrigation area, Northwest China. Expo Health 10(4):241–258. https://doi.org/10.1007/s12403-17-0258-6
Li P, He S, Yang N, Xiang G (2018c) Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: implications to sustainable groundwater quality management on the Loess Plateau. Environ Earth Sci 77(23):775. https://doi.org/10.1007/s12665-2018-7968-3
Li P, He X, Li Y, Xiang G (2019a) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess plateau: a case study of Tongchuan, Northwest China. Expo Health 11(2):95–107. https://doi.org/10.1007/s12403-018-0278-x
Li P, He X, Guo W (2019b) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an City on the Loess Plateau of northwest China. Hum Ecol Risk Assess 25(1–2):11–31. https://doi.org/10.1080/10807039.2018.1553612
Liu G, Wang J, Zhang E, Hou J, Liu X (2016) Heavy metal speciation and risk assessment in dry land and paddy soils near mining areas at southern China. Environ Sci Pollut R 23(9):8709–8720. https://doi.org/10.1007/s11356-016-6114-6
Liu B, Ai S, Zhang W, Huang D, Zhang Y (2017) Assessment of the bioavailability, bioaccessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW China. Sci Total Environ 609(17):822–829. https://doi.org/10.1016/j.scitotenv.2017.07.215
Mashal K, Salahat M, Al-Qinna M, Al-Degs Y (2015) Spatial distribution of cadmium concentrations in street dust in an arid environment. Arab J Geosci 8(5):3171–3182. https://doi.org/10.1007/s12517-014-1367-1
Ministry of Environmental Protection of P.R. China (2004) Technical specifications for environmental monitoring of groundwater (HJ/T 164–2004). National standards for environmental protection of China. (in Chinese)
Ministry of Environmental Protection of the P.R. China (2014) Technical guidelines for risk assessment of contaminated sites, (HJ 25.3–2014). China Environmental Science Press, Beijing (in Chinese)
Ministry of Health of the P.R. China, Standardization Administration of the P.R. China (2006) Standards for drinking water quality (GB 5749–2006). China Standard Press, Beijing (in Chinese)
Mosley L, Daly R, Palmer D, Yeates P, Dallimore C, Biswas T, Simpson S (2015) Predictive modelling of pH and dissolved metal concentrations and speciation following mixing of acid drainage with river water. Appl Geochem 59:1–10. https://doi.org/10.1016/j.apgeochem.2015.03.006
Nguyen V, Bang S, Viet P, Kim K (2009) Contamination of groundwater and risk assessment for arsenic exposure in Ha Nam Province. Vietnam Environ Int 35(3):472. https://doi.org/10.1016/j.envint.2008.07.014
Ogunbanjo O, Onawumi O, Gbadamosi M, Ogunlana A, Anselm O (2016) Chemical speciation of some heavy metals and human health risk assessment in soil around two municipal dumpsites in Sagamu, Ogun state, Nigeria. Chem Spec Bioavailab 28(1–4):142–151. https://doi.org/10.1080/09542299.2016.1203267
Olujimi O, Oputu O, Fatoki O, Opatoyinbo O, Aroyewun O, Baruani J (2015) Heavy metals speciation and human health risk assessment at an illegal gold mining site in Igun, Osun State. J Health Pollut 5(8):19–32. https://doi.org/10.5696/i2156-9614-5-8.19
Piper A (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25(1):27–39. https://doi.org/10.1016/0197-0186(84)90023-8
Ruby M, Davis A, Schoof R, Eberle S, Sellstone C (1996) Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ Sci Technol 30(2):422–430. https://doi.org/10.1021/es950057z
Reis A, Patinha C, Noack Y, Robert S, Dias A, Silva E (2014) Assessing the human health risk for aluminium, zinc and lead in outdoor dusts collected in recreational sites used by children at an industrial area in the western part of the Bassin Minier de Provence, France. J Afr Earth Sci 99(278):724–734. https://doi.org/10.1016/j.jafrearsci.2013.08.001
Song N, Ma Y (2017) The toxicity of HCrO4- and CrO42- to barley root elongation in solution culture: pH effect and modelling. Chemosphere 171:537–543. https://doi.org/10.1016/j.chemosphere.2016.12.050
Shi Y (2016) Study on the rational allocation of various water resources in Gaobeidian City, Jiangxi. Build Mater 7:127–128 (in Chinese)
Stefansson A, Gunnarsson I, Kaasalainen H, Arnorsson S (2015) Chromium geochemistry and speciation in natural waters, Iceland. Appl Geochem 62:200–206. https://doi.org/10.1016/j.apgeochem.2014.07.007
Skevas T (2020) Evaluating alternative policies to reduce pesticide groundwater pollution in Dutch arable farming. J Environ Plan Manag 63(4):733–750. https://doi.org/10.1080/09640568.2019.1606618
Su Z, Wu J, He X, Elumalai V (2020) Temporal changes of groundwater quality within the groundwater depression cone and prediction of confined groundwater salinity using Grey Markov model in Yinchuan area of northwest China. Expo Health. https://doi.org/10.1007/s12403-020-00355-8
Tipping E (1994) Whamc-a chemical equilibrium model and computer code for waters, sediments, and soils incorporating a discrete site/electrostatic model of ion-binding by humic substances. Comput Geosci 20(6):973–1023. https://doi.org/10.1016/0098-3004(94)90038-8
Wang D, Wu J, Wang Y, Ji Y (2020a) Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: analysis, assessment, and management. Expo Health 12:307–322. https://doi.org/10.1007/s12403-019-00314-y
Wang L, Huang T, Yang G, Lu C, Dong F, Li Y, Guan W (2020b) The precursor-guided hydrothermal synthesis of CuBi2O4/WO3 heterostructure with enhanced photoactivity under simulated solar light irradiation and mechanism insight. J Hazard Mater 381:120956. https://doi.org/10.1016/j.jhazmat.2019.120956
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36(1):169–182. https://doi.org/10.1007/s10653-013-9537-8
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, Mid-West China. Expo Health 8(3):311–329. https://doi.org/10.1007/s12403-015-0170-x
Wu J, Zhou H, He S, Zhang Y (2019) Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ Earth Sci 78(15):446. https://doi.org/10.1007/s12665-019-8471-1
Wu J, Zhang Y, Zhou H (2020) Groundwater chemistry and groundwater quality index incorporating health risk weighting in Dingbian County, Ordos basin of northwest China. Geochemistry. https://doi.org/10.1016/j.chemer.2020.125607
Yang K, Im J, Jeong S, Nam K (2015) Determination of human health risk incorporating experimentally derived site-specific bioaccessibility of arsenic at an old abandoned smelter site. Environ Res 137:78–84. https://doi.org/10.1016/j.envres.2014.11.019
Zhai Y, Zhao X, Teng Y, Xiao L, Zhang J (2017) Groundwater nitrate pollution and human health risk assessment by using HHRA model in an agricultural area, NE China. Ecotoxicol Environ Saf 137:130–142. https://doi.org/10.1016/j.ecoenv.2016.11.010
Zhang Y, Chen J, Shi W, Zhang D, Zhu T, Li X (2017) Establishing a human health risk assessment methodology for metal species and its application of Cr6+ in groundwater environments. Chemosphere 189:525–537. https://doi.org/10.1016/j.chemosphere.2017.08.175
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77(7):273. https://doi.org/10.1007/s12665-018-7456-9
Zhou Y, Li P, Chen M, Dong Z, Lu C (2020a) Groundwater quality for potable and irrigation uses and associated health risk in southern part of Gu’an County, North Chinas Plain. Environ Geochem Health. https://doi.org/10.1007/s10653-020-00553-y
Zhou Y, Li P, Xue L, Dong Z, Li D (2020b) Solute geochemistry and groundwater quality for drinking and irrigation purposes: a case study in Xinle City, North China. Geochemistry. https://doi.org/10.1016/j.chemer.2020.125609
Acknowledgements
This subject is supported by the Fundamental Research Funds for the Central Universities, CHD (Grant Nos. 300102299505 and 300102290501) and Projects supported by the youth science and technology fund of Hebei GEO University (Grant No. QN201508) and Natural Science Foundation of Education Department in Hebei Province (Grant No. D2019403194). The authors are grateful to the anonymous reviewers and the editors who helped us in improving the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Ning, J., Li, L. et al. Health Risk Assessment of Groundwater in Gaobeidian, North China: Distribution, Source, and Chemical Species of the Main Contaminants. Expo Health 12, 427–446 (2020). https://doi.org/10.1007/s12403-020-00365-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-020-00365-6