Abstract
The study area is a part of the North China Plain, where groundwater is heavily abstracted for drinking and irrigation purposes. Groundwater quality is adversely affected due to rapid economic development and urbanization. Therefore, the purposes of this study were to evaluate the suitability of groundwater for drinking and irrigation purposes and to quantify the associated human health risks in the southern part of Gu’an County, North China Plain. The matter-element extension method based on entropy weight was used to evaluate the water quality for drinking, while sodium adsorption ratio, sodium percentage (%Na), residual sodium carbonate and magnesium hazard were used to evaluate the water quality for irrigation. Non-carcinogenic and carcinogenic health risks via different exposure ways were evaluated for different age groups. The study found that the quality of both deep and shallow groundwater in this area was generally suitable for drinking. Deep water quality has better quality than the shallow water. However, 8.70% and 73.92% of water samples pose non-carcinogenic health risks on adults and children, respectively. Children and adults are also at cancer risk due to Cr6+ and As in drinking groundwater in this area. The main responsible parameters for non-carcinogenic risk are Cr6+, F− and Fe, and Cr6+ is also responsible for carcinogenic risk. These toxic elements are mainly from industries. Therefore, deterioration of groundwater quality can be prevented by strengthening the sewage management of various industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an important water resource to maintain natural ecosystems and support social and economic development (Holland et al. 2015; Lu et al. 2019; Wang et al. 2019). Rapid economic development, however, has led to deterioration of groundwater quality in some regions (Hua et al. 2015; Li et al. 2017a; Wichelns and Qadir 2015). The existing research indicated that groundwater quality deterioration is controlled by both geogenic and anthropogenic factors (Neshat et al. 2014; Zhang et al. 2018; He and Wu 2019a; Kaur et al. 2019), and the groundwater pollution can be disastrous to human health and sustainable societal development (Zhou et al. 2016; Li et al. 2019a; Li and Wu 2019a, b). For example, He et al. (2019a) investigated the major ion chemistry of groundwater in Wuqi County of the Chinese Loess Plateau, finding that the human in the area are at high risk due to the pollution of nitrate and Cr6+. Li et al. (2019b) noted that fluoride contributes the most to the total health risk in Tongchuan County, Northwest China. The Gu’an County, one of the most water-scarce regions in the world, is also facing the serious groundwater quality deterioration, which may also induce health risk to the residents.
The study area is located in the south of Gu’an County in the North China Plain, including Peng County, Qugou Town, Niutou Town, Lirangdian Town and Mazhuang Town, with an area of about 390 km2. It is an important zone in the Beijing–Tianjin–Hebei region (Liu 2011; Ding 2012). In 2015, Gu'an is rated as one of the counties with strong county economy. The electronic information industry, automobile parts industry and modern equipment manufacturing industry are the primary industries in the region, which may produce iron, manganese, fluorine and nitrate pollution. According to media reports, the waste discharged by a color printing company in the Niutou Town contained toluene, butanone and other organic compounds, as well as lead and other heavy metals. At the same time, the small- and medium-sized rivers in the study area are relatively developed, which are not only used for diverting waterlogging, irrigation and drainage waters, but also for the discharge of rural sewage and industrial wastewater (Yu 2016). Definitely, the hydraulic connection between the contaminated river and the shallow groundwater is an important source of groundwater pollution (Li et al. 2016). Liu et al. (2018) reviewed that the surface water pollution caused by human activities was the main source of volatile phenols, As, Hg and Cr6+ in the groundwater in and around the study area. In addition, agricultural and domestic pollution sources lead to the continuous increase in ammonia nitrogen, nitrate and nitrite in groundwater (Liu et al. 2018). However, groundwater is the main source of water for drinking, agricultural irrigation and industrial production in this area. And the southern region is a concentrated area of tourism and leisure, conference and exhibition, high-end pension and other industries, as well as a concentrated area of high-quality residential quarters. Exposure to groundwater contaminated by above pollutants may cause diarrhea, jaundice, cholera, dysentery or fluorosis (Zhang 2014; Chica-Olmo et al. 2014; Su et al. 2017; Singh et al. 2018). Therefore, it is necessary to assess the groundwater quality and health risks in the region.
Many methods have been used to evaluate water quality, such as WOI method (Şener et al. 2017), fuzzy mathematics method (Karmakar and Mujumdar 2006; Dahiya et al. 2007), TOPSIS approach (Li et al. 2012; Gorgij et al. 2019) and grey cluster analysis method (Gonçalves and Alpuim 2011). Su et al. (2019) proposed a set pair analysis-Markov chain model for groundwater quality evaluation and prediction. Almost in the same time, Tian and Wu (2019) integrated the set pair analysis and the game theory to form a novel approach for groundwater quality assessment. Each method has its own advantages and disadvantages. For example, comprehensive evaluation via fuzzy mathematics can fully reflect the groundwater quality, and the membership degree of each pollution element with respect to the standard limits can be objectively calculated to avoid human influence. However, the calculation processes are too complicated, and the results cannot be compared. Matter-element extension analysis has been widely used in the comprehensive evaluation of environmental quality (Tang et al. 2009; Li et al. 2017b, c), ecological security (Ou et al. 2017; Yue et al. 2018), land grade (Gong et al. 2012) and water quality assessment (Li et al. 2018a). Compared with other groundwater quality evaluation methods, the matter-element extension evaluation model is not only suitable for multi-index comprehensive evaluation, but also can transform the incompatible problem into the compatible problem by means of matter-element transformation and structural transformation (Jin et al. 2012). In addition, entropy is a thermodynamic physical concept, which represents the effective utilization of heat energy in the process of heat work conversion. In information theory, entropy reflects the degree of information disorder. The larger the entropy is, the lower the degree of order is, and the greater the uncertainty is. Therefore, entropy is widely used to determine the weight value, which is called entropy weight method (Wu et al. 2015; Li et al. 2013). Based on the matter-element extension theory proposed by Cai (1994), Li et al. (2016) used the matter-element extension method based on entropy weight to evaluate the water quality and found that this modified approach is more credible and practical than other traditional methods. This method can avoid artificial interference and fully reflect the internal relationship between various indicators. In addition to traditional water quality assessment, health risk assessment due to exposure to contaminated water has also been widely carried out by researchers (Adimalla et al. 2019; Adimalla and Li 2019; Karunanidhi et al. 2019a). These assessment studies on health risk are mainly based on the risk assessment model proposed by the United States Environmental Protection Agency (USEPA). However, China has constructed a country-specific health risk assessment system (Cushman et al. 2001), which has the following steps: hazard identification, dose–effect analysis, exposure evaluation and risk characterization (Li et al. 2008). This paper applied this analysis approach to identify the degree of groundwater pollution in the study area and its possible risks to human health.
The main aims of this study were to: (1) analyze the hydrochemical characteristics of the groundwater, (2) assess the suitability of groundwater quality for drinking and irrigation purposes, and (3) evaluate the health risks for adults and children, respectively, by considering different exposure pathways. This study identified the main factors affecting the groundwater quality in this area and analyzed the possible sources in detail, providing the necessary knowledge for the decision-makers to manage and regulate groundwater in this area.
Study area
Location and climate
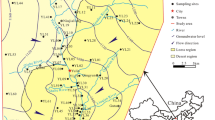
The study area is located in the plain area to the east of the Taihang Mountains. It is situated between the longitudes 116° 06′ E to 116° 28′ E and latitudes 39° 07′ N to 39° 31′ N, covering an area of 696 km2 (Fig. 1). The general topography descends from northwest to southeast with the slope of less than 1‰. The ground surface elevation is about 10–30 m above the mean sea level. The climate is humid and semi-arid with distinct seasonal variation. The precipitation decreased from northwest to southeast with the average annual precipitation of 515.4–546.3 mm (Fig. 2) and the average annual evaporation of about 1315.9 mm.
Geology and hydrogeology
The outcropping stratum in the area is the Quaternary loose deposits with a thickness of 350–500 m. The deposits genetically originate from alluvial and pluvial processes. In the northwest, Langfang fault is developed with the strike of NE-NEE. Niutuo town fault is developed in the southeast with scattered fissures in the south. The media are mainly silty clay which is rich in calcium carbonate.
The study area belongs to the Yongding River groundwater system. The Quaternary deposits can be divided into four aquifers. The first and second aquifers are the shallow groundwater aquifers composed of fine sand and silty sand, ranging from 30 to 50 m in thickness. The depth of the aquifer bottom is within 80–120 m below the ground surface and the groundwater level depth ranges within 8–12 m (Fig. 3). The third aquifer is deep groundwater aquifer, with the bottom depth of 350–385 m below the ground surface. The groundwater level depth is less than 15 m in the northwest, and it gradually increases towards the southeast. The fourth aquifer is mainly composed of medium and fine sand with a depth of 420–520 m and the thickness ranging from 20 to 40 m. The groundwater in this group is not exploited due to great aquifer depth.
The shallow groundwater is recharged in the study area by atmospheric precipitation, followed by agricultural irrigation, surface water infiltration and lateral inflow, while is discharged by mainly manual mining, evaporation and lateral outflow. The dynamic of the shallow groundwater is characterized by infiltration and exploitation with the hydraulic gradient ranging from 0.8 to 1.7‰ (Fig. 3). The recharge to deep groundwater is mainly in the form of lateral inflow, while the discharge is mainly in the form of artificial drainage with minor lateral outflow. The hydraulic gradient of the deep groundwater is 0.8–2.2‰ (Fig. 3).
The main rivers in the area are the Mangniu River and Friendship River. Mangniu River is a tributary of the Yongding River. It starts from the south of Xima village, Gu’an County, and flows south to Nanbai village where it joins with Taiping River and then flows east. The river bottom is 25–55 m in width, with a length of 18.1 km, and the drainage area covers 754 km2. The Friendship River, also known as the Xiongguba New River, is mainly used to drain excessive rainfall. Groundwater flows generally from the northwest to the southeast. Due to the excessive use of groundwater for agriculture, a groundwater funnel is formed around the Mazhuang town, resulting in the change in groundwater flow direction and convergence of groundwater flow to the funnel center (Fig. 4). In recent years, the groundwater level depth in the funnel center is gradually deepening, and the funnel area is increasing year by year.
Materials and methods
Sample collection and analysis
A total of 23 samples consisting of 16 shallow groundwater samples (80–120 m in depth) and 7 deep groundwater samples (150–350 m in depth) were collected from pumping wells in this study. All samples were collected from the sediment of the Quaternary system. The lithology of shallow water aquifer is mainly fine sand and fine powder sand, while the lithology of deep aquifer is mainly medium sand and fine sand. The sampling locations were selected randomly in a way that they represent different geological formations and landuse patterns at varying topography of the study area. The locations of water samples were recorded by GPS and are shown in Fig. 1. The pH and electrical conductivity (EC) of the groundwater were measured on site (Table 1), while other water quality indicators were measured in the laboratory. Samples were collected in pre-cleaned white polyethylene bottles (Table 1), and the wells were pumped for several minutes before sampling to avoid the influence of stagnant water in the wells. After collection, the samples were labeled and sealed and were then immediately transported to the laboratory for further analysis. Further, fixative was added as required before packaging and all the parameters within a specified time to complete the test (Ministry of Environmental Protection of P.R. China 2009). For examples, groundwater samples for the K+, Na+, Fe and Mn need to add HNO3, for NH4+ and As need to add H2SO4, and for Cr6+ need to add Na(OH) as fixing agent. The specific addition requirements are shown in Table 1.The specific sampling requirements, storage conditions, test methods, instruments and models of each indicator are shown in Table 1. Samples for the total hardness, TDS, SO42−, Cl−, HCO3−, NO3−, NO2− and F− shall be kept at low temperature and away from light potassium. To examine the precision and the accuracy of the data, the analyzed parameters were checked for consistency and accuracy using standard approaches to make sure that all the data obtained are reliable (Duraisamy et al. 2019; Sharma et al. 2020). The charge balance errors were within ± 5%, and the recovery ratio was within ± 10%, indicating that the measurement accuracy in the study was reasonably good (Kumar et al. 2020).
Entropy-based matter-element extension analysis
Cai (1994) and Cai et al. (2000) proposed the matter-element extension analysis method, and then it was used in groundwater evaluation (Jin et al. 2012; He et al. 2011). According to the theory of the matter-element extension, a system is considered as a set of matter elements, and each element consists of objects, features and values that participate in a series of transformation processes (Tang et al. 2009; Gong et al. 2012). The main concept of matter-element extension analysis is as follows:
Matter-element extension method defines M to represent the matter, and in this study it refers to the groundwater quality. C is defined as the characteristics of the matter, and V is the value of the characteristics. Thus, they form an ordered triad \(R = \left[ {M,C_{i} ,V_{i} } \right]\) as the basic form to describe the matter, which is called a matter-element (Peng et al. 2009). If the matter has n characteristics denoted as ci, it should be described by n corresponding values expressed vi. The matter-element then can be written as Eq. (1):
where ci represents the physicochemical parameters of the water sample and vi represents the value of parameters in this study. Where i denotes the number of the water quality parameter, and \(i = 1,2, \ldots ,n.\). When the matter-element is used to represent the standard of water quality grade, Eq. (1) is converted to the classical matter-element matrix (\(R^{c}\)) and is called classical domain (Eq. 2.). If the water quality has m standard intervals, there will be m classical domains.
In Eq. 2, Nj, \(C_{{\text{i}}}\) and \(V_{ij}^{c}\) represent water quality in grade j, physicochemical parameters used in the assessment and the standard value range of the jth level for each parameter, respectively, where, \(i = 1,2, \ldots ,n\), \(j = 1,2, \ldots ,m\). The classical domain represents the standard value range for the ith physicochemical parameter and the jth grade. Based on the classical domain (\(R^{c}\)), a section domain (\(R^{s}\)) can be further defined, as shown in Eq. (3):
where P represent the number of grades and \(\left[ {\begin{array}{*{20}c} {a_{i}^{s} ,} & {b_{i}^{s} } \\ \end{array} } \right]\) denotes the value range of grade to which the physicochemical parameters belong. Based on above definitions, the matter-element of groundwater quality evaluation in the study can be expressed as follows:
On the basis of the above, the correlation degree and correlation function are established. Let Kj(vi) be the correlation degree between the matter vi to be evaluated and the corresponding standard jth grade. The correlation function can be expressed as follows:
where \(\rho (x_{j} ,x_{ij}^{0} )\) is the distance between \(x_{j}\) and classical domain, and \(\rho (x_{j} ,x_{j}^{p} )\) is the distance between \(x_{j}\) and section domain. Assume that the range of magnitude is (aij0, bij0) and the module of interval \(x_{ij}^{0}\) is \(\left| {\begin{array}{*{20}c} {a_{ij}^{0} ,} & {b_{ij}^{0} } \\ \end{array} } \right|\), then the distance from \(x_{j}\) to interval \(x_{ij}^{0}\) is:
The distance between \(x_{j}\) and \(x_{j}^{p}\) is given by Eq. (7):
If \(K_{j} (v_{i} ) = \max \left\{ {K_{j} \left( {v_{1} } \right),K_{j} \left( {v_{2} } \right), \ldots ,K_{j} \left( {v_{n} } \right)} \right\},\;j = 1,2, \ldots ,m.\), \(v_{i}\) is rated as grade j, the comprehensive correlation degree can be given as follows:
where\(w_{i}\) represents the weight of each parameter. If \(K_{j} (P) = \max \left\{ {K_{j} \left( P \right),K_{j} \left( P \right), \ldots ,K_{j} \left( P \right)} \right\},\;j = 1,2, \ldots ,m.\), then the water quality of the sample belongs to grade j. If \(K_{j} (P) \ge 1\), the evaluated object exceeds the upper limit of the standard. If \(0 \le K_{j} (P) \le 1\), the object conforms to the requirements of the membership degree of the standard, and a higher value suggests the higher proximity to upper limit of the standard. If \(- 1 \le K_{j} (P) \le 0\), the object does not meet the requirements of the standard, though it may be converted to conform to the standard. If \(K_{j} (P) \le - 1\), the evaluated objects neither meet the requirements of standard nor can be transformed into standard objects (Tang et al. 2009).
Above procedure is highly constrained by the determination of the weight for each water quality parameter. Entropy is a common method to determine the weights of parameters (Wu et al. 2015; Li et al. 2016). It is a method to determine the weight of an index by a judgment matrix composed of the evaluation index under objective conditions. Assume there are m water samples with n different evaluation parameters each to be evaluated, an eigenvalue matrix X can be constructed as follows, where xij signifies the value of the parameter:
The indicators can be divided into the following 4 categories based on their characteristics: efficiency type, cost type, fixed type and interval type (Tian and Wu 2019). The initial eigenvalue matrix can be converted to the standardized matrix Y as follows:
For efficiency-type parameters,
For cost-type parameters,
For efficiency-type parameters, a higher parameter value represents good water quality, and for cost-type parameters, the lower values reflect good quality.
After transformation, the standardized matrix can be expressed as:
The following scale matrix P can be constructed from the standard level matrix:
where \(p_{ij} = \frac{{y_{ij} }}{{\sum\nolimits_{i = 1}^{m} {y_{ij} } }}\) and m represents the total number of water sample points. Entropy weights are calculated using the following formulas:
where \(e_{j}\) is the information entropy and \(w_{j}\) is the entropy weight of parameter j, respectively. When \(p_{ij} = 0\), \(p_{ij} \ln p_{ij} = 0\).
Health risk assessment
Health risk assessment evaluates the harmful effects of exposure to chemicals in the contaminated environment on humans (Giri and Singh 2015; Liu et al. 2010). Groundwater poses health risks to human health mainly through drinking and skin contact. Therefore, health risks of drinking and skin contact were evaluated separately in this study for children and adults, whose physiology is different. Specifically, the risks of F−, NO3−, NO2−, Fe ion (Fe2+, Fe3+), NH4+, Cr6+, Mn and As were considered in this study.
The non-carcinogenic risk from drinking and skin contact was calculated as follows (He et al. 2020a; Li et al. 2019a, b; Sharma et al. 2020; Wu et al. 2019, 2020):
For drinking water intake:
where C, IR, EF, ED, BW, AT and RfD represent the pollutant concentration in groundwater (mg/L), the daily ingestion rate of water (L/day), the exposure frequency (day/a), the exposure duration (a), average weight (kg), average exposure time (day) and the reference dose of contaminant (mg/(kg day)), respectively. The value of EF is taken as 365 day/a. IR, ED, BW and AT and RfD are 1.5 L/day, 30 a, 70 kg and 10,950 days, respectively, for adults, and 0.7 L/day, 12 a, 15 kg, and 4380 day, respectively, for children. RfDoral for F−, NO3−, NO2−, Fe ions (Fe2+, Fe3+), NH4+, Cr6+, Mn and As are 0.04, 1.6, 0.1, 0.3, 0.97, 0.003, 0.14 and 0.0003 mg/(kg day), respectively, (Ministry of Environmental Protection of the P.R. China 2014; Li et al. 2016).
For non-carcinogenic risk from skin contact (Li et al. 2017b):
where EV, DA, SA, K, t, CF and H are the daily exposure frequency (= 1 in this study) (Ministry of Environmental Protection of the P.R. China 2014), the exposure dose (mg/cm2), skin surface area (cm2), skin permeability (= 0.001 cm/h), contact duration (= 0.4 h/day), a conversion factor (= 0.001), average height. ABSgi represents the parameter for gastrointestinal absorption. The ABSgi value for all parameters is 1 except Cr6+, which has the value of 0.025 (Ministry of Environmental Protection of the P.R. China 2014; USEPA 2015; He and Wu 2019a). H is 165.3 cm for adults and 99.4 cm for children.
The total non-carcinogenic risk was calculated as follows:
where HI is non-carcinogenic risk index, which indicates the total non-carcinogenic risk. HI > 1 indicates unacceptable risk (Ministry of Environmental Protection of the P.R. China 2014; Wu and Sun 2016).
The carcinogenic risk from drinking water and skin contact was calculated as follows (Ministry of Environmental Protection of the P.R. China 2014):
For the carcinogenic risk from drinking water intake (He and Li 2020; Kumar et al. 2020):
where SF is the slope factor of carcinogenic risk (mg/(kg day)), and SF for Cr6+ is 0.5 (mg/(kg day))−1 and for As is 1.5 (mg/(kg day))−1 (Ministry of Environmental Protection of the P.R. China 2014). Similarly, the total carcinogenic risk was determined as follows:
where CRtotal represents the total carcinogenic risk and CRtotal > 1 × 10−6 indicates a possible potential carcinogenic risk.
Results and discussion
Hydrochemical parameters
The physicochemical parameters of groundwater were statistically analyzed and compared with the WHO standards (WHO 2011). The results are given in Table 2.
The pH is between 7.45 and 8.24, with an average of 7.92, indicating slightly alkaline groundwater (Talib et al. 2019). Mean TDS and TH are 339.20 mg/L and 182.83 mg/L, respectively, which are lower than the WHO standards (WHO 2011). The average cation and anion concentrations are lower than the Chinese drinking water quality standard limits, except for HCO3−. Concentration of HCO3− is between 202.36 and 609.54 mg/L and is higher than the standard in 34.78% of the samples. This may be because the groundwater is slightly alkaline, so the main form of carbonic acid is HCO3−, which is consistent with the theory of carbonic acid equilibrium (Lakshmanan et al. 2003). The abundance of anions and cations in the research area is Na+, Ca2+ and Mg2+, with Na+ content being the most abundant, whereas HCO3− is the main anion followed by Cl− and SO42− (Table 2). In order to further analyze the reasons for the high Na+ content in the groundwater, the relationship diagram between the major ions was drawn, as shown in Fig. 5. The points of Cl− and Na+ + K+ are located below 1:1 (Fig. 5a), which indicates that Cl− content is lower than Na+. Because Cl− in groundwater is usually stable and is not easy to react with other ions, or absorbed by plants and soil, it is assumed that there are other sources of Na+ except the dissolution of halite, such as cation exchange or the dissolution of albite. Meanwhile, industrial and agricultural pollution in the study area may also be an important factor in the increase in Na+. Generally, Ca2+ in the groundwater comes from the dissolution of gypsum and carbonate rocks. Therefore, the content of Ca2+ should be higher than that of HCO3− and SO42−, respectively. According to Fig. 5b, c, the content of HCO3− is higher than that of Ca2+. The discussion above has indicated ion exchange in groundwater, which was discussed further (Fig. 5d). Almost all of the points are on the 1:1 line, and mostly on the upper left, which means that the main ions in groundwater have been affected by ion alternate adsorption, expressions are as follows:
Trace metals are usually in low contents in groundwater. In this study, only the Mn and Fe concentrations among are higher than the standard, which range within 0.07–0.62 mg/L and 0.08–6.51 mg/L, respectively.
Hydrochemical types of groundwater
The hydrochemical types of groundwater are controlled by anions and cations, which are influenced by land use/land cover (Aravinthasamy et al. 2019a; He et al. 2019b; He and Wu 2019b). The hydrochemical characteristics of groundwater were studied using the Piper diagram (He and Li 2019), and the ions are expressed in milliequivalents per liter (meq/L). The groundwater recharge is abundant and the groundwater circulation is quick in the study area, which leads to a single HCO3 hydrochemical type. According to Fig. 6, the hydrochemical types in shallow groundwater are mainly HCO3–Na·Ca and HCO3–Na·Mg·Ca types, while those in deep groundwater are HCO3–Na·Ca and HCO3–Na types. The deep and shallow groundwaters have the similar hydrochemical characteristics. However, the shallow water hydrochemical types change to HCO3·SO4–Na near the groundwater funnel where groundwater is heavily extracted in Dongtaoyuan village of Mazhuang town (Fig. 4). Figure 6 also shows that alkali earth metals and weak acidic anions are predominant in the area over alkali metals (Ca2+ + Mg2+ > Na+ + K+) and strong acidic anions (HCO3− > Cl− + SO42−), respectively.
Groundwater quality assessment for drinking purpose
According to the national standards (Ministry of Health of the P.R. China and Standardization Administration of the P.R. China 2006), groundwater quality is divided into the following five grades: excellent (grade I), good (grade II), fair (grade III), poor (grade IV) and very poor (grade V). All the groundwater samples were assessed using the entropy matter-element extension analysis method, and the results are shown in Table 3.
There are 11 (68.75%) of the 16 shallow water samples belong to grade I which is suitable for various uses, while 4 samples (25%) are classified into grade II and 1 (6.25%) is sorted into grade III (Table 3). In contrast, all 7 deep water samples are of excellent water quality. In general, most of the groundwater is suitable for drinking in the study area.
The spatial distribution of groundwater quality is shown in Fig. 7. There are 5 water samples with non-excellent water quality that are mainly distributed in the east and southeast. The quality of these water samples is mainly affected by Fe, Mn, TH, NH4+, Cr6+ and NO2−, while three grade II water samples are affected by Fe and Mn that are distributed in and around Niutuo town, which has iron, locomotive accessories, fishing gear and other industries. There are many industrial parks such as Gu'an High-tech park, Daqinghe park and Airport industrial park in the north of Gu'an. One grade II water sample is affected by NH4+ and NO2− in Dongtaoyuan village, Mazhuang town, due to agricultural activities. The industrial area near Nansong village has grade III water quality due to the presence of Cr6+. The groundwater in this area is from northwest to southeast, so the groundwater pollution in the study area may be partly from the upstream area.
In summary, the deep and shallow water quality in the study area is generally good. However, the shallow water in the east part is relatively poor. The main influencing factors are Fe, Mn, Cr6+ from industrial pollution, and NH4+ and NO2− from agricultural pollution. According to the industrial plan of Gu'an County from 2016 to 2030, Niutuo County is a hot spring new town. Therefore, it will be a concentrated area for residents. The deterioration of groundwater quality may bring great harm to residents' health. Thus, strong management, effective treatment of wastewater or zero discharge system are effective ways of preventing the water quality from deterioration in this region. In addition, health risk may not be ignored due to existence of some pollutants in groundwater, and it is mandatory to carry out the health risk assessment study.
Groundwater quality assessment for irrigation purpose
According to the industrial plan of Gu'an County from 2016 to 2030, the southern area of Gu'an is the modern ecological agriculture sector. Groundwater is the main source of irrigation. Therefore, it is necessary to evaluate the quality of irrigation water with groundwater in this area. The sodium adsorption ratio (SAR), sodium percentage (%Na), residual sodium carbonate (RSC) and magnesium hazard (MH), together with USSL chart and Wilcox chart, were used to evaluate the suitability of groundwater for irrigation (Li et al. 2018a, b; USSL 1954; Karunanidhi et al. 2013). SAR, %Na and RSC are used to measure the alkali damage, while MH is used to measure the magnesium damage. Equations for each method are listed below, and ions are expressed in meq/L.
Sodium adsorption ratio can measure the alkali damage to crops and is widely used to determine the suitability of groundwater for irrigation (Bouderbala 2017). If the SAR value is too high, soil alkalization will be caused, which has a great impact on the evaluation of the suitability of groundwater irrigation. The calculation formula of SAR is as follows (Zhou et al. 2020):
The minimum, maximum and average SAR are 0.76, 4.74 and 2.12, respectively (Table 4). SAR for all water samples are less than 18, indicating that water in the study area is suitable for irrigation. A USSL diagram was drawn based on the relationship between EC and SAR (He and Li 2019, Fig. 8). Four water samples fall in the C2S1 region, whereas 18 samples fall in the C3S1 region, suggesting that deep groundwater in the study area is suitable for irrigation. Only 1 water sample in the shallow groundwater is not suitable for irrigation due to high EC value in the C4S1 area, as it could cause salt damage (Alam 2014).
%Na was used to evaluate the suitability of groundwater for irrigation. Large %Na in irrigation water can reduce the permeability of soil, affecting plant growth. %Na is calculated as follows:
According to %Na, 5 water samples (21.74%) are not suitable for irrigation, including 1 in deep groundwater. According to the Wilcox diagram (Fig. 9), 21 samples (91.30%) including 7 deep water sample points are located in excellent to the permissible area. Two shallow groundwater samples (8.70%) located in the southeast are not suitable for irrigation because of the high EC.
RSC (residual sodium carbonate) indicates the degree of alkali damage and is calculated as follows:
According to Table 4, the minimum, maximum and average RSC for the 23 groundwater samples are − 2.21, 4.04 and 1.19, respectively, with only one shallow groundwater sample (4.35%) exceeding 2.5, indicating unsuitability for irrigation. Long-term use of water with high RSC for irrigation can affect vegetation growth (Vasanthavigar et al. 2012; Zaidi et al. 2015).
Increase in Mg2+ relative to Ca2+ content leads to an increase in aggregates in soil, resulting in the dispersion of clay particles and damage to soil structure due to water conductivity reduction. Magnesium hazard (MH) is used to characterize this factor as follows:
The minimum and maximum of MH in 23 water samples are 32.34 and 61.77, respectively. Among them, 18 water samples (78.26%), including 7 deep groundwater water samples, can be used for irrigation.
In general, the quality of deep groundwater is better than the shallow groundwater for irrigation. Although the shallow groundwater is generally suitable for irrigation according to SAR, some of them may not be suitable for irrigation according to the results of %Na, RSC, MH, USSL chart and Wilcox chart. Shallow groundwater in the study area may produce salinity damage locally, and, therefore, adequate protection measures should be undertaken. The high Na+ content in groundwater is the key factor that causes the irrigation water to fail to meet the standards. Combined with the above analysis of the water chemical composition, Na+ is mainly affected by ion exchange, which causes its content to increase, which shows that the aqueous medium in the study area has been contaminated with Na+ to a certain extent, and the specific source of sodium contamination needs to be further determined in conjunction with the soil pollution survey results in the area, and reasonable measures should be taken accordingly.
Health risk assessment
Non-carcinogenic risks for adults through ingestion of drinking water and skin exposure are shown in Table 5. Regarding shallow groundwater (W1-W16), HItotal ranges from 0.270 to 1.232, with an average of 0.691 for adults, and about 12.5% study sites show higher HItotal values than the acceptable limit (HItotal > 1). About 87% of the study sites are showing the unacceptable non-carcinogenic risk of health hazards (HItotal > 1) for children. Furthermore, regarding deep groundwater (W17–W23), it poses significantly lower non-carcinogenic risks than the shallow groundwater. HItotal varies within 0.39–0.84 for adults and 0.72–1.48 for children, which indicates no risks to adults. The spatial distribution of the non-carcinogenic risk to adults and children indicates significant health risk of shallow groundwater in northeast part of the study area (Fig. 10a, b). However, the high-risk for both adults and children are also associated with poor deep groundwater quality in the southeast part (Fig. 10c, d). What is more, F−, Fe and As are the key factors of the health risk for adult in shallow groundwater (He et al. 2020b), and Cr6+ contributes greater to the health risk in the deep groundwater for adults (Fig. 11a). It also found that the high health risk to children could be attributed to the exceptionally high levels of F−, and Fe and As in shallow groundwater, and As in deep groundwater (Fig. 11b). These factors have been proved by many scholars to be the main cause of health risks in various regions (Karunanidhi et al. 2019b; Kaur et al. 2019; Aravinthasamy et al. 2019a, b). Therefore, certain measures, such as controlling pollution and lowering F−, Fe, As and Cr6+ levels in the groundwater, must be employed to reduce the non-carcinogenic health risk (Li et al. 2016). Furthermore, greater attention should be paid to the source of Fe in the groundwater of W8, and W11. According to our field survey, it may be caused by some industrial pollution.
Carcinogenic risk assessment was evaluated by taking into account of the carcinogenic effects Cr6+ and As. As shown in Table 6, CRtotal ranges between 1.66 × 10−4 and 9.71 × 10−4, with an average of 4.48 × 10−4 for adults, whereas CRtotal is between 2.69 × 10−4 and 1.49 × 10−3, with a mean of 7.05 × 10−4 for children. Both ranges are above the standard level (1 × 10−6) recommended by the Ministry of Environmental Protection of P.R. China (2014), which indicates that the high potential carcinogenic risk from groundwater to both adults and children. Similarly, the studies conducted in adjacent regions by Zhao (2010) also reported the significant risk from heavy metals in groundwater. The risk distributions in shallow and deep groundwater for both adults and children were demonstrated in Fig. 12. The results show that samples with higher health threat to both adults and children in shallow groundwater are distributed in the southeast part of the study area (Fig. 12a, b), and in deep groundwater are mainly distributed in the southern and eastern sides of the study area (Fig. 12c, d), while being lower in other regions. With the groundwater flowing from northwest to southeast, the health risk increased gradually (Fig. 12), which demonstrated the strong relationship and relatively consistent trend between the health risk and the spatial distribution of the groundwater flow. Moreover, children are more susceptible to the effect of Cr6+ and As compared with adults, and the carcinogenic risks are significantly lower in deep groundwater compared with shallow groundwater (Fig. 13a, b). Obviously, Cr6+ plays a dominant role for the carcinogenic risk in both shallow and deep groundwater (Fig. 13). According to our survey, most Cr6+ in the groundwater may come from the electroplating factories and other factories nearby. Therefore, measures to strengthen the management of effluents discharge and improve the capacity of sewage treatment must be carried out in this area.
From the discussion above, it is clear that although the water quality is generally good for irrigation and drinking, human health risk, especially the carcinogenic risk, remains. In addition, the risk assessment has some sources of uncertainty, generally from parameter uncertain. The statistical averages were adopted for IR, ED, BW and AT, and therefore the results represent the average risk values for adults and children. The true risk to individuals may vary. Moreover, other toxic contaminants that could cause risks to human, such as pesticide (Mittal et al. 2014), were not included in this paper. All of these may increase the uncertainty of the assessment. However, the results are still meaningful for the decision makers to make their decisions to protect the groundwater quality.
Conclusions
In this study, 23 groundwater samples were collected and analyzed for 20 physicochemical parameters. The matter-element extension analysis model based on entropy weight was used to comprehensively evaluate the groundwater quality, while the health risk was assessed according to the age of the population and exposure pathways. The major findings of this study are:
The groundwater in the study area is weakly alkaline. The abundance of ions is Na+ > Ca2+ > Mg2+ for cations and HCO3− > SO42− > Cl− for anions. In general, the water quality in the study area is generally good and can be used for drinking. Fe ions (Fe2+ and Fe3+), NO2−–N, Cr6+ and TDS are contributed by industrial production, agricultural production and natural processes.
Although SAR indicates all the shallow groundwater samples are suitable for irrigation, some shallow groundwater samples may not be suitable for irrigation due to salinity hazard. However, the deep groundwater quality is generally better than the shallow groundwater quality in the study area.
For adults, only 12.5% of the 16 shallow groundwater samples are above the acceptable potential non-carcinogenic risk level, while 87% of the samples exceed the allowable level for children in shallow groundwater. The non-carcinogenic risk is mainly posed by F−, Fe and As. Regarding deep groundwater, no non-carcinogenic risk is observed for adults, but Cr6+ contributes to non-negligible non-carcinogenic risk for children. The CRtotal values for both adults and children are higher than the recommended limit values (< 10−6) in shallow and deep groundwater, which indicates that potential carcinogenic risks are non-negligible. Therefore, adequate measures should be implemented to reduce the concentration of toxic parameters that cause health risk in groundwater.
References
Adimalla, N., & Li, P. (2019). Occurrence, health risks and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Human and Ecological Risk Assessment, 25(1–2), 81–103. https://doi.org/10.1080/10807039.2018.1480353.
Adimalla, N., Li, P., & Qian, H. (2019). Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: A special emphasis on human health risk assessment (HHRA). Human and Ecological Risk Assessment, 25(5), 1107–1124. https://doi.org/10.1080/10807039.2018.1460579.
Alam, F. (2014). Evaluation of hydrogeochemical parameters of groundwater for suitability of domestic and irrigational purposes: A case study from central Ganga plain, India. Arabian Journal of Geosciences, 7(10), 4121–4131. https://doi.org/10.1007/s12517-013-1055-6.
Aravinthasamy, P., Karunanidhi, D., Subramani, T., Anand, B., Roy, P. D., & Srinivasamoorthy, K. (2019b). Fluoride contamination in groundwater of the Shanmuganadhi River basin (south India) and its association with other chemical constituents using geographical information system and multivariate statistics. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125555.
Aravinthasamy, P., Karunanidhi, D., Subramani, T., Srinivasamoorthy, K., & Anand, B. (2019a). Geochemical evaluation of fluoride contamination in groundwater from Shanmuganadhi River basin, South India: Implication on human health. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00452-x.
Bouderbala, A. (2017). Assessment of groundwater quality and its suitability for domestic and agricultural uses in Low-Isser plain, Boumedres, Algeria. Arabian Journal of Geosciences, 10(15), 333. https://doi.org/10.1007/s12517-017-3119-5.
Cai, W. (1994). Matter-element model and its application. Science and Technology. Beijing: Academic Press. (in Chinese).
Cai, W., Yang, C., & Lin, C. (2000). Extension engineering method. Beijing: Science Press. (in Chinese).
Chica-Olmo, M., Luque-Espinar, J., Rodriguez-Galiano, V., Pardo-Igúzquiza, E., & Chica-Rivas, L. (2014). Categorical indicator kriging for assessing the risk of groundwater nitrate pollution: the case of Vega de Granada aquifer (SE Spain). Science of the Total Environment, 470–471, 229–239. https://doi.org/10.1016/j.scitotenv.2013.09.077.
Cushman, D. J., Driver, K. S., & Ball, S. D. (2001). Risk assessment for environmental contamination: An overview of the fundamentals and application of risk assessment at contaminated sites. Canadian Journal of Civil Engineering, 28(S1), 155–162. https://doi.org/10.1139/l00-086.
Dahiya, S., Singh, B., Gaur, S., Garg, V., & Kushwaha, H. (2007). Analysis of groundwater quality using fuzzy synthetic evaluation. Journal of Hazardous Material, 147(3), 938–946. https://doi.org/10.1016/j.jhazmat.2007.01.119.
Ding, J. (2012). Langfang city management and economic development research. Journal of North China Institute of Aerospace Engineering., 04, 47–49. (in Chinese).
Duraisamy, S., Govindhaswamy, V., Duraisamy, K., Krishinaraj, S., Balasubramanian, A., & Thirumalaisamy, S. (2019). Hydrogeochemical characterization and evaluation of groundwater quality in Kangayam taluk, Tirupur district, Tamil Nadu, India, using GIS techniques. Environmental Geochemistry and Health, 41(2), 851–873. https://doi.org/10.1007/s10653-018-0183-z.
Giri, S., & Singh, A. (2015). Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environmental Monitoring and Assessment, 187(3), 63. https://doi.org/10.1007/s10661-015-4265-4.
Gonçalves, A. M., & Alpuim, T. (2011). Water quality monitoring using cluster analysis and linear models. Environmetrics, 22(8), 933–945. https://doi.org/10.1002/env.1112.
Gong, J., Liu, Y., & Chen, W. (2012). Land suitability evaluation for development using a matter-element model: A case study in Zengcheng, Guangzhou, China. Land Use Policy, 29(2), 464–472. https://doi.org/10.1016/j.landusepol.2011.09.005.
Gorgij, A., Wu, J., & Moghadam, A. (2019). Groundwater quality ranking using the improved entropy TOPSIS method: A case study in Azarshahr Plain aquifer, east Azerbaijan, Iran. Human and Ecological Risk Assessment, 25(1–2), 176–190. https://doi.org/10.1080/10807039.2018.1564235.
He, S., & Li, P. (2019). A MATLAB based graphical user interface (GUI) for quickly producing widely used hydrogeochemical diagrams. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125550.
He, S., Li, P., Wu, J., Elumalai, V., & Adimalla, N. (2019b). Groundwater quality under land use/land cover changes: A temporal study from 2005 to 2015 in Xi’an, northwest China. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2019.1684186.
He, S., & Wu, J. (2019a). Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, northwest China. Exposure and Health, 11(2), 125–137. https://doi.org/10.1007/s12403-018-0289-7.
He, S., & Wu, J. (2019b). Relationships of groundwater quality and associated health risks with land use/land cover patterns: A case study in a loess area, northwest China. Human and Ecological Risk Assessment, 25(1–2), 354–373. https://doi.org/10.1080/10807039.2019.1570463.
He, X., & Li, P. (2020). Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): Occurrence, sources and health risks. Exposure and Health. https://doi.org/10.1007/s12403-020-00344-x.
He, X., Li, P., Ji, Y., Wang, Y., Su, Z., & Elumalai, V. (2020b). Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: occurrence, distribution and management. Exposure and Health. https://doi.org/10.1007/s12403-020-00347-8.
He, X., Li, P., Wu, J., Wei, M., Ren, X., & Wang, D. (2020a). Poor groundwater quality and high potential health risks in the Datong Basin, northern China: Research from published data. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00520-7.
He, X., Wu, J., & He, S. (2019a). Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Human and Ecological Risk Assessment, 25(1–2), 32–51. https://doi.org/10.1080/10807039.2018.1531693.
He, Y., Dai, A., Zhu, J., He, H., & Li, F. (2011). Risk assessment of urban network planning in china based on the matter-element model and extension analysis. International Journal of Electrical Power & Energy Systems, 33(3), 775–782. https://doi.org/10.1016/j.ijepes.2010.12.037.
Holland, R., Scott, K., Flörke, M., Brown, G., & Eigenbrod, F. (2015). Global impacts of energy demand on the freshwater resources of nations. Proceedings of the National Academy of Sciences, 112(48), E6707–E6716. https://doi.org/10.1073/pnas.1507701112.
Hua, S., Liang, J., Zeng, G., Xu, M., Zhang, C., & Yuan, Y. (2015). How to manage future groundwater resource of china under climate change and urbanization: An optimal stage investment design from modern portfolio theory. Water Research, 85, 31–37. https://doi.org/10.1016/j.watres.2015.08.007.
Jin, J., Qian, H., Chen, Y., & Xi, W. (2012). Assessment of groundwater quality based on matter element extension model. Journal of Chemistry, 2013, 715647. https://doi.org/10.1155/2013/715647.
Karmakar, S., & Mujumdar, P. (2006). Grey fuzzy optimization model for water quality management of a river system. Advances in Water Resources, 29(7), 1088–1105.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Roy, P., & Srinivasamoorthy, K. (2019b). Risk of fluoride-rich groundwater on human health: Remediation through managed aquifer recharge in a hard rock terrain, South India. Natural Resources Research. https://doi.org/10.1007/s11053-019-09592-4.
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Wu, J., & Srinivasamoorthy, K. (2019a). Potential health risk assessment for fluoride and nitrate contamination in hard rock aquifers of Shanmuganadhi River basin, South India. Human and Ecological Risk Assessment, 25(1–2), 250–270.
Karunanidhi, D., Vennila, G., Suresh, M., & Subramanian, S. (2013). Evaluation of the groundwater quality feasibility zones for irrigational purposes through GIS in Omalur Taluk, Salem District, South India. Environmental Science and Pollution Research, 20(10), 7320–7333.
Kaur, G., Kumar, R., Mittal, S., Sahoo, P., & Vaid, U. (2019). Ground/drinking water contaminants and cancer incidence: A case study of rural areas of South West Punjab, India. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2019.170514.
Kumar, R., Mittal, S., Sahoo, P. K., & Sahoo, S. K. (2020). Source apportionment, chemometric pattern recognition and health risk assessment of groundwater from southwestern Punjab, India. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00518-1.
Lakshmanan, E., Kannan, R., & Kumar, M. (2003). Major ion chemistry and identification of hydrogeochemical processes of ground water in a part of Kancheepuram district, Tamil Nadu, India. Environmental Geosciences, 10(4), 157–166.
Li, B., Yang, G., Wan, R., & Hörmann, G. (2017c). Dynamic water quality evaluation based on fuzzy matter–element model and functional data analysis, a case study in Poyang Lake. Environmental Science and Pollution Research, 24(23), 19138–19148.
Li, P., Feng, W., Xue, C., Tian, R., & Wang, S. (2017b). Spatiotemporal variability of contaminants in lake water and their risks to human health: A case study of the Shahu Lake tourist area, northwest China. Exposure and Health, 9(3), 213–225. https://doi.org/10.1007/s12403-016-0237-3.
Li, P., He, S., He, X., & Tian, R. (2018a). Seasonal hydrochemical characterization and groundwater quality delineation based on matter element extension analysis in a paper wastewater irrigation area, Northwest China. Exposure and Health, 10(4), 241–258. https://doi.org/10.1007/s12403-17-0258-6.
Li, P., He, S., Yang, N., & Xiang, G. (2018b). Groundwater quality assessment for domestic and agricultural purposes in Yan’an City, northwest China: Implications to sustainable groundwater quality management on the Loess Plateau. Environmental Earth Sciences, 77(23), 775. https://doi.org/10.1007/s12665-2018-7968-3.
Li, P., He, X., & Guo, W. (2019b). Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: A case study in Yan’an City on the Loess Plateau of northwest China. Human and Ecological Risk Assessment, 25(1–2), 11–31. https://doi.org/10.1080/10807039.2018.1553612.
Li, P., He, X., Li, Y., & Xiang, G. (2019a). Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: A case study of Tongchuan, Northwest China. Exposure and Health, 11(2), 95–107. https://doi.org/10.1007/s12403-018-0278-x.
Li, P., Li, X., Meng, X., Li, M., & Zhang, Y. (2016). Appraising groundwater quality and health risks from contamination in a semiarid region of northwest China. Exposure and Health, 8(3), 361–379. https://doi.org/10.1007/s12403-016-0205-y.
Li, P., Qian, H., Wu, J., & Chen, J. (2013). Sensitivity analysis of TOPSIS method in water quality assessment: I. Sensitivity to the parameter weights. Environmental Monitoring and Assessment, 185, 2453–2461. https://doi.org/10.1007/s10661-012-2723-9.
Li, P., Tian, R., Xue, C., & Wu, J. (2017a). Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environmental Science and Pollution Research, 24(15), 13224–13234. https://doi.org/10.1007/s11356-017-8753-7.
Li, P., & Wu, J. (2019a). Sustainable living with risks: Meeting the challenges. Human and Ecological Risk Assessment, 25(1–2), 1–10. https://doi.org/10.1080/10807039.2019.1584030.
Li, P., & Wu, J. (2019b). Drinking water quality and public health. Exposure and Health, 11(2), 73–79. https://doi.org/10.1007/s12403-019-00299-8.
Li, P., Wu, J., & Qian, H. (2012). Groundwater quality assessment based on rough sets attribute reduction and TOPSIS method in a semi-arid area, China. Environmental Monitoring and Assessment, 184(8), 4841–4854. https://doi.org/10.1007/s10661-011-2306-1.
Li, Z., Bi, E., Zhang, S., Yin, M., Ma, L., Wang, W., et al. (2008). Method for health risk assessment of groundwater pollution. South-to-North Water Diversion and Water Conservancy Technology, 6(6), 47–51. (in Chinese).
Liu, C., Zhang, X., Li, H., Pu, C., & Zhu, X. (2018). Groundwater quality assessment and analysis in Langfang City. Environmental Chemistry, 37(6), 1399–1409. (in Chinese).
Liu, J. (2011). Analysis of the variation trend of groundwater quality in Langfang. South-to-North Water Diversion and Water Conservancy Technology, A01, 50–52. (in Chinese).
Liu, N., Ni, T., Xia, J., Dai, M., He, C., & Lu, G. (2010). Non-carcinogenic risks induced by metals in drinking source water of Jiangsu Province, China. Environmental Monitoring and Assessment, 177(1–4), 449–456.
Lu, C., Guo, F., Yan, Q., Zhang, Z., Li, D., Wang, L., et al. (2019). Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic activity under simulated solar light degradation of tetracycline. Journal of Alloys and Compounds, 811, 151976.
Ministry of Environmental Protection of P.R. China. (2009). Water quality: Technical regulation of the preservation and handling of samples, (HJ 493-2009). Beijing: National Standards for Environmental Protection of China. (in Chinese).
Ministry of Environmental Protection of the P.R. China. (2014). Technical guidelines for risk assessment of contaminated sites, (HJ 25.3-2014). Beijing: China Environmental Science Press. (in Chinese).
Ministry of Health of the P.R. China, Standardization Administration of the P.R. China. (2006). Standards for drinking water quality (GB 5749-2006). Beijing: China Standard Press. (in Chinese).
Mittal, S., Kaur, G., & Vishwakarma, G. S. (2014). Effects of environmental pesticides on the health of rural communities in the Malwa Region of Punjab, India: A review. Human and Ecological Risk Assessment, 20(2), 366–387.
Neshat, A., Pradhan, B., Pirasteh, S., & Shafri, H. Z. M. (2014). Estimating groundwater vulnerability to pollution using a modified drastic model in the Kerman agricultural area, Iran. Environmental Earth Sciences, 71(7), 3119–3131.
Ou, Z., Zhu, Q., & Sun, Y. (2017). Regional ecological security and diagnosis of obstacle factors in underdeveloped regions: A case study in Yunnan Province, China. Journal of Mountain Science, 14(5), 870–884.
Peng, S. J., Xu, J., Tao, Y. Q., & Cheng, M. J. (2009). Matter-elements model and application for prediction of coal and gas outburst. Journal of Coal Science and Engineering, 15(3), 273–277.
Şener, Ş., Şener, E., & Davraz, A. (2017). Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Science of the Total Environment, 584–585, 131–144.
Sharma, S., Kumar, R., Sahoo, P. K., & Mittal, S. (2020). Geochemical relationship and translocation mechanism of arsenic in rice plants: A case study from health prone south west Punjab, India. Groundwater for Sustainable Development, 10(4), 100333.
Singh, U. K., Ramanathan, A. L., & Subramanian, V. (2018). Groundwater chemistry and human health risk assessment in the mining region of East Singhbhum, Jharkhand, India. Chemosphere, 204, 501–513. https://doi.org/10.1016/j.chemosphere.2018.04.060.
Su, F., Wu, J., & He, S. (2019). Set pair analysis-Markov chain model for groundwater quality assessment and prediction: A case study of Xi’an City, China. Human and Ecological Risk Assessment, 25(1–2), 158–175. https://doi.org/10.1080/10807039.2019.1568860.
Su, H., Kang, W., Xu, Y., & Wang, J. (2017). Evaluation of groundwater quality and health risks from contamination in the north edge of the Loess Plateau, Yulin City, Northwest China. Environmental Earth Sciences, 76(13), 1–21.
Talib, M., Tang, Z., Shahab, A., Siddique, J., Faheem, M., & Fatima, M. (2019). Hydrogeochemical characterization and suitability assessment of groundwater: A case study in Central Sindh, Pakistan. International Journal of Environmental Research and Public Health, 16(5), 886. https://doi.org/10.3390/ijerph16050886.
Tang, J., Wang, C., Lin, N., Li, Z., & Mao, Z. (2009). Application of matter-element model in soil nutrient evaluation of ecological fragile region. Chinese Geographical Science, 19(2), 168–176.
Tian, R., & Wu, J. (2019). Groundwater quality appraisal by improved set pair analysis with game theory weightage and health risk estimation of contaminants for Xuecha drinking water source in a loess area in Northwest China. Human and Ecological Risk Assessment, 25(1–2), 132–157. https://doi.org/10.1080/10807039.2019.1573035.
USEPA (Unite States Environmental Protection Agency). (2015). Regional screening levels (RSLs)—generic tables. Retrieved March 15, 2016, from https://www.epa.gov/risk/regional-screening-levels-rsls-generictables-november-2015.
USSL (United States Salinity Laboratory). (1954). Diagnosis and improvement of saline and alkali soils, Agriculture handbook 60 (pp. 69–81). Washington: US Department of Agriculture (USDA).
Vasanthavigar, M., Srinivasamoorthy, K., & Prasanna, M. V. (2012). Evaluation of groundwater suitability for domestic, irrigational, and industrial purposes: A case study from Thirumanimuttar river basin, Tamilnadu, India. Environmental Monitoring & Assessment, 184(1), 405–420.
Wang, D., Wu, J., Wang, Y., & Ji, Y. (2019). Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: Analysis, assessment, and management. Exposure and Health. https://doi.org/10.1007/s12403-019-00314-y.
WHO. (2011). Guidelines for drinking water quality (4th ed.). Geneva: World Health Organization.
Wichelns, D., & Qadir, M. (2015). Achieving sustainable irrigation requires effective management of salts, soil salinity, and shallow groundwater. Agricultural Water Management, 157, 31–38.
Wu, J., Li, P., Qian, H., & Chen, J. (2015). On the sensitivity of entropy weight to sample statistics in assessing water quality: statistical analysis based on large stochastic samples. Environmental Earth Sciences, 74(3), 2185–2195. https://doi.org/10.1007/s12665-015-4208-y.
Wu, J., & Sun, Z. (2016). Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, Mid-west China. Exposure and Health, 8(3), 311–329. https://doi.org/10.1007/s12403-015-0170-x.
Wu, J., Zhang, Y., & Zhou, H. (2020). Groundwater chemistry and groundwater quality index incorporating health risk weighting in Dingbian County, Ordos basin of northwest China. Geochemistry. https://doi.org/10.1016/j.chemer.2020.125607.
Wu, J., Zhou, H., He, S., & Zhang, Y. (2019). Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environmental Earth Sciences, 78(15), 446. https://doi.org/10.1007/s12665-019-8471-1.
Yu, X. (2016). The study on the biodiversity protection measures of the rivers in Gu’an County. Master thesis, Beijing Forestry University, Beijing (in Chinese).
Yue, D., Zeng, J., Yang, C., Zou, M., Li, K., Chen, G., et al. (2018). Ecological risk assessment of the Gannan Plateau, Northeastern Tibetan Plateau. Journal of Mountain Science, 15(6), 105–118.
Zaidi, F. K., Nazzal, Y., Jafri, M. K., Naeem, M., & Ahmed, I. (2015). Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: a case study from northwestern Saudi Arabia. Environmental Monitoring and Assessment, 187, 607.
Zhang, X. W. (2014). Analyses of groundwater resources exploitation situation and the environment geological problems in Langfang. Groundwater, 36(3), 47–48. (in Chinese).
Zhang, Y., Wu, J., & Xu, B. (2018). Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest china. Environmental Earth Sciences, 77(7), 273. https://doi.org/10.1007/s12665-018-7456-9.
Zhao, X. (2010). Water quality health-hazard risk assessment on drinking water supply sources in Hebei Province. Journal of Anhui Agricultural Sciences, 26, 14614–14617. (in Chinese).
Zhou, Y., Li, P., Xue, L., Dong, Z., & Li, D. (2020). Solute geochemistry and groundwater quality for drinking and irrigation purposes: a case study in Xinle City, North China. Geochemistry. https://doi.org/10.1016/j.chemer.2020.125609.
Zhou, Y., Wei, A., Li, J., Yan, L., & Li, J. (2016). Groundwater quality evaluation and health risk assessment in the Yinchuan region, northwest China. Exposure and Health, 8(3), 443–456.
Acknowledgements
The corresponding author is grateful for the financial support granted by the National Natural Science Foundation of China (41602238 and 41761144059), the Special Funds for Basic Scientific Research of Central Colleges (300102299301), the Fok Ying Tong Education Foundation (161098), the China Postdoctoral Science Foundation (2015M580804, 2016M590911, 2016T090878 and 2017T100719), the Shaanxi Postdoctoral Science Foundation (2015BSHTDZZ09 and 2016BSHTDZZ03), and the Ten Thousand Talent Program (W03070125). The other authors are thankful to the support from the Open Fund of the Key Laboratory of Subsurface Hydrology and Ecological Effects in Arid Region of the Ministry of Education (300102299505 and 33001002290501), the Natural Science Foundation of Education Department in Hebei Province (D2019403194) and the Water Conservancy Research and Promotion Plan Project of Hebei Province in 2018 (2018S01). The authors are grateful to the anonymous reviewers and the editors who helped us in improving the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Y., Li, P., Chen, M. et al. Groundwater quality for potable and irrigation uses and associated health risk in southern part of Gu’an County, North China Plain. Environ Geochem Health 43, 813–835 (2021). https://doi.org/10.1007/s10653-020-00553-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00553-y