Abstract
Aquaculture makes a significant contribution in the production of protein-rich food for human consumption. Aquaculture practices encounter many challenges, and one of the most devastating problems is disease outbreaks caused by microbial pathogens. To control disease outbreaks, several chemotherapeutics and antibiotics were used indiscriminately, which in turn leads to residual problems in the surrounding environment affecting higher animals and also humans. Immunostimulants are considered as an alternative for antibiotics, which will boost the immune system of the cultured organism, thus effectively countering the assault of pathogens. The use of plant materials as immunostimulant will be an ecofriendly approach for the control of pathogens. The botanicals present in the plants have a key role in enhancing the fish immunity. This review focuses on the importance of plant material as immunostimulant in the control of diseases in aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish farming is the principal form of aquaculture in the world and growing more rapid food animal-producing sector (FAO 2002, 2003). Increasing demands on wild fisheries by commercial fishing operations have caused widespread overfishing. Fish farming offers an alternative solution to the increasing market demand for fish and fish protein. Pisciculture has been employed to compensate the shortage of animal protein all over the world. It has the potential to create huge job opportunities, provided fish cultivation is done on a scientific basis. Disease is the single most important limiting factor in the progressive growth of aquaculture industries. Several studies conducted on the modulation of fish immune system in order to prevent the outbreak of disease were reviewed by Sakai (1999).
The immune systems of aquatic vertebrates, such as those of higher animals, are sensitive to immune challenges by environmental stresses. High-density fish farming and chemical contaminants in water, especially chronic exposure to toxic pollutants, can lead to decreased resistance to viral, bacterial and parasitic diseases. Micro-organisms and metazoans are still engaged in a never-ending arms race, which has led to the evolution of protection mechanisms in vertebrates and pathogenicity mechanisms in microbes. It has been suggested that the diverse evasive strategies of micro-organisms to their host have acted as inducers of vertebrate defence evolution (Castro and Gonzaa’lez 2001). Fishes are one of the most primitive vertebrates, which possess non-specific defence mechanisms of the invertebrates such as phagocytic mechanisms of macrophages and granular leucocytes. They were also the first animals to develop both cellular and humoral immune responses mediated by lymphocytes. The main lymphoid organs of fish are the thymus, anterior kidney and spleen. In fishes, non-specific immunity is considered as the first line of defence and represents a considerable part of the immune response (Dalmo et al. 1997). The epidermal secretions, anti-microbial proteins, lysozyme, phosphatases and trypsin, their amount and activity depend on the species (Fast et al. 2002).
In aquaculture, antibiotics have been used mainly for therapeutic purposes and also as prophylactic agents. Antibiotics are drugs of natural or synthetic origin, with the capacity to kill or inhibit the growth of micro-organisms. Aquaculture faces serious issues due to various adverse effects of antibiotics such as accumulation in the tissue and immunosuppression (Gudding et al. 1999). Chemotherapeutics and antibiotics are mainly administered in the culture practices through medicated feeds or food materials. This practice may result in antibiotics entering into the environment by leaching from uneaten feeds, unabsorbed parts in manure or aquatic animals (Robinson et al. 2007). The options adept in aquaculture for controlling and preventing diseases were vaccines, antibiotics, probiotics, genetically improved stocks, immunostimulants, husbandry practices and water treatments as given in Table 1.
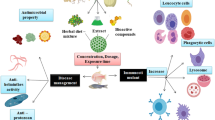
Use of antibiotics in fish production increases the prevalence of antibiotic resistance in human diseases. For reducing the usage of antibiotics, immunostimulant is one of the useful tools in aquaculture. Moreover, due to the availability of limited vaccines in few countries and their pathogen-specific protective action, much attention has been directed towards the use of immunostimulants in aquaculture to control infectious diseases. An immunostimulant is a chemical drug, stressor or action that increases the non-specific defence mechanism or the specific immune response. Sajid et al. (2011) have given an overall review about immunostimulant activity of glucan, chitosan, levamisole and herbal plant products. Various herbal products such as from Hygrophila spinosa, Withania somnifera, Zingiber officinale, Solanum trilobatum, Andrographis paniculata, Psoralea corylifolia, Eclipta erecta, Ocimum sanctum, Picrorhiza kurroa, Phyllanthus niruri, Tinospora cordifolia, purified Silajit and cod-liver oil have the characteristics of growth promotion, anti-stress, immunostimulation and anti-bacterial. These preparations had a prerogative influence on the Penaeus larviculture (Citarasu et al. 1998, 2002). Citarasu et al. (2006) used five herbal plants Cynodon dactylon, Aegle marmelos, T. cordifolia, P. kurroa and Eclipta alba extracts incorporated into basal diet formula for testing white spot syndrome virus infection in Penaeus monodon. The aim of this study is to review the positive effect of plant materials on the fish immunity for the control of microbial diseases.
Immunostimulants
Immunostimulants, also known as immunostimulators, are substances (drugs and nutrients) that stimulate the immune system by inducing activation or increasing activity of any of its components. The immunostimulants could increase the resistance of fish to infectious diseases by enhancing non-specific defence mechanism. Immunostimulants can be administered by injection, bathing or orally, with the latter appearing to be the most practicable (Jeney and Anderson 1993; Sakai 1999; Yin et al. 2006). It will boost the potency of the vaccine, thereby decreasing the dose necessary for the same effect (Jeney and Anderson 1993). The immunomodulation of larval fish has been proposed as a potential method for improving larval survival by increasing the innate responses until its adaptive immune response is sufficiently developed for effective response against the pathogen. To this end, it has been proposed that the use of immunostimulants as a dietary supplement to larval fish could be of considerable benefit in boosting the animal’s innate defences with little detriment to the developing animal (Bricknell and Dalmo 2005). Galeotti (1998) suggested that in vitro screening methods should be used to elucidate the mechanisms of immunostimulation and then in vivo methods should be used to establish whether the benefits occur in live fish. Total serum IgM levels of fish fed with the assayed immunostimulant-supplemented diets were statistically higher than those of fish fed with a non-supplemented diet (Cuesta et al. 2004). The addition of various food additives such as vitamins, carotenoids and herbal remedies to the fish feed has been tested in aquaculture. Reducing the stress response, increasing the activity of innate parameters and improving disease resistance (Amar et al. 2004; Puangkaew et al. 2004; Cerezuela et al. 2009; Yin et al. 2009) are the overall beneficial effects. In vivo and in vitro effects are the two different immune response measuring factors. There are many studies reporting a variety of substances including synthetic (Rao et al. 2006), bacterial (Goetz et al. 2004), animal and plant products (Hardie et al. 1991; Rao et al. 2006; Ardo et al. 2008) can be used as immunostimulants to enhance non-specific immune system of cultured fish species. Various types of plant active compound substances such as saponin (Ninomiya et al. 1995), glycyrrhizin (Jang et al. 1995), aloe (Kim et al. 1999) and azadirachtin (Logambal and Michael 2000, 2001; Harikrishnan et al. 2009a, b) have been reported to enhance the innate immunity in fishes. Some plants are the rich sources of compounds such as volatile oils, saponins, phenolics, tannins, alkaloids, polysaccharides and polypeptides. These natural plant products have various activities such as anti-stress, appetizer, tonic, anti-microbials and immunostimulants (Citarasu et al. 2002). Interest in the use of immunostimulants as an alternative to the drugs, chemicals and antibiotics currently being used to control fish diseases is growing, partially because immunostimulants, in contrast to vaccines, enhance the innate (or non-specific) immune response (Galeotti 1998; Sakai 1999). For the past decade, genomic organization and characterization of immune related genes and their expression through various immunostimulants (zymozan, peptidoglycan, β-glucan) were studied to understand the molecular mechanism behind the immune system of crustaceans (Rattanachaia et al. 2005; Vaseeharan et al. 2006, 2011; Lin et al. 2007; Sivakama valli and Vaseeharan 2012).
Leave extract as immunostimulant
Recently, in aquaculture, scores of plant extracts have been tested and used with good results in the control of bacterial and viral diseases. Fourteen herbs have been tested against Aeromonas hydrophila infection in Oreochromis niloticus, among them, the ethanol extract of Psidium guajava has been found to have highest anti-microbial activity (Pachanawan et al. 2008). The stimulation of specific and non-specific immunity and the protection against fish pathogen A. hydrophila in O. mossambicus by ethanol and petroleum ether extracts of T. cordifolia were observed (Sudhakaran et al. 2006). Salah and Mohamed (2008) studied the effect of Echinacea on the growth rate and disease resistance in Nile tilapia. The experiment was carried out on 1200 Nile tilapia, reared in earth ponds. A controlled infection with Pseudomonas fluorescens was also carried out by intraperitoneal inoculation. The test group showed a significant increase in body weight gain, specific growth rate, haematocrit values, lysozyme activity and total leucocytic count. The survival rate was significantly enhanced in the experimental group prior to and post-inoculation. Astragalus membranaceus extract significantly enhanced the phagocytic activity of leucocytes isolated from Nile tilapia within 1 week of being provided to the fish, and this increased activity was maintained during the entire experiment (Yin et al. 2006; Ardo et al. 2008). Carp fed with A. membranaceus extract showed enhanced survival compared with control fish, following a challenge with A. hydrophila (Yin et al. 2009). Feeding O. niloticus with two herbal extracts (A. membranaceus and Lonicera japonica) alone or in combination significantly enhanced phagocytic and respiratory burst activity of blood phagocytic cells in treatment groups compared with control group (Ardo et al. 2008). In O. mossambicus, the acetone extract of O. sanctum was found to enhance the anti-sheep red blood cell (SRBC; sheep erythrocytes) antibody response (Hemapriya 1997), while the water extract stimulated both the specific and non-specific immune mechanisms (Logambal et al. 2000). Leaves of O. sanctum contain water-soluble phenolic compounds and various other constituents, such as eugenol, methyl eugenol and caryophyllene (Chopra et al. 1956). Administration of O. sanctum leaf extract to tilapia O. mossambicus, simultaneously with or after vaccination, resulted in changes both the magnitude of antibody response and the day of peak antibody response increased protection against experimental infection with A. hydrophila (Logambal et al. 2000). The effects of water- and hexane-soluble fractions of S. trilobatum on the non-specific immune mechanisms and disease resistance of tilapia found that all doses of water-soluble fraction significantly enhanced the production of reactive oxygen and decreased the percentage mortality, following a challenge with A. hydrophila (Divyagnaneswari et al. 2008). The addition of plant extracts of four Chinese herbs (Rheum officinale, A. paniculata, Isatis indigotica and L. japonica) to the feed of crucian carp resulted in increased phagocytosis of the white blood cells (Chen et al. 2003). Dip treatment in goldfish with Azadirachta indica aqueous leaf extract exhibited a significant increase in serum glucose, cholesterol and total protein (Harikrishnan et al. 2009b). Dip treatment of C. carpio with aqueous leaf extract of A. indica significantly increased serum protein levels and protected the fish from A. hydrophila infection (Harikrishnan et al. 2003). Methanolic extracts of the herbals O. sanctum, W. somnifera and Myristica fragrans herbs significantly improved the immune parameters such as phagocytic activity, serum bactericidal activity, albumin–globulin (A/G) ratio and leucocrit against Vibrio harveyi challenge in juvenile grouper, Epinephalus tauvina larviculture (Sivaram et al. 2004). The acetone extracts of four plants C. dactylon, A. marmelos, W. somnifera and Z. officinale were screened for their inhibitory activity against seven fish Vibrio pathogens V. alginolyticus, V. parahaemolyticus, V. mimicus, V. campbelli, V. vulnificus, V. harveyi and P. damselae, and these extracts were mixed with fish feed in proper ratio; as a result, there was an enhancement in leucocrit, phagocytic and lysozyme activities in the blood of O. mossambicus fed with experimental diet compared with the control diet-fed fish (Immanuel et al. 2009). Five herbs Acalypha indica, H. spinosa, P. kurooa, T. cordifolia and Z. officinale were selected to screen for the in vitro immunostimulant activity against the shrimp pathogen Vibrio harveyi, and the herbal extract improved the total haemocyte count (THC), phagocytosis, phenol oxidase (PO) haemagglutinin activity and bacterial clearance activity in Fenneropenaeus indicus (Raja Rajeswari et al. 2012). Yin et al. (2009) reported that the complementary vaccine with Ganoderma and Astragalus to carp showed a significantly enhanced respiratory burst activity of phagocytic cells as well as enhanced phagocytosis and lysozyme activities in plasma. The specific immune response was also increased, although there were no significant differences between the vaccinated group not fed with herb extracts and the vaccinated fish fed with herb extracts. The herbal plant extracts of A. indica, C. dactylon, W. somnifera, Z. officinalis and P. kurooa having anti-viral and immunostimulant characteristics, which offer better growth and immunostimulation and act as anti-viral during the dual administration against the WSSV infection in Penaeus monodon (Yogeeswaran et al. 2012). Fish fed with both herbs and vaccine showed best survival against infection with A. hydrophila. The herbal immunostimulants Emblica officinalis, C. dactylon and Adhathoda vasica improved the immune system and reduced microbial infection in the goldfish Carassius auratus (Minomol 2005). Punitha et al. (2008) screened the herbal plant extracts of C. dactylon, Piper longum P. niruri, Tridax procumbens and Z. officinalis testing immunostimulant activity in grouper E. tauvina against V. harveyi infection, and the petroleum ether extract is very effective against vibrio pathogens in in vitro screening. Herbal extracts have a potential application as an immunostimulant in fish culture, primarily because they can be easily prepared, are inexpensive and act against a broad spectrum of pathogens. Most of the herbs and herbal extracts can be given orally, which is the most convenient method of immunostimulation. However, the effect is dose dependent, and there is always a potential for overdosing (Yin et al. 2006). It has been observed that the plant extract might act directly on the immunopoietic cells as immunostimulant (Jeney and Anderson 1993).
Plant parts as immunostimulant
Many parts of the plant materials possess medicinal properties. Numerous plant materials are widely used in aquaculture for preventing diseases by controlling the pathogenic microbes and enhancing the immunity. A. hydrophila infection in rainbow trout (Oncorhyncus mykiss) was controlled by garlic (Nya and Austin 2007). At an inclusion level of 0.5 and 1.0 mg/g feed, a 4 % reduction in mortality was observed than the control group. Garlic can help in the control of bacteria and fungi and increase the welfare of fish (Corzo-Martinez et al. 2007). Nya and Austin (2009) used ginger to control an experimental infection of A. hydrophila in rainbow trout, and mortality was reduced to zero compared with the control group. They have also recorded the enhancement of growth rate, feed conversion and protein efficiency in the rainbow trouts fed with ginger. Several medicinal-plant-based materials were administered as immunostimulants in various fish species against pathogens and are given in Table 2. Hemapriya (1997) reported that the acetone extract of P. emblica enhanced the anti-SRBC antibody response in tilapia, while Balasubramani and Michael (2002) found that both crude extracts and a water-soluble fraction of P. emblica fruit had a stimulatory effect on the immune response of tilapia. P. emblica fruit pulp contains large proportion of vitamin C, which has also been identified as an immunostimulant (Li and Lovell 1985). Achyranthes aspera seed was incorporated into the diet of Labeo rohita, rohu fingerlings, and the results indicated that A. aspera seed stimulated immunity and increased resistance to A. hydrophila infection in fish (Rao et al. 2006). Dorucu et al. (2009) reported that black cumin seed extract enhances the total immunoglobulin level in Oncorhynchus mykiss after 3 weeks feeding period. Natural immunostimulants are biocompatible, biodegradable and safe for both the environment and human health. Moreover, they possess an added nutritional value (Ortuno et al. 2002). Rainbow trout fed with Z. officinale (ginger) extract had significantly higher extracellular activity of phagocytic cells in blood and in trout fed with nettle, and mistletoe extracts increased the production of extracellular superoxide anion (Dugenci et al. 2003).
Essential oil as immunostimulant
In aquaculture practice, pathogenic microbes are controlled by essential oils, and they also act as one of the immunostimulants. The carbonyl group of cinnamaldehyde is thought to be responsible for anti-microbial action by binding to cellular proteins and preventing them from functioning properly (Wendakoon and Sakaguchi 1995). Pongsak and Parichat (2010) have studied the cinnamon oil potential to control Streptococcus iniae infection on Nile tilapia. They studied and compared inhibitory capacity of four essential oils: Citrus hystrix (leech lime), Cymbopogon citratus (lemon grass stems), Curcuma longa (turmeric) and Cinnamomum verum (cinnamon). The highest inhibitory activity was observed in cinnamon oil. As for the growth parameters, there was no significant difference observed between the control group and the experimental groups. Diet supplemented with Zataria multiflora essential oil enhanced common carp immunity to some extent even though fish cannot express its potential immunity during low temperature. Recently, Vaseeharan et al. (2012) reported the inhibitory activity of essential oils from medicinal plants against Pseudomonas spp. isolated from aquatic environments, and its control efficiency was visualized under confocal laser scanning microscopy. The Z. multiflora essential oil had the potential for enhancing innate immune system of carp under temperature stress (Soltani et al. 2010). Oral administration of Quil A saponin increased leucocyte migration in yellowtail fish (Ninomiya et al. 1995). Some effective components such as thymol and terpinene present in the oils have the properties in stimulating the fish immunity.
Herbal drugs as immunostimulators
Resveratrol (RESV; trans-3,5,40-trihydroxystilbene), a natural polyphenol, was first isolated in 1940 as a constituent of the roots of white hellebore, but since then, it has been found in various plants, including grapes, berries and peanuts (Khanna et al. 2007). It was found that RESV strongly inhibited intracellular and extracellular myeloperoxidase (MPO) activity, behaving as a non-competitive and reversible inhibitor, and also induced a decrease in MPO mRNA levels in Turbot Psetta maxima neutrophils (Castro et al. 2008). Papaya leaf meal contains an enzyme, namely papain, which increases the protein digestion, food conversion ratio, specific growth rate and weight gain in 16 % unsoaked papaya meal diet fed to P. monodon post-larvae (Penaflorida 1995). Edahiro et al. (1991) reported that yellowtail fish treated orally with glycyrrhizin showed increased protection against Edwardsiella seriola infection, although lysozyme activity of blood and phagocytic activities of macrophages were not enhanced. Glycyrrhizin is a glycosylated saponin, containing one molecule of glycyrrhetinic acid, which has anti-inflammatory and anti-tumour activities, mediated by its immunomodulatory activities (Wada et al. 1987; Zhang et al. 1990). Livol (IHF-1000) is a herbal growth promoter containing different plant ingredients such as Boerhavia diffusa, Solanum nigrum, Terminalia arjuna, Colosynth and black salt and has been found to significantly improve digestion, thereby leading to better growth, production and health in cultivable fishes (Shadakshari 1993; Unnikrishnan 1995; De Bolle et al. 1996; Jayaprakas and Euphrasia 1996). The herbal extracts from Astragalus membranaceus, Portulaca oleracea, Flavescent sophora and A. paniculata act as an anti-stressor and induce the immunological parameters such as serum lysozyme activity, SOD, NOS and levels of total serum protein, globulin and albumin in Cyprinus carpio (Wu et al. 2007). Azadirachtin, a triterpenoid derived from A. indica, enhanced respiratory burst activities, the leucocyte count and the primary and secondary antibody response against SRBC in tilapia (Logambal and Michael 2000, 2001).
Conclusion
Antibiotics, chemotherapeutants and vaccines are expensive and lead to many adverse effects such as bioaccumulation and multi-resistance species development in the environment. Plant materials have a potential application as an immunostimulant in fish culture, primarily because they are not expensive and act against a broad spectrum of pathogens. The preparation of plant extract is much easier and inexpensive. Many plant products are used as anti-bacterial and anti-viral materials. The use of plant products as immunostimulants in fish culture systems may also be of environmental value due to its biodegradability. The enhancement compounds present in the leaf extracts such as phenolics, polyphenols, alkaloids, quinones, terpenoids, lectines and polypeptides have shown to be very effective alternatives to antibiotics and other synthetic compounds. Plant phenolics, polysaccharides, proteoglycans and flavonoids play a major role in preventing or controlling infectious microbes. In many studies, the usage of plant products as immunostimulant has revealed that they increase the immune responses, survival and growth rate of the fish. Due to the beneficial effect of plant material as immunostimulants, it can be used in fish farming as alternatives to vaccines, antibiotics and chemical drugs. For getting better growth and diseases management in aquaculture, attention has to be focused around many medicinal plants and drugs derived from medicinal plants, and these can be used easily as feed supplementation. In future, purification and experimental evaluation of active compounds from the herbal plants are needed for the effective control of disease-causing agents in aquaculture. Further, aquaculture practice with medicinal plant derivatives can get healthy natural protein-rich food for consumers and will be profitable for aquaculture farmers.
References
Amar EC, Kiron V, Satoh S, Watanabe T (2004) Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunol 16:527–537
Ardo L, Yin G, Xu P, Varadi L, Zigeti GS, Jeney Z, Jeney G (2008) Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture 275:26–33
Balasubramani SP, Michael RD (2002) Immunomodulation by the fruit extract of Indian Gooseberry, Phyllanthus emblica (Linn) in Oreochromis mossambicus (Peters). MSc thesis. The American College, Madurai
Bricknell Ian, Dalmo RA (2005) The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol 5:457–472
Castro HA, Gonzaa′lez SR (2001) Los microbios y el hombre, enemigos o amigos? Acta Bioquim Clin L 35:37–61
Castro R, Lamas J, Morais P, Sanmartı′n ML, Orallo F, Leiro J (2008) Resveratrol modulates innate and inflammatory responses in fish leucocytes. Vet Immunol Immunopathol 126:9–19
Cerezuela R, Cuesta A, Meseguer J, Ángeles Esteban M (2009) Effects of dietary vitamin D3 administration on innate immune parameters of seabream (Sparus aurata L.). Fish Shellfish Immunol 26:243–248
Chen X, Wu Z, Yin J, Li L (2003) Effects of four species of herbs on immune function of Carassius auratus gibelio. J Fish Sci China 10:36–40
Chopra RN, Chopra IC, Handa KL, Kapur LD (1956) Chopra’s indigenous drugs of India. UN Dhur and Sons, Calcutta
Citarasu T, Immanuel G, Marian MP (1998) Effects of feeding Artemia enriched with stresstol and cod liver oil on growth and stress resistance in the Indian white shrimp Penaeus indicus post larvae. Asian Fish Sci 12:65–75
Citarasu T, Babu MM, Sekar RJ, Marian PM (2002) Developing Artemia enriched herbal diet for producing quality larvae in Penaeus monodon, Fabricius. Asian Fish Sci 15:21–32
Citarasu T, Sivaram V, Immanuel G, Rout N, Murugan V (2006) Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol 21:372–384
Corzo-Martinez M, Corzo N, Villamiel M (2007) Biological properties of onions and garlic. Trends Food Sci Tech 18:609–625
Cuesta A, Meseguer J, Esteban MA (2004) Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.). Vet Immunol Immunopathol 101:203–210
Dalmo RA, Ingebrigtsen K, Boegwald J (1997) Non-specific defence mechanisms in fish with particular reference to the reticulo-endothelial system. J Fish Dis 20:241–273
De Bolle MF, Osborn RW, Goderis IJ, Noe L, Acland D, Hart CA, Torrekens S, Van Leuven F, Broekart NF (1996) Antimicrobial properties from Mirablis jalapa and Amaranthus caudalus: expression, processing, localization and biological activity in transgenic tobacco. Plant Mol Biol 31:993–1008
Divyagnaneswari M, Christybapita D, Michael D (2008) Enhancement of nonspecific immunity and disease resistance in Oreochromis mossambicus by Solanum trilobatum leaf fractions. Fish Shellfish Immunol 23:249–259
Dorucu M, Ozesen Colak S, Ispir U, Altinterim B, Celayir Y (2009) The effect of black cumin seeds, Nigella sativa, on the immune response of rainbow trout, Oncorhynchus mykiss. Mediterr Aqua J 2:1–7
Dugenci SK, Arda N, Candan A (2003) Some medicinal plants as immunostimulant for fish. J Ethnopharmacol 88:99–106
Edahiro T, Hamaguchi M, Kusuda R (1991) Suppressive effect of glycyrrhizin against streptococcal infection promoted by feeding oxidized lipids to yellowtail Seriola quinqueradiata. Suisanzoshoku 39:21–27
FAO (2002) The state of world fisheries and aquaculture. ISBN 92-5-104842-8, Rome
FAO newsrooms (2003) www.fao.org/english/newsroom/news/2003/14203-en-html
Fast MD, Sims DE, Burka JF, Mustafa A, Ross NW (2002) Skin morphology and humoral non-specific defence parameters of mucus and plasma in rainbow trout, coho and Atlantic salmon. Comp Biochem Physiol 132:645–657
Galeotti M (1998) Some aspects of the application of immunostimulants and a critical review of methods for their evaluation. J Appl Ichthyol 14:189–199
Goetz FW, Lliev DB, McCauley LAR, Liarte CQ, Tort LB, Planas JV, Mackenzie S (2004) Analysis of genes isolated from lipopolysaccharide stimulated rainbow trout (Oncorhynchus mykiss) macrophages. Mol Immunol 41:1199–1210
Gudding R, Lillehaug A, Evensen A (1999) Recent developments in fish vaccinology. Vet Immunol Immunopathol 72:203–212
Hardie LJ, Fletcher TC, Secombes JC (1991) The effect of dietary vitamin C on the immune response of the Atlantic salmon (Salmo salar L.). Aquaculture 95:201–214
Harikrishnan P, Nisha RM, Balasundaram C (2003) Hematological and biochemical parameters in common carp, Cyprinus carpio, following herbal treatment for Aeromonas hydrophila infection. Aquaculture 222:41–50
Harikrishnan R, Balasundaram C, Dharaneedharan S, Moon YG, Kim MC, Kim JS, Heo MS (2009a) Effect of plant active compounds on immune response and disease resistance in Cirrhina mrigala infected with fungal fish pathogen, Aphanomyces invadans. Aquac Res 40:1170–1181
Harikrishnan R, Balasundaram C, Kim MC, Kim JS, Han YJ, Heo MS (2009b) Innate immune response and disease resistance in Carassius auratus by triherbal solvent extracts. Fish Shellfish Immunol 27:508–515
Hemapriya VS (1997) Immunostimulatory effect of leaf extracts of few medicinal plants in Oreochromis mossambicus (Peters). MSc thesis. The American College, Madurai
Immanuel G, Uma RP, Iyapparaj P, Citarasu T, Punitha Peter SM, Michael Babu M, Palavesam A (2009) Dietary medicinal plant extracts improve growth, immune activity and survival of tilapia Oreochromis mossambicus. J Fish Biol 74:1462–1475
Jang SI, Marsden MJ, Kim YG, Choi MS, Secombes CJ (1995) The effect of glycyrrhizin on rainbow trout, Oncorhynchus mykiss (Walbaun), leucocyte responses. J Fish Dis 18:307–315
Jayaprakas V, Euphrasia J (1996) Growth performance of Labeo rohita (Ham.) to Livol (IHF-1000), a herbal product. Proc Indian Natl Sci Acad B 63:1–10
Jeney G, Anderson DP (1993) Enhanced immune response and protection in rainbow trout to Aeromonas salmonicida bacterin following prior immersion in immunostimulants. Fish Shellfish Immunol 33:51–58
Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB (2007) Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol 7:344–351
Kim KH, Hwang YJ, Bai SC (1999) Resistance to Vibrio alginolyticus in juvenile rockfish (Sebastes schlegeli) fed diets containing different doses of aloe. Aquaculture 180:13–21
Li Y, Lovell T (1985) Elevated levels of dietary ascorbic acid increased immune response in channel cat fish. J Nutr 115:123–131
Lin YC, Vaseeharan B, Ko CF, Chiou TT, Chen JC (2007) Molecular cloning and characterization of a protease inhibitor gene alpha 2-macroglobulin (α2-M) from the haemocytes of giant tiger shrimp Penaeus monodon. Mol Immunol 44:1065–1074
Logambal SM, Michael RD (2000) Immunostimulatory effect of azadirachtin in Oreochromis mossambicus (Peters). Indian J Exp Biol 38:1092–1096
Logambal SM, Michael RD (2001) Azadirachtin—an immunostimulant for Oreochromis mossambicus. J Aquac Trop 16:339–347
Logambal SM, Venkalalakshmi S, Michael RD (2000) Immunostimulatory effect of leaf extract of Ocimum sanctum Linn. in Oreochromis mossambicus (Peters). Hydrobiologia 430:113–120
Minomol M (2005) Culture of Gold fish Carassius auratus using medicinal plants having immunostimulant characteristics. M. Phil Dissertation, MS University, India
Ninomiya M, Hatta H, Fujiki M, Kim M, Yamamoto TR (1995) Enhancement of chemotactic activity of yellowtail (Seriola quinqueradiata) leucocytes by oral administration of Quillaja saponin. Fish Shellfish Immunol 5:325–327
Nya EJ, Austin B (2007) Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32:963–970
Nya EJ, Austin B (2009) Use of dietary ginger, Zingiber officinale Roscoe, as an immunostimulant to control Aeromonas hydrophila infections in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32:971–977
Ortuno J, Cuesta A, Rodriguez A, Angeles Eesteban M, Meseguer J (2002) Oral administration of yeast, Saccharomyces cerevisiae, enhances the celluler innate immune response of gilthead seabream, (Sparus aurata L.). J Vet Immunol Immunopathol 85:41–50
Pachanawan A, Phumkhachorn P, Rattanachaikunsopon P (2008) Potential of Psidium guajava supplemented fish diets in controlling Aeromonas hydrophila infection in tilapia (Oreochromis niloticus). J Biosci Bioeng 106:419–424
Penaflorida VD (1995) Effect of papaya leaf meal on the Penaeus monodon post larvae. Israeli J Aquac Bamidgeh 47:25–33
Pongsak R, Parichat P (2010) Potential of cinnamon (Cinnamomum verum) oil to control Streptococcus iniae infection in tilapia (Oreochromis niloticus). Fish Sci 76:287–293
Puangkaew J, Kiron V, Somamoto T, Okamoto N, Satoh S, Takeuchi T, Watanabe T (2004) Nonspecific immune response of rainbow trout (Oncorhynchus mykiss Walbaum) in relation to different status of vitamin E and highly unsaturated fatty acids. Fish Shellfish Immunol 16:25–39
Punitha SMJ, Babu MM, Sivaram V, Shankar VS, Dhas SA, Mahesh TC, Immanuel G, Citarasu T (2008) Immunostimulating influence of herbal biomedicines on nonspecific immunity in Groupers Epinephelus tauvina juvenile against Vibrio harveyi infection. Aquac Int 16:511–523
Raja Rajeswari P, Velmurugan S, Michael Babu M, Albin Dhas S, Kesavan K, Citarasu T (2012) A study on the influence of selected Indian herbal active principles on enhancing the immune system in Fenneropenaeus indicus against Vibrio harveyi infection. Aquac Int 20:1009–1020
Rao YV, Das BK, Pradhan J, Chakrabarti R (2006) Effect of Achyranthes aspera on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. Fish Shellfish Immunol 20:263–273
Rattanachaia A, Hironoa I, Ohiraa T, Takahashib Y, Aokia T (2005) Peptidoglycan inducible expression of a serine proteinase homologue from kuruma shrimp (Marsupenaeus japonicus). Fish Shellfish Immunol 18:39–48
Robinson I, Junqua G, Coillie RV, Thomas O (2007) Trends in the detection of pharmaceutical products, and their impact and mitigation in water and wastewater in North America. Anal Bioanal Chem 387:1143–1151
Sajid M, Prabjeet S, Munir HS, Khusheeba M (2011) Emerging role of immunostimulants in combating the disease outbreak in aquaculture. Int Aquat Res 3:147–163
Sakai M (1999) Current research status of fish immunostimulant. Aquaculture 172:63–92
Salah MA, Mohamed (2008) Echinacea as immunostimulatory agent in nile tilapia (Oreochromis niloticus) via earthen pond experiment. In: 8th international symposium on tilapia in aquaculture 2008, pp 1033–1040
Shadakshari GS (1993) Effect of bioboost forte, Livol and Amchemin AQ on growth and body composition of common carp, Cyprinus carpio (Linn.). M.F.Sc. Thesis, University of Agriculture Sciences, Bangalore, p 155
Shalaby AM, Khattab YA, Abdel Raman AM (2006) Effects of garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). J Venom Animals Toxins Incl Trop Dis 12(2):195
Sivakama valli J, Vaseeharan B (2012) cDNA cloning, characterization and expression of lipopolysaccharide and β-1, 3-glucan binding protein (LGBP) gene from the Indian white shrimp Fenneropenaeus indicus. Comp Biochem Physiol Part A 163:74–81
Sivaram V, Babu MM, Citarasu T, Immanuel G, Murugadass S, Marian MP (2004) Growth and immune response of juvenile greasy groupers (Epinephelus tauvina) fed with herbal antibacterial active principle supplemented diets against Vibrio harveyi infections. Aquaculture 237:9–20
Soltani M, Sheikhzadeh N, Mousavi HAE, Zargar A (2010) Effects of Zataria multiflora essential oil on innate immune responses of common carp (Cyprinus carpio). J Fish Aquat Sci 5:191–199
Sudhakaran DS, Srirekha P, Devasree LD, Premsingh S, Michael RD (2006) Immunostimulatory effect of Tinospora cordifolia Miers leaf extract in Oreochromis mossambicus. Indian J Exp Biol 44:726–732
Unnikrishnan G (1995) Effect of Livol on growth, food utilization and body composition of the Indian major carp, Catla catla (Ham.). M.Sc. Dissertation, University of Kerala, India, p 34
Vaseeharan B, Lin YC, Ko CF, Chen JC (2006) Cloning and characterization of a serine proteinase from the haemocytes of mud crab Scylla serrata. Fish Shellfish Immunol 21:20–31
Vaseeharan B, Shanthi S, Prabhu NM (2011) A novel clip domain serine proteinase (SPs) gene from the haemocytes of Indian white shrimp Fenneropenaeus indicus. Fish Shellfish Immunol 30(3):980–985
Vaseeharan B, Manju S, Ramasamy P (2012) Inhibitory activity of essential oils from medicinal plants against Pseudomonas spp. isolated from aquatic environments. Aqua Res 1(9)
Wada T, Arima T, Nagashima H (1987) Natural killer activity in patients with chronic hepatitis treated with OK432, interferon, adenine arabinoside and glycyrrhizin. Gastroenterol Jpn 22:312–321
Wendakoon CN, Sakaguchi M (1995) Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J Food Protect 58:280–283
Wu G, Yuan C, Shen M, Tang J, Gong Y, Li D, Sun F, Huang C, Han X (2007) Immunological and biochemical parameters in carp (Cyprinus carpio) after Qompsell feed ingredients for long-term administration. Aquac Res 38:246–255
Yin G, Jeney G, Ra′cz T, Pao X, Jeney Z (2006) Effect of two Chinese herbs (Astragalus radix and Scutellaria radix) on non-specific immune response of tilapia, Oreochromis niloticus. Aquaculture 253:39–47
Yin G, Ardo′ L, Thompson KD, Adams A, Jeney Z, Jeney G (2009) Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immunol 26:140–145
Yogeeswaran A, Velmurugan S, Punitha SMJ, Michael Babu M, Selvaraj T, Kumaran T, Citarasu T (2012) Protection of Penaeus monodon against white spot syndrome virus by inactivated vaccine with herbal immunostimulants. Fish Shellfish Immunol 32:1058–1067
Zhang YH, Yoshida T, Isobe K, Rahman MJ, Nagase F, Ding L, Nakashima I (1990) Modulation by glycyrrhizin of the cell-surface expression of H-2 class 1 antigens on murine tumor cell lines and normal cell populations. Immunology 70:405–410
Acknowledgments
The author wishes to thank the Department of Science and Technology (DST) and the Ministry of Science and Technology, Government of India. RT gratefully acknowledges the INSPIRE grant (DST, New Delhi) for the financial assistance rendered (Ref no Dy.No.100 / IFD / 5665).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaseeharan, B., Thaya, R. Medicinal plant derivatives as immunostimulants: an alternative to chemotherapeutics and antibiotics in aquaculture. Aquacult Int 22, 1079–1091 (2014). https://doi.org/10.1007/s10499-013-9729-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-013-9729-3