Abstract
Groundwater is a vital source for domestic and irrigation purposes in the loess area of Northwest China where climate is arid. However, the quality of groundwater in this area is deteriorating due to intensive industrial and agricultural activities, and this has a great adverse impact on human health. In order to better understand the pollution status of groundwater and the health risks to local residents, comprehensive water quality index was applied to assess the quality of drinking water in Yulin City, Northwest China, and sodium adsorption ratio, sodium percentage, residual sodium carbonate and permeability index were used to evaluate the quality of irrigation water. Moreover, the health risks caused by ingestion of groundwater were evaluated using the model proposed by the Ministry of Environmental Protection of the PR China. The results show that all groundwater samples for irrigation will not induce soil salinization, but more than half of them are not suitable for drinking, and Fe, Mn, TH, Mg2+ and NO3–N are the common contaminants which are mainly from natural processes, industrial and agricultural activities. The health risk assessment indicates that children face greater non-carcinogenic risk than adults. The order of contribution of contaminants to non-carcinogenic risk is NO3 − > As > F− > Fe > Mn > Ba2+ > Cr6+ > Zn > NO2 −. The average carcinogenic risk of carcinogens (Cr6+ and As) is 1.17 × 10−4 and 1.37 × 10−4 for adults and children, respectively, which surpasses the permissible level (1 × 10−6) stipulated by the Ministry of Environmental Protection of the PR China. Hence, effective measures are highly demanded to manage groundwater pollution and reduce the risks to human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As an important part of water resource as well as the major source of agricultural, industrial and domestic water, groundwater plays a significant role in securing resident life, supporting socioeconomic development and maintaining ecological balance (Su et al. 2014a, b). It is estimated that groundwater consumption in the world accounted for about 50, 40 and 20% of the amount of domestic water, industrial water and irrigation water, respectively (Tai et al. 2012). In China, groundwater is used as the main drinking water source in about two-thirds of the city (Zhang et al. 2009). Especially in the arid and semiarid area, groundwater is often the only source of drinking water supply (Li 2016). In recent years, with the expansion of urban scale, the growth of population and the rapid development of economy, domestic and industrial wastewater is discharged, a variety of garbage is piled up at random, and pesticides and fertilizers are excessively used, posing a significant adverse effect on groundwater quality (Li et al. 2012, 2016a; Qian et al. 2012; Wu et al. 2014a). The most common contaminants in groundwater include arsenic (Alam et al. 2015), fluorine (Li et al. 2014a), nitrogen (Suthar et al. 2009) and organic contaminants (Hu et al. 2016). In particular, the large-scale land-creation projects in the loess regions of Northwest China have changed the vulnerable groundwater environment (Li et al. 2014b), and the creation of the “Silk Road economic belt” between China and Central Asia in 2013 may cause deterioration of groundwater quality (Li et al. 2017a, b). Therefore, it is essential to carry out groundwater quality research, especially in these areas where groundwater quality is influenced significantly by human activities (Li 2016; Li et al. 2017b).
In recent decades, a number of scholars have conducted in-depth researches on the dynamics of groundwater pollution and have attained significant results (Wen et al. 2013; Li et al. 2016b; Rasool et al. 2016). Chidambaram et al. (2014) investigated the metal contamination of groundwater in an industrial concentration district of Tamil Nadu, finding that the major metals that cause the deterioration of groundwater quality are Cr6+, Mn, Zn, Fe, As, Cu, Cd and Pb, and these toxic pollutants are mainly from local industrial activities. Hudak (2010) carried out a detailed study on the potential sources of groundwater contamination in a portion of the Trinity Aquifer of USA and found that the contaminants are mainly derived from agriculture and oil production. Their study provides guidance for other groundwater researchers to investigate the local groundwater quality. Li et al. (2016b) assessed groundwater quality and provided some measures for the protection and management of groundwater in Hua County, China. Their detailed study shows that human interference is the main cause of deterioration of groundwater quality, and their recommendations may be useful for local groundwater managers to manage contaminated groundwater.
Recent years, many scholars have used a variety of methods for their respective research areas of the groundwater quality evaluation (Li et al. 2010; Wu et al. 2011, 2014b). Fagbote et al. (2013) applied entropy-weighted method to evaluate the groundwater quality in the farm settlements in Western Nigeria. The same method was also adopted by Li et al. (2014c) to assess groundwater quality in an industrial park in China. Mohebbi et al. (2013) described the overall situation of groundwater quality in urban areas of Iran using an improved water quality assessment method based on the Canadian water quality index. Yidana et al. (2010) evaluated the groundwater quality in Hashtgerd plain by means of multivariate statistical techniques. These statistical techniques include factor analysis, cluster analysis, discriminant analysis and principal component analysis. Li et al. (2016c) carried out an assessment of groundwater quality and health risks caused by the contaminants in a traditional agricultural region of Northwest China. Similarly, the health risk assessment of hazardous substances in groundwater was carried out by Batayneh (2012), Chen et al. (2016) and Ryu et al. (2007) independently to quantify the impact of different hazardous substances on human health. These studies indicated that the main way to pose carcinogenic and non-carcinogenic risks was to ingest contaminated groundwater. Al-Rawabdeh et al. (2014) utilized an integrated model to evaluate the quality of contaminated groundwater in Houston County, Minnesota. This integrated model is a combination of Geographic Information Systems (GIS) and DRASTIC model recommended by the United States Environmental Protection Agency (USEPA). Dahiya et al. (2007) proposed an approach based on fuzzy sets theory to eliminate the adverse effects of uncertainty factors on groundwater quality evaluation in imprecise environment. In addition, electrical resistivity tomography (Rao et al. 2013), neural network (Sirat 2013), set pair analysis (Li et al. 2011) and matter element extension analysis (Li et al. 2016c) have been widely used in the identification of groundwater pollutants and the evaluation of groundwater quality. Hence, it is confirmed that these methods are more trustworthy for groundwater quality assessment.
Groundwater is exploited in the loess area of Northwest China for various purposes such as domestic, agriculture and industry uses (Qian et al. 2013). Yulin City is located in the north edge of the Loess Plateau of China. Loess is a homogeneously porous media. The pores among the loess are quite small, which makes the loess formation difficult to yield large quantity of groundwater, which make groundwater much more precious in the loess area. In Yulin City, groundwater is heavily used for drinking and irrigation by local residents due to the lack of fresh surface water, which burdens the pressure of groundwater quantity in this area. Particularly, groundwater quality is deteriorating at an alarming rate due to intensive human activities. According to the statistical data of 2010, the discharge of wastewater and waste residue in this area was 4798.75 × 104 t and 1462.36 × 104 t, respectively (Zhu 2012). The random discharge of these pollutants has resulted in the deterioration of the local groundwater quality. Drinking contaminated groundwater can cause great health risks to local residents. However, the extent of groundwater pollution and associated health risks has not been reported. Therefore, the purposes of this research are (1) to analyze groundwater hydrogeochemical characteristics and formation mechanisms; (2) to evaluate the groundwater quality for drinking and irrigation purposes; and (3) to assess the health risks caused by drinking water intake pathway to adults and children. This study will provide substantial scientific information which helps to improve the groundwater quality management and protection for human health.
Study area
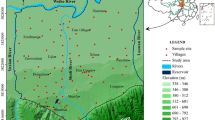
The study area is situated in the northern part of Shaanxi Province within latitude 38°05′34.2″–39°19′43.1″N and longitude 109°34′10.2″–109°55′13.1″E. This area belongs to the transition zone of the Loess Plateau and Mu Us desert. It is bounded by Shenmu County in the north, Hengshan County in the south, Wushen County in the west, and Jia County and Mizhi County in the east, covering about 1,300 km2 (Fig. 1). The area belongs to the arid and semiarid continental climate zone. According to the observational data of Yulin meteorological station from 1978 to 2010, the annual average temperature is 8.1 °C. The average annual precipitation is 368.9 mm, 63% of which concentrated in July, August and September. The average annual evaporation is 1195.5 mm, which is 2.89 times higher than the annual precipitation. The Yuxi River which runs through the middle of the study area from north to south is the biggest river in the study area (Fig. 1). The average annual runoff is 3.711 × 108 m3. It has nine tributaries from north to south: Baihe River, Qiqiu River, Wudao River, Erdao River, Toudao River, Qinhe River, Shahe River, Qingyun River and Liuqian River. Because of industrial and domestic wastewater discharge, almost all of the rivers are severely polluted and thus cannot be used for drinking and irrigation.
The aquifer in the study area is classified into the loose rock pore aquifer, loose rock fracture aquifer and clastic rock fracture aquifer. This study focuses on the loose rock pore aquifer which is mainly composed of the Quaternary aeolian sands, alluvial sands and gravels. The thickness of this aquifer ranges from 10 to 70 m, and the yield of a single well ranges from 47.58 to 1824 m3/d. The groundwater in the study area receives recharge from many sources including precipitation infiltration, lateral flow and irrigation infiltration. Groundwater runoff is dominated by topography, geomorphology and the geological structure of the aquifer. The flow direction of groundwater in the study area is generally from both east and west to the Yuxi River (Fig. 1). Evaporation, springs and artificial abstraction constitute the major components of groundwater discharge.
Materials and methods
Sample collection and analysis
For this study, sixty-one groups of groundwater samples were collected from monitoring wells during September 2011. Before sample collection, every well was pumped for a few minutes to ensure the elimination of the adverse effects of stagnant water. And then groundwater samples were collected using 1.5-L polyethylene bottles which were thoroughly washed three times using the well water before sampling. Besides, pH and electrical conductivity (EC) of groundwater were measured by the German-made multifunctional portable tester (Multi-340i/SET) at each sampling site. All of the sampling sites, as shown in Fig. 1, were accurately recorded by a handheld GPS. All the collected samples were transported immediately to the Groundwater Mineral Water and Environmental Monitoring Centre of Ministry of Land and Resources in China for physiochemical analysis. The collection, preservation, transportation and measurement of groundwater samples were carried out in accordance with the standard methods prescribed by APHA (2012).
Each groundwater sample was tested for major ions (K+, Na+, Ca2+, Mg2+, Cl−, SO4 2− and HCO3 −), nitrate (NO3–N), nitrite (NO2–N), fluoride (F−), silicate (H2SiO3 −), total hardness (TH), total dissolved solids (TDS), chemical oxygen demand (CODMn) and heavy metals (Fe, Mn, Zn, Ba2+, Cr6+ and As). K+ and Na+ were measured using flame atomic absorption spectrophotometry; Ca2+, Mg2+ and TH were analyzed using ethylene diamine tetraacetic acid (EDTA) titration; Cl−, HCO3 − and Ba2+ were determined using titration; SO4 2− and F− were tested by ion chromatography; NO3–N was analyzed by phenol disulfonic acid spectrophotometry; NO2–N was determined by spectrophotometry; H2SiO3 − was determined by silicon molybdenum blue spectrophotometry; TDS was tested using drying and weighing; CODMn was determined by dichromate method; Fe, Mn, Zn and Cr6+ were determined by means of inductively coupled plasma atomic emission spectrometry; and As was measured by atomic fluorescence spectrometry. In the process of analysis of the hydrogeochemical parameters of groundwater, duplicates were completed to ensure the reliability of the analysis results.
Groundwater quality evaluation
Drinking water quality evaluation
According to the comprehensive water quality index (CWQI) stipulated by the Quality Standard for Groundwater of China, groundwater quality can be classified into five ranks: rank I (excellent), rank II (good), rank III (medium), rank IV (poor) and rank V (extremely poor) (Bureau of Quality and Technical Supervision of China 1993; Li et al. 2014d). Based on the standard, the specific calculation steps of CWQI are as follows:
where F i denotes the evaluation value of each parameter; (F i )mean and (F i )max represent the mean and maximum values of F i , respectively; n represents the number of parameters selected for water quality assessment. The classification of groundwater quality based on CWQI is shown in Table 1.
Irrigation water quality evaluation
Groundwater irrigation is the main cause of soil salinization–alkalization in arid and semiarid areas (Li et al. 2013a, b). In the study area, groundwater is the only source of irrigation water. Therefore, irrigation water quality evaluation was necessary in order to inform the local agricultural managers of the degree of soil salinization–alkalization posed by groundwater irrigation. Sodium adsorption ratio (SAR) is an important index to measure the soil alkali hazard caused by irrigation water (Xiao et al. 2016; Wu et al. 2017). The SAR is calculated by formula (3):
where Na+, Mg2+ and Ca2+ are expressed in meq/L. The classification of irrigation water quality based on SAR is shown in Table 2.
Sodium percentage (%Na) is widely used to assess the suitability of groundwater quality for irrigation purpose (Golekar et al. 2013a). %Na is calculated as follows:
where K+, Na+, Mg2+ and Ca2+ are expressed in meq/L. The classification of irrigation water quality based on the %Na is as follows: (1) excellent (<20), (2) good (20–40), (3) permissible (40–60), (4) doubtful (60–80), (5) unsuitable (>80).
The residual sodium carbonate (RSC) is used to identify the hazard impacts of high bicarbonate and carbonate groundwater on the soil. The RSC is calculated using formula (5):
where CO3 2−, HCO3 −, Mg2+ and Ca2+ are expressed in meq/L. The quality of irrigation water based on the RSC can be classified as (Golekar et al. 2014): (1) good (<1.25), (2) doubtful (1.25–2.5), (3) unsuitable (>2.5).
Permeability index (PI) is widely used to evaluate the effects on soil permeability of Na+, Ca2+, Mg2+ and HCO3 − in groundwater which is long term used for irrigation purposes (Doneen 1964). The PI is computed as follows:
where Na+, Mg2+, Ca2+ and HCO3 − are expressed in meq/L. Based on the PI values, irrigation water quality is classified into three classes: excellent (class I), acceptable (class II) and unsuitable (class III).
Health risk assessment
Health risk assessment provides an important reference for groundwater environmental protection and management (Wu and Sun 2016). Human is usually exposed to contaminants through ingestion, inhalation and skin contact. Normally, drinking water intake and skin contact are the main pathways to pose health risks to human for groundwater. Many studies have shown that the health risks of contaminated groundwater through skin contact can be ignored in comparison with the drinking water intake (Liu et al. 2011). Due to inadequate valuable data for the assessment of the health risk caused by skin contact, therefore, this study only evaluated the health risks from drinking water intake pathway using the models proposed by the Ministry of Environmental Protection of the PR China (2014). In this study, NO3 −, NO2 −, F−, Fe, Mn, Zn, Ba2+, Cr6+ and As were used as the parameters for health risk assessment. The health risks caused by drinking water intake can be calculated as follows:
The non-carcinogenic risk through drinking water intake is calculated by formulas (7) and (8):
where Intakeoral represents the average daily exposure dose through ingestion of groundwater [mg/(kg day)], C w is the concentration of target pollutants in groundwater (mg/L), IR represents groundwater ingestion rate (L/d, IR = 1.5 L/day for adults and 0.7 L/day for children in this study), EF represents the exposure frequency (days/year, EF = 365 days/year), ED represents the exposure duration (years, ED = 30 years for adults and 12 years for children), BW represents the average body weight (kg, BW = 56.8 kg for adults and 15.9 kg for children in this study), and AT represents average exposure time (days, AT = 10,950 days for adults and 4380 days for children), HQoral and RfDoral represent the hazard quotient and reference dosage, respectively, for non-carcinogenic pollutants through drinking water intake [mg/(kg d)]. In the present study, the RfDoral values of NO3 −, NO2 −, F−, Fe, Mn, Zn, Ba2+, Cr6+ and As are 1.6, 0.1, 0.04, 0.3, 0.14, 0.3, 0.2, 0.003 and 0.0003 mg/(kg d), respectively.
According to the health risk assessment standard recommended by the Ministry of Environmental Protection of the PR China (2014), the acceptable limit of HQ is 1. HQ > 1 means the non-carcinogenic risk is beyond the acceptable limit. Cr6+ and As can also cause carcinogenic risks in addition to non-carcinogenic risks. The carcinogenic risks caused by Cr6+ and As through drinking water intake are expressed as (Ministry of Environmental Protection of the PR China 2014):
In this formula, CR indicates the carcinogenic risk. CR value of more than 1 × 10−6 is considered harmful for human health. SF represents the slope factor of carcinogenic pollutants (mg/(kg d), SF = 0.5 mg/(kg d) for Cr6+ and 1.5 mg/(kg d) for As). The exposure duration (ED) for carcinogenic risk is 70 years for adults and children.
Results and discussion
Groundwater chemistry
General hydrogeochemical characteristics
The results of statistical analysis of all collected groundwater samples in the study area are given in Table 3. pH is an important hydrogeological parameter indicating whether groundwater is suitable for drinking (Li and Qian 2011). The national standards prescribe that the acceptable pH range for drinking water is 6.5–8.5. As shown in Table 3, the pH is in the range of 7.34–8.47 with a mean of 7.51, which suggests that the groundwater is slightly alkaline in nature over the study area. The pH values of all the samples are within the acceptable limits. As a significant index for analyzing groundwater quality, national standards stipulate that the maximum of EC cannot exceed 2500 μs/cm for drinking purposes. The EC concentration in the study area ranges from 239 to 1907 μs/cm with an average of 576.92 μs/cm, indicating that all groundwater locations are within the prescribed limits.
TDS is usually used to ascertain whether groundwater is suitable for human consumption (Li et al. 2014c). Groundwater with TDS < 1000 mg/L is considered suitable for drinking. The level of TDS in the study area ranges from 164.90 to 1097.00 mg/L with a mean of 369.58 mg/L, demonstrating that TDS can meet the requirements of drinking water after mixing. Only two groundwater samples (YL33 and YL57) exceed the permissible limit, and they are observed near the sewage discharge in the downstream of the Yuxi River, which indicates that domestic wastewater entering the aquifer through the unsaturated zone is the main cause of deterioration of groundwater quality. TH represents the sum of the evaporites (Ca2+ and Mg2+) dissolved in groundwater. The TH in the study area is in the range of 116.3–636.30 mg/L with a mean of 246.25 mg/L. National standards set 450 mg/L as the allowable limit for TH in groundwater for drinking purposes. Seven groundwater samples (11.48% of all collected samples) are classified as extremely hard water (TH > 450 mg/L) which are unfit for drinking. These samples are mainly distributed in the valley of Yuxi River and Liuqian River, suggesting that these two contaminated rivers have severely affected the quality of groundwater (Zhu 2012). TDS and TH are often combined to make a simple classification of groundwater quality (Brindha et al. 2016). As shown in Fig. 2, a total of thirteen samples (21.34% of all samples) are classified as soft fresh water type. Forty-six groundwater samples (75.41% of all samples) fall in hard fresh water type, and the remaining (only two samples) are hard brackish water type.
K+ in groundwater is one of the essential trace elements to maintain human health (He and MacGregor 2008). In the study area, the level of K+ ranges from 0.69 to 51.3 mg/L, with a mean of 2.26 mg/L. Na+ in the total collected samples is in the range of 8.95–226.00 mg/L with an average of 32.66 mg/L and a standard deviation of 33.65. National standards stipulate that the permissible level of Na+ for drinking is 200 mg/L. One groundwater sample (YL57) exceeds the permissible limit. This sample is located in the downstream area of Yuxi River, suggesting that the polluted river has a certain impact on the quality of groundwater. Ca2+ and Mg2+ are also necessary for humans, but excessive intake will have negative impacts on human health. In this study, Ca2+ is in the range of 26.10–163.90 mg/L with an average of 65.07 mg/L, signifying that all concentrations are within the acceptable limit. The Mg2+ concentration ranges between 5.62 and 77.97 mg/L with a mean of 20.33 mg/L. These concentrations are acceptable for drinking. Overall, the abundance of cations based on the average values is as follows: Ca2+ > Na+ > Mg2+ > K+. For anions, the concentrations of Cl−, SO4 2− and HCO3 − are in the range of 2.79–358.90, 10.36–238.30 and 143.90–558.40 mg/L, respectively. National drinking water quality standards stipulate that the acceptable limit of Cl− and SO4 2− is 250 mg/L. In the study area, only one sample (YL57) exceeds the acceptable limit for Cl− and all samples are within the limit for SO4 2−. Based on mean concentrations, the abundance of anions is HCO3 − > SO4 2− > Cl−.
NO3–N is an effective indicator usually used to measure the extent of groundwater contamination by agricultural activities (Golekar et al. 2013b; Wu et al. 2013a). National standards prescribe that NO3–N concentration should be less than 20 mg/L in drinking water. In this study, five samples have NO3–N concentration higher than the standard limit. It is believed that NO3–N pollution of groundwater in this area is closely related to agricultural activities. Each year, large quantities of fertilizer and pesticide are used to ensure the plant production and a large amount of groundwater is used for irrigation. Soluble NO3–N can easily reach the aquifer through the vadose zone with sufficient irrigation water infiltration, increasing the concentration of NO3–N in groundwater.
F− is an essential trace element for human health, but the long-term intake of excessive fluoride can lead to fluorosis (Adimalla and Venkatayogi 2017; Li et al. 2014a). The WHO standards stipulate that the optimal level of F− in drinking water should be less than 1.5 mg/L, while the Chinese national standards set the optimal level to be less than 1.0 mg/L. In this study, the concentration of F− ranges from 0.34 to 1.10 mg/L, with a mean of 0.25 mg/L (Table 3). Only one groundwater sample (YL34) is not suitable for drinking due to the high concentration of F−. This sample is located in the loess area in the southeast of the study area, indicating that F− may be contributed by fluorine-containing minerals, such as muscovite, biotite and fluorite (Regional Hydrogeologic Investigation Team of Shaanxi 1980).
Heavy metals such as Fe, Mn, Zn and Ba2+ are necessary for humans in trace amount, but excessive intake can have a negative impact on health (Gao and Chen 2012). As shown in Table 3, the concentrations of Fe, Mn, Zn, Ba2+, Cr6+ and As are in the range of 0–5.74, 0–1.57, 0–0.12, 0.027–0.340, 0–0.008 and 0–0.039 mg/L, respectively. All metals except Zn, Ba2+ and Cr6+ exceed the acceptable limits for drinking water. Twenty samples (32.79% of all samples) exceed the acceptable limit of Fe for drinking purposes, eighteen samples (29.51% of all samples) are higher than the acceptable level for Mn, and three samples exceed the acceptable limit for As. High Fe and Mn concentrations are observed mostly in the desert area of the study area (Fig. 5). Intensive industrial activities may be the main cause of excessive Fe and Mn in groundwater. Besides, slow groundwater runoff (the average hydraulic gradient is 2.05‰) in this area also provides a good environment for the accumulation of soluble Fe and Mn.
Groundwater types
Piper trilinear diagram (Piper 1944) can directly reflect the hydrogeochemical characteristics and types of groundwater. The relative concentrations of the major ions of groundwater samples in the study area are shown in Fig. 3. For cations, almost all the samples are plotted in zones B and D of the left triangle, signifying that the hydrochemical types of groundwater in the study area are mainly the “calcium” and “no dominant” type. For anions, samples are mostly plotted in zone F of the right triangle, indicating that the groundwater type mainly correlates with the “bicarbonate and carbonate” type. This type of groundwater is primarily a result of dissolution of carbonate minerals (Li et al. 2014c). Fifty-eight groundwater samples (more than 95% of all samples) are plotted in zone 2 of the upper diamond, suggesting that HCO3 −, Ca2+ and Mg2+ are the predominant ions in the groundwater of the study area. Samples in zones 1 and 4 show that SO4 2− and Cl− are the major anions, and Na+ is the predominant cation for the sample in zone 4.
Groundwater chemistry evolution mechanisms
In order to analyze the evolution mechanism of natural water chemistry, Gibbs (1970) designed two semilog diagrams which are now known as the Gibbs diagrams. Although these diagrams were originally applied to the analysis of surface water, they are now also widely used to characterize the formation mechanism of groundwater chemistry (Li et al. 2016d). The main mechanisms are classified into 3 types by the Gibbs diagrams: evaporation dominance, rock dominance and precipitation dominance (Wu et al. 2013b). Two semilog diagrams show the characteristic regions of these three mechanisms: One is the relationship between TDS and Na+/(Na++Ca2+), and the other one is the ratios of TDS with Cl−/(Cl−+HCO3 −). As shown in Fig. 4, all groundwater samples in the study area are plotted in the middle part of the diagrams, suggesting that rock weathering plays a significant role in the evolution of groundwater chemistry. The molar ratio of HCO3 − to SiO2 is often used to approximately determine the types of partial minerals involved in water–rock interactions (Kortatsi et al. 2008). When HCO3 −/SiO2 < 5, the chemical constituents of groundwater are mainly derived from the dissolution of silicate minerals; when HCO3 −/SiO2 > 10, the chemical constituents are from the dissolution of carbonate minerals; when 5 < HCO3 −/SiO2 < 10, the chemical constituents come from both. In this study, fifty-three groundwater samples (86.89% of all samples) have HCO3 −/SiO2 ratio higher than 10, suggesting that the main constituents of these groundwater samples are contributed by carbonate minerals. The HCO3 −/SiO2 ratio of the remaining samples (13.11% of all samples) ranges from 8.20 to 9.95, indicating that the dissolution of carbonate and silicate minerals is the source of these chemical constituents.
Groundwater quality assessment
Drinking water quality
In the study area, groundwater is a vital source of drinking water for local residents. The overall groundwater quality was evaluated using the CWQI to determine the suitability for human consumption, and the results are presented in Table 4. As shown in Table 4, F values of all analyzed samples vary from 0.71 to 7.30, ranging from excellent quality to extremely poor quality. Thirty groundwater samples (49.18% of all samples) are classified as excellent and good-quality water (ranks I and II) which is suitable for various purposes. Twenty-eight samples are of poor-quality water (rank IV), and three samples belong to extremely poor-quality water (rank V). The ranks IV and V account for 45.90 and 4.92% of all collected samples, respectively, indicating that more than half of groundwater samples are not suitable for human consumption. And the common contaminants in these samples are Fe, Mn, TH, Mg2+ and NO3–N, which are mainly from natural processes, industrial and agricultural activities. The serious pollution is delineated in Fig. 5. As shown in Fig. 5, the samples with poor and extremely poor-quality water are mainly observed in the valley of the Yuxi River, which indicates that the polluted river water into the aquifer is the main reason for the deterioration of groundwater quality. Moreover, as the discharge area, strong evaporation also affects the suitability of groundwater for drinking purpose. The samples with excellent and good quality are mostly distributed in the tributaries of the Yuxi River. In these areas, the groundwater flow is fast (the average hydraulic gradient is 10‰), indicating a short water–rock interaction time, and therefore less minerals are dissolved in groundwater. In general, groundwater quality in the study area is unsatisfactory for human consumption. Long-term intake of this groundwater causes serious threats to human health. Therefore, groundwater should be pretreated before being consumed by local residents.
Irrigation water quality
Groundwater in the study area is also extracted for irrigation in addition to human consumption. Therefore, it is necessary to perform irrigation water quality assessment in order to determine its safety to soil and plants. Many scholars often use the salinity and alkalinity of groundwater to determine its suitability for agricultural irrigation (Thilagavathi et al. 2012). EC is an effective parameter to measure salinity. Generally, groundwater with EC less than 2250 μs/cm will not cause salinity hazard when used for irrigation. As shown in Table 3, EC ranges from 239.00 to 1907.00 μs/cm, with an average of 576.92 μs/cm, indicating that groundwater in the study area is suitable for irrigation. SAR and %Na are often used to quantify the saline/alkali hazards caused by irrigation water. Table 5 shows the SAR values are in the range of 0.27–4.89 with a mean of 0.86, signifying an excellent groundwater quality for irrigation. %Na varies from 8.30 to 57.97 with an average of 21.25, suggesting the suitability of groundwater for irrigation. Groundwater used for irrigation in the study area will not induce saline/alkali hazards. Groundwater with RSC higher than 2.25 is considered unsuitable for irrigation. In this study, RSC values are observed in the range of −9.27 to 0.74 meq/L with a mean of −0.97 meq/L, suggesting that all the samples are suitable for agricultural use. Long-term use of such groundwater for irrigation may not threaten the health of soil and plants.
The US salinity diagram (Richards 1954) and the Wilcox (1948) diagram are widely used to evaluate the suitability of groundwater for irrigation purpose. As shown in Fig. 6a, forty-seven samples fall in C1S1 and C2S1, signifying an excellent and good groundwater quality for irrigation. Fourteen samples are plotted in C3S1 and one sample in C3S2, indicating that the groundwater is low alkalinity and high salinity which is acceptable for irrigation. According to the Wilcox diagram (Fig. 6b), sixty groundwater samples are plotted in zones of “excellent to good” and “good to permissible,” indicating the suitability of groundwater for irrigation. Only one sample is observed in “permissible to doubtful” zone with %Na less than 60, suggesting that there would be no sodium hazard if used for irrigation. According to the PI values, twenty-one samples (34.43% of all samples) are observed in class I and forty (65.57% of all samples) in class II (Fig. 7), suggesting that all groundwater samples are suitable for irrigation.
Overall, the long-term use of groundwater in the study area for irrigation may not induce soil salinization and may not affect soil permeability and plant growth.
Health risk assessment
The health risks of non-carcinogens (NO3 −, NO2 −, F−, Fe, Mn, Zn and Ba2+) and carcinogens (Cr6+ and As) for adults and children through drinking water intake were assessed using the approach described in the present study. The assessment results for adults and children are shown in Tables 6 and 7, respectively. For adults, the greatest non-carcinogenic risk through drinking water intake (HQoral) is caused by NO3 −, ranging from 0.00 to 7.86 with a mean of 0.59. And As contributes the second non-carcinogenic risk, with a mean of 0.24. Moreover, NO2 − contributes the least non-carcinogenic risk, with the maximum value of 4.23 × 10−3. As shown in Fig. 8a, NO3 − is the largest contributor to the total non-carcinogenic risk (53.99% of total non-carcinogenic risk), followed by As (22.07% of total non-carcinogenic risk) and F− (15.11% of total non-carcinogenic risk). The remaining contaminants (NO2 −, Fe, Mn, Zn, Ba2+ and Cr6+) contribute only 8.83% of the total non-carcinogenic risk, signifying that NO3 −, As and F− are the most influential factors to the health of adults. The order of non-carcinogenic risk is NO3 − > As > F− > Fe > Mn > Ba2+ > Cr6+ > Zn > NO2 −. For children, the greatest contributor to non-carcinogenic risks is also NO3 −, ranging within 0.00–13.10 with a mean of 0.98 which is almost 1.67 times than that for adults. The order of non-carcinogenic risk for children is the same as that for adults: NO3 − > As > F− > Fe > Mn > Ba2+ > Cr6+ > Zn > NO2 − (Fig. 8c). Fe and Mn are the most common metal contaminants in the groundwater of the study area, induced by the natural processes and industrial activities. However, Fe and Mn contribute only 4.31 and 2.37% of the total non-carcinogenic risk, respectively, to adults and children. The reason is that Fe and Mn are essential trace elements in human body and are harmful only in the long-term intake of excessive concentration. The average hazard quotient through drinking water intake (HQoral) for adults and children is 1.09 and 1.82, respectively, indicating that children in the study area are more vulnerable to contaminants than adults. In general, adults and children in the study area face high non-carcinogenic risk.
As indicated in Tables 6 and 7, the carcinogenic risk (CRoral) caused by As is in the range of 0.00–1.54 × 10−3 with an average of 1.08 × 10−4 for adults; risk (CRoral) from Cr6+ ranges from 0.00 to 1.06 × 10−4 with an average of 8.44 × 10−6, suggesting that the health risk caused by As is larger than Cr6+ (Fig. 8b). The total carcinogenic risk (CRtotal) for adults ranges from 0.00 to 1.54 × 10−3 with a mean of 1.17 × 10−4, indicating that the risk is almost two orders of magnitude higher than the recommended level (1 × 10−6) of the Ministry of Environmental Protection of the PR China (2014). For children, As contributes more risk (58.80% of the total carcinogenic risk) than Cr6+ (41.20% of the total carcinogenic risk) (Fig. 8d), because the average concentration of As in groundwater is higher than Cr6+. The total carcinogenic risk (CRtotal) for children is in the range of 0.00 to 1.14 × 10−3 with an average of 1.37 × 10−4, signifying that the total carcinogenic risks (CRtotal) of As and Cr6+ exceed the acceptable limit (1 × 10−6). Therefore, the groundwater in the study area needs pretreatment before being consumed by local residents.
At present, with the rapid development of agriculture and industry, groundwater pollution is becoming more and more serious, causing various diseases such as lung, prostate and bladder cancer (Golekar et al. 2013c). Therefore, many similar groundwater risk assessment researches have been reported in China and other countries in the world (Cai et al. 2015; Giri and Singh 2015; Navoni et al. 2014; Ni et al. 2009; Su et al. 2013; Yang et al. 2012). In order to better understand the health risk of groundwater pollution in the study area, the health risks in the loess area of Northwest China are compared (Table 8).
Li et al. (2016c) performed a detailed study on health risk of groundwater contamination for local residents by direct ingestion in the Weining plain, Northwest China. They found that the hazard quotient through drinking water intake (HQoral) ranged from 0.35 to 2.94 with an average value of 1.38, and the total carcinogenic risk (CRtotal) of As and Cr6+ was between 1.06 × 10−5 and 7.53 × 10−4 with an average of 5.15 × 10−5, indicating that the carcinogenic risk was much greater in the study area. Ni et al. (2010) assessed the health risk caused by ingestion of groundwater in Mingshan County, a city in Northwest China. They found that the non-carcinogenic risk was in the range of 0.07438–3.69643 with a mean of 1.44500, and the carcinogenic risk ranged from 1.0 × 10−5 to 2.2 × 10−4 with an average of 1.4 × 10−5. This suggests that the carcinogenic risk of groundwater pollution in the study area is higher than that in Mingshan County. Wei et al. (2008) assessed the health risk induced through drinking pathway in Yinchuan City, Northwest China. They found the carcinogenic risk was between 2.14 × 10−5 and 8. 47 × 10−5, and the average human health risk was 5.48 × 10−5. The carcinogenic risk in this study is twice higher than that in Yinchuan City, indicating that carcinogenic pollutants have more negative influences on human health in the study area. Similarly, in other loess areas of Northwest China, many studies on the health risks associated with groundwater consumption also show that the present study area is among the most risky areas (Li and Qian 2011; Li et al. 2014c; Su et al. 2016, 2017; Wu et al. 2017). Therefore, urgent action should be taken to guarantee the safety of drinking water for local residents.
Conclusions
In the present study, sixty-one groups of groundwater samples were collected and twenty-two physiochemical parameters were analyzed. Statistical analysis and Piper diagrams were applied to characterize the general groundwater chemistry. Gibbs diagrams were used to study the formation mechanisms of groundwater chemistry. CWQI was used to assess overall groundwater quality; EC, SAR, %Na, RSC and PI were applied to assess the suitability of groundwater for irrigation purposes. The health risk assessment model recommended by the Ministry of Environmental Protection of the PR China was used to estimate the health risks caused by ingestion of groundwater to adults and children. The following conclusions can be drawn:
-
1.
The pH value of groundwater in the study area varies from 7.34 to 8.47 signifying alkalinity. TDS and TH are in the range of 164.90–1097.00 and 116.3–636.30 mg/L, respectively. The abundance of anions and cations is HCO3 − > SO4 2− > Cl− and Ca2+ > Na+ > Mg2+ > K+, respectively, which makes the predominant type of groundwater is HCO3-Ca·Mg type. Gibbs diagrams indicate that rock weathering and water–rock interactions control the formation mechanisms of groundwater chemistry. The main pollutants in groundwater are TH, Mg2+ and NO3–N, indicating that groundwater in the study area needs to be pretreated before being used for drinking purposes by local residents. Trace elements are necessary for human body, but the excess will have a negative impact on health. Fe, Mn and As contamination occurs in some areas due to natural and anthropogenic factors.
-
2.
The assessment result of the overall groundwater quality in the study area based on CWQI shows that 49.18% of the groundwater samples are classified as excellent and good-quality water which is suitable for various purposes. More than half of groundwater samples (50.82% of the samples) are classified as poor and extremely poor-quality water. Fe, Mn, TH, Mg2+ and NO3–N are the common contaminants in these samples, which are mainly from natural processes, industrial and agricultural activities. EC, SAR, %Na, RSC, US salinity diagram, Wilcox diagram and PI signify that groundwater in the study area for irrigation will not induce soil salinization and will not affect soil permeability and plant growth.
-
3.
Contaminated groundwater in the study area has a negative impact on the health of the local residents through drinking water intake. The total non-carcinogenic risk is in the range of 0.11–7.89 for adults. The non-carcinogenic risk is higher for children, ranging from 0.18 to 13.16. The order of contribution of contaminants to non-carcinogenic risk for adults and children is NO3 − > As > F− > Fe > Mn > Ba2+ > Cr6+ > Zn > NO2 −. The total carcinogenic risks of carcinogens (Cr6+ and As) for adults and children range from 0.00 to 1.54 × 10−3 and 0.00 to 1.14 × 10−3, with averages of 1.17 × 10−4 and 1.37 × 10−4, respectively, which exceed the permissible level (1 × 10−6) stipulated by the Ministry of Environmental Protection of the PR China. Hence, effective measures are highly demanded to manage groundwater pollution and reduce the risks to human health.
References
Adimalla N, Venkatayogi S (2017) Mechanism of fluoride enrichment in groundwater of hard rock aquifers in Medak, Telangana State, South India. Environ Earth Sci 76:45. doi:10.1007/s12665-016-6362-2
Alam MO, Shaikh WA, Chakraborty S, Avishek K, Bhattacharya T (2015) Groundwater arsenic contamination and potential health risk assessment of gangetic plains of Jharkhand, India. Expo Health 8(1):125–142. doi:10.1007/s12403-015-0188-0
Al-Rawabdeh AM, Al-Ansari NA, Al-Taani AA, Al-Khateeb FL, Knutsson S (2014) Modeling the risk of groundwater contamination using modified DRASTIC and GIS in Amman-Zerqa Basin, Jordan. Cent Eur J Chem Eng 4(3):264–280. doi:10.2478/s13531-013-0163-0
American Public Health Association (2012) Standard methods for the examination of water and wastewater, vol 22. American Public Health Association, Washington
Batayneh AT (2012) Toxic (aluminum, beryllium, boron, chromium and zinc) in groundwater: health risk assessment. Int J Environ Sci Te 9(1):153–162. doi:10.1007/s13762-011-0009-3
Brindha K, Pavelic P, Sotoukee T, Douangsavanh S, Elango L (2016) Geochemical characteristics and groundwater quality in the Vientiane Plain, Laos. Expo Health. doi:10.1007/s12403-016-0224-8
Bureau of Quality and Technical Supervision of China (1993) National Standard of the People’s Republic of China: Quality Standard for Groundwater, GB/T 14848-93 (in Chinese)
Cai L-M, Xu Z-C, Qi J-Y, Feng Z-Z, Xiang T-S (2015) Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 127:127–135. doi:10.1016/j.chemosphere.2015.01.027
Chen J, Wu H, Qian H (2016) Groundwater nitrate contamination and associated health risk for the rural communities in an agricultural area of Ningxia, Northwest China. Expo Health 8(3):349–359. doi:10.1007/s12403-016-0208-8
Chidambaram S, Prasad MBK, Prasanna MV, Manivannan R, Anandhan P (2014) Evaluation of metal pollution in groundwater in the industrialized environs in and around Dindigul, Tamilnadu, India. Expo Health 7(3):307–317. doi:10.1007/s12403-014-0150-6
Dahiya S, Singh B, Gaur S, Garg VK, Kushwaha HS (2007) Analysis of groundwater quality using fuzzy synthetic evaluation. J Hazard Mater 147(3):938–946. doi:10.1016/j.jhazmat.2007.01.119
Doneen LD (1964) Notes on water quality in agriculture. Published as a water science and engineering paper 4001, Department of Water Sciences and Engineering, University of California
Fagbote EO, Olanipekun EO, Uyi HS (2013) Water quality index of the ground water of bitumen deposit impacted farm settlements using entropy weighted method. Int J Environ Sci Technol 11(1):127–138. doi:10.1007/s13762-012-0149-0
Gao XL, Chen CTA (2012) Heavy metal pollution status in surface sediments of the coastal Bohai Bay. Water Res 46(6):1901–1911. doi:10.1016/j.watres.2012.01.007
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090. doi:10.1126/science.170.3962.1088
Giri S, Singh AK (2015) Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ Monit Assess 187(3):63. doi:10.1007/s10661-015-4265-4
Golekar RB, Baride MV, Patil SN (2013a) Assessment of surface and waste water quality for irrigation suitability: a case study of Jalgaon Urban area, Maharashtra (India). Der Chem Sin 4(2):177–181
Golekar RB, Abhay Salokhe, Nirmal Kamble, Baride MV, Patil SN, Pathare SV (2013b) Hydrogeochemistry of subsurface and surface water from bank of Jayanti stream, Kolhapur area, Maharashtra (India). Int J Geom Geosci 4(1):136
Golekar RB, Patil SN, Baride MV (2013c) Human health risk due to trace element contamination in groundwater from the Anjani and Jhiri river catchment area in northern Maharashtra, India. Earth Sci Res J 17(1):17–23
Golekar RB, Bartakke VV, Patil SN, Baride MV (2014) Groundwater quality assessment from Tarali river sub basin of Krishna river basin, western Maharashtra (India). Int J Adv Geosci 2(1):8–12. doi:10.14419/ijag.v2i1.1696
He FJ, MacGregor GA (2008) Beneficial effects of potassium on human health. Physiol Plant 133:725–735. doi:10.1111/j.1399-3054.2007.01033.x
Hu Y, Qi S, Yuan L, Liu H, Xing X (2016) Assessment of organochlorine pesticide contamination in waterbirds from an agricultural region, Central China. Environ Geochem Health. doi:10.1007/s10653-016-9891-4
Hudak PF (2010) Solutes and potential sources in a portion of the Trinity aquifer, Texas, USA. Carbonates Evaporites 25(1):15–20. doi:10.1007/s13146-010-0005-6
Kortatsi B, Tay C, Anornu G, Hayford E, Dartey G (2008) Hydrogeochemical evaluation of groundwater in the lower Offin basin, Ghana. Environ Geol 53(8):1651–1662. doi:10.1007/s00254-007-0772-0
Li P (2016) Groundwater quality in western China: challenges and paths forward for groundwater quality research in western China. Expo Health 8(3):305–310. doi:10.1007/s12403-016-0210-1
Li P, Qian H (2011) Human health risk assessment for chemical pollutants in drinking water source in Shizuishan City, Northwest China. Iran J Environ Health Sci Eng 8(1):41–48
Li P, Qian H, Wu J (2010) Groundwater quality assessment based on improved water quality index in Pengyang County, Ningxia, Northwest China. J Chem 7(S1):S209–S216. doi:10.1155/2010/451304
Li P, Qian H, Wu J (2011) Application of set pair analysis method based on entropy weight in groundwater quality assessment–a case study in Dongsheng City, Northwest China. J Chem 8(2):851–858. doi:10.1155/2011/879683
Li P, Wu J, Qian H (2012) Groundwater quality assessment based on rough sets attribute reduction and TOPSIS method in a semi-arid area, China. Environ Monit Assess 184:4841–4854. doi:10.1007/s10661-011-2306-1
Li P, Wu J, Qian H (2013a) Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ Earth Sci 69(7):2211–2225. doi:10.1007/s12665-012-2049-5
Li P, Qian H, Wu J, Zhang Y, Zhang H (2013b) Major ion chemistry of shallow groundwater in the Dongsheng Coalfield, Ordos Basin, China. Mine Water Environ 32(3):195–206. doi:10.1007/s10230-013-0234-8
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014a) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145. doi:10.1007/s12665-013-2691-6
Li P, Qian H, Wu J (2014b) Accelerate research on land creation. Nature 510(7503):29–31. doi:10.1038/510029a
Li P, Qian H, Wu J (2014c) Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ Geochem Health 36(4):693–712. doi:10.1007/s10653-013-9590-3
Li P, Wu J, Qian H (2014d) Hydrogeochemistry and quality assessment of shallow groundwater in the southern part of the Yellow River Alluvial Plain (Zhongwei Section), China. Earth Sci Res J 18(1):27–38. doi:10.15446/esrj.v18n1.34048
Li P, Feng W, Xue C, Tian R, Wang S (2016a) Spatiotemporal variability of contaminants in lake water and their risks to human health: a case study of the Shahu Lake tourist area, northwest China. Expo Health. doi:10.1007/s12403-016-0237-3
Li P, Wu J, Qian H (2016b) Hydrochemical appraisal of groundwater quality for drinking and irrigation purposes and the major influencing factors: a case study in and around Hua County, China. Arab J Geosci 9(1):1–17. doi:10.1007/s12517-015-2059-1
Li P, Li X, Meng X, Li M, Zhang Y (2016c) Appraising groundwater quality and health risks from contamination in a semiarid region of Northwest China. Expo Health 8(3):361–379. doi:10.1007/s12403-016-0205-y
Li P, Wu J, Qian H, Zhang Y, Yang N, Jing L, Yu P (2016d) Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger desert, northwest China. Expo Health 8(3):331–348. doi:10.1007/s12403-016-0193-y
Li P, Qian H, Zhou W (2017a) Finding harmony between the environment and humanity: an introduction to the thematic issue of the Silk Road. Environ Earth Sci 76(3):105. doi:10.1007/s12665-017-6428-9
Li P, Tian R, Xue C, Wu J (2017b) Progress, opportunities and key fields for groundwater quality research under the impacts of human activities in China with a special focus on western China. Environ Sci Pollut Res 24(15):13224–13234. doi:10.1007/s11356-017-8753-7
Liu N, Ni TH, Xia J, Dai MZ, He CY, Lu GF (2011) Non-carcinogenic risks induced by metals in drinking source water of Jiangsu Province, China. Environ Monit Assess 177(1):449–456. doi:10.1007/s10661-010-1646-6
Ministry of Environmental Protection of the PR China (2014) Technical guidelines for risk assessment of contaminated sites (HJ 25.3-2014). China Environmental Science Press, Beijing (in Chinese)
Mohebbi MR, Saeedi R, Montazeri A, Vaghefi KA, Labbafi S, Oktaie S, Abtahi M, Mohagheghian A (2013) Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI). Ecol Indic 30(30):28–34. doi:10.1016/j.ecolind.2013.02.008
Navoni JA, De Pietri D, Olmos V, Gimenez C, Bovi Mitre G, de Titto E, Villaamil Lepori EC (2014) Human health risk assessment with spatial analysis: study of a population chronically exposed to arsenic through drinking water from Argentina. Sci Total Environ 499:166–174. doi:10.1016/j.scitotenv.2014.08.058
Ni FQ, Liu GD, Ren HZ, Yang SC, Jian Y, Lu XY, Yang M (2009) Health risk assessment on rural drinking water safety—a case study in Rain City District of Ya’an City of Sichuan Province. J Water Resour Protect 01(2):128–135. doi:10.4236/jwarp.2009.12017
Ni F Q, Liu G D, Tan Y S, Deng Y (2010) Spatial variation of health risk of groundwater for drinking water supply in Mingshan County, Ya’an City, China. Water Sci Eng 3(4):454–466. doi:10.3882/j.issn.1674-2370.2010.04.008
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Trans Am Geophys Union 25(6):914–928. doi:10.1029/TR025i006p00914
Qian H, Li P, Howard KWF, Yang C, Zhang X (2012) Assessment of groundwater vulnerability in the Yinchuan Plain, Northwest China using OREADIC. Environ Monit Assess 184(6):3613–3628. doi:10.1007/s10661-011-2211-7
Qian H, Li P, Wu J, Zhou Y (2013) Isotopic characteristics of precipitation, surface and ground waters in the Yinchuan Plain, Northwest China. Environ Earth Sci 70(1):57–70. doi:10.1007/s12665-012-2103-3
Rao GT, Rao VVSG, Padalu G, Dhakate R, Sarma VS (2013) Application of electrical resistivity tomography methods for delineation of groundwater contamination and potential zones. Arab J Geosci 7(4):1373–1384. doi:10.1007/s12517-013-0835-3
Rasool A, Farooqi A, Masood S, Hussain K (2016) Arsenic in groundwater and its health risk assessment in drinking water of Mailsi, Punjab, Pakistan. Hum Ecol Risk Assess 22(1):187–202. doi:10.1080/10807039.2015.1056295
Regional Hydrogeologic Investigation Team of Shaanxi (1980) Report of regional hydrogeologic investigation for Shenmu. Regional Hydrogeologic Investigation Team of Shaanxi, Shenmu (in Chinese)
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Agricultural hand book, vol 60. U.S. Dept. of Agriculture, Washington, p 160
Ryu H, Han JK, Jung JW, Bae B, Nam K (2007) Human health risk assessment of explosives and heavy metals at a military gunnery range. Environ Geochem Health 29(4):259–269. doi:10.1007/s10653-007-9101-5
Sirat M (2013) Neural network assessment of groundwater contamination of US Mid-continent. Arab J Geosci 6:3149–3160. doi:10.1007/s12517-012-0570-1
Su XS, Wang H, Zhang Y (2013) Health risk assessment of nitrate contamination in groundwater: a case study of an agricultural area in northeast China. Water Resour Manag 27(8):3025–3034. doi:10.1007/s11269-013-0330-3
Su XS, Xu W, Du SH (2014a) In situ infiltration test using a reclaimed abandoned river bed: managed aquifer recharge in Shijiazhuang City, China. Environ Earth Sci 71(12):5017–5025. doi:10.1007/s12665-013-2893-y
Su XS, Xu W, Du SH (2014b) Responses of groundwater vulnerability to artificial recharge under extreme weather conditions in Shijiazhuang City, China. J Water Supply 63(3):224–238. doi:10.2166/aqua.2013.132
Su H, Kang W, Xu Y, Wang J (2016) Assessment of groundwater quality and health risk in the oil and gas field of Dingbian County, northwest China. Expo Health. doi:10.1007/s12403-016-0234-6
Su H, Kang W, Xu Y, Wang J (2017) Assessing groundwater quality and health risks of nitrogen pollution in the Shenfu mining area of Shaanxi Province, Northwest China. Expo Health. doi:10.1007/s12403-017-0247-9
Suthar S, Bishnoi P, Singh S, Mutiyar PK, Nema AK, Patil NS (2009) Nitrate contamination in groundwater of some rural areas of Rajasthan, India. J Hazard Mater 171(1–3):189–199. doi:10.1016/j.jhazmat.2009.05.111
Tai T, Wang J, Wang Y, Bai L (2012) Groundwater pollution risk evaluation method research progress in our country. J Beijing Norm Univ Nat Sci 06:648–653 (in Chinese)
Thilagavathi R, Chidambaram S, Prasanna MV, Thivya C, Singaraja C (2012) A study on groundwater geochemistry and water quality in layered aquifers system of Pondicherry region, southeast India. Appl Water Sci 2(4):253–269. doi:10.1007/s13201-012-0045-2
Wei JM, Mi WB, He TH (2008) Assessment of the safety status for the drinking water supply sources and its management strategies in Yinchuan City. J Water Resour Water Eng 19(3):65–68 (in Chinese)
Wen D, Zhang F, Zhang E, Wang C, Han S, Zheng Y (2013) Arsenic, fluoride and iodine in groundwater of China. J Geochem Explor 135:1–21. doi:10.1016/j.gexplo.2013.10.012
Wilcox LV (1948) The quality of water for irrigation use. US Dept of Agriculture Tech Bull 1962, Washington
Wu H (2014) Groundwater vulnerability and health risk assessment in Yinchuan Beijiao water resource. A Dissertation submitted for the Degree of Master, Chang’an Univeristy, Xi’an (in Chinese)
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8(3):311–329. doi:10.1007/s12403-015-0170-x
Wu J, Li P, Qian H (2011) Groundwater quality in Jingyuan County, a semi-humid area in Northwest China. J Chem 8(2):787–793. doi:10.1155/2011/163695
Wu J, Li P, Qian H (2013a) Variations of hydrogeochemical characteristics of shallow groundwater caused by agricultural activities. Asian J Chem 25(13):7441–7444. doi:10.14233/ajchem.2013.14808
Wu J, Li P, Qian H (2013b) Environmental chemistry of groundwater near an industrial area, Northwest China. Asian J Chem 25(17):9795–9799. doi:10.14233/ajchem.2013.15355
Wu J, Li P, Qian H, Fang Y (2014a) Assessment of soil salinization based on a low-cost method and its influencing factors in a semi-arid agricultural area, northwest China. Environ Earth Sci 71(8):3465–3475. doi:10.1007/s12665-013-2736-x
Wu J, Li P, Qian H, Duan Z, Zhang X (2014b) Using correlation and multivariate statistical analysis to identify hydrogeochemical processes affecting the major ion chemistry of waters: case study in Laoheba phosphorite mine in Sichuan, China. Arab J Geosci 7(10):3973–3982. doi:10.1007/s12517-013-1057-4
Wu J, Xue C, Tian R, Wang S (2017) Lake water quality assessment: a case study of Shahu Lake in the semi-arid loess area of northwest China. Environ Earth Sci 76:232. doi:10.1007/s12665-017-6516-x
Xiao C, Liu J, Liang X, Du SH (2016) Hydrogeochemistry characteristics of groundwater and its suitability for water supply and irrigation in Jilin City, China. Arab J Geosci 9(6):1–11. doi:10.1007/s12517-016-2447-1
Yang M, Fei Y, Ju Y, Ma Z, Li H (2012) Health risk assessment of groundwater pollution-a case study of typical city in North China Plain. J Earth Sci 23(3):335–348. doi:10.1007/s12583-012-0260-7
Yidana SM, Banoeng-Yakubo B, Akabzaa TM (2010) Analysis of groundwater quality using multivariate and spatial analyses in the Keta basin, Ghana. J Afr Earth Sci 58(2):220–234. doi:10.1016/j.jafrearsci.2010.03.003
Zhang R, Li HX, Wu X, Fan F et al (2009) Assessment of water quality in drinking water in rural areas, China. J Environ Health 26:3–4
Zhu L (2012) Research of groundwater pollution and vulnerability in the city of Yulin and its surrounding areas. A dissertation submitted for the Degree of Master, Northwest Univeristy, Xi’an (in Chinese)
Acknowledgements
This research is funded by the State Key Program of National Natural Science Foundation of China (41630639), the Land and Resources Survey Project of China (1212011220982), the Major Program of National Natural Science Foundation of China (91025019) and the Joint Foundation of Key Laboratory of Institute of Hydrogeology and Environmental Geology, Chinese Academy of Geological Sciences (KF201607). Anonymous reviewers and the editor are sincerely acknowledged for their constructive comments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is a part of a Topical Collection in Environmental Earth Sciences on Water resources development and protection in loess areas of the world, edited by Drs. Peiyue Li and Hui Qian.
Rights and permissions
About this article
Cite this article
Su, H., Kang, W., Xu, Y. et al. Evaluation of groundwater quality and health risks from contamination in the north edge of the Loess Plateau, Yulin City, Northwest China. Environ Earth Sci 76, 467 (2017). https://doi.org/10.1007/s12665-017-6781-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-017-6781-8