Abstract
This research was conducted to understand the seasonal characteristics of water quality for domestic purpose in Hancheng City of the Guanzhong plain, China. The health risks were also assessed using the water quality monitoring data collected from the Hancheng Center for Disease Control and Prevention. For this study, 48 samples were collected from the drinking water distribution system (chlorinated water and terminal tap water) in the dry and wet seasons, and were analyzed for pH, total hardness (TH), total dissolved solids (TDS), Cl−, SO42−, F−, NH4-N, NO3-N, Cr6+, As, Hg and Mn. The water quality was assessed using the entropy water quality index (EWQI) and the results show that above 80% of the water samples are of good quality which is suitable for drinking and other domestic purposes. The potential non-carcinogenic risks of Cr6+, As, F−, and NO3-N and carcinogenic risks of Cr6+ and As to consumers were assessed by the model recommended by the US Environmental Protection Agency (USEPA). The non-carcinogenic health risks in the dry season are higher than the risks in the wet season for both adults and children. Water quality indicators considered in the risk assessment contribute with different degrees to the total non-carcinogenic risk during the dry and wet seasons. The order of the average non-carcinogenic risk values of the chlorinated water and terminal tap water in the dry season was F− > As > NO3-N > Cr6+, while that in the wet season was F− > NO3-N > Cr6+ > As. People face higher carcinogenic risk in the wet season in terms of terminal tap water consumption, while they face higher carcinogenic risk in the dry season in terms of the chlorinated water. Children face almost twice higher the carcinogenic risks than the adults.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an important source of drinking water in China (Li et al. 2018a; Su et al. 2019). In China, approximately two-thirds of cities utilize groundwater as a primary water supply (Zhang et al. 2020). At the same time, groundwater is also an indispensable water resource for irrigation, industrial production, urbanization and other activities related to social development (He and Wu 2019a; He et al. 2019a; Li et al. 2016a; Oişte 2014; Velis et al. 2017; Zhang et al. 2018). However, with the continuous population growth and rapid economic development, the demand for clean fresh water has increased and the water crisis is stressed (Su et al. 2019). Particularly, groundwater over-extraction and wastewater effluents affect the quality and availability of groundwater resources (Mohabansi et al. 2011). Chemical pollutants from human activities such as industry and agriculture cause water pollution problems that directly threaten or pose potential risks to human health (Li and Wu 2019; Milovanovic 2007). According to the WHO (2011), the water as the pollutant carrier can induce 70% of the diseases, and in the world 20% of cancers are associated with water pollution.
Among the various pollutants, long-term exposure to nitrate contaminated water is risky to consumers, especially to infants who are usually prone to a disease known as “blue baby syndrome” caused by exposure to nitrate contaminated drinking water (Mencio et al. 2016; Tian and Wu 2019). Recently, He et al. (2019b) and Li et al. (2019a) reported in their research that children were associated with higher risk than adults when they experienced the similar exposure. In addition, waterborne diseases caused by fluoride are usually regionally endemic (Mahvi et al. 2012). Long-term consumption of water with fluoride exceeding the standard limits (1 mg/L for the Chinese drinking standards and 1.5 mg/L for the WHO guidelines) will lead to dental and skeletal fluorosis (Ali et al. 2016, 2018, 2019; Edmunds and Smedley 2013). Fluoride concentration in drinking water is not only usually controlled by natural factors, such as fluorine-bearing minerals (Adimalla et al. 2018), but is also influenced by anthropogenic contamination (Borgnino et al. 2013; Li et al. 2014a, 2019b). Arsenic (As) and hexavalent chromium (Cr6+) are toxic to human beings. They can cause irreversible damage to the human body (Kumar et al. 2010; He and Wu 2019b; He et al. 2019b; He and Li 2020). For example, arsenic in drinking water can cause various skin diseases, neurological and digestive disorders, and cancer. Long-term exposure to Cr6+ may cause sensitive and fragile skin and respiratory tract (El-Sikaily et al. 2007; He et al. 2020a). Liu et al. (2003) stated that arsenic had been identified as a major risk factor for blackfoot disease. In the study by Shen et al. (2010), a linear relationship between fluoride concentration and the prevalence of dental fluorosis has been identified. In addition, Li et al. (2016b, 2018b, 2019a, b) have done tremendous work on groundwater quality and evaluated health risks, particularly in semi-arid regions of northwest China. All of these studies have proved that securing drinking water quality safety is important for safeguarding the human health and maintaining the sustainable development of society.
The study area, Hancheng City, is located in the Guanzhong Plain of northwest China and belongs to the arid and semi-arid climate zone. There were many villages in this city suffering from endemic diseases many years ago. For example, the drinking water fluorosis was a typical endemic disease in this city because of high F− concentration in drinking groundwater. Several villages had also suffered from high-As groundwater. About 17 years ago, the provincial Department of Water Resource and the local Centers for Disease Control and Prevention carried out some water quality improvement projects and began to supply high-quality groundwater in the study area. As a result, the endemic diseases have almost been cured till now. Regular annual water quality monitoring has been carried out since then. However, the assessment of drinking water quality and associated health risk has not been conducted in this study area. Therefore, this study was conducted (1) to delineate the physicochemical water quality parameters of drinking water in dry and wet seasons, (2) to evaluate drinking water quality using entropy water quality index (EWQI), and (3) to assess the health risks of F−, As, Cr6+, NO3-N to adults and children through oral drinking water intake pathway. This study provides a reference for further strengthening local drinking water supply management and water quality monitoring.
Study Area
Location and Climate
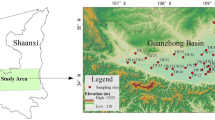
Hancheng City is located on the west bank of the Yellow River in the northeast of Shaanxi Province (Fig. 1). It is a part of the Guanzhong basin, and is 244 km away from Xi'an, the provincial capital city of Shaanxi Province. Hancheng is situated to the west of the Yellow River, and is adjacent to Huanglong County in the west, Yichuan in the north, and Heyang in the south. It ranges from east longitude 110°07′19″ to 110°37′24″, and north latitude 35°18′50″ to 35°52′08″. The maximum distance from east to west is 42.2 km, and the maximum width from south to north is 50.7 km, covering an area of 1621 km2. It has a population of 426,000, including six towns and two administrative districts.
Hancheng experiences warm temperate semi-humid to semi-arid climate, which is a continental monsoon climate with distinct seasons (Fig. 2). According to the amount of precipitation, three seasons are divided: pre-monsoon (February to May), monsoon (June to September), and post-monsoon (October to the following January). The annual average temperature is 13.5 °C. The hottest month is July with an average temperature of 27.1 °C and the coldest month is January with the average temperature of − 0.6 °C. The precipitation in Hancheng is greatly affected by the monsoon rainfall belt in eastern China. The annual average rainfall in this area is 559.7.7 mm, with 63.56% of it concentrated in the monsoon season (wet season hereafter) and 21.26% in the pre-monsoon season (dry season hereafter). Evaporation rate in the study area is high. The annual evaporation rate in this area is over 1800 mm, with approximately 63% occurring in the months of April to August.
Hydrogeology
Hancheng is characterized by a variety of topographies, including mountains, rivers and plains. The study area can be divided into four different areas according to the geology, geomorphology, and conditions of groundwater occurrence and circulation: the Yellow River and its tributaries area, the loess tableland area, the hilly area and the limestone karst area.
The Yellow River and its tributaries area includes the Yellow River floodplain, secondary and tertiary terraces of the Yellow River, and the primary and secondary terraces of the tributaries. The upper layer of the secondary and tertiary terraces of the Yellow River is composed of loess, while the lower part consists of fluvial and lacustrine deposits. The tributaries are overlain with loess in the primary and secondary terraces, and the lower part is alluvial–lacustrine facies. Groundwater occurs in the pores of unconsolidated deposits, and pores and fissures of loose rocks. The groundwater level depth varies with the terrain. It is usually less than 7 m in the Yellow River floodplain, while it increases to 30–50 m in the terraces of the Yellow River and 20 m in the tributary terraces.
The loess tableland area is composed of river and lake facies deposits in the lower section of the profile, which is covered with loess of over 100 m thick in the upper section. The surface elevation is high in the west and low in the east, but the general landform is relatively flat. The groundwater level depth in the loess tableland is more than 30–100 m, and the specific yield of the well is usually less than 10 m3/h (Li et al. 2019b).
The hilly area is distributed in the northwest of the city, accounting for 70% of the total area of the city. This area is mainly composed of Cambrian, Ordovician, Carboniferous, Permian, Triassic sandstone and coal-bearing strata. Groundwater mainly occurs in sandstone pores and fractures, and can be classified into unconfined water and confined water.
The limestone karst area is locally distributed in the study area where Ordovician and Cambrian strata are formed. Ordovician and Cambrian strata have a big productivity of groundwater. However, the karst groundwater is rarely used as it is usually of poor quality with high total hardness and high salinity.
Precipitation, irrigation infiltration and lateral recharge are the main sources of groundwater recharge. The groundwater flows from northwest to southeast as per the general topography. The groundwater level depth in the study area is relatively shallow, so evaporation is one of the main discharge routes. Tectonic springs are formed in local areas, which is a way of groundwater discharge. In addition, mining activities in this area are intense, and mine water discharge and artificial abstraction are important ways of groundwater discharge.
Materials and Methods
Sample Collection and Analysis
For this study, 48 water samples were collected from the drinking water distribution system in both dry (May) and wet (September) seasons in 2018. These sampling sites cover all the six towns and two districts administrated by the city. Twenty-four chlorinated water samples (12 in the dry season and 12 in the wet season) were collected from the water treatment plants where raw water (groundwater) is chlorinated for water treatment before the water is supplied to consumers, and the other 24 samples (also 12 in the dry season and 12 in the wet season) were collected from the pipeline terminals (tap water samples) where consumers can use the chlorinated water. Each of the terminal tap water sampling locations is adjacent to its corresponding chlorinated water sampling location, so they were labeled using the same sample numbers (1 to 12). The dry season samples were labeled as KW1 to KW12, and wet season samples were labeled as FW1 to FW12, respectively. The sampling locations were recorded using portable GPS device and are shown in Fig. 1.
Each water sample was collected using a 2.5-L white pre-cleaned plastic bottle and two 1000-mL glass jars. Before sampling, all containers were washed thoroughly with the water to be collected. Portable pH meter was used to measure the pH on site. The samples analyzed for arsenic (As), nitrate (NO3–N) and ammonia nitrogen (NH4–N) were added with H2SO4 solution to maintain their stability before analysis, and the samples for the analysis of Mn and Cr6+ were added with HNO3 solution. After collection, all water samples were sent to the Hancheng Center for Disease Control and Prevention for physicochemical analysis. Analyzed physicochemical indices include total dissolved solids (TDS), total hardness (TH), SO42−, Cl−, trace metals (Hg, Mn, As and Cr6+), nitrate (NO3–N), ammonia nitrogen (NH4–N), and fluoride (F–). TDS was determined through traditional drying and weighing approach, and EDTA titrimetric method was used to determine the content of total hardness (TH). NO3-N was measured by thymol spectrophotometry. F– was analyzed by fluorine spectrophotometry, Mn was measured by atomic absorption spectrophotometry. Hg and As were measured by atomic absorption method. Cr6+ was analyzed by diphenylcarbohydrazide spectrophotometric method. During the analysis, replicates were introduced to ensure the accuracy of the analysis. The relative errors of the replicates were within ± 5%, indicating acceptable analytical accuracy.
Entropy Water Quality Index
Water quality index (WQI) was first developed by Horton in 1965 (Horton 1965), for which ten commonly used water quality variables such as dissolved oxygen, pH, alkalinity and chloride were used for the calculation of the WQI (Shweta et al. 2013; Amiri et al. 2014). Since then WQI has been widely accepted and applied in various water quality studies worldwide. The entropy concept was first introduced by Shannon (1948), and it was integrated into traditional WQI by Li et al. (2010) and formulated the entropy water quality index (EWQI, Li et al. 2018c; Wu et al. 2017).
Due to the clear concept and objective weight determination, the EWQI has been widely applied by scholars in groundwater quality assessment (Amiri et al. 2014; Li et al. 2018c; Su et al. 2017, 2018; Wu et al. 2011, 2013). The steps of calculating the EQWI are as follows (Li et al. 2010; Wang et al. 2019).
The initial matrix Xij (i = 1,2,…,m; j = 1,2,…,n) should be established based on the results of the physicochemical analysis as follows, where m represents total number of samples to be assessed and n means the number of water quality indicators for each of the samples.
The initial data should be normalized according to Eq. (2), where yij is the normalized data value, (xij)jmax and (xij)jmin are the maximum and minimum values for indicator j. After standardization the standardized matrix Y can be obtained as Eq. (3).
After the standardization, the information entropy for indicator j (ej) can be achieved according to Eqs. (4) and (5). The constant 10–4 in Eq. (4) is the correction factor (Tian and Wu 2019).
After the ej is obtained by Eq. (5), the entropy weight of indicator j (wj) can be calculated as per Eq. (6). The quality rating scale (qj) can be calculated by Eq. (7), where Cj is the concentration of water quality indicator j (mg/L) and Sj represents the standard limit of each indicator (mg/L).
Finally, EWQI can be obtained by Eq. (8).
According to Li et al. (2014b), water quality can be classified into five ranks based on EWQI, ranging from excellent to extremely poor quality and the classification standards are listed in Table 1.
Human Health Risk Assessment
Health risk assessment is a method that links pollutant loads with human health, and then evaluates the potential hazards of contaminants to the health of humans (Giri and Singh 2015; Yu and Tang 2013; Wu et al. 2020). According to the United States Environmental Protection Agency (USEPA), there are four steps in the health risk assessment, including hazard identification, dose–response assessment, exposure assessment and risk characterization (Li et al. 2014b; Momot and Synzynys 2005). A complete health risk assessment should consider all exposure pathways and all possible contaminants in a given environment (USEPA 1989). In this study, however, only the exposure pathway of drinking water intake was considered (Adimalla et al. 2019a, b, 2018), for the most of the contaminants in water have little effect on human health through other exposure pathways as indicated by other water research (Chen et al. 2017; Li et al. 2019b). This research considered the pollutants Cr6+, As, F−, and NO3-N for non-carcinogenic risk assessment, and considered Cr6+ and As for carcinogenic risk assessment. The chronic daily intake per unit weight through oral exposure pathway [mg/(kg day)] can be calculated by Eq. (9), where C is the concentration of the contaminants in water (mg/L), IR represents the water ingestion rate (L/day, IR = 1.5 L/day for adults and 0.7 L/day for children), EF is the exposure frequency (days/year, EF = 365 days/year), ED denotes the exposure duration (years, ED = 30 years for adults and 6 years for children), BW indicates the average body weight (kg, BW = 60 kg for adults and 15 kg for children), and AT means the average exposure time (days, AT = 10,950 days for adults and 2190 days for children).
The non-carcinogenic hazard quotient (HQ) through oral pathway can be calculated by Eq. (10), and j is the water quality indicator. In this equation, RfDoral means the reference dosage for non-carcinogenic through oral intake exposure pathway. RfDoral = 0.003 mg/(kg day) for Cr6+, 0.04 mg/(kg day) for F−, 0.0003 mg/(kg day) for As and 1.6 mg/(kg day) for NO3-N (USEPA 2019).
The non-carcinogenic hazard index (HI) can be calculated by Eq. (11)
Cr6+ and As are also major risk factors for chemical carcinogens (Cabral Pinto and Ferreira da Silva 2019). The carcinogenic risk can be calculated by Eq. (12), where SF is the slope factor of the carcinogenic contaminants mg/(kg day), which is 1.5 mg/(kg day) for As, and 0.5 mg/(kg day) for Cr6+ (USEPA 2019). For carcinogenic risk, the exposure duration (ED) in Eq. (9) should be 70 years for adults and children due to the life-long effects of the carcinogenic risk. Correspondingly, the AT value should be 25,550 days for adults and children.
Result and Discussion
Physicochemical Water Quality Parameters
Chlorinated Water
The physicochemical parameters of the chlorinated water samples in the dry season are listed in Table 2. As Table 2 shows, pH values for water samples are slightly alkaline, ranging from 7.07 to 7.73 in the dry season and 7.29 to 7.85 in the wet season. According to the standards set by China (Ministry of Health of the P.R. China and Standardization Administration of the P.R. China 2017), the acceptable pH range for drinking water is 6.5–8.5. Therefore, all the chlorinated water samples in both dry and wet seasons are suitable for drinking purpose with respect to pH. It is also noticeable that the water has a slightly higher pH in the wet season than the dry season due to stronger water–rock interactions in the wet season.
TDS represents the water salinity (Li et al. 2017; Wu et al. 2019). TDS of the chlorinated water ranges from 305 to 662 mg/L with an average value of 400 mg/L in the dry season and varies from 240 to 1100 mg/L with an average value of 563 mg/L in the wet season with one sample (FW8) exceeding the permissible TDS limit for drinking water (1000 mg/L). Higher TDS is observed in the wet season probably due to the greater evaporation rate and the high concentration of SO42– in the wet season.
TH reflects the total concentration of Ca2+ and Mg2+ in water, which is an important indicator for water quality monitoring (Jin et al. 2012; Li et al. 2018c). Water can be classified as extremely soft water (TH < 75 mg/L), soft water (75 < TH < 150 mg/L), moderately hard water (150 < TH < 300 mg/L), hard water (300 < TH < 450 mg/L), and extremely hard water (TH > 450 mg/L). Water with high TH will affect its suitability for domestic uses. The permissible limit of TH for drinking purpose is 450 mg/L according to the Chinese national standard (Ministry of Health of the P.R. China and Standardization Administration of the P.R. China 2017). In this study, TH ranges from 164.9 to 539.3 mg/L in the dry season and varies from 127.5 to 323.0 mg/L in the wet season. Among the chlorinated water samples collected in the dry season, one water sample exceeds the acceptable limit of TH for drinking (KW10). One sample (KW8) is classified as hard water, and the rest 10 samples are classified as moderately hard water. In the wet season, all water samples are below the standard limit for drinking purpose. Among all the chlorinated water samples in the wet season, one sample is classified as soft water (FW1), two samples are hard water (FW8, FW11), and the rest nine samples are moderately hard water.
SO42− and Cl– are common water quality indicators, and generally they do not have health concerns (WHO 2011). However, they may affect the taste of drinking water, and sometimes they are indicators of anthropogenic water pollution (Lin et al. 2018). The Chinese drinking water quality standards (Ministry of Health of the P.R. China and Standardization Administration of the P.R. China 2017) set the acceptable limits for both SO42− and Cl− at 250 mg/L. In this study, SO42− ranges from 5 to 167 mg/L in the dry season and 46 to 290 mg/L in the wet season. Cl− ranges from 11.2 to 166.6 mg/L in the dry season and 11.2 to 127.1 mg/L in the wet season. In this study, only one water sample collected in the wet season exceeds the permissible limit of SO42− (FW9). SO42− shows higher concentration in the wet season than the dry season due to the higher evaporation rate and stronger water–rock interactions in the wet season, while Cl– shows lower concentration in the wet season, probably because of the low Cl− in the recharge water such as precipitation.
Nitrogen and its compounds play an important role in agricultural production (Zhang et al. 2018). The concentration of nitrate is commonly used to reflect the nonpoint agricultural pollution in agricultural areas (Chen et al. 2016; Barros et al. 2012). The maximum permissible limits of NH4-N and NO3-N are 0.5 and 20 mg/L, respectively, according to the Chinese groundwater quality standard. In this study, NH4-N concentration in the chlorinated water is low in the dry season (0.01 mg/L), while NO3-N ranges from 0.70 to 5.35 mg/L in the dry season, which indicates that the chlorinated water is suitable for domestic uses in the dry season. In the wet season, NH4-N and NO3-N of the samples are in the ranges of 0.06 to 0.24 mg/L and 0.25 to 29.70 mg/L, respectively. One sample exceeds the permissible limit of NO3-N for domestic uses (FW11).
Fluoride (F−) is an essential element for human health at low concentrations (Adimalla and Venkatayogi 2017; Odiyo and Makungo 2018; He et al. 2020b), but is toxic at high levels (Fordyce et al. 2007; Li et al. 2014a). Generally, the desirable concentration of F− in drinking water ranges from 0.5 to 1.0 mg/L (Wu et al. 2015). WHO (2011) has set the maximum acceptable level of F− in drinking water as 1.5 mg/L, while the Chinese national standard limit is 1.0 mg/L (Ministry of Health of the P.R. China and Standardization Administration of the P.R. China 2017). In this study, F− ranges from 0.03 to 0.07 mg/L in the dry season and 0.19 to 0.60 mg/L in the wet season. As can be seen from the table, all the chlorinated water samples are within the acceptable limit of F– for drinking purpose. This has to be attributed to the water quality improvement projects carried out by the Shaanxi Provincial Department of Water Resources and the Hancheng Centers for Disease Control and Prevention in this area, which have helped a lot to reduce the prevalence of drinking water fluorosis. In general, there are two ways of reducing the fluoride content in drinking water: (1) find a new source of water with low fluoride content (2) use physical and chemical methods such as precipitation–flocculation, ion exchange, lime softening and other measures to reduce the fluoride concentration. However, finding a new source of water with low fluoride is the most convenient, economical, and effective way to control drinking water fluorosis. Therefore, the local government drilled new wells and supplied low fluoride groundwater to residents.
Cr6+ is toxic to human health and is a source of carcinogens to human health (He and Li 2020). The maximum permissible limit of Cr6+ for drinking purpose is 0.005 mg/L (Ministry of Health of the P.R. China and Standardization Administration of the P.R. China 2017). In this study, the Cr6+ concentration in the chlorinated water samples ranges from 0.002 to 0.012 mg/L in the dry season and 0.002 to 0.031 mg/L in the wet season. One sample (KW7) in the dry season and two samples (FW4, FW10) in the wet season exceed the permissible limit of Cr6+ for drinking purpose. All the chlorinated water samples show low concentrations of As, Hg and Mn. They are below the acceptable limits for drinking water.
Terminal Tap Water
The statistical analysis results for the terminal tap water samples are listed in Table 3. Similar to the chlorinated water, TDS of the terminal tap water is higher in the wet season. Especially, one terminal tap water sample in the wet season exceeds the acceptable limit of TDS for drinking (FW9). TH of the terminal tap water is in the range of 168.4–348.3 mg/L in the dry season and 138.9–350.9 mg/L in the wet season. All water samples are considered suitable for domestic purpose with respect to TH in both dry and wet seasons.
For anions, SO42− ranges from 5 to 165 mg/L in the dry season and 84 to 260 mg/L in the wet season. None of the samples in the dry season exceeds the limit of SO42− for drinking purpose, but one terminal tap water sample exceeds the acceptable limit of SO42− (FW9) in the wet season. Cl− ranges from 12.9 to 172.3 mg/L and 11.1 to 124.9 mg/L in the dry and wet seasons, respectively, and all terminal tap samples in the dry and wet seasons are below the permissible limit of Cl− for drinking purpose. The concentration of NO3-N ranges from 0.62 to 9.10 mg/L in the dry season, and 0.25 to 29.70 mg/L in the wet season with one sample (FW11) exceeding the standard limit. The concentration of NH4-N in the dry season samples is low (0.01 mg/L), and ranges from 0.06 to 0.34 mg/L in the wet season. In this study, the concentration of F– collected in the dry season ranges from 0.04 to 0.70 mg/L, and Cr6+ ranges from 0.002 to 0.011 mg/L. In wet season, F– and Cr6+ range from 0.20 to 0.56 mg/L and 0.002 to 0.033 mg/L, respectively. All terminal tap water samples are suitable for drinking with respect to F–, while some samples (KW7, FW1, FW4, FW10 and FW11) have higher Cr6+ concentration than the permissible limit. With respect to other trace metals such as As, Hg and Mn, the concentrations are within the acceptable limits for drinking purpose.
In this study, the sampling locations of the terminal tap water are adjacent to the corresponding chlorinated water sampling locations, so in the dry and wet seasons, the physicochemical water quality parameters of the terminal tap water and the corresponding chlorinated water samples taken from the same sites generally show similar trends. The mean pH, TDS, SO42–, Cr6+, NH4-N and NO3-N values of both the chlorinated water and the terminal tap water are higher in the wet season than in the dry season, indicating the stronger impacts of climate and agricultural activities in the wet season. While the mean concentrations of TH, Cl–, F–, As, Hg and Mn are lower in the wet season than in the dry season, suggesting the dilution effects of recharge water on the source water. With respect to water quality indices in a given season, some rules can also be observed. For water samples collected in the dry season, the mean levels of pH, TH, TDS, SO42–, As and F– are lower in the terminal tap water, while the mean concentrations of Cl– and NO3-N become higher in the tap water compared to those in the chlorinated water. With respect to water samples in the wet season, the mean concentrations of all water quality indices except pH are higher in the terminal tap water than those in the chlorinated water.
Water Quality Assessment Using EWQI
In this study, the water quality parameters of As, Cr6+, NO3-N, F−, TH and TDS were selected to perform the overall water quality assessment using the EWQI, and the calculation results of the EWQI for chlorinated water samples and terminal tap water samples are shown in Table 4.
EWQI values of all the chlorinated water samples collected in the dry season range from 24.42 to 44.53. Three samples are classified as excellent-quality water (rank 1) and 9 samples are classified as good-quality water (rank 2), which are suitable for various purposes without treatment (Su et al. 2018). The assessment results for the wet season samples range from 25.03 to 61.90 based on EWQI. Eleven samples are classified as good-quality water (rank 2) and one sample is medium-quality water (rank 3) that can be used for drinking purpose after some initial treatment. The study results show that the chlorinated water quality in both dry and wet seasons is generally suitable for drinking purpose. In addition, the water quality in the dry season is generally better than that in the wet season except at the No. 1, 7 and 12 sampling sites. The reasons may be the high temperature and high evaporation rate during the wet season, which is responsible for evaporation and concentration effect of the groundwater. In addition, the rise of surface water level and groundwater level in the wet season makes it easier for the surface water, which is vulnerable to environmental pollution such as animal excrement, human waste and so on, to infiltrate into groundwater.
In Table 4, the EWQI values of all the collected terminal tap water samples in the dry season are from 23.83 to 42.55. All the terminal tap water samples collected in the dry season belong to good-quality category (rank 2), except one sample being classified as excellent-quality water (rank 1). In the wet season, EWQI values for the collected terminal tap water samples range from 23.20 to 66.37, including one excellent-quality water sample (rank 1), ten good-quality water samples (rank 2) and one medium-quality water sample (rank 3). The results show that all the terminal tap water samples collected in both wet and dry seasons are of good quality for domestic purpose. However, similar to the chlorinated water, the water quality of the terminal tap in the dry season is generally better than that in the wet season except at the sampling sites 5, 6 and 12. Drinking water is transported through pipes, and high temperature will also affect the reproduction of microorganisms in the pipes, then an increase in bacteria, microorganisms, leads to an increase in metabolites, thus affecting comprehensive quality of water. The main factors affecting water quality are TDS and NO3-N. The concentration of NO3-N in different seasons is related to the agricultural activities. Maize and wheat were planted in the study area mainly after May, and therefore, the concentration of groundwater NO3-N in the wet season was higher than that in the dry season. TDS is a measure of groundwater salinity. TDS is higher in the wet season may be attributed to the evaporation and concentration effect of groundwater.
Overall, the water quality of both the chlorinated water and the terminal tap water is good for domestic purposes. For both the chlorinated water and the terminal tap water, water quality in the dry season is better than that in the wet season. This indicates that climatic factors can have significant impacts on groundwater quality. Comparison of the chlorinated water and the terminal tap water shows that the chlorinated water has relatively better quality than the terminal tap water. According to the investigation, most of the chlorinated water has water supply and disinfection equipment, but most of the terminal tap water will not be further sterilized. In addition, the quality of the terminal tap water is greatly affected by the material and the year in use of the pipelines. The chlorinated water is corrosive, and it is likely to lead to the increase of Fe, Mn and microorganisms in the terminal tap water. High temperature and microbial growth will consume residual chlorine, so the amount of chlorine for disinfection should be properly adjusted, and appropriately raised in the dry season and reduced in the wet season to ensure good water quality. The terminal tap water should also be filtered using home-use devices before being consumed.
Health Risk Assessment
Risk assessment uses relevant models and study area specificities to identify and quantify the potential risks to human health due to exposure to contaminants via various exposure pathways (Varol and Davraz 2016). Many previous studies (Adimalla and Li 2019; Adimalla and Wu 2019) have shown that there will be potential health risks even the concentrations of water quality indices are within the drinking water standard limits (He and Li 2020). Therefore, health risk assessment is an important and supplementary to traditional water quality assessment. In the study area, previous endemic diseases include Kashin-Beck disease, waterborne fluorosis, and drinking water arsenism (Yousefi et al. 2019; He et al. 2020b). Therefore, Cr6+, As, F− and NO3-N were selected to perform the non-carcinogenic health risks, and Cr6+ and As were used for quantifying the carcinogenic health risks for adults and children through drinking water intake in the study area.
Chlorinated water
Health risk assessment results of the chlorinated water are shown in Table 5. As shown in Table 5, the non-carcinogenic hazard index (HI) of the chlorinated water in the dry season ranges from 0.313 to 0.798 with a mean of 0.456 for adults, indicating that the total non-carcinogenic risk of all chlorinated water samples is within the acceptable limit (HI = 1) for adults. With respect to each individual water quality parameter, the non-carcinogenic HQ values of Cr6+, As, F− and NO3-N for adults are in the ranges of 0.017–0.100, 0.042–0.508, 0.156–0.431 and 0.011–0.084, respectively, which suggested that adult consumers are in low health risk with respect to exposure to these water quality indices in the dry season. In the wet season, the HI values range from 0.200 to 0.722 with a mean of 0.329. The risks of all chlorinated water samples in the wet season are considered low to adults and are within the acceptable limit value (HI = 1). The ranges of HQ for Cr6+, As, F− and NO3-N are 0.017–0.258, 0.042–0.042, 0.119–0.375 and 0.004–0.464 in the wet season.
With respect to the non-carcinogenic health risk to children due to intake of the chlorinated water, the HI values in the dry season range from 0.585 to 1.490 with a mean of 0.852. Risk of two samples (KW11 and KW12) exceeds the acceptable limit (HI = 1). The non-carcinogenic HQ values of Cr6+, As, F− and NO3-N range from 0.031 to 0.187, 0.078 to 0.949, 0.291 to 0.805 and 0.020 to 0.156, respectively, which suggests that the non-carcinogenic risk of individual contaminant is acceptable to children in the dry season. In the wet season, HI varies from 0.373 to 1.348 with an average value of 0.614. The risk of one sample (FW11) exceeds the permissible limit. HQ values for Cr6+, As, F− and NO3-N are in the ranges of 0.031–0.482, 0.078–0.078, 0.222–0.700 and 0.007–0.866, respectively, for children.
The greatest non-carcinogenic risks to adults and children in the dry season are caused by F− which contributes 53.97% to the value of HI, while the Cr6+ contributes the least to the total non-carcinogenic risk. As and NO3-N contribute 32.87% and 8.00% to the total non-carcinogenic risks, respectively. In the wet season, HQ of F− is highest for both adults and children (57.34%) and followed by NO3-N (16.60%), Cr6+ (13.36%) and As (12.70%). In summary, the average non-carcinogenic risks to adults and children are in the order of F– > As > NO3-N > Cr6+ in the dry season and F– > NO3-N > Cr6+ > As in the wet season (Fig. 3).
The carcinogenic risks due to exposure to As and Cr6+ are shown in Table 5. As shown in Table 5, the total carcinogenic risk (CR) for adults in the dry season ranges from 4.38E−05 to 2.54E−04 with an average of 1.03E−04, while that in the wet season varies from 4.38E−05 to 4.07E−04 with an average of 8.45E−05. With respect to children, the total carcinogenic risk in the dry season ranges from 8.17E−05 to 4.74E−04 with an average value of 1.92E−04, and in the wet season the CR value ranges from 8.17E−05 to 7.58E−04 with an average of 1.58E−04. The results show that the carcinogenic risk values in dry and wet seasons are higher than the acceptable limit of 1E−06 (Ministry of Environmental Protection of the People’s Republic of China 2014) for both adults and children. As per the average values of CR, As contributes 65.60% to the total CR, and Cr6+ accounts for 34.40% of the total CR in the dry season. Cr6+ accounts for 77.75% of the CR and As accounts for 22.25% of the CR in the wet season.
In summary, adults and children face higher non-carcinogenic and carcinogenic health risks in the dry seasons than in the wet seasons, because higher F− and As concentrations were observed in the dry season than in the wet season. In addition, children face higher health risks than adults in both dry and wet seasons, which is in accordance with previous research (Wu and Sun 2016).
Terminal Tap Water
The health risk estimated for the terminal tap water is shown in Table 6. In the dry season, the total non-carcinogenic risk of the terminal tap water for adults ranges from 0.254 to 0.721 with a mean value of 0.421, indicating that the total non-carcinogenic risk of all terminal tap water samples is within the permissible limit value (HI = 1) for adults. The non-carcinogenic HQ values of Cr6+, As, F− and NO3-N for adults are in the range of 0.017–0.092, 0.042–0.533, 0.027–0.438 and 0.010–0.142, respectively. Similarly, in the wet season, the total non-carcinogenic risk of the terminal tap water for adults ranges from 0.193 to 0.847 with a mean value of 0.351. All the terminal tap water samples show lower risk value than the acceptable limit (HI = 1) for adults in the wet season. The non-carcinogenic HQ values of Cr6+, As, F− and NO3-N in the wet season range within 0.017–0.275, 0.042–0.042, 0.131–0.350 and 0.004–0.464, respectively.
With respect to children, the HI value in the dry season ranges from 0.473 to 1.347 with a mean value of 0.785. The HI values in three samples (KW1, KW11 and KW12) surpass the acceptable limit, indicating a relatively higher risk to children than adults. The non-carcinogenic HQ values of Cr6+, As, F− and NO3-N range from 0.031 to 0.171, 0.078 to 0.996, 0.050 to 0.817 and 0.018 to 0.265, indicating that the risk is acceptable regarding the individual water quality parameter. In the wet season, the HI value for children ranges from 0.361 to 1.582 with a mean value of 0.655. The HI values of two samples (FW10 and FW11) in the wet season are higher than the acceptable limit. The range of HQ for Cr6+, As, F− and NO3-N is 0.031–0.513, 0.078–0.078, 0.233–0.653 and 0.007–0.866 in the wet season for children. The total non-carcinogenic risk in the dry season is contributed mostly by F− (56.51%), while is least contributed by Cr6+ (5.46%). As and NO3-N contribute 29.00% and 9.03% to the total non-carcinogenic risks, respectively. In the wet season, HI is contributed mostly by F− (52.69%) and followed by NO3-N (17.83%), Cr6+ (17.61%) and As (11.87%). According to the average values of HQ, the non-carcinogenic risk of the terminal tap water to adults and children is F− > As > NO3-N > Cr6+ in the dry season and F− > NO3-N > Cr6+ > As in the wet season (Fig. 4). The order is the same to that of the chlorinated water.
In terms of the carcinogenic risk (CR) for adults in the dry season, the values range from 4.38E−05 to 2.65E−04 with an average of 8.91E−05, while in the wet season, the value of total CR ranges from 4.38E−05 to 4.32E−04 with an average of 1.12E−04. Similarly, in the dry season, the total CR for children ranges from 8.17E−05 to 4.95E−04 with an average value of 1.66E−04, while in the wet season, the total CR value ranges from 8.17E−05 to 8.05E−04 with a mean of 2.08E−04. As shown above, the carcinogenic health risks are higher than the acceptable level of 1E−06 (Ministry of Environmental Protection of the People’s Republic of China 2014) in both dry and wet seasons for adults and children. In addition, the carcinogenic risk is higher in the wet season than in the dry season for both adults and children as per the mean CR values. Children face higher carcinogenic risk than adults in both seasons, and children bear almost twice the total carcinogenic risks than the adults, because children are more sensitive to the health risk damage of these contaminants, which is similar to the results of previous research (He and Wu 2019a). This is due to the physiological differences between children and adults. According to the average values of CR, As (61.39% of the total CR) contributes more to the total carcinogenic risks than Cr6+ (38.61% of the total CR) in the dry season, while Cr6+ (83.18% of the total CR) contributes to a greater degree to the total carcinogenic risk than As (16.82% of the total CR) in the wet season.
In summary, the non-carcinogenic risk caused by individual water quality indices of the terminal tap water is generally within the acceptable level in both seasons with respect to both adults and children. However, adults and children face higher non-carcinogenic risk of the terminal tap water in the dry season according to the mean HI values, because the concentrations of F− and As are higher in the dry season. As for the non-carcinogenic HQ values, the HQ values of As and F− are higher in the dry season, while the values of F− and NO3-N are higher in the wet season. Both adults and children face higher carcinogenic risk in the wet season than in the dry season in terms of terminal tap water intake.
Comparison of the health risk via the intake of the chlorinated water and the terminal tap water in the dry and wet seasons shows that the order of the non-carcinogenic health risks of the chlorinated water and the terminal tap water showed consistent rules, and the order is F− > As > NO3-N > Cr6+ in the dry season and F− > NO3-N> Cr6+ > As in the wet season. The non-carcinogenic HQ values of As and F− are higher in the dry season and the values of Cr6+ and NO3-N are higher in the wet season for both the chlorinated water and the terminal tap water. In the dry season, the average non-carcinogenic HI value of the chlorinated water is higher than that of the terminal tap water, while opposite results are observed in the wet season. Similarly, in the dry season, the average total CR value of the chlorinated water is higher than that of the terminal tap water for adults and children, while in the wet season, the average total CR value of the chlorinated water is lower than that of the terminal tap water.
Improving the rural environment and drinking water hygiene is closely related to the physical health of the residents. It is an inevitable requirement for realizing the harmonious development of human and environment. China has invested a lot of funds, manpower and material resources to prevent and cure the endemic fluorine disease and improve the drinking water quality. Some achievements have been made, and the prevalence of fluorosis has decreased after the water quality improvement projects were carried out. However, to maintain the effectiveness of prevention and treatment in the long run, two aspects must be considered.
From the perspective of governmental departments, they need to support more water improvement projects and find high-quality water source for domestic supply. What is even more important is that the protection, maintenance and management of water supply facilities must be strengthened to maintain the performance of these water improvement projects. At the same time, regular monitoring of more drinking water quality parameters should be carried out, as the overall water quality is controlled by many individual parameters. From the perspective of the local residents, household filters and water boiling should be encouraged to improve the terminal tap water quality before consumption. This will be effective in reducing the health risks.
Conclusions
In this study, based on the water quality monitoring data in Hancheng City in 2018, the seasonal changes of water quality and the potential health risks to human health through oral exposure pathway in the study area were assessed. The main conclusions are as follows.
-
(1)
For the chlorinated water and the terminal tap water, the average concentration of pH, TDS, SO42−, NH4-N, Cr6+ and NO3-N is greater in the wet season than in the dry season, while the mean concentrations of TH, Cl−, F−, As, Hg and Mn are higher in the dry season. For water samples collected in the dry season, the mean levels of pH, TH, TDS, SO42− and F– are higher in the terminal tap water, while the mean concentrations of Cl− and NO3-N become higher in the tap water compared to those in the chlorinated water. With respect to water samples in the wet season, the mean concentrations of all water quality indices except pH are higher in the terminal tap water than those in the chlorinated water.
-
(2)
Above 80% of the water samples are of good quality which is suitable for drinking and other purposes according to the EWQI. The water quality in the dry season is better than that in the wet season. The evaporation and concentration effect of groundwater is an important reason responsible for the relatively poorer water quality in the wet season. The water level rise in both surface water and groundwater in the wet season makes it easier for pollutants to enter the groundwater, resulting in the deterioration of the source water. Human activities, such as pesticide residues and fertilizer application in the wet season, may also affect the comprehensive water quality.
-
(3)
The individual non-carcinogenic risk of Cr6+, As, F− and NO3-N is within the acceptable level. For adults, the HI values of all the chlorinated water and terminal tap water in the dry and wet seasons are within the acceptable limit. For children, the HI values of the chlorinated water KW11, KW12 and the terminal tap water KW1, KW11 and KW12 in the dry season exceed the permissible limit, while in the wet season, one chlorinated water sample (FW11) and two terminal tap water samples (FW10, FW11) surpass the acceptable limit. This indicates that attention is required to reduce the total non-carcinogenic risks to ensure the safety of children though the HQ values of individual contaminants are below the acceptable limit.
-
(4)
The carcinogenic risks of the chlorinated water and the terminal tap water to both adults and children in both seasons should not be overlooked. Children bear almost twice the carcinogenic risks than adults. Although the endemic disease had been eliminated by improving the drinking water quality, the carcinogenic health risk still exists and needs particular attention.
References
Adimalla N, Li P (2019) Occurrence, health risks, and geochemical mechanisms of fluoride and nitrate in groundwater of the rock-dominant semi-arid region, Telangana State, India. Hum Ecol Risk Assess 25(1–2):81–103. https://doi.org/10.1080/10807039.2018.1480353
Adimalla N, Venkatayogi S (2017) Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environ Earth Sci 76(1):45. https://doi.org/10.1007/s12665-016-6362-2
Adimalla N, Wu J (2019) Groundwater quality and associated health risks in a semi-arid region of south India: Implication to sustainable groundwater management. Hum Ecol Risk Assess 25(1–2):191–216. https://doi.org/10.1080/10807039.2018.1546550
Adimalla N, Vasa SK, Li P (2018) Evaluation of groundwater quality, Peddavagu in Central Telangana (PCT), South India: an insight of controlling factors of fluoride enrichment. Model Earth Syst Environ 4(2):841–852. https://doi.org/10.1007/s40808-018-044
Adimalla N, Qian H, Li P (2019a) Entropy water quality index and probabilistic health risk assessment from geochemistry of groundwaters in hard rock terrain of Nanganur County, South India. Geochemistry. https://doi.org/10.1016/j.chemer.2019.125544
Adimalla N, Li P, Qian H (2019b) Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: A special emphasis on human health risk assessment (HHRA). Hum Ecol Risk Assess 25(5):1107–1124. https://doi.org/10.1080/10807039.2018.1460579
Ali S, Thakur SK, Sarkar A, Shekhar S (2016) Worldwide contamination of water by fluoride. Environ Chem Lett 14:291–315. https://doi.org/10.1007/s10311-016-0563-5
Ali S, Shekhar S, Bhattacharya P, Verma G, Gurav T, Chandrashekhar AK (2018) Elevated fluoride in groundwater of Siwani Block, Western Haryana, India: a potential concern for sustainable water supplies for drinking and irrigation. Groundw Sustain Dev 7:410–420. https://doi.org/10.1016/j.gsd.2018.05.008
Ali S, Fakhri Y, Golbini M, Thakur SK, Alinejad A, Parseh I, Shekhar S, Bhattacharya P (2019) Concentration of fluoride in groundwater of India: a systematic review, meta-analysis and risk assessment. Groundw Sustain Dev 9:100224. https://doi.org/10.1016/j.gsd.2019.100224
Amiri V, Rezaei M, Sohrabi N (2014) Groundwater quality assessment using entropy weighted water quality index (EWQI) in Lenjanat, Iran. Environ Earth Sci 72(9):3479–3490. https://doi.org/10.1007/s12665-014-3255-0
Barros R, Isidoro D, Aragüés R (2012) Irrigation management, nitrogen fertilization and nitrogen losses in the return flows of La Violada irrigation district (Spain). Agr Ecosyst Environ 155:161–171. https://doi.org/10.1016/j.agee.2012.04.004
Borgnino L, Garcia MG, Bia G, Stupar YV, Le Coustumer P, Depetris PJ (2013) Mechanisms of fluoride release in sediments of Argentina’s central region. Sci Total Environ 443:245–255. https://doi.org/10.1016/j.scitotenv.2012.10.093
Cabral Pinto MMS, Ferreira da Silva EA (2019) Heavy metals of Santiago Island (Cape Verde) alluvial deposits: baseline value maps and human health risk assessment. Int J Environ Res Public Health 16(1):2. https://doi.org/10.3390/ijerph16010002
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, Northwest China. Expo Health 8(3):349–359. https://doi.org/10.1007/s12403-016-0208-8
Chen J, Qian H, Wu H, Gao Y, Li X (2017) Assessment of arsenic and fluoride pollution in groundwater in Dawukou area, northwest China, and the associated health risk for inhabitants. Environ Earth Sci 76:314. https://doi.org/10.1007/s12665-017-6629-2
Currell M, Cartwright I, Raveggi M, Han D (2011) Controls on elevated fluoride and arsenic concentrations in groundwater from the Yuncheng Basin, China. Appl Geochem 26(4):540–552. https://doi.org/10.1016/j.apgeochem.2011.01.012
Edmunds WM, Smedley PL (2013) Fluoride in natural waters. In: Selinus O (ed) Essentials of medical geology. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4375-5_13
El-Sikaily A, El Nemr A, Khaled A, Abdelwehab O (2007) Removal of toxic chromium from wastewater using green algae Ulva lactuca and its activated carbon. J Hazard Mater 148(1–2):216–228. https://doi.org/10.1016/j.jhazmat.2007.01.146
Fordyce FM, Vrana K, Zhovinsky E, Povoroznuk V, Toth G, Hope BC, Iljinsky U, Baker J (2007) A health risk assessment for fluoride in central Europe. Environ Geochem Health 29(2):83–102. https://doi.org/10.1007/s10653-006-9076-7
Giri S, Singh AK (2015) Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ Monit Assess 187(3):63. https://doi.org/10.1007/s10661-015-4265-4
He X, Li P (2020) Surface water pollution in the middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): occurrence, sources and health risks. Expo Health. https://doi.org/10.1007/s12403-020-00344-x
He S, Wu J (2019a) Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, northwest China. Expo Health 11(2):125–137. https://doi.org/10.1007/s12403-018-0289-7
He S, Wu J (2019b) Relationships of groundwater quality and associated health risks with land use/land cover patterns: a case study in a loess area, northwest China. Hum Ecol Risk Assess 25(1–2):354–373. https://doi.org/10.1080/10807039.2019.1570463
He X, Wu J, He S (2019a) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess 25(1–2):32–51. https://doi.org/10.1080/10807039.2018.1531693
He X, Wu J, Guo W (2019b) Karst spring protection for the sustainable and healthy living: the examples of Niangziguan spring and Shuishentang spring in Shanxi, China. Expo Health 11(2):153–165. https://doi.org/10.1007/s12403-018-00295-4
He X, Li P, Wu J, Wei M, Ren X, Wang D (2020a) Poor groundwater quality and high potential health risks in the Datong Basin, northern China: research from published data. Environ Geochem Health. https://doi.org/10.1007/s10653-020-00520-7
He X, Li P, Ji Y, Wang Y, Su Z, Elumalai V (2020b) Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: occurrence, distribution and management. Expo Health. https://doi.org/10.1007/s12403-020-00347-8
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–305
Jin J, Qian H, Chen Y, Xi W (2012) Assessment of groundwater quality based on matter element extension model. J Chem 2013:715647. https://doi.org/10.1155/2013/715647
Kumar A, Adak P, Gurian PL, Lockwood JR (2010) Arsenic exposure in US public and domestic drinking water supplies: a comparative risk assessment. J Eposure Sci Environ Epidemiol 20(3):245–254. https://doi.org/10.1038/jes.2009.24
Li P, Wu J (2019) Drinking water quality and public health. Expo Health 11(2):73–79. https://doi.org/10.1007/s12403-019-00299-8
Li P, Qian H, Wu J (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, northwest China. E-J Chem 7(S1):S209–S216. https://doi.org/10.1155/2010/451304
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014a) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145. https://doi.org/10.1007/s12665-013-2691-6
Li P, Wu J, Qian H (2014b) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36(4):693–712. https://doi.org/10.1007/s10653-013-9590-3
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016a) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo Health 8(3):331–348. https://doi.org/10.1007/s12403-016-0193-y
Li P, Zhang Y, Yang N, Jing L, Yu P (2016b) Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist Town of China. Expo Health 8(2):239–252. https://doi.org/10.1007/s12403-016-0198-6
Li P, Feng W, Xue CT, Tian R, Wang S (2017) Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake tourist area, Northwest China. Expo Health 9(3):213–225. https://doi.org/10.1007/s12403-016-0237-3
Li P, He S, He X, Tian R (2018a) Seasonal hydrochemical characterization and groundwater quality delineation based on matter element extension analysis in a paper wastewater irrigation area, northwest China. Expo Health 10(4):241–258. https://doi.org/10.1007/s12403-017-0258-6
Li P, He S, Yang N, Xiang G (2018b) Groundwater quality assessment for domestic and agricultural purposes in Yan’an city, northwest China: implications to sustainable groundwater quality management on the Loess Plateau. Environ Earth Sci 77(23):775. https://doi.org/10.1007/s12665-018-7968-3
Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K (2018c) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, Northwest China. Mine Water Environ 37(2):222–237. https://doi.org/10.1007/s10230-017-0507-8
Li P, He X, Guo W (2019a) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an city on the loess plateau of northwest China. Hum Ecol Risk Assess 25(1–2):11–31. https://doi.org/10.1080/10807039.2018.1553612
Li P, He X, Li Y, Xiang G (2019b) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: a case study of Tongchuan, Northwest China. Expo Health 11(2):95–107. https://doi.org/10.1007/s12403-018-0278-x
Lin X, Yang A, Chen F (2018) Measurement and evaluation of residual disinfection by products in tap water from Xiamen. IOP Conf Ser: Earth Environ Sci 146(1):012015. https://doi.org/10.1088/1755-1315/146/1/012015
Liu C, Lin K, Kuo Y (2003) Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci Total Environ 313(1–3):77–89. https://doi.org/10.1016/s0048-9697(02)00683-6
Mahvi AH, Ghanbarian M, Ghanbarian M, Khosravi A, Ghanbarian M (2012) Determination of fluoride concentration powdered milk in Iran 2010. Br J Nutr 107(7):1077–1079. https://doi.org/10.1017/S0007114511003941
Mencio A, Mas-Pla J, Otero N, Regas O, Boy-Roura M, Puig R, Bach J, Domenech C, Zamorano M, Brusi D, Folch A (2016) Nitrate pollution of groundwater; all right, but nothing else? Sci Total Environ 539:241–251. https://doi.org/10.1016/j.scitotenv.2015.08.151
Milovanovic M (2007) Water quality assessment and determination of pollution sources along the Axios/Vardar River, Southeastern Europe. Desalination 213(1–3):159–173. https://doi.org/10.1016/j.desal.2006.06.022
Ministry of Environmental Protection of the People’s Republic of China (2014) Technical guidance for risk assessment of contaminated sites, (HJ 25.3-2014). China Environmental Science Press, Beijing (in Chinese)
Ministry of Health of the P.R. China, Standardization Administration of the P.R. China (2017) Standards for groundwater quality (GB14848–2017). China Standard Press, Beijing (in Chinese)
Mohabansi N, Tekade P, Bawankar S (2011) Physico-chemical Parameters of Textile Mill Effluent Hinganghat, Dist. Wardha (M.S.). Curr World Environ 6(1):165–168. https://doi.org/10.12944/CWE.6.1.24
Momot O, Synzynys B (2005) Toxic aluminium and heavy metals in groundwater of middle Russia: health risks assessment. Int J Environ Res Public Health 2(2):214–218. https://doi.org/10.3390/ijerph2005020003
Odiyo JO, Makungo R (2018) Chemical and microbial quality of groundwater in siloam village, implications to human health and sources of contamination. Int J Environ Res Public Health 15(2):317. https://doi.org/10.3390/ijerph15020317
Oişte AM (2014) Groundwater quality assessment in urban environment. Int J Environ Sci Technol 11(7):2095–2102. https://doi.org/10.1007/s13762-013-0477-8
Shannon CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423
Shen Z, Guo H, Xu G, Wang C (2010) Abnormal Groundwater Chemistry and Endemic Disease. Chinese Journal of Nature 7:83–89 (in Chinese)
Shweta T, Bhavtosh S, Prashant S, Rajendra D (2013) Water quality assessment in terms of water quality index. Am J Resour 1(3):34–38. https://doi.org/10.12691/ajwr-1-3-3
Su H, Kang W, Xu Y, Wang J (2017) Assessment of groundwater quality and health risk in the oil and gas field of Dingbian County, Northwest China. Expo Health 9:227–242. https://doi.org/10.1007/s12403-016-0234-6
Su H, Kang W, Xu Y, Wang J (2018) Assessing groundwater quality and health risks of nitrogen pollution in the Shenfu mining area of Shaanxi Province, northwest China. Expo Health 10:77–97. https://doi.org/10.1007/s12403-017-0247-9
Su F, Wu J, He S (2019) Set pair analysis-Markov chain model for groundwater quality assessment and prediction: a case study of Xi’an City, China. Hum Ecol Risk Assess 25(1–2):158–175. https://doi.org/10.1080/10807039.2019.1568860
Tian R, Wu J (2019) Groundwater quality appraisal by improved set pair analysis with game theory weightage and health risk estimation of contaminants for Xuecha drinking water source in a loess area in northwest China. Hum Ecol Risk Assess 25(1–2):176–190. https://doi.org/10.1080/10807039.2019.1573035
Umamageswari TSR, Sarala Thambavani D, Liviu M (2019) Hydrogeochemical processes in the groundwater environment of Batlagundu block, Dindigul district, Tamil Nadu: conventional graphical and multivariate statistical approach. Appl Water Sci 9:14. https://doi.org/10.1007/s13201-018-0890-8
USEPA (1989) Risk assessment guidance for superfund, volume 1: human health evaluation manual (part A). Office of Emergency and Remedial Response, Washington DC
USEPA (2019) Regional screening levels (RSLs)-generic tables. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables. Accessed 20 February, 2020
Varol S, Davraz A (2016) Evaluation of potential human health risk and investigation of drinking water quality in Isparta city center (Turkey). J Water Health 14(3):471–488. https://doi.org/10.2166/wh.2015.187
Velis M, Conti KI, Biermann F (2017) Groundwater and human development: synergies and trade-offs within the context of the sustainable development goals. Sustain Sci 12(6):1007–1017. https://doi.org/10.1007/s11625-017-0490-9
Wang D, Wu J, Wang Y, Ji Y (2019) Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: analysis, assessment, and management. Expo Health. https://doi.org/10.1007/s12403-019-00314-y
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8(3):311–329. https://doi.org/10.1007/s12403-015-0170-x
Wu J, Li P, Qian H (2011) Groundwater quality in Jingyuan County, a semi-humid area in Northwest China. E-J Chem 8:787–793. https://doi.org/10.1155/2011/163695
Wu J, Li P, Qian H (2013) Environmental chemistry of groundwater near an industrial area, northwest China. Asian J Chem 25(17):9795–9799. https://doi.org/10.14233/ajchem.2013.15355
Wu J, Li P, Qian H (2015) Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ Earth Sci 73(12):8575–8588. https://doi.org/10.1007/s12665-015-4018-2
Wu J, Xue C, Tian R, Wang S (2017) Lake water quality assessment: a case study of Shahu Lake in the semiarid loess area of northwest China. Environ Earth Sci 76:232. https://doi.org/10.1007/s12665-017-6516-x
Wu J, Zhou H, He S, Zhang Y (2019) Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ Earth Sci 78:446. https://doi.org/10.1007/s12665-019-8471-1
Wu J, Zhang Y, Zhou H (2020) Groundwater chemistry and groundwater quality index incorporating health risk weighting in Dingbian County, Ordos basin of northwest China. Geochemistry. https://doi.org/10.1016/j.chemer.2020.125607
Yousefi M, Asghari BF, Zuccarello P, Conti OG, Ejlali A, Mohammadi AA, Ferrante M (2019) Spatial distribution variation and probabilistic risk assessment of exposure to fluoride in ground water supplies: a case study in an endemic fluorosis region of northwest Iran. Int J Environ Res Public Health 16(4):564. https://doi.org/10.3390/ijerph16040564
Yu W, Tang D (2013) Application of TOPSIS model based on vague set entropy in the evaluation of groundwater quality. Adv Mater Res 712–715:452–456. https://doi.org/10.4028/www.scientific.net/AMR.712-715.452
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77(7):273. https://doi.org/10.1007/s12665-018-7456-9
Zhang Q, Miao L, Wang H, Hou J, Li Y (2020) How rapid urbanization drives deteriorating groundwater quality in a provincial capital of China. Pol J Environ Stud 29(1):441–450. https://doi.org/10.15244/pjoes/103359
Acknowledgements
This research has been financially supported by the National Natural Science Foundation of China (Grant Nos. 41602238 and 41761144059), the Young Stars Program granted by the Shaanxi Provincial Department of Science and Technology (Grant No. 2016KJXX-29), the Fundamental Research Funds for the Central Universities of CHD (Grant No. 300102299301), the Fok Ying Tong Education Foundation (Grant No. 161098), the China Postdoctoral Science Foundation (Grant Nos. 2015M580804, 2016M590911, 2016T090878 and 2017T100719), the Shaanxi Postdoctoral Science Foundation (Grant Nos. 2015BSHTDZZ09 and 2016BSHTDZZ03), and the Ten Thousand Talent Program (Grant No. W03070125). We are grateful to the anonymous reviewers and the editor for their useful and constructive comments. The Hancheng Center for Disease Control and Prevention is also acknowledged for the assistance in data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest has been reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, Y., Wu, J., Wang, Y. et al. Seasonal Variation of Drinking Water Quality and Human Health Risk Assessment in Hancheng City of Guanzhong Plain, China. Expo Health 12, 469–485 (2020). https://doi.org/10.1007/s12403-020-00357-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-020-00357-6