Abstract
The escalation of antibiotic resistance has revitalized bacteriophage (phage) therapy. Recently, phage therapy has been gradually applied in medicine, agriculture, food, and environmental fields due to its distinctive features of high efficiency, specificity, and environmental friendliness compared to antibiotics. Likewise, phage therapy also holds great promise in controlling pathogenic bacteria in aquaculture. The application of phage therapy instead of antibiotics to eliminate pathogenic bacteria such as Vibrio, Pseudomonas, Aeromonas, and Flavobacterium and to reduce fish mortality in aquaculture has been frequently reported. In this context, the present review summarizes and analyzes the current status of phage therapy in aquaculture, focusing on the key parameters of phage application, such as phage isolation, selection, dosage, and administration modes, and introducing the strategies and methods to boost efficacy and restrain the emergence of resistance. In addition, we discussed the human safety, environmental friendliness, and techno-economic practicability of phage therapy in aquaculture. Finally, this review outlines the current challenges of phage therapy application in aquaculture from the perspectives of phage resistance, phage-mediated resistance gene transfer, and effects on the host immune system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria-derived diseases are the leading cause of high fish mortality and tremendous economic losses in aquaculture. Antibiotics have been mainly used for therapeutic and prophylactic purposes in aquaculture (Vaseeharan and Thaya 2014). However, antibiotics may enter into the environment by leaching from uneaten feeds, the excreted fraction with manure since antibiotics are mainly administered through the dip-coated feed (Robinson et al. 2007). To the present knowledge, the residues of antibiotic components and the spread of antibiotic resistance genes (ARGs) are universal in aquaculture and pose significant health threats (Fang et al. 2019; Gao et al. 2018; Guo et al. 2019; Han et al. 2020). Furthermore, broad-spectrum antibiotics can jeopardize the indigenous microorganisms and beneficial microbiota. Recently, studies have shown that the addition of antibiotics, even below the dose approved by FDA, can irreversibly influence the diversity and community structure of the gut microflora of fish (Saenz et al. 2019).
In recent years, advances in antibiotic alternatives for fish disease control have emerged. Some novel vaccines (Vinay et al. 2018; Yan et al. 2021; Zhang et al. 2021a) have been trialed in aquaculture, and promisingly, a vaccine injection of salmonids in particular is performed on a large-scale. However, given the complexity of pathogens and the difficulties of vaccine administration in aquaculture (Micoli et al. 2021), vaccination is still debatable. Probiotics, live microbial food additives for regulating host gut homeostasis (Fuller 1989), appear to be an effective biological control agent owing to their salient nature of positively affecting host metabolic activity and increasing host resistance to pathogens (Dawood et al. 2019). Nonetheless, the rapid colonization and proliferation of probiotics in a given environment have always been an insurmountable gap due to the competition for niches and nutrients with another microbiota in the environment (Van Hien et al. 2021). These impediments, coupled with the escalating issue of antibiotic resistance, have made it urgent to explore novel and efficient antibiotic alternatives in aquaculture.
Bacteriophages (phages), the most abundant organisms on the planet (Suttle 2007), are known as natural killers of bacteria (Domingo-Calap and Delgado-Martinez 2018). The phage-based biocontrol method, or phage therapy, is characterized by high efficiency, specificity, and environmental friendliness (Choudhury et al. 2017; Sieiro et al. 2020) and has gradually become the protagonist of a post-antibiotic era (Altamirano and Barr 2019). Nowadays, phage therapy has been heavily explored in medicine (Onsea et al. 2019; Ooi et al. 2019), agriculture (Kahn et al. 2019; Svircev et al. 2018), food (Clavijo et al. 2019; Luiz Vaz et al. 2020), and environmental fields (Ayyaru et al. 2018; Sun et al. 2019). Similarly, phage therapy has great potential in aquaculture against pathogenic bacteria (Le and Kurtboke 2019). Recently, many successful cases of phage therapy for preventive and therapeutic purposes in aquaculture have been reported, using phages targeting Vibrio sp. Va-F3 (Chen et al. 2019), Vibrio coralliilyticus (Chen et al. 2019; Kim et al. 2019a), Vibrio alginolyticus (Tuan Son et al. 2020), Pseudomonas aeruginosa (Cafora et al. 2019), Aeromonas hydrophila (Cao et al. 2020; Tuan Son et al. 2018), and Flavobacterium columnare (Laanto et al. 2015) and other fish pathogens.
Although phage therapy in aquaculture has made great progress in laboratory research, there are still many problems to be solved before practical application. Due to the complexity of application scenarios, some of the key parameters in phage therapy, such as phage selection, dose, and administration method, are often customized, but a systematic review and analysis of relevant content are currently lacking. In addition, it is necessary to introduce some novel approaches that may be used to improve the efficacy of phage therapy in aquaculture. Furthermore, the environmental health, food safety, and techno-economic practicability of phage therapy in aquaculture, which is an ongoing concern for us, has also been rarely mentioned in other literature. In such a context, this review spotlights these issues and presents some challenges and prospects of phage therapy in aquaculture, intending to provide new insights into phage therapy and accelerate its application in aquaculture.
Rationale for phage therapy in aquaculture
The earliest cognition of phages can be dated back to “the cholera period.” Ernest Hanbury Hankin, a British bacteriologist, believed that there was a mysterious substance that could kill Vibrio cholerae in the Ganges River, India, and Frederick W. Twort first confirmed the existence of phages in 1915. However, scientific data on phage-based pathogen control is limited at the time. In particular, the discovery of penicillin, a broad-spectrum antibiotic, in 1928, exacerbated the long stagnation of phage therapy research. Until recently, it was gradually revived and received widespread attention with the advent of the antibiotic crisis.
Phages can replicate through the lytic, lysogenic, chronic, and pseudolysogenic cycles (Mirzaei and Maurice 2017), but chronic and pseudolysogenic cycles remain poorly described. In the lytic cycle, phage utilizes the substrates within the host to produce progeny phages after invading the host and, hence, leading to host lysis and death. As for the lysogenic cycle, phages typically integrate their DNA into the genome of the host and replicate synchronously with the host replication but ultimately leading to the death of the host as well. Phage therapy, as a means of preventing or treating diseases, mainly exploits the lytic effect of virulent phages to reduce the density of pathogenic bacteria and thereby reduce or avoid the incidence of disease occurrence. Although cases of lysogenic phage for therapy are still relatively rare, lysogenic phages in the natural environment can enter into the lytic cycle induced by temperature (Chu et al. 2011), pH (Miller-Ensminger et al. 2020) and UV (Zhang et al. 2020) to regulate microflora. Notably, unlike chemical bacteriostatic agents such as antibiotics, phages can lyse hosts continuously to produce progeny phages to maintain phage titers in the environment.
There is an order of magnitude more phages than bacteria on the planet, namely, 1031 or more (Guemes et al. 2016; Mokili et al. 2012), widespread in seawater (Ignacio-Espinoza et al. 2020; Wu et al. 2020), marine sediments (Engelhardt et al. 2014, 2015; Lachnit et al. 2019), soils (Jin et al. 2019; Kuzyakov and Mason-Jones 2018), and artificial ecosystems (Brown et al. 2019). Additionally, phages for therapeutic use in aquaculture can stably survive in a wide range of pH (5–9), temperature (4–37 °C), and salinity (0.1–3.5%) (Chandrarathna et al. 2020; Kim et al. 2019a; Nikapitiya et al. 2020a), covering common aquaculture environments. Of particular interest is the fact that these phages can survive for extended periods even used in vivo in aquatic economic organisms. For example, phages can maintain survival activity in the complex biological environment of the rainbow trout intestines and spleens for at least 4 days (Christiansen et al. 2014). Strong environmental tolerance reduces the frequency of phage administration, facilitating their practical application. Compared with other biochemical agents, phage therapy has the following unparalleled advantages: (1) high efficiency against drug-resistant bacteria; (2) strong specificity to hosts; (3) low direct toxicity to eukaryotes; and (4) flexible administration modes.
Pre-application preparation
Since the antibiotic crisis, especially since the twenty first century, the number of phage therapy-related publications has been increasing dramatically with each passing day, with a rate of > 1400 publications/year in the last decade (Fig. 1A, B). Inappropriate choice, poor preparation, and decay of phages before application are recognized as the three main factors in the failure of phage application (Gill and Hyman 2010). Furthermore, the possibility of phage formulation determines the potential of phage application in moving from small trails to field applications. This section reviews and summarizes the preparation steps for phage application in aquaculture (Fig. 2), aiming to provide theoretical support for phage application.
A number of phage therapy-related publications and sequenced phages targeting fish pathogenic bacteria in the last 100 years (A) and in the last decade (B) demonstrate widespread interest in phage therapy. The bar indicates the number of Web of Science searching for the publications related to phage therapy (TS = (phage therap* OR bacteriophage therap* OR phage control* OR bacteriophage control* OR phage cure* OR phage treat*)) and NCBI released complete phage genomes associated with fish pathogens (Aeromonas, Edwardsiella, Flavobacterium, Francisella, Photobacterium, Piscirickettsia, Pseudomonas, Tenacibaculum, Vibrio, Yersinia, Lactococcus, Renibacterium, Streptococcus, Acinetobacter, Shewanella, Plesiomonas, Stenotrophomonas, and Kocuria) from 1920 to 2019
Phage isolation
Phages and their corresponding hosts tend to concomitance, so it is common to isolate phages from sources with high host abundance (Kim et al. 2020; Richards et al. 2021). For example, Richards et al. (2021) isolated 16 novel Vibrio coralliilyticus and Vibrio tubiashii phages from seawater. Also, Liu et al. (2020) isolated 5 Aeromonas hydrophila phages from fish ponds and polluted rivers, not from sewage. These suggest that the source of isolation is critical. By 2019, over 2,000 phages targeting fish pathogens have been isolated, and over 90% of these phages were isolated in the last decade (Fig. 1A, B) (complete genomes of phages targeting fish pathogens released by the National Center Biotechnology for Information).
For phage isolation, the most classical method is the double-layer agar (DLA) method. This method places a semi-solid medium containing the host bacteria and phages on top of a solid plate to visualize phage plaques. Clear plaques are most likely to be formed by virulent phages and appropriate for subsequent applications, while turbid ones are mostly formed by temperate phages. Interestingly, broad-spectrum phages are more common in environments with low bacterial concentrations owing to their ability to infect more potential hosts, while high host concentrations stimulate the growth of phages with strong host specificity (Gill and Hyman 2010). Accordingly, this survival strategy of phages undoubtedly promotes the selection for narrow-spectrum phages due to the high host density in the DLA method.
Several novel methods for phage isolation have been developed in recent years, such as ultrafiltration- or adsorption-based separation methods, and concentration methods by enrichment from water, soil, and sediment (Nair et al. 2021). Nafarrate et al. (2020) used Bolton selective broth to enrich phages for 48 h at 42 ℃ to isolate Campylobacter-specific phages without shaking. This method achieved a low detection limit and high recovery rate compared to other isolation methods with different parameter settings, demonstrating this method to be a simple, reproducible, and efficient approach for the isolation of Campylobacter phages. In addition to typical strong-specific phages, polyvalent phages (Ngiam et al. 2021; Yu et al. 2016) and macrophages (Saad et al. 2019) can also be selectively isolated by modified isolation methods.
Currently, almost all phage isolation methods are host-dependent. However, a typical paradigm that only 1% of bacteria are cultivable (Whitman et al. 1998) suggests that the vast majority of hosts in the natural environment are unculturable, let alone corresponding phages. Vibrio, Flavobacterium, Aeromonas, and Pseudomonas are the hosts with the most isolated phage counterparts, as well as the pathogens with the most phage therapy applications in aquaculture (Culot et al. 2019). Fortunately, cultivation techniques for uncultured pathogenic bacteria are relatively well-developed (Lewis et al. 2021), which is conducive to phage-based disease control in aquaculture.
Phage characterization and selection
Clarifying the fundamental properties of phages is a prerequisite for screening phages as safe biocontrol agents. Some basic but critical features, such as phage morphology, host range, one-step growth curve, the optimal multiplicity of infection (MOI), environmental tolerance, detailed genome analysis, and in vitro lysis effect, are closely related to phage selection. For example, optimal MOI can guide the phage-to-host ratio in actual production, and in vitro lysis effect can guide phage dosage. In fact, studies have shown that there are significant differences in the protective effects of different MOIs on fish (Kim et al. 2019a; Laanto et al. 2015). More detailed selection criteria of phages for therapeutic use have been discussed in several literatures (El Haddad et al. 2019; Gill and Hyman 2010; Luong et al. 2020).
Theoretically, an ideal phage candidate for therapy should be strictly lytic, polyvalent, environment-tolerant, and free of antibiotic resistance and virulence genes and can coexist with other phages (to form phage cocktails). The ability of phages to infect bacteria in biofilms should also be considered, since biofilm is a common form of aggressive resistance of pathogenic bacteria to therapeutic agents and thus a challenging aspect for treatment. Some phages can destroy the biofilm formed by single- or multi-species bacteria effectively (Forti et al. 2018; Gonzalez et al. 2017; Kim et al. 2019b), while others may promote biofilm formation by increasing aggregation, surface adhesion, and production of bacteria fimbria to create barriers (Hansen et al. 2019; Lacqua et al. 2006). Although phage selection is a critical step to the success of phage therapy trials, uniform standards have not yet been met, and much work remains to be done.
Phage preservation
To maintain the phage activity for a long-time, a suitable preservation method should be selected. In general, the phage titer slightly decreases over several months with commonly used protective agents such as chloroform and SM buffers, as well as with cryopreservation. For example, Srinivasan and Ramasamy preserved the isolated Vibrio vulnificus phages VV1–VV4 in chloroform (7%) or DMSO (7%), which showed that the infectivity of all phages was not affected at − 40 °C for up to 30 days in both preservation methods (Srinivasan and Ramasamy 2017). González-Menéndez et al. (2018) compared the titer of Staphylococcus aureus phages phiIPLA-RODI and phiIPLA-C1C under different preservation conditions. They found that lower temperatures (− 80 °C and − 196 °C) or lyophilization allow phages to exhibit good viability, and encapsulation facilitates preservation and transportation, but repeated freezing and thawing cycles will inactivate part of the stored phage particles (González-Menéndez et al. 2018). Other than this, the results of Leung et al. (2017) showed that a powder containing ≥ 40% trehalose exhibited good phage PEV2 storage performance within 12 months. In another analogous study, pullulan–trehalose encapsulated phages can remain infectious for up to 3 months at ambient conditions (Leung et al. 2018).
Phage preparation
Most phages currently isolated that target fish pathogenic bacteria are more poorly tolerated at low pH owing to the nature of their protein capsids (Le Thanh et al. 2021; Tan et al. 2021; Xu et al. 2021), while the pH values in the gastrointestinal tract of fish vary from 2 to 7 (Kihara et al. 2012). Therefore, for intestinal disorders, it is particularly essential to coat or encapsulate phages to minimize titer loss as the phages pass through the digestive tract when administrated orally. Huang and Nitin (2019) developed a phage-based edible whey protein isolate (WPI) coating on fish feed; the results demonstrate that this feed loaded with coated phages enhances phage stability and reduces host bacteria concentration in a simulated gastrointestinal environment compared to feed loaded uncoated phages (validation with T7 phage and Vibrio phage and their corresponding hosts). In a similar study, Salmonella phage SPT 015 microencapsulated with a 3: 1 ratio of WPI and trehalose can survive over 90% of exposure to pH 1.5 for 5 h, while non-encapsulated phages could not survive at the same conditions (Petsong et al. 2021). Furthermore, studies on liposome- or alginate/CaCO3-encapsulated phages can enhance the tolerance of phages under a low pH environment compared to non-encapsulated phages which has also been reported (Colom et al. 2017, 2015).
Customized phage therapy
Several parameters, such as dosage and mode of phage administration, are crucial in phage therapy, and optimizing such parameters allows to reduce the costs as well as to enhance the therapeutic efficacy and accelerate the widespread use of phage therapy in aquaculture. The following section discusses the latest researches on these parameters. For reference, laboratory findings under different parameters are listed in Table 1.
Broad-spectrum phages or phage cocktails
Aquaculture environments contain multiple species of bacteria that are pathogenic to aquatic organisms, and the same species of bacteria also include several pathogenic serotypes, which impedes the widespread use of a single narrow-spectrum phage. In such a context, more efforts have been dedicated to broad-spectrum phages and phage cocktails.
Broad-spectrum phages, phages that can infect multiple distinct hosts within one genus or in multiple genera (de Jonge et al. 2019), can efficiently lyse not only host bacteria but also background pathogens that are ubiquitous in farming and natural environment (Kalatzis et al. 2016; Rorbo et al. 2018). For example, Vibrio phage KVP40, a broad-spectrum phage, was able to not only postpone death or reduce mortality in the challenge group but also lower background mortality in the non-challenge control group by lysing background pathogenic hosts (Rorbo et al. 2018), highlighting the prevention use of phage KVP40 in aquaculture. Phages capable of lysing multiple serotypes have also been reported. Phage PH669 can lyse multiple strains of O3 and O4 serotypes in Vibrio parahaemolyticus (Hu et al. 2021), while the broader host range of phage vB_SPuM_SP116 and phage vB_SalS-LPSTLL can lyse 9 (Bao et al. 2019) and 11 (Guo et al. 2021) different types of serotypes in Salmonella, respectively. However, the acquisition of broad-spectrum phages remains challenging due to the differences in phage survival strategies (see part 3.1) and imperfection of isolation methods (Ngiam et al. 2021; Yu et al. 2016).
Phage cocktails, the mixture of multiple phages, can expand the host range and reduce the frequency of bacterial resistance development (Peters et al. 2020), as bacteria that are resistant to certain phages can also be killed by other phages. In general, the efficacy of phage cocktails is more potent than that of a single phage (Chen et al. 2019, 2018; Forti et al. 2018; Mateus et al. 2014). For example, a phage cocktail containing phage VP-1, VP-2, and VP-3 delayed the formation of phage resistance and improved the effect of inactivation against Vibrio parahaemolyticus compared to single phage therapy (Mateus et al. 2014). Moreover, Chen et al. (2019) isolated five broad-spectrum phages with strong lytic capacity from aquaculture wastewater and the intestinal tract of a diseased shrimp to construct a phage cocktail for inactivation of Vibrio sp. Va-F3. The inhibitory effect of the phage cocktail on Vibrio sp. Va-F3 was significantly better than any single phage in vitro, and the in vivo protection of shrimp can be comparable to antibiotics (survival rates reach 91.4 and 91.6% in 7 days, respectively) (Chen et al. 2019). Encouragingly, phage cocktails containing phage PVP1 and PVP2 were as effective as antibiotics in controlling Vibrio parahaemolyticus in sea cucumber Apostichopus japonicus at doses of MOI = 10 and MOI = 100 (Ren et al. 2019). These highlight the advantages of phage cocktails in the control of bacterial diseases in aquaculture (fish and other aquatic organisms). However, it has been shown that cocktails containing excessive species of phages constructed for a wider host range may produce new host specificity (Essoh et al. 2013), and the optimal phage cocktail formulation is modifiable and should not be overly complex (Chan et al. 2013).
Dosage
Although it is challenging to determine the MOI in the actual disease context, the determination of the optimal phage dosage to be administrated can ensure therapeutic efficiency while saving costs in the laboratory study. Underdose of therapeutic phages may decrease the efficacy or induce phage resistance in bacteria (Kim et al. 2012), while overdose may cause hosts to resist phage infection by forming aggregates or biofilms and increase costs (Tan et al. 2015). Water flow and environmental stresses in the aquaculture ecosystem are responsible for the loss of therapeutic phages, while phages can hijack hosts to continuously produce progeny phages. These factors, together with the administration dosage, co-determine the phage titer in the aquaculture environment.
Typically, MOI provides an essential reference for the dosage administered. Dang et al. (2021) studied the protective efficacy of different MOIs of phage PVN02 against hemorrhagic septicemia in striped catfish Pangasianodon hypophthalmus via oral administration. They found that the mortality rate of striped catfish reduced by 60% at the phage dose of log 6.2 ± 0.09 compared to the phage-free group and striped catfish mortality was negatively correlated with phage dosage (Dang et al. 2021). A similar pattern that the higher the phage dose, the lower the mortality was also observed in the control of acute hepatopancreatic necrosis disease (AHPND) caused by Vibrio parahaemolyticus in the shrimp aquaculture industry (Ding et al. 2020). In contrast, phage pVco-14 can inactivate Vibrio coralliilyticus favorably at low MOI (MOI = 0.1) for oyster larvae protection (Kim et al. 2019a). Interestingly, in our previous study of phage therapy for Edwardsiellosis in zebrafish, the optimal MOI for phages against pathogenic bacteria in vitro and in vivo would be different since the fish gastrointestinal tract is a complex environment containing multiple microorganisms and chemical substances (unpublished data). This further emphasizes the indispensable line of clinical validation of phage therapy in aquaculture. The dose of phage administrated tends to be personalized due to the influence of water quality, fish species, and other factors and requires extensive field trials for ongoing optimization.
Administration mode
The mode of phage administration is also a crucial factor affecting the efficacy of phage therapy. Several modes of administration, such as oral administration by soaking feed; directly adding phages in the culture system, intraperitoneal or intramuscular injections; and immersion, have been reported for phage therapy in aquaculture (Donati et al. 2021; Kokkari et al. 2018; Prasad et al. 2011).
In a flow-through experimental system close to the real-life rearing environment, bath treatment achieves the optimal protection for rainbow trout when the first symptoms of columnaris disease were observed (Kunttu et al. 2021). However, the volume of water requiring phage treatment in practical aquaculture is generally quite large. Pre-infection administration for prophylactic purposes is currently the prevailing approach in aquaculture, particularly for injection administration to avoid secondary hurt to the fish, but cases of phages for post-infection treatment have also shown good results (Kunttu et al. 2021; Wu et al. 2021). This administration method resembles vaccine injection, with a single mode of dosing and difficulty in operation. Other than this, oral phage has been demonstrated to be more efficient than the injection route in the control of skin syndrome of sea cucumber caused by Vibrio cyclitrophicus (Li et al. 2016). Despite the fact that gastric acid is a negative factor for orally administered phages, as far as the literature is concerned, most phages can reach the intestine through the gastric barrier (Donati et al. 2021; Xue et al. 2020). Donati et al. (2021) adopted three different modes of administration, namely, oral, bath, and injection, to study the phage efficacy against Flavobacterium psychrophilum and migration rate in different organs of rainbow trout. The Flavobacterium psychrophilum phage can be detected consistently in the intestine and determined sporadically in the spleen, brain, and kidney via oral administration (Donati et al. 2021). This finding broadly provides evidence for previous observations that phages can be detected in the intestine, spleen, brain, and kidney post-oral administration (Christiansen et al. 2014). Of great concern is that phages loaded onto the feed will be re-released into the aqueous environment (Huang and Nitin 2019), which is advantageous for oral administration because some of the pathogens are freely in the water or can form biofilms on rearing equipment.
Upgrading phage therapy to boost the efficacy
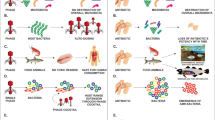
Most current methods aimed to boost the phage efficacy and avoid the emergence of phage resistance broadly follow at least one of three main strategies. They either rely on combination with other antimicrobial drugs for co-inhibition (phage–antibiotic synergy), or enhance the efficacy of phage therapy by customized modification of the phage (genetically engineered phages), or utilize its gene expression products (phage lysis proteins). Methods that fall into these three categories are reviewed and discussed in the sections below (Fig. 3).
Phage–antibiotic synergy
The phenomenon that phage–antibiotic combinations can enhance therapeutic efficacy was discovered as early as 1945 (Himmelweit 1945) but failed to receive sufficient attention. Until 2007, Comeau et al. (2007) observed plaque expansion in β-lactam antibiotics, and the phenomenon was then officially designated as phage–antibiotic synergy (PAS) and gradually regained attention.
Phage–antibiotic synergy has been shown to effectively inhibit multiple antibiotic-resistant bacteria (Jo et al. 2016a, 2016b; Ryan et al. 2012). The co-existence of a sub-lethal dose of antibiotics with phages and hosts may cause cocci swelling or bacilli filamentation and, simultaneously, increase the content of mRNA encoding phage polymerase and delay the lysis time after infection to obtain a greater phage burst size and a larger plaque (Kim et al. 2018). Likewise, Kamal and Dennis (2015) mixed Burkholderia cepacia complex (Bcc) and Bcc phages KS12 and KS14 with antibiotics and found that the host cells developed a chain-like arrangement, an elongated morphology, as well as a clustered arrangement, and the size of the plaque and phage titer increased with increasing antibiotic concentration. Although to our knowledge there are currently no studies reported on the application of phage–antibiotic combination in aquaculture, the remarkable results of PAS in other fields herald its great potential in aquaculture. For example, in the treatment of Bcc infected Galleria mellonella larvae, the survival rate of Bcc phage KS12 combined with low concentrations of the meropenem group was significantly higher than that of KS12 or antibiotics alone (Kamal and Dennis 2015). However, discordant opinions have also appeared that the combination of sub-MIC streptomycin with phages not only enhances antibiotic resistance in bacteria but also leads to the extinction of phages (Cairns et al. 2017). Although this anomalous result may be due to the fact that streptomycin is a common mutagen, this finding still remains alarming. In particular, when combining phages with antibiotics against pathogens in aquaculture, the selection of phages and antibiotics and their mixing ratio, and even the order of addition, should be fully considered (Tagliaferri et al. 2019).
Genetically engineered phages
Genetic engineering as an emerging technology allows the insertion of antibiotic sensitive genes into the genome of temperate phages and further integration of these genes into resistant bacteria using their lysogenic properties and hence restores antibiotic sensitivity of bacteria (Edgar et al. 2012) or customizes the tail recognition structure of virulent phages to broaden their host range (Mahichi et al. 2009). Yehl et al. (2019) altered and expanded the host range by modifying complementarity-determining regions (CDRs) in the phage tail fiber, wherein phage pB10 was effective in inhibiting pathogen growth in a mouse skin infection model without phage resistance. Notably, genetically engineered phages for human therapy were first reported with good results in 2019 (Abbasi 2019). Despite the fact that the world is currently conservative on genetic engineering and genetically modified phages are still confined in the laboratory, it may be a potential tool against pathogenic bacteria in aquaculture.
Phage lysis protein
A method for direct utilization of phage lysis proteins, mainly virion-associated phage hydrolase proteins and cell envelope digesting proteins, has been proposed in recent years to broaden the host range (Drulis-Kawa et al. 2015). These proteins can also synergize with other antimicrobial agents to reduce the risk of virulence factor transfer and resistance mutations in bacteria (Duarte et al. 2021; Grabowski et al. 2021; Letrado et al. 2018; Linden et al. 2021; Schmelcher et al. 2012). Virion-associated peptidoglycan hydrolase plays a major role in bacterial surface receptor recognition in the first step of the infection. Cell envelope digesting proteins, mainly holins and endolysins, disrupt the cell structure after completing the assembly of progeny phages, thereby releasing the progeny phages. Although the cell envelope digesting proteins destroy cellular structure from inside the cell, it has been shown that externally administered endolysins can still lyse Gram-negative bacteria slowly by osmosis (Briers and Lavigne 2015). LysVPMS1, endolysins with a broad lytic activity, has been proven to be effective biocontrol agents (Angelica Zermeno-Cervantes et al. 2018). In addition, although phage PSP01 lyses the host bacteria Plesiomonas shigelloides specifically, the combination of HolPSP and LysPSP-1, holin and endolysin of phage PSP01, exhibited a synergetic effect on the lysis of E. coli (Zhang et al. 2021b). Notably, protein therapy is particularly powerful toward Gram-positive bacteria such as Staphylococci and Streptococci (Linden et al. 2021). However, phage lysis proteins may generate neutralizing antibodies to compromise their efficacy due to their proteinaceous nature (Fischetti 2010). Furthermore, whether these proteins are sufficiently tolerated in complex aquaculture environments and the acidic digestive system of the fish are also questions worth investigating in depth.
Food safety, environmental benefits, and techno-economic analysis of phage therapy
Food safety
There is currently no well-developed methodology to assess the safety of phage therapy in food-related industries, but theoretically, it is harmless to human health for the following reasons. Firstly, phages are abundant and ecologically important in aquatic ecosystems (Kavagutti et al. 2019) and widespread in the digestive tract of mammals (Letarov et al. 2010) and are part of the normal microbiota of water environments and human guts. Secondly, phages have been widely used in the food industry for foodborne pathogen detection, active food packaging, postharvest, and processed foodstuff biopreservation (O’Sullivan et al. 2019) and have a significant role in facilitating the development of the food industry. Thirdly, the phages used in aquaculture specifically target only pathogenic bacteria, not the normal human microbiota, and should not pose a threat to human health.
Actually, since the product that anti-Listeria, i.e., Listex™, was first generally recognized as safe (GRAS) by the US Food Drug and Administration (FDA) in 2006, an increasing number of phage-related products have been GRAS in recent years. To date, at least seven biotechnology companies have devoted to phage therapy for pathogenic bacteria control in aquaculture and have developed several safe, simple, and effective commercial phage products to target and destroy pathogenic bacteria (for instance, CUSTUS®YRS for Yersinia ruckeri control, LUXON for Vibrio parahaemolyticus control). More detailed information is available at https://www.bacteriophage.news.
Environmental friendliness
Pathogenic bacteria are prevalent in intensive marine aquaculture systems and may influence the initial microbial communities and threaten the safety of fish in natural water bodies (Wang et al. 2018). However, a common chemical disinfectant can disturb the bacterial communities in aquaculture even at low concentrations (Saenz et al. 2019; Teitge et al. 2020). Given this situation, the utilization of phages in aquaculture may be a promising method to prevent the disruption of normal microbiota in farmed and further natural water bodies due to its strong specificity. Notably, it has been previously demonstrated that phage therapy has less environmental impact than chemotherapy in fish farming plants (Almeida et al. 2009). On the other hand, a large number of ARGs, such as sul1, tetG, intl1, tetX, and tetW, have also been detected in large-scale freshwater farming systems, forming a huge reservoir of ARGs (Shen et al. 2020). Theoretically, the use of phage therapy in aquaculture can reduce antibiotic consumption, thereby lowering the frequency of ARG production in aquaculture water and reducing the spread of ARGs in natural environments. In fact, polyvalent phage YSZ-1 alone or in combination with biochar can significantly reduce the abundance of antibiotic-resistant pathogenic bacteria E. coli K-12 and Plesiomonas aeruginosa PAO1 and their carried ARGs (tetM, tetQ, tetW, ampC, and fosA) in a soil–lettuce system (Ye et al. 2018). Our present study also found that phage treatment for Edwardsiellosis can significantly reduce the abundance of floR gene in zebrafish excreta and associated feeding water environment compared to florfenicol treatment (unpublished data).
Technically efficient and cost-saving
The discovery of novel antibiotics at the current stage is increasingly difficult, requiring a combination of new strategies such as molecular biology, genomics, and metabolomics, and the speed of development remains severely lagging the pace of the emergence of drug-resistant bacteria especially in aquaculture, which consumes substantial antibiotics. By contrast, phage-based antimicrobial agents are renowned for their technical simplicity and economical saving, which greatly reduces the difficulty of development and shortens the application cycle of phage therapeutic agents. For example, the estimated cost of large-scale phage production and cost of biopolymer phage coating formation containing 5% WPI and 2.5% glycerol was $4.41 × 10−13 per phage particular (Krysiak-Baltyn et al. 2018) and 0.001 cents per fish feed pellets (Huang and Nitin 2019), respectively. Regarding antibiotics, oxytetracycline (OTC), the most widely used in aquaculture, costs about 1.5 cents per day per kg fish body weight (calculated at $200/kg OTC and 75 mg OTC/d/kg fish body weight). Therefore, phage therapy can be technically simpler and economically more economical than antibiotic therapy in aquaculture.
Challenges and perspectives
Bacterial resistance to phages
Phages, like antibiotics, can also cause bacteria to develop resistance mutations in their interaction with the host, although ten times slower than antibiotics, which may lead to serious therapeutic concerns (Kaur et al. 2021) and thus a major barrier to successful phage therapy (Borin et al. 2021). Bacteria have evolved multiple defense mechanisms to prevent phage infection, including (i) the adsorption-blocking mechanisms; (ii) the super infection exclusion system; (iii) the R-M and CRISPR-Cas system; (iv) the abortive infection (Abi) systems and some chemical defense; or (v) the cyclic oligonucleotide-based anti-phage signaling system (Bernheim and Sorek 2020; Hampton et al. 2020; Labrie et al. 2010). Likewise, phages have also evolved counter-defense mechanisms such as anti-CRISPR proteins, evading Abi system, modification of restriction sites, and adapting host receptors (Harrington et al. 2017; Safari et al. 2020; Shin et al. 2017), to reinfect bacteria in a long arms race with bacteria. One idea of “training” phages to combat resistance by exploiting their natural property of co-evolution with the host has been proposed and exhibited an enhanced suppression effect on the host and delayed resistance mutations, demonstrating its feasibility for robust phage therapy (Borin et al. 2021). On the other hand, multiple studies have shown that pathogenic bacteria that are resistant to phages can gradually reduce their adaptability to the environment or diminish pathogenicity when mutations occur in cell surface structures that act as both bacterial virulence factors and phage adsorption receptors (Geisinger and Isberg 2015; Kortright et al. 2019; Leon and Bastias 2015). In addition, the emergence of phage resistance can also restore the sensitivity of antibiotic-resistant bacteria to antibiotics (Chan et al. 2016; Gordillo Altamirano et al. 2021).
Phage-mediated resistance gene transfer
Phages as reservoirs of ARGs in the environment can promote ARG transmission across strains through transduction (Calero-Caceres et al. 2019; Chevallereau et al. 2021; Larranaga et al. 2018; Yang et al. 2018). It has been shown that phage-mediated resistance gene transfer is prevalent in full-scale hospital wastewater treatment plants (Manoharan et al. 2021) and that phage-mediated resistance gene transfer had a comparable frequency to that of plasmid under certain circumstances in a laboratory study (Sun et al. 2021). In the aquaculture environment, for the current studies, only a limited mobilization from bacteria to phages or vice versa in aquaculture plants (Colombo et al. 2016) and a significantly lower content of antibiotic resistome in phages than in plasmid in prawn mariculture environment were observed (Zhao 2021). The impacts of using phage therapy in aquaculture on the abundance of ARGs and the changes in the contribution of phage-mediated HGT in fish excreta and associated feeding water environment are even more completely unknown. It is worth noting that the control of resistance gene flow in the environment through phage intervention has also been reported (Parmar et al. 2017). In such a context of unclear phage ecology role in an aquaculture environment, extensive experiments to verify the contribution of phages to horizontal gene transfer (HGT) are needed.
Can phages ameliorate the host immune system?
The interaction between phages and the aquatic host immune system is interesting but poorly investigated. The immune system of a healthy body is constantly in dynamic balance, and proper inflammation helps to eliminate detrimental stimuli and restore health, but excessive or uncontrolled inflammation may damage healthy tissues (Kumar et al. 2004). MJG, a novel phage with positive therapeutic effects against Aeromonas hydrophila infection in rainbow trout, has been proven to ameliorate the immune response of rainbow trout by regulating IL-8 and IL-1β levels (Cao et al. 2020). Likewise, phage ZHF can alleviate the immune response of turbot caused by Aeromonas salmonicida and reduce mortality of turbot (Xu et al. 2021). Nikapitiya and colleagues’ research highlights the relative mRNA expression level of CXCL-8a, IL-1β, TNF-α, IL-6, IL-10, and SOD-1 in the intestine was within the safety threshold after feeding phage ETP-1-enriched artemia compared to the control group (Nikapitiya et al. 2020b). These results are promising, but the impact of phages on the host immune system is multi-pathway and multi-faceted, and there is still a relative paucity of research to draw arbitrary conclusions.
Conclusions
Collectively, phage therapy has unparalleled advantages over other chemotherapeutics, such as high efficiency, specificity, and environmental friendliness, with great potential for application in aquaculture. This technology is expected to partially or even completely substitute antibiotics as the mainstream of biomedicine in the future. Nonetheless, most of the current research on phage therapy is limited to the laboratory stage—only a few commercially available products (Altamirano and Barr 2019; Kortright et al. 2019). Ideally, phages for therapeutic and prophylactic applications should be able to inactivate pathogenic bacteria effectively without affecting beneficial microbiota, preferably with a low resistance mutation rate and no risk of horizontal transfer of virulence genes, as well as be easy for mass production and storage, which is what we strive for.
References
Abbasi J (2019) Patient receives first genetically engineered phage treatment. JAMA-J Am Med Assoc 322:107–107. https://doi.org/10.1001/jama.2019.9394
Akmal M, Rahimi-Midani A, Hafeez-ur-Rehman M, Hussain A, Choi T-J (2020) Isolation, characterization, and application of a bacteriophage infecting the fish pathogen Aeromonas hydrophila. Pathogens 9:215. https://doi.org/10.3390/pathogens9030215
Almeida A, Cunha A, Gomes NCM, Alves E, Costa L, Faustino MAF (2009) Phage therapy and photodynamic therapy: low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar Drugs 7:268–313. https://doi.org/10.3390/md7030268
Altamirano FLG, Barr JJ (2019) Phage therapy in the postantibiotic era. Clin Microbiol Rev 32:e00066-e118. https://doi.org/10.1128/cmr.00066-18
Angelica Zermeno-Cervantes L, Makarov R, Omar Lomeli-Ortega C, Francisco Martinez-Diaz S, Salvador Cardona-Felix C (2018) Recombinant LysVPMS1 as an endolysin with broad lytic activity against Vibrio parahaemolyticus strains associated to acute hepatopancreatic necrosis disease. Aquacult Res 49:1723–1726. https://doi.org/10.1111/are.13577
Ayyaru S, Choi J, Ahn Y-H (2018) Biofouling reduction in a MBR by the application of a lytic phage on a modified nanocomposite membrane. Environ Sci-Wat Res 4:1624–1638. https://doi.org/10.1039/c8ew00316e
Bao H, Shahin K, Zhang Q, Zhang H, Wang Z, Zhou Y, Zhang X, Zhu S, Stefan S, Wang R (2019) Morphologic and genomic characterization of a broad host range Salmonella enterica serovar Pullorum lytic phage vB_SPuM_SP116. Microb Pathog 136:103659. https://doi.org/10.1016/j.micpath.2019.103659
Bernheim A, Sorek R (2020) The pan-immune system of bacteria: antiviral defence as a community resource. Nat Rev Microbiol 18:113–119. https://doi.org/10.1038/s41579-019-0278-2
Borin JM, Avrani S, Barrick JE, Petrie KL, Meyer JR (2021) Coevolutionary phage training leads to greater bacterial suppression and delays the evolution of phage resistance. Proc Natl Acad Sci U S A 118:e2104592118. https://doi.org/10.1073/pnas.2104592118
Briers Y, Lavigne R (2015) Breaking barriers: expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol 10:377–390. https://doi.org/10.2217/fmb.15.8
Brown MR, Baptista JC, Lunn M, Swan DL, Smith SJ, Davenport RJ, Allen BD, Sloan WT, Curtis TP (2019) Coupled virus - bacteria interactions and ecosystem function in an engineered microbial system. Water Res 152:264–273. https://doi.org/10.1016/j.watres.2019.01.003
Cafora M, Deflorian G, Forti F, Ferrari L, Binelli G, Briani F, Ghisotti D, Pistocchi A (2019) Phage therapy against Pseudomonas aeruginosa infections in a cystic fibrosis zebrafish model. Sci Rep 9:1527. https://doi.org/10.1038/s41598-018-37636-x
Cairns J, Becks L, Jalasvuori M, Hiltunen T (2017) Sublethal streptomycin concentrations and lytic bacteriophage together promote resistance evolution. Philos Trans R Soc B-Biol Sci 372:20160040. https://doi.org/10.1098/rstb.2016.0040
Calero-Caceres W, Ye M, Luis Balcazar J (2019) Bacteriophages as environmental reservoirs of antibiotic resistance. Trends Microbiol 27:570–577. https://doi.org/10.1016/j.tim.2019.02.008
Cao Y, Li S, Han S, Wang D, Zhao J, Xu L, Liu H, Lu T (2020) Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 523:735193. https://doi.org/10.1016/j.aquaculture.2020.735193
Chan BK, Abedon ST, Loc-Carrillo C (2013) Phage cocktails and the future of phage therapy. Future Microbiol 8:769–783. https://doi.org/10.2217/fmb.13.47
Chan BK, Sistrom M, Wertz JE, Kortright KE, Narayan D, Turner PE (2016) Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci Rep 6:26717. https://doi.org/10.1038/srep26717
Chandrarathna HPSU, Nikapitiya C, Dananjaya SHS, De Silva BCJ, Heo G-J, De Zoysa M, Lee J (2020) Isolation and characterization of phage AHP-1 and its combined effect with chloramphenicol to control Aeromonas hydrophila. Braz J Microbiol 51:409–416. https://doi.org/10.1007/s42770-019-00178-z
Chen L, Fan J, Yan T, Liu Q, Yuan S, Zhang H, Yang J, Deng D, Huang S, Ma Y (2019) Isolation and characterization of specific phages to prepare a cocktail preventing Vibrio sp. Va-F3 infections in shrimp (Litopenaeus vannamei). Front Microbiol 10:2337. https://doi.org/10.3389/fmicb.2019.02337
Chen L, Yuan S, Liu Q, Mai G, Yang J, Deng D, Zhang B, Liu C, Ma Y (2018) In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front Microbiol 9:1476. https://doi.org/10.3389/fmicb.2018.01476
Chevallereau A, Pons BJ, van Houte S, Westra ER (2021) Interactions between bacterial and phage communities in natural environments. Nat Rev Microbiol 20:49–62. https://doi.org/10.1038/s41579-021-00602-y
Choudhury TG, Nagaraju VT, Gita S, Paria A, Parhi J (2017) Advances in bacteriophage research for bacterial disease control in aquaculture. Rev Fish Sci Aquac 25:113–125. https://doi.org/10.1080/23308249.2016.1241977
Christiansen RH, Dalsgaard I, Middelboe M, Lauritsen AH, Madsen L (2014) Detection and quantification of Flavobacterium psychrophilum-specific bacteriophages in vivo in rainbow trout upon oral administration: implications for disease control in aquaculture. Appl Environ Microbiol 80:7683–7693. https://doi.org/10.1128/aem.02386-14
Chu T-C, Murray SR, Hsu S-F, Vega Q, Lee LH (2011) Temperature-induced activation of freshwater cyanophage AS-1 prophage. Acta Histochem 113:294–299. https://doi.org/10.1016/j.acthis.2009.11.003
Clavijo V, Baquero D, Hernandez S, Farfan JC, Arias J, Arevalo A, Donado-Godoy P, Vives-Flores M (2019) Phage cocktail SalmoFREE (R) reduces Salmonella on a commercial broiler farm. Poult Sci 98:5054–5063. https://doi.org/10.3382/ps/pez251
Colom J, Cano-Sarabia M, Otero J, Arinez-Soriano J, Cortes P, Maspoch D, Llagostera M (2017) Microencapsulation with alginate/CaCO3: a strategy for improved phage therapy. Sci Rep 7:41441. https://doi.org/10.1038/srep41441
Colom J, Cano-Sarabia M, Otero J, Cortes P, Maspoch D, Llagostera M (2015) Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl Environ Microbiol 81:4841–4849. https://doi.org/10.1128/aem.00812-15
Colombo S, Arioli S, Guglielmetti S, Lunelli F, Mora D (2016) Virome-associated antibiotic-resistance genes in an experimental aquaculture facility. FEMS Microbiol Ecol 92:fiw003. https://doi.org/10.1093/femsec/fiw003
Comeau AM, Tetart F, Trojet SN, Prere M-F, Krisch HM (2007) Phage-antibiotic synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2:e799. https://doi.org/10.1371/journal.pone.0000799
Culot A, Grosset N, Gautier M (2019) Overcoming the challenges of phage therapy for industrial aquaculture: a review. Aquaculture 513:734423. https://doi.org/10.1016/j.aquaculture.2019.734423
Dang THO, Xuan TTT, Duyen LTM, Le NP, Hoang HA (2021) Protective efficacy of phage PVN02 against haemorrhagic septicaemia in striped catfish Pangasianodon hypophthalmus via oral administration. J Fish Dis 44:1255–1263. https://doi.org/10.1111/jfd.13387
Dawood MAO, Koshio S, Abdel-Daim MM, Van Doan H (2019) Probiotic application for sustainable aquaculture. Rev Aquac 11:907–924. https://doi.org/10.1111/raq.12272
de Jonge PA, Nobrega FL, Brouns SJJ, Dutilh BE (2019) Molecular and evolutionary determinants of bacteriophage host range. Trends Microbiol 27:51–63. https://doi.org/10.1016/j.tim.2018.08.006
Ding TY, Sun HZ, Pan Q, Zhao FY, Zhang ZZ, Ren HY (2020) Isolation and characterization of Vibrio parahaemolyticus bacteriophage vB_VpaS_PG07. Virus Res 286:198080. https://doi.org/10.1016/j.virusres.2020.198080
Domingo-Calap P, Delgado-Martinez J (2018) Bacteriophages: protagonists of a post-antibiotic era. Antibiotics-Basel 7:66. https://doi.org/10.3390/antibiotics7030066
Donati VL, Dalsgaard I, Sundell K, Castillo D, Er-Rafik M, Clark J, Wiklund T, Middelboe M, Madsen L (2021) Phage-mediated control of Flavobacterium psychrophilum in aquaculture: in vivo experiments to compare delivery methods. Front Microbiol 12:628309. https://doi.org/10.3389/fmicb.2021.628309
Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B (2015) Bacteriophages and phage-derived proteins - application approaches. Curr Med Chem 22:1757–1773. https://doi.org/10.2174/0929867322666150209152851
Duarte AC, Fernandez L, De Maesschalck V, Gutierrez D, Campelo AB, Briers Y, Lavigne R, Rodriguez A, Garcia P (2021) Synergistic action of phage phiIPLA-RODI and lytic protein CHAPSH3b: a combination strategy to target Staphylococcus aureus biofilms. npj Biofilms Microbomes 7:39. https://doi.org/10.1038/s41522-021-00208-5
Edgar R, Friedman N, Molshanski-Mor S, Qimron U (2012) Reversing bacterial resistance to antibiotics by phage-mediated delivery of dominant sensitive genes. Appl Environ Microbiol 78:744–751. https://doi.org/10.1128/aem.05741-11
El Haddad L, Harb CP, Gebara MA, Stibich MA, Chemaly RF (2019) A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Clin Infect Dis 69:167–178. https://doi.org/10.1093/cid/ciy947
Engelhardt T, Kallmeyer J, Cypionka H, Engelen B (2014) High virus-to-cell ratios indicate ongoing production of viruses in deep subsurface sediments. ISME J 8:1503–1509. https://doi.org/10.1038/ismej.2013.245
Engelhardt T, Orsi WD, Jorgensen BB (2015) Viral activities and life cycles in deep subseafloor sediments. Environ Microbiol Rep 7:868–873. https://doi.org/10.1111/1758-2229.12316
Essoh C, Blouin Y, Loukou G, Cablanmian A, Lathro S, Kutter E, Hoang VuT, Vergnaud G, Pourcel C (2013) The susceptibility of Pseudomonas aeruginosa strains from cystic fibrosis patients to bacteriophages. PLoS ONE 8:e60575. https://doi.org/10.1371/journal.pone.0060575
Fang H, Huang K, Yu J, Ding C, Wang Z, Zhao C, Yuan H, Wang Z, Wang S, Hu J, Cui Y (2019) Metagenomic analysis of bacterial communities and antibiotic resistance genes in the Eriocheir sinensis freshwater aquaculture environment. Chemosphere 224:202–211. https://doi.org/10.1016/j.chemosphere.2019.02.068
Fischetti VA (2010) Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol 300:357–362. https://doi.org/10.1016/j.ijmm.2010.04.002
Forti F, Roach DR, Cafora M, Pasini ME, Horner DS, Fiscarelli EV, Rossitto M, Cariani L, Briani F, Debarbieux L, Ghisotti D (2018) Design of a broad-range bacteriophage cocktail that reduces Pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob Agents Chemother 62:e02573-e2617. https://doi.org/10.1128/aac.02573-17
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66:365–378
Gao Q, Li Y, Qi Z, Yue Y, Min M, Peng S, Shi Z, Gao Y (2018) Diverse and abundant antibiotic resistance genes from mariculture sites of China’s coastline. Sci Total Environ 630:117–125. https://doi.org/10.1016/j.scitotenv.2018.02.122
Geisinger E, Isberg RR (2015) Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11:e1004691. https://doi.org/10.1371/journal.ppat.1004691
Gill JJ, Hyman P (2010) Phage choice, isolation, and preparation for phage therapy. Curr Pharm Biotechnol 11:2–14. https://doi.org/10.2174/138920110790725311
Gonzalez-Menendez E, Fernandez L, Gutierrez D, Rodriguez A, Martinez B, Garcia P (2018) Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS ONE 13:e0205728. https://doi.org/10.1371/journal.pone.0205728
Gonzalez S, Fernandez L, Belen Campelo A, Gutierrez D, Martinez B, Rodriguez A, Garcia P (2017) The behavior of Staphylococcus aureus dual-species biofilms treated with bacteriophage phiIPLA-RODI depends on the accompanying microorganism. Appl Environ Microbiol 83:e02821-e2916. https://doi.org/10.1128/aem.02821-16
Gordillo Altamirano F, Forsyth JH, Patwa R, Kostoulias X, Trim M, Subedi D, Archer SK, Morris FC, Oliveira C, Kielty L, Korneev D, O'Bryan MK, Lithgow TJ, Peleg AY, Barr JJ (2021) Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat Microbiol 6:157-+. https://doi.org/10.1038/s41564-020-00830-7
Grabowski L, Lepek K, Stasilojc M, Kosznik-Kwasnicka K, Zdrojewska K, Maciag-Dorszynska M, Wegrzyn G, Wegrzyn A (2021) Bacteriophage-encoded enzymes destroying bacterial cell membranes and walls, and their potential use as antimicrobial agents. Microbiol Res 248:126746. https://doi.org/10.1016/j.micres.2021.126746
Guemes AGC, Youle M, Cantu VA, Felts B, Nulton J, Rohwer F (2016) Viruses as winners in the game of life. In: Enquist LW (ed) Annual Review of Virology 3:197–214. https://doi.org/10.1146/annurev-virology-100114-054952
Guo X, Feng C, Gu E, Tian C, Shen Z (2019) Spatial distribution, source apportionment and risk assessment of antibiotics in the surface water and sediments of the Yangtze Estuary. Sci Total Environ 671:548–557. https://doi.org/10.1016/j.scitotenv.2019.03.393
Guo Y, Li J, Islam MS, Yan T, Zhou Y, Liang L, Connerton IF, Deng K, Li J (2021) Application of a novel phage vB_SalS-LPSTLL for the biological control of Salmonella in foods. Food Res Int 147:110492. https://doi.org/10.1016/j.foodres.2021.110492
Hampton HG, Watson BNJ, Fineran PC (2020) The arms race between bacteria and their phage foes. Nature 577:327–336. https://doi.org/10.1038/s41586-019-1894-8
Han QF, Zhao S, Zhang XR, Wang XL, Song C, Wang SG (2020) Distribution, combined pollution and risk assessment of antibiotics in typical marine aquaculture farms surrounding the Yellow Sea. North China Environ Int 138:105551. https://doi.org/10.1016/j.envint.2020.105551
Hansen MF, Lo Svenningsen S, Roder HL, Middelboe M, Burmolle M (2019) Big impact of the tiny: bacteriophage-bacteria interactions in biofilms. Trends Microbiol 27:739–752. https://doi.org/10.1016/j.tim.2019.04.006
Harrington LB, Doxzen KW, Ma E, Liu J-J, Knott GJ, Edraki A, Garcia B, Amrani N, Chen JS, Cofsky JC, Kranzusch PJ, Sontheimer EJ, Davidson AR, Maxwell KL, Doudna JA (2017) A broad-spectrum inhibitor of CRISPR-Cas9. Cell 170:1224–1233. https://doi.org/10.1016/j.cell.2017.07.037
Van Hien D, Soltani M, Ringo E (2021) In vitro antagonistic effect and in vivo protective efficacy of Gram-positive probiotics versus Gram-negative bacterial pathogens in finfish and shellfish. Aquaculture 540:736581. https://doi.org/10.1016/j.aquaculture.2021.736581
Higuera G, Bastias R, Tsertsvadze G, Romero J, Espejo RT (2013) Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture 392:128–133. https://doi.org/10.1016/j.aquaculture.2013.02.013
Himmelweit F (1945) Combined action of penicillin and bacteriophage on Staphylococci. Lancet 249:104–105
Hu Z, Chen X, Chen W, Li P, Bao C, Zhu L, Zhang H, Dong C, Zhang W (2021) Siphoviridae phage PH669 capable of lysing some strains of O3 and O4 serotypes in Vibrio parahaemolyticus. Aquaculture 545:737192. https://doi.org/10.1016/j.aquaculture.2021.737192
Huang K, Nitin N (2019) Edible bacteriophage based antimicrobial coating on fish feed for enhanced treatment of bacterial infections in aquaculture industry. Aquaculture 502:18–25. https://doi.org/10.1016/j.aquaculture.2018.12.026
Ignacio-Espinoza JC, Ahlgren NA, Fuhrman JA (2020) Long-term stability and red queen-like strain dynamics in marine viruses. Nat Microbiol 5:265-+. https://doi.org/10.1038/s41564-019-0628-x
Jin M, Guo X, Zhang R, Qu W, Gao B, Zeng R (2019) Diversities and potential biogeochemical impacts of mangrove soil viruses. Microbiome 7:58. https://doi.org/10.1186/s40168-019-0675-9
Jo A, Ding T, Ahn J (2016a) Synergistic antimicrobial activity of bacteriophages and antibiotics against Staphylococcus aureus. Food Sci Biotechnol 25:935–940. https://doi.org/10.1007/s10068-016-0153-0
Jo A, Kim J, Ding T, Ahn J (2016b) Role of phage-antibiotic combination in reducing antibiotic resistance in Staphylococcus aureus. Food Sci Biotechnol 25:1211–1215. https://doi.org/10.1007/s10068-016-0192-6
Kahn LH, Bergeron G, Bourassa MW, De Vegt B, Gill J, Gomes F, Malouin F, Opengart K, Ritter GD, Singer RS, Storrs C, Topp E (2019) From farm management to bacteriophage therapy: strategies to reduce antibiotic use in animal agriculture. Ann N Y Acad Sci 1441:31–39. https://doi.org/10.1111/nyas.14034
Kalatzis PG, Bastias R, Kokkari C, Katharios P (2016) Isolation and characterization of two lytic bacteriophages, phi St2 and phi Grn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS ONE 11:e0151101. https://doi.org/10.1371/journal.pone.0151101
Kamal F, Dennis JJ (2015) Burkholderia cepacia complex phage-antibiotic synergy (PAS): antibiotics stimulate lytic phage activity. Appl Environ Microbiol 81:1132–1138. https://doi.org/10.1128/aem.02850-14
Katharios P, Kalatzis PG, Kokkari C, Sarropoulou E, Middelboe M (2017) Isolation and characterization of a N4-like lytic bacteriophage infecting Vibrio splendidus, a pathogen of fish and bivalves. PLoS ONE 12:e0190083. https://doi.org/10.1371/journal.pone.0190083
Kaur G, Agarwal R, Sharma RK (2021) Bacteriophage therapy for critical and high-priority antibiotic-resistant bacteria and phage cocktail-antibiotic formulation perspective. Food Environ Virol 13:433–446. https://doi.org/10.1007/s12560-021-09483-z
Kavagutti VS, Andrei A-S, Mehrshad M, Salcher MM, Ghai R (2019) Phage-centric ecological interactions in aquatic ecosystems revealed through ultra-deep metagenomics. Microbiome 7:135. https://doi.org/10.1186/s40168-019-0752-0
Kihara M, Abe Y, Kaga H (2012) Acidity and pepsin-like protease activity of the gastric juice of farmed pacific bluefin tuna Thunnus orientalis. Aquaculture Science 60:285–286. https://doi.org/10.11233/aquaculturesci.60.285
Kim HJ, Giri SS, Kim SG, Kim SW, Kwon J, Lee SB, Park SC (2020) Isolation and characterization of two bacteriophages and their preventive effects against pathogenic Vibrio coralliilyticus causing mortality of pacific oyster (Crassostrea gigas) larvae. Microorganisms 8:926. https://doi.org/10.3390/microorganisms8060926
Kim HJ, Jun JW, Giri SS, Chi C, Yun S, Kim SG, Kim SW, Kang JW, Han SJ, Kwon J, Oh WT, Park SC (2019a) Application of the bacteriophage pVco-14 to prevent Vibrio coralliilyticus infection in Pacific oyster (Crassostrea gigas) larvae. J Invertebr Pathol 167:107244. https://doi.org/10.1016/j.jip.2019.107244
Kim JH, Son JS, Choi YJ, Choresca CH, Shin SP, Han JE, Jun JW, Kang DH, Oh C, Heo SJ, Park SC (2012) Isolation and characterization of a lytic Myoviridae bacteriophage PAS-1 with broad infectivity in Aeromonas salmonicida. Curr Microbiol 64:418–426. https://doi.org/10.1007/s00284-012-0091-x
Kim M, Jo Y, Hwang YJ, Hong HW, Hong SS, Park K, Myung H (2018) Phage-antibiotic synergy via delayed lysis. Appl Environ Microbiol 84:e02085-e2118. https://doi.org/10.1128/aem.02085-18
Kim SG, Jun JW, Giri SS, Yun S, Kim HJ, Kim SW, Kang JW, Han SJ, Jeong D, Park SC (2019b) Isolation and characterisation of pVa-21, a giant bacteriophage with anti-biofilm potential against Vibrio alginolyticus. Sci Rep 9:6284. https://doi.org/10.1038/s41598-019-42681-1
Kokkari C, Sarropoulou E, Bastias R, Mandalakis M, Katharios P (2018) Isolation and characterization of a novel bacteriophage infecting Vibrio alginolyticus. Arch Microbiol 200:707–718. https://doi.org/10.1007/s00203-018-1480-8
Kortright KE, Chan BK, Koff JL, Turner PE (2019) Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. https://doi.org/10.1016/j.chom.2019.01.014
Krysiak-Baltyn K, Martin GJO, Gras SL (2018) Computational modelling of large scale phage production using a two-stage batch process. Pharmaceuticals 11:31. https://doi.org/10.3390/ph11020031
Kumar R, Clermont G, Vodovotz Y, Chow CC (2004) The dynamics of acute inflammation. J Theor Biol 230:145–155. https://doi.org/10.1016/j.jtbi.2004.04.044
Kunttu HMT, Runtuvuori-Salmela A, Middelboe M, Clark J, Sundberg L-R (2021) Comparison of delivery methods in phage therapy against Flavobacterium columnare infections in rainbow trout. Antibiotics-Basel 10:914. https://doi.org/10.3390/antibiotics10080914
Kuzyakov Y, Mason-Jones K (2018) Viruses in soil: nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol Biochem 127:305–317. https://doi.org/10.1016/j.soilbio.2018.09.032
Laanto E, Bamford JKH, Ravantti JJ, Sundberg L-R (2015) The use of phage FCL-2 as an alternative to chemotherapy against columnaris disease in aquaculture. Front Microbiol 6:829. https://doi.org/10.3389/fmicb.2015.00829
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. https://doi.org/10.1038/nrmicro2315
Lachnit T, Dafforn KA, Johnston EL, Steinberg P (2019) Contrasting distributions of bacteriophages and eukaryotic viruses from contaminated coastal sediments. Environ Microbiol 21:1929–1941. https://doi.org/10.1111/1462-2920.14340
Lacqua A, Wanner O, Colangelo T, Martinotti MG, Landini P (2006) Emergence of biofilm-forming subpopulations upon exposure of Escherichia coli to environmental bacteriophages. Appl Environ Microbiol 72:956–959. https://doi.org/10.1128/aem.72.1.956-959.2006
Larranaga O, Brown-Jaque M, Quiros P, Gomez-Gomez C, Blanch AR, Rodriguez-Rubio L, Muniesa M (2018) Phage particles harboring antibiotic resistance genes in fresh-cut vegetables and agricultural soil. Environ Int 115:133–141. https://doi.org/10.1016/j.envint.2018.03.019
Le ST, Kurtboke J (2019) Bacteriophages as biocontrol agents in aquaculture. Microbiol Aust 40:37–41. https://doi.org/10.1071/ma19003
Le Thanh D, Ky LB, Bui The H, Mursalim MF, Kayansamruaj P, Senapin S, Rodkhum C, Ha Thanh D (2021) Characterization and protective effects of lytic bacteriophage pAh6.2TG against a pathogenic multidrug-resistant Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Transbound Emerg Dis. https://doi.org/10.1111/tbed.14321
Leon M, Bastias R (2015) Virulence reduction in bacteriophage resistant bacteria. Front Microbiol 6:343. https://doi.org/10.3389/fmicb.2015.00343
Letarov AV, Golomidova AK, Tarasyan KK (2010) Ecological basis for rational phage therapy. Acta Naturae 2:60–71. https://doi.org/10.32607/20758251-2010-2-1-60-71
Letrado P, Corsini B, Diez-Martinez R, Bustamante N, Yuste JE, Garcia P (2018) Bactericidal synergism between antibiotics and phage endolysin Cpl-711 to kill multidrug-resistant pneumococcus. Future Microbiol 13:1215–1223. https://doi.org/10.2217/fmb-2018-0077
Leung SSY, Parumasivam T, Gao FG, Carter EA, Carrigy NB, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan H-K (2017) Effects of storage conditions on the stability of spray dried, inhalable bacteriophage powders. Int J Pharm 521:141–149. https://doi.org/10.1016/j.ijpharm.2017.01.060
Leung V, Szewczyk A, Chau J, Hosseinidoust Z, Groves L, Hawsawi H, Anany H, Griffiths MW, Ali MM, Filipe CDM (2018) Long-term preservation of bacteriophage antimicrobials using sugar glasses. ACS Biomater Sci Eng 4:3802–3808. https://doi.org/10.1021/acsbiomaterials.7b00468
Lewis WH, Tahon G, Geesink P, Sousa DZ, Ettema TJG (2021) Innovations to culturing the uncultured microbial majority. Nat Rev Microbiol 19:225–240. https://doi.org/10.1038/s41579-020-00458-8
Li Z, Zhang J, Li X, Wang X, Cao Z, Wang L, Xu Y (2016) Efficiency of a bacteriophage in controlling Vibrio infection in the juvenile sea cucumber Apostichopus japonicus. Aquaculture 451:345–352. https://doi.org/10.1016/j.aquaculture.2015.09.024
Linden SB, Alreja AB, Nelson DC (2021) Application of bacteriophage-derived endolysins to combat streptococcal disease: current state and perspectives. Curr Opin Biotechnol 68:213–220. https://doi.org/10.1016/j.copbio.2021.01.012
Liu J, Gao S, Dong Y, Lu C, Liu Y (2020) Isolation and characterization of bacteriophages against virulent Aeromonas hydrophila. BMC Microbiol 20:141. https://doi.org/10.1186/s12866-020-01811-w
Luiz Vaz CS, Voss-Rech D, Alves L, Coldebella A, Brentano L, Trevisol IM (2020) Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella Enteritidis in broiler chickens. Vet Microbiol 240:108527. https://doi.org/10.1016/j.vetmic.2019.108527
Luong T, Salabarria A-C, Roach DR (2020) Phage therapy in the resistance era: where do we stand and where are we going? Clin Ther 42:1659–1680. https://doi.org/10.1016/j.clinthera.2020.07.014
Mahichi F, Synnott AJ, Yamamichi K, Osada T, Tanji Y (2009) Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol Lett 295:211–217. https://doi.org/10.1111/j.1574-6968.2009.01588.x
Manoharan RK, Srinivasan S, Shanmugam G, Ahn Y-H (2021) Shotgun metagenomic analysis reveals the prevalence of antibiotic resistance genes and mobile genetic elements in full scale hospital wastewater treatment plants. J Environ Manage 296:113270. https://doi.org/10.1016/j.jenvman.2021.113270
Mateus L, Costa L, Silva YJ, Pereira C, Cunha A, Almeida A (2014) Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture 424:167–173. https://doi.org/10.1016/j.aquaculture.2014.01.001
Micoli F, Bagnoli F, Rappuoli R, Serruto D (2021) The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol 19:287–302. https://doi.org/10.1038/s41579-020-00506-3
Miller-Ensminger T, Garretto A, Stark N, Putonti C (2020) Mimicking prophage induction in the body: induction in the lab with pH gradients. PeerJ 8:e9718. https://doi.org/10.7717/peerj.9718
Mirzaei MK, Maurice CF (2017) Menage a trois in the human gut: interactions between host, bacteria and phages. Nat Rev Microbiol 15:397–408. https://doi.org/10.1038/nrmicro.2017.30
Mokili JL, Rohwer F, Dutilh BE (2012) Metagenomics and future perspectives in virus discovery. Curr Opin Virol 2:63–77. https://doi.org/10.1016/j.coviro.2011.12.004
Nafarrate I, Mateo E, Amarita F, Martinez de Maranon I, Lasagabaster A (2020) Efficient isolation of Campylobacter bacteriophages from chicken skin, analysis of several isolation protocols. Food Microbiol 90:103486. https://doi.org/10.1016/j.fm.2020.103486
Nair A, Ghugare GS, Khairnar K (2021) An appraisal of bacteriophage isolation techniques from environment. Microb Ecol. https://doi.org/10.1007/s00248-021-01782-z
Ngiam L, Schembri MA, Weynberg K, Guo J (2021) Bacteriophage isolated from non-target bacteria demonstrates broad host range infectivity against multidrug-resistant bacteria. Environ Microbiol 23:5569–5586. https://doi.org/10.1111/1462-2920.15714
Nikapitiya C, Chandrarathna HPSU, Dananjaya SHS, De Zoysa M, Lee J (2020a) Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 63:14–23. https://doi.org/10.1016/j.biologicals.2019.12.006
Nikapitiya C, Dananjaya SHS, Edirisinghe SL, Chandrarathna HPSU, Lee J, De Zoysa M (2020b) Development of phage delivery by bioencapsulation of Artemia nauplii with Edwardsiella tarda phage (ETP-1). Braz J Microbiol 51:2153–2162. https://doi.org/10.1007/s42770-020-00324-y
O'Sullivan L, Bolton D, McAuliffe O, Coffey A (2019) Bacteriophages in food applications: from foe to friend. In: Doyle MP, McClements DJ (eds). Annu Rev Food Sci Technol 10:151–172. https://doi.org/10.1146/annurev-food-032818-121747
Onsea J, Soentjens P, Djebara S, Merabishvili M, Depypere M, Spriet I, De Munter P, Debaveye Y, Nijs S, Vanderschot P, Wagemans J, Pirnay J-P, Lavigne R, Metsemakers W-J (2019) Bacteriophage application for difficult-to-treat musculoskeletal infections: development of a standardized multidisciplinary treatment protocol. Viruses-Basel 11:891. https://doi.org/10.3390/v11100891
Ooi ML, Drilling AJ, Morales S, Fong S, Moraitis S, Macias-Valle L, Vreugde S, Psaltis AJ, Wormald P-J (2019) Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to Staphylococcus aureus. JAMA Otolaryngol-Head Neck Surg 145:723–729. https://doi.org/10.1001/jamaoto.2019.1191
Parmar KM, Hathi ZJ, Dafale NA (2017) Control of multidrug-resistant gene flow in the environment through bacteriophage intervention. Appl Biochem Biotechnol 181:1007–1029. https://doi.org/10.1007/s12010-016-2265-7
Peters TL, Song Y, Bryan DW, Hudson LK, Denes TG (2020) Mutant and recombinant phages selected from in vitro coevolution conditions overcome phage-resistant Listeria monocytogenes. Appl Environ Microbiol 86:e02138-e2220. https://doi.org/10.1128/aem.02138-20
Petsong K, Benjakul S, Vongkamjan K (2021) Optimization of wall material for phage encapsulation via freeze-drying and antimicrobial efficacy of microencapsulated phage against Salmonella. J Food Sci Technol-Mysore 58:1937–1946. https://doi.org/10.1007/s13197-020-04705-x
Prasad Y, Arpana KD, Sharma AK (2011) Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. J Environ Biol 32:161–168
Ren HY, Li Z, Xu YP, Wang LL, Li XY (2019) Protective effectiveness of feeding phage cocktails in controlling Vibrio parahaemolyticus infection of sea cucumber Apostichopus japonicus. Aquaculture 503:322–329. https://doi.org/10.1016/j.aquaculture.2019.01.006
Richards GP, Watson MA, Madison D, Soffer N, Needleman DS, Soroka DS, Uknalis J, Baranzoni GM, Church KM, Polson SW, Elston R, Langdon C, Sulakvelidze A (2021) Bacteriophages against Vibrio coralliilyticus and Vibrio tubiashii: isolation, characterization, and remediation of larval oyster mortalities. Appl Environ Microbiol 87:e00008-21. https://doi.org/10.1128/aem.00008-21
Robinson I, Junqua G, Van Coillie R, Thomas O (2007) Trends in the detection of pharmaceutical products, and their impact and mitigation in water and wastewater in North America. Anal Bioanal Chem 387:1143–1151. https://doi.org/10.1007/s00216-006-0951-y
Rorbo N, Ronneseth A, Kalatzis PG, Rasmussen BB, Engell-Sorensen K, Kleppen HP, Wergeland HI, Gram L, Middelboe M (2018) Exploring the effect of phage therapy in preventing Vibrio anguillarum infections in cod and turbot larvae. Antibiotics-Basel 7:42. https://doi.org/10.3390/antibiotics7020042
Ryan EM, Alkawareek MY, Donnelly RF, Gilmore BF (2012) Synergistic phage-antibiotic combinations for the control of Escherichia coli biofilms in vitro. FEMS Immunol Med Microbiol 65:395–398. https://doi.org/10.1111/j.1574-695X.2012.00977.x
Saad AM, Soliman AM, Kawasaki T, Fujie M, Nariya H, Shimamoto T, Yamada T (2019) Systemic method to isolate large bacteriophages for use in biocontrol of a wide-range of pathogenic bacteria. J Biosci Bioeng 127:73–78. https://doi.org/10.1016/j.jbiosc.2018.07.001
Saenz JS, Marques TV, Coelho Barone RS, Possebon Cyrino JE, Kublik S, Nesme J, Schloter M, Rath S, Vestergaard G (2019) Oral administration of antibiotics increased the potential mobility of bacterial resistance genes in the gut of the fish Piaractus mesopotamicus. Microbiome 7:24. https://doi.org/10.1186/s40168-019-0632-7
Safari F, Sharifi M, Farajnia S, Akbari B, Ahmadi MKB, Negahdaripour M, Ghasemi Y (2020) The interaction of phages and bacteria: the co-evolutionary arms race. Crit Rev Biotechnol 40:119–137. https://doi.org/10.1080/07388551.2019.1674774
Schmelcher M, Donovan DM, Loessner MJ (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. https://doi.org/10.2217/fmb.12.97
Shen X, Jin G, Zhao Y, Shao X (2020) Prevalence and distribution analysis of antibiotic resistance genes in a large-scale aquaculture environment. Sci Total Environ 711:134626. https://doi.org/10.1016/j.scitotenv.2019.134626
Shin J, Jiang F, Liu J-J, Bray NL, Rauch BJ, Baik SH, Nogales E, Bondy-Denomy J, Corn JE, Doudna JA (2017) Disabling Cas9 by an anti-CRISPR DNA mimic. Sci Adv 3:e1701620. https://doi.org/10.1126/sciadv.1701620
Sieiro C, Areal-Hermida L, Pichardo-Gallardo A, Almuina-Gonzalez R, de Miguel T, Sanchez S, Sanchez-Perez A, Villa TG (2020) A hundred years of bacteriophages: can phages replace antibiotics in agriculture and aquaculture? Antibiotics-Basel 9:493. https://doi.org/10.3390/antibiotics9080493
Silva YJ, Moreirinha C, Pereira C, Costa L, Rocha RJM, Cunha A, Gomes NCM, Calado R, Almeida A (2016) Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with phage AS-A. Aquaculture 450:225–233. https://doi.org/10.1016/j.aquaculture.2015.07.025
Srinivasan P, Ramasamy P (2017) Morphological characterization and biocontrol effects of Vibrio vulnificus phages against vibriosis in the shrimp aquaculture environment. Microb Pathog 111:472–480. https://doi.org/10.1016/j.micpath.2017.09.024
Sun M, Ye M, Zhang Z, Zhang S, Zhao Y, Deng S, Kong L, Ying R, Xia B, Jiao W, Cheng J, Feng Y, Liu M, Hu F (2019) Biochar combined with polyvalent phage therapy to mitigate antibiotic resistance pathogenic bacteria vertical transfer risk in an undisturbed soil column system. J Hazard Mater 365:1–8. https://doi.org/10.1016/j.jhazmat.2018.10.093
Sun R, Yu P, Zuo P, Alvarez PJJ (2021) Bacterial concentrations and water turbulence influence the importance of conjugation versus phage-mediated antibiotic resistance gene transfer in suspended growth systems. ACS Environ Au. https://doi.org/10.1021/acsenvironau.1c00027
Suttle CA (2007) Marine viruses - major players in the global ecosystem. Nat Rev Microbiol 5:801–812. https://doi.org/10.1038/nrmicro1750
Svircev A, Roach D, Castle A (2018) Framing the future with bacteriophages in agriculture. Viruses-Basel 10:218. https://doi.org/10.3390/v10050218
Tagliaferri TL, Jansen M, Horz H-P (2019) Fighting pathogenic bacteria on two fronts: phages and antibiotics as combined strategy. Front Cell Infect Microbiol 9:22. https://doi.org/10.3389/fcimb.2019.00022
Tan CW, Rukayadi Y, Hasan H, Abdul-Mutalib N-A, Jambari NN, Hara H, Thung TY, Lee E, Radu S (2021) Isolation and characterization of six Vibrio parahaemolyticus lytic bacteriophages from seafood samples. Front Microbiol 12:616548. https://doi.org/10.3389/fmicb.2021.616548
Tan D, Dahl A, Middelboe M (2015) Vibriophages differentially influence biofilm formation by Vibrio anguillarum strains. Appl Environ Microbiol 81:4489–4497. https://doi.org/10.1128/aem.00518-15
Teitge F, Peppler C, Steinhagen D, Jung-Schroers V (2020) Effect of disinfection with peracetic acid on the microbial community of a seawater aquaculture recirculation system for Pacific white shrimp (Litopenaeus vannamei). J Fish Dis 43:991–1017. https://doi.org/10.1111/jfd.13207
Tuan Son L, Southgate PC, O’Connor W, Vu SV, Kurtboeke DI (2020) Application of bacteriophages to control Vibrio alginolyticus contamination in oyster (Saccostrea glomerata) larvae. Antibiotics-Basel 9:415. https://doi.org/10.3390/antibiotics9070415
Tuan Son L, Thi Hien N, Hong Phuong V, Van Cuong D, Hong Loc N, Minh Trung T, Trong Tuan T, Southgate PC, Kurtboke DI (2018) Protective effects of bacteriophages against Aeromonas hydrophila causing motile Aeromonas septicemia (MAS) in striped catfish. Antibiotics-Basel 7:16. https://doi.org/10.3390/antibiotics7010016
Vaseeharan B, Thaya R (2014) Medicinal plant derivatives as immunostimulants: an alternative to chemotherapeutics and antibiotics in aquaculture. Aquacult Int 22:1079–1091. https://doi.org/10.1007/s10499-013-9729-3
Veyrand-Quiros B, Gomez-Gil B, Lomeli-Ortega CO, Escobedo-Fregoso C, Millard AD, Tovar-Ramirez D, Balcazar JL, Quiroz-Guzman E (2020) Use of bacteriophage vB_Pd_PDCC-1 as biological control agent of Photobacterium damselae subsp. damselae during hatching of longfin yellowtail (Seriola rivoliana) eggs. J Appl Microbiol 129:1497–1510. https://doi.org/10.1111/jam.14744
Vinay TN, Bhat S, Choudhury TG, Paria A, Jung M-H, Kallappa GS, Jung S-J (2018) Recent advances in application of nanoparticles in fish vaccine delivery. Rev Fish Sci Aquac 26:29–41. https://doi.org/10.1080/23308249.2017.1334625
Wang J-H, Lu J, Zhang Y-X, Wu J, Zhang C, Yu X, Zhang Z, Liu H, Wang W-H (2018) High-throughput sequencing analysis of the microbial community in coastal intensive mariculture systems. Aquacult Eng 83:93–102. https://doi.org/10.1016/j.aquaeng.2018.10.001
Whitman WB, Coleman DC, Wiebe WJ (1998) Prokaryotes: the unseen majority. Proc Natl Acad Sci U S A 95:6578–6583. https://doi.org/10.1073/pnas.95.12.6578
Wu L, Tian Y, Pang M, Yang Z, Bao H, Zhou Y, Sun L, Wang R, Zhang H (2021) A novel vibriophage vB_VhaS_PcB-1G capable of inhibiting virulent Vibrio harveyi pathogen. Aquaculture 542:736854. https://doi.org/10.1016/j.aquaculture.2021.736854
Wu S, Zhou L, Zhou Y, Wang H, Xiao J, Yan S, Wang Y (2020) Diverse and unique viruses discovered in the surface water of the East China Sea. BMC Genomics 21:441. https://doi.org/10.1186/s12864-020-06861-y
Xu Z, Jin P, Zhou X, Zhang Y, Wang Q, Liu X, Shao S, Liu Q (2021) Isolation of a virulent Aeromonas salmonicida subsp. masoucida bacteriophage and its application in phage therapy in turbot (Scophthalmus maximus). Appl Environ Microbiol 87:e01468-e1521. https://doi.org/10.1128/aem.01468-21
Xue Y, Zhai S, Wang Z, Ji Y, Wang G, Wang T, Wang X, Xi H, Cai R, Zhao R, Zhang H, Bi L, Guan Y, Guo Z, Han W, Gu J (2020) The Yersinia phage X1 administered orally efficiently protects a murine chronic enteritis model against Yersinia enterocolitica infection. Front Microbiol 11:351. https://doi.org/10.3389/fmicb.2020.00351
Yan Y, Liu Y, Mo Z, Li J, Liu S, Gao Y, Li G, Li J (2021) Development of Aeromonas salmonicida subsp. masoucida vaccine in turbot and evaluation of protection efficacy under field conditions. Aquaculture 544:737035. https://doi.org/10.1016/j.aquaculture.2021.737035
Yang Y, Shi W, Lu S-Y, Liu J, Liang H, Yang Y, Duan G, Li Y, Wang H, Zhang A (2018) Prevalence of antibiotic resistance genes in bacteriophage DNA fraction from Funan River water in Sichuan, China. Sci Total Environ 626:835–841. https://doi.org/10.1016/j.scitotenv.2018.01.148
Ye M, Sun M, Zhao Y, Jiao W, Xia B, Liu M, Feng Y, Zhang Z, Huang D, Huang R, Wan J, Du R, Jiang X, Hu F (2018) Targeted inactivation of antibiotic-resistant Escherichia coli and Pseudomonas aeruginosa in a soil-lettuce system by combined polyvalent bacteriophage and biochar treatment. Environ Pollut 241:978–987. https://doi.org/10.1016/j.envpol.2018.04.070
Yehl K, Lemire S, Yang AC, Ando H, Mimee M, Torres MDT, de la Fuente-Nunez C, Lu TK (2019) Engineering phage host-range and suppressing bacterial resistance through phage tail fiber mutagenesis. Cell 179:459-+. https://doi.org/10.1016/j.cell.2019.09.015
Yu P, Mathieu J, Li M, Dai Z, Alvarez PJJ (2016) Isolation of polyvalent bacteriophages by sequential multiple-host approaches. Appl Environ Microbiol 82:808–815. https://doi.org/10.1128/aem.02382-15
Zhang J, Hu Y, Sun Q, Li X, Sun L (2021a) An inactivated bivalent vaccine effectively protects turbot (Scophthalmus maximus) against Vibrio anguillarum and Vibrio harveyi infection. Aquaculture 544:737158. https://doi.org/10.1016/j.aquaculture.2021.737158
Zhang J, Xu H, Yang H, Li J, Xiao S, Hu S, Yan F, Xia L, Zhang Y (2021b) Screening of a Plesiomonas shigelloides phage and study of the activity of its lysis system. Virus Res 306:198581. https://doi.org/10.1016/j.virusres.2021.198581
Zhang Y, Liao Y-T, Salvador A, Sun X, Wu VCH (2020) Prediction, diversity, and genomic analysis of temperate phages induced from shiga toxin-producing Escherichia coli strains. Front Microbiol 10:3093. https://doi.org/10.3389/fmicb.2019.03093
Zhao Z (2021) Comparison of microbial communities and the antibiotic resistome between prawn mono- and poly-culture systems. Ecotoxicol Environ Saf 207:111310. https://doi.org/10.1016/j.ecoenv.2020.111310
Funding
This work was financially supported by the National Key Research and Development Program of China (No. 2019YFE0122400) and the Research Ability Improvement Project for Outstanding Young Teachers of University of Chinese Academy of Sciences (No. Y95401FXX2).
Author information
Authors and Affiliations
Contributions
Conceptualization and editing, Ruyin Liu; visualization and original preparation, Ganghua Han; validation, Zong Li and Shujuan Cun; supervision, Bin Hao, Jianping Zhang, and Xinchun Liu.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, R., Han, G., Li, Z. et al. Bacteriophage therapy in aquaculture: current status and future challenges. Folia Microbiol 67, 573–590 (2022). https://doi.org/10.1007/s12223-022-00965-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-022-00965-6