Abstract
The rise of antibiotic-resistant bacterial strains is a global concern in many sectors, such as aquaculture, as described in chapter “The Rise and Fall of Antibiotics in Aquaculture.” To counter this phenomenon, several alternatives or complement to antibiotics have been investigated. Here, we will look at one of those proposed strategies that of using bacteria-specific viruses, called bacteriophages, or commonly phages. Since their discovery in the early 1900s, bacteriophage treatments have had a fleeting popularity in Western countries due to several scientific reasons as well as in some cases, political motives. Only recently, with the appearance of multidrug-resistant bacterial strains, a new craze for phage therapy appeared in Western countries. In an aquaculture context, some studies have shown promising results for the treatment of fish diseases using phages. More specifically, the experimentations with phage cocktail against A. salmonicida, infectious agent of furunculosis in salmonids, both in vitro and in vivo, provide an interesting foundation for future alternative treatments. However, since phages and bacteria are evolving entities, this biological war is far from over. The presence of phage-resistance mechanisms in bacteria and other technical aspects of phage therapy in aquaculture are factors to consider before having any applicable treatments.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 The Enemy of My Enemy Is My Friend
Viruses are biological entities capable of infecting the entire kingdom of life, including bacteria. Bacterial viruses are called bacteriophages or more commonly phages, and they were co-discovered independently by Frederick Twort (1915) and Félix d’Hérelle (1917). Rapidly after their discovery, studies on the therapeutic potential of phages, also called phagotherapy, were published (McKinley 1923). Félix d’Hérelle was the pioneer of the phagotherapy, using phages against dysentery but also against cholera and bubonic plague (Chanishvili 2012). However, the lack of standardized methods including proper controls has resulted in several conflicting studies on the potential of phages in a therapeutic context (Summers 2012). In addition, phages usually have a narrow host range (limited to a few bacterial strains is some cases), which makes their use highly targeted, unlike antibiotics, which have a much broader spectrum (Summers 2012). As indicated in chapter “The Rise and Fall of Antibiotics in Aquaculture,” the Second World War was a decisive moment in the discovery and use of antibiotics and thus a breaking point for the use of phages in several countries, with the exception of the Soviet Union and Germany (Cisek et al. 2017).
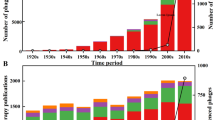
Nevertheless, even today, phage therapy is still used in several Eastern European countries, such as the Georgia, Poland, and Russia (Abedon et al. 2011). The largest institute dedicated to phage therapy is in Georgia. The Eliava Institute is a historic center founded by the Georgian microbiologist George Eliava in collaboration with Félix d’Hérelle (Sulakvelidze and Alavidze 2001). This unique phage therapy clinic offers highly specialized and personalized treatments for ears, throat, nose, urologic, and gynecologic human infections. With the growing crisis of antibiotic-resistant bacteria, the use of phages in a therapeutic or biocontrol context has been rediscovered (Abedon 2014). This growing interest of phages in a therapeutic context can be illustrated by the increasing number of articles about “phage therapy” that are indexed by Web of Science (Fig. 1).
A recent clinical study has shown excellent success rates for treating chronic otitis caused by Pseudomonas aeruginosa with phages (Wright et al. 2009). In addition, a European initiative, PhagoBurn (http://www.phagoburn.eu), is currently investigating phage therapy against Escherichia coli and P. aeruginosa. From a commercial point of view, several companies are interested in a therapeutic application of phages, and some products based on these viruses are already approved and marketed in Western countries (Sarhan and Azzazy 2015). For example, in Canada, it is possible to use the product AgriPhage-CMM, which contains phages against Clavibacter michiganensis subsp. michiganensis, the etiological agent causing canker of tomatoes. Phage products are also registered in the USA for food applications (Bai et al. 2016), including among others ListShield (against Listeria monocytogenes), EcoShield (against E. coli O157: H7), and SalmoFresh (against Salmonella enterica).
2 The Biology of Phages
Similarly to the majority of antibiotic molecules (before some artificial chemical modifications), phages are naturally found in the environment, particularly in bacteria-colonized ecosystems. Phages can be found at a titer of up to 2.5 × 108 viral particles per milliliter of water in natural environments (Bergh et al. 1989). In addition, it is now well known that phages play important ecological roles in controlling bacterial populations and participating in the regulation of several biogeochemical cycles (Bratbak et al. 1990; Proctor and Fuhrman 1990; Suttle 2007; Sime-Ngando 2014).
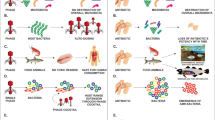
Inside the bacterial host cells, a phage can remain in at least four forms leading to different evolutionary strategies: as a replicating virus during the lytic cycle, as an unstable carrier state termed pseudolysogeny, as a prophage with complete genome during the lysogeny state, or as a defective cryptic prophage (Golais et al. 2013). To simplify, we often talk about the lytic cycle in opposition to the lysogenic state (Fig. 2). A lytic cycle comprises five major steps (Sulakvelidze and Alavidze 2001; Drulis-Kawa et al. 2012): (I) adsorption of the phage on the surface of the bacterium, (II) injection of the phage genetic material in the host bacterium, (III) phage genome replication and host cell machinery redirection for viral particle production, (IV) phage assembly, and (V) host cell lysis. For a temperate phage, steps I and II occur as for a lytic phage; however, during lysogeny the phage DNA is inserted into the bacterial chromosome (Sulakvelidze and Alavidze 2001). At this stage, the phage DNA is replicated along with the bacterial DNA and is called a prophage. Any given stress can cause excision of the prophage, allowing it to continue its cycle in steps III, IV, and V as for a lytic phage. It should be noted that some prophages, including those found in the bacteria Leptospira, can replicate their DNA in an extrachromosomal manner similar to that of plasmids (Girons et al. 2000; Zhu et al. 2015).
In a therapeutic context, strictly lytic phages are favored because the only outcome is killing the phage-infected bacterium. Indeed, temperate phages can cause some undesirable effects. During the lysogenic cycle, phage DNA becomes an integral part of the bacterial genome and can therefore make its genes usable by the bacterium (De Paepe et al. 2014). Several bacterial genomes are known to contain prophages, and in some cases, the percentage of phage DNA can reach more than 20% of the total genome (Hatfull and Hendrix 2011; Casjens 2003). Prophages are recognized for potentially conferring certain characteristics such as causing an increased in the fitness of the bacterium, protecting against phage infections, as well as enhancing virulence through lysogenic conversion factors (Brüssow et al. 2004). The bacterium E. coli O157:H7 is a known example of bacteria with toxin genes (Shiga toxins) from a prophage (Plunkett et al. 1999).
While strictly lytic phages remain by far the favorite option for eradication purposes, recent works expand the horizon of phagotherapy. Indeed, some groups have begun to explore the possibility of using temperate phages naturally present in bacteria or completely engineered (Monteiro et al. 2018) as alternative options. The temperate phage therapy may be useful in some cases where it is hard to isolate lytic phages against some specific pathogenic bacteria. For example, there is a lack of virulent phages that target Clostridium difficile, an anaerobic pathogen (Hargreaves and Clokie 2014). To overcome this problem, the combination of temperate and lytic phages was used. The cocktail caused the complete lysis of C. difficile in vitro and prevented the appearance of lysogens (Nale et al. 2016).
There are also other biological aspects to consider when using phages in a therapeutic context. For example, the host range of the lytic phage and the ability of the targeted bacteria to evolve a defense mechanism are crucial parameters. Phages tend to be specific to one or a few bacterial species or even a few strains (Hyman and Abedon 2010). This can be problematic in a therapeutic context since it requires knowing precisely the bacterium causing the infection, which can still be challenging. However, it is often suggested that a mixture of phages, in the form of a cocktail, could increase the bacterial host range (Chan and Abedon 2012; Chan et al. 2013). For example, a metagenomic method has revealed that the Intesti-bacteriophage cocktail, initially developed by d’Hérelle and now produced by the Eliava Institute, contains about 23 phages, allowing to target a large number of different bacteria (Zschach et al. 2015).
Bacteria can protect themselves against phage infection by several mechanisms. It is possible to categorize these mechanisms in various classical groups: the inhibition of phage adsorption, the blocking of the entry of phage genetic material, the degradation of phage nucleic acids (CRISPR-Cas and restriction-modification systems), and cell death, the latter known as bacterial abortive infection (Abi) systems (Labrie et al. 2010). However, a plethora of additional and novel phage defense mechanisms are still been identified (Doron et al. 2018; Kronheim et al. 2018). Phages, unlike antibiotics, are dynamic biological entities that can evolve to counteract bacterial protection mechanisms (Samson et al. 2013). Again, it is proposed that the use of a cocktail of phages would be able to decrease the level of resistance to these phages (Lu and Koeris 2011; Abuladze et al. 2008). However, it is recommended to minimize the number of viruses and rigorously test the cocktail to verify that phages have no antagonistic effects and that there is no recombination capability between phage genomes (Mateus et al. 2014; Klumpp and Loessner 2013).
3 Phagotherapy in the Digital Age: What if We Could Make a Custom Phage?
As noted in chapter “The Rise and Fall of Antibiotics in Aquaculture,” several antibiotic molecules are naturally produced by various organisms, either to protect themselves or to compete for resources. As reviewed elsewhere (Wright et al. 2014; Nicolaou and Rigol 2018), chemists have used, modified, and recreated these molecules in order to have active, safe, bioavailable, optimized, and economically viable compounds. It is interesting that the chemistry of antibiotics, which arguably began in the laboratory of Paul Ehrlich, was born in the early 1900s, as was the discovery of phages by Twort and d’Hérelle. However, it was not until several important discoveries in molecular biology were made before phage could be modified.
Ironically, phages played a crucial role in the discovery of key enzymes used in molecular biology, such as restriction enzymes (Smith and Welcox 1970) and ligases (Weiss and Richardson 1967). The first genomes sequenced were those of phage MS2 (single-stranded RNA) (Fiers et al. 1976), ΦX174 (single-stranded DNA) (Sanger et al. 1977) and λ (double-stranded DNA) (Sanger et al. 1982). More recently, it has been shown that the CRISPR-Cas system, an adaptive defense of bacteria against exogenous DNAs such as phage genomes and plasmids (Barrangou et al. 2007; Marraffini and Sontheimer 2008), can be used to make targeted double-strand DNA breakage and thus be a powerful tool for genome editing (Jinek et al. 2012). Now, thanks to this system, it is even possible to modify phage genomes very precisely (Martel and Moineau 2014; Lemay et al. 2017).
In addition to modifying an existing genome, it is now possible, thanks to the advances made in molecular biology and bioinformatics, to de novo create synthetic genomes and thus reconstitute organisms. This allowed the team of J. Craig Venter in 2003 to artificially recreate, from oligonucleotides, the genome of the phage ΦX174 (infecting E. coli) and to generate infectious virions from this synthetic genome (Smith et al. 2003). Although the creation of synthetic genomes must be ethically accepted (Cho et al. 1999), it is clear that this new discipline has the potential to radically change many facets of science and medicine, including, of course, phage therapy (Kilcher et al. 2018; Kilcher and Loessner 2018).
In this sense, given the advances in genome engineering and synthetic biology, it would be possible to modify/create a phage having features ideally designed for a therapeutic context, such as a larger host range and no lysogenic cycle (Barbu et al. 2016; Brown et al. 2017). For example, it has already been possible to modify the host range of phage T2 (infecting E. coli) by changing its long tail fiber genes, 37 and 38, by those of phage IP008, which naturally has a much larger host spectrum than the one of T2 (Mahichi et al. 2009). Similarly, another study demonstrated that by swapping the same genes of phage T2 with those of phage PP01, infecting specifically the pathogenic E. coli O157:H7, the modified phage T2 was also able to infect this bacterium (Yoichi et al. 2005). Since it is possible to more closely control the genes present in phage genomes, it is also realistic to think that synthetic biology will make it easier to satisfy safety requirements for the approval process with the different agencies. Like the antibiotic molecules currently available, it is possible to believe that in the near future, natural, semisynthetic, and synthetic phage products will emerge in the market of antimicrobial compounds.
4 Phage Therapy in Aquaculture
As with all other living things, aquatic animals are subject to diseases. In addition, as discussed in chapter “The Rise and Fall of Antibiotics in Aquaculture,” it is increasingly difficult to sustainably treat certain bacterial diseases because of the rise of antibiotic-resistant strains. This is why, in recent years, several studies have investigated the potential of phage therapy in the aquatic environments (Gon Choudhury et al. 2017). For example, phages have been tested to control infections caused by Vibrio (vibriosis disease) (Wang et al. 2017; Kalatzis et al. 2018), Flavobacterium columnare (columnaris disease) (Prasad et al. 2011; Laanto et al. 2015), Thalassomonas loyana (white plague coral disease) (Atad et al. 2012), and Aeromonas salmonicida subsp. salmonicida (furunculosis disease) (Imbeault et al. 2006; Silva et al. 2016). For a little over a year now, it is possible for fish farmers to obtain the product BAFADOR®, a new bacteriophage-based preparation, to fight two of the most common pathogens responsible for mortality in farmed fish, Aeromonas hydrophila and Pseudomonas fluorescens (globalaginvesting.com). This preparation can be used both prophylactically to increase the resistance of the fish and eels and therapeutically in case of infection (Schulz et al. 2019).
While these studies all show promising results, the fact remains that there are significant challenges inherent to aquaculture that must be considered. One of these challenges is undoubtedly the method of phage delivery (Gon Choudhury et al. 2017). Unlike humans or large animals, with which it is possible to use the oral or intracutaneous routes, the administration of drugs has additional constraints with fish. Several ways of administration were already been proposed. Among these, there is the immersion of fish in a solution containing phages, the addition of viruses in the feed or even their addition directly in water. Finally, other ways also include the anal intubation or the injection of phages. However, these last two methods require important handling of fish that may be difficult to put in place in a fish-farming context. In addition the phage delivery via the parenteral route, i.e., intraperitoneal injection in fish, against Flavobacterium resulted in immediate distribution of phages to the circulation system and organs, but no significant reduction of fish mortality was detected (Castillo et al. 2012). By the oral route, the immersion, or directly in circulation system, the phage treatment is likely to be diluted in water (Christiansen et al. 2014). Although it is possible to add phages to food (Nakai and Park 2002; Park and Nakai 2003; Jun et al. 2013), it is well known that fish compete for it (Cuenco et al. 1985); phage intake would consequently be unequal between individuals, especially for those who have less energy to feed oneself like sick fish.
Some other aspects must be challenged with phage therapy in aquaculture. One of them is the bacterial community that is very variable over seasons and years, showing a higher complexity during warm seasons. On the other hand, it seems that the diversity of pathogenic bacteria showed lower seasonal variation as reported for Vibrio genus (Pereira et al. 2011a, b). It was suggested that the spring season is the best time to apply phages and that an annual water monitoring is needed. It is also important to consider that the chemical disinfection use in the farms is often a source of disturbance and variation of the microbial community.
It is crucial to identify the main pathogenic bacteria and to choose the best phage cocktail. This information must be rapidly known to avoid the spreading of the infectious agents. The culture-independent in situ hybridization using specific probes provides an overview of the real proportion of cultivable and non-cultivable pathogenic bacteria (Taylor-Brown et al. 2017), or multiplex PCR approaches can be used to diagnostic in a short time (Nishiki et al. 2018). In fact, an annual follow-up of the bacterial diversity could be a good way to use the phages in preventive treatment instead of curative in aquaculture context.
Finally, the phage therapy in aquaculture could be possible if phages show a good survival time in water system according to the chosen method of administration, have only a moderated impact on the overall bacterial community structure, and provide desired specificity and efficiency for the targeted pathogenic bacteria (Pereira et al. 2011a, b).
5 Toward Phagotherapy to Control Furunculosis
The bacterium A. salmonicida subsp. salmonicida is the etiologic agent of furunculosis, a worldwide disease affecting salmonids (Dallaire-Dufresne et al. 2014). Historically, the control of furunculosis is through vaccination and antibiotic therapy. While vaccination implies heavy fish handling and high cost, the use of antibiotics was, and still is, the preferred method for treating furunculosis. However, more and more cases of A. salmonicida subsp. salmonicida strains resistant or even multiresistant to antibiotics are listed (Vincent et al. 2014, 2016a; Trudel et al. 2016; Bartkova et al. 2017). With increasing access to high-throughput sequencing technologies, it is now possible to effectively investigate the determinants of resistance to different antimicrobial compounds (Chan 2016). In the case of A. salmonicida subsp. salmonicida and as discussed in chapter “The Rise and Fall of Antibiotics in Aquaculture,” several recent studies have shown that plasmids are important vectors in the spread of antibiotic resistance genes. A study has shown, for example, that two plasmids, pSN254b and pAB5S9b, can provide resistance to all antibiotics approved by the Veterinary Drugs Directorate (VDD) of Health Canada to treat infected fish (Trudel et al. 2016). It is therefore clear that effective alternatives to antibiotics are needed to control furunculosis.
Several independent studies showed in vitro that phage therapy might be one of the alternatives to antibiotics against furunculosis. Many lytic phages infecting A. salmonicida subsp. salmonicida have been found and characterized (Vincent et al. 2017a). Importantly, some of these phages can infect a large number of bacterial strains without distinction of geographical origin or other parameters. Although it is often considered preferable to have phages with a large host range to facilitate treatment, it is also important to avoid using phages that can lyse non-targeted species or strains. In the case of A. salmonicida, this seems to be guaranteed by a biological barrier imposed by the bacterium itself. The strains of the subspecies salmonicida are psychrophilic, so they cannot grow at 37 °C (Dallaire-Dufresne et al. 2014). However, several mesophilic strains of A. salmonicida have recently been characterized, although there is no subspecies attribution yet (Vincent et al. 2016a, b, 2017b, 2019). This biological dichotomy also reflected in phage sensitivity, where mesophilic strains are resistant to phages infecting psychrophilic ones (including strains of other psychrophilic subspecies of A. salmonicida) (Table 1). It is interesting to note that the mesophilic strain A527 is an exception since it is sensitive to phage 44RR2.8t.2, isolated from a strain of subspecies salmonicida. This suggests that the phage receptor 44RR2.8t.2 may be present in strain A527, although still unknown.
Another major concern with the use of phages in aquaculture is how these bacterial viruses are maintained under aquaculture conditions. Three phages infecting A. salmonicida subsp. salmonicida were found to persist in water similar to that found in aquaculture for a long period of time (Fig. 3). While the phages were diluted in water, still a considerable number of virions remained detectable in spite of the absence of host bacteria to ensure their replication. It should also be noted that phages can replicate in the presence of their host, allowing a potential for autodosing and thus minimizing the impact of dilution. Another study has also evaluated the persistence of A. salmonicida subsp. salmonicida phages with brook trout by reinfecting fish with doses of A. salmonicida subsp. salmonicida. After 7 days, the phages remained in aquariums and allowed decrease of the pathogen population (Imbeault et al. 2006).
Detection by qPCR of A. salmonicida phage DNA from a phage cocktail in water circulation system in time. Bacteriophages were amplified separately at an initial concentration of 1 × 107 UFP/ml and pooled into the same circulating water system. Time 0 was taken 1 h after the water was completely recircularized. Three water samples were then taken each week. The amount of residual phage DNA was then analyzed by qPCR in triplicate
The emergence of bacteriophage-insensitive mutants (BIM) during phage infection has been reported in some studies in aquaculture context (Hossain et al. 2012; Tan et al. 2015), but the mechanisms of phage resistance are not yet completely understood. The presence of quorum-sensing-regulated phage defense mechanisms depending on population cell density and mutation of the phage receptor seem to be probable strategies to resist phage infection (Hossain et al. 2012). In A. salmonicida subsp. salmonicida, some phage-resistant bacteria also emerged after phage treatment including successive streak-plating steps (Moreirinha et al. 2018). A significant modification in the expression of intracellular proteins was observed when compared with the phage-sensitive bacteria. These proteins would have molecular function associated in phage replication. This decrease of proteins in the host cell prevented the phage from completing its lytic cycle. Fortunately, phages have also developed strategies to overcome bacterial resistance (Samson et al. 2013) making this constant battle very interesting for therapeutic purpose.
The use of a phage cocktail rather than a single phage for therapeutic purpose has been mentioned in the previous section to avoid bacterial resistance, at least, to decrease it. Although mixing several phages in a same cocktail can make it more effective or synergistic, it can also result in antagonistic effects. A cocktail combining two specific phages against A. salmonicida showed significantly higher antimicrobial activity than other cocktails (with three, four, or five phages) and individual phages (Chen et al. 2018).
Fish immune responses are also possible (Khan Mirzaei et al. 2016) if there are bacterial debris present in the phage cocktail (Abedon 2014; Dufour et al. 2016). It would consequently be crucial to develop a protocol for the production and purification of A. salmonicida subsp. salmonicida phages. Attempts to optimize such general processes have already been made (Bourdin et al. 2014; Lipinski et al. 2016). Finally, the determination of an experimental animal model is of great importance in the study of fish infection to avoid variations and to be close to the reality (Romero et al. 2016).
What about the in vivo trials in aquaculture context against furunculosis? To date, only few publications mention the use of phages against furunculosis (Imbeault et al. 2006; Silva et al. 2016). One of them demonstrated that phage therapy can increase the survival of infected rainbow trout model against an infection with A. salmonicida subsp. salmonicida (Kim et al. 2015). The intramuscular administration of single phage dose at MOI of 10,000 against A. salmonicida subsp. salmonicida showed notable protective effects such as increasing the survival rates. For all the smallest MOI, the bacterial growth was markedly retarded up to 12 h after phage inoculation but started to increase gradually after 24 h. Furthermore, no development of fish humoral immunity was shown in this study. These results demonstrated that some phages could be considered as alternative biological control agents against A. salmonicida subsp. salmonicida infections in rainbow trout, but the required concentration of phages (MOI) is very high. Future works will have to focus on isolating and characterizing phages with high replication rate at lower doses of infection and to test other administration routes.
The early stage of fish growth is particularly important because traditional antibiotic treatments or vaccination are not effective. The implementation of approaches to control furunculosis in juvenile fish is essential to ensure the sustainability of production, and phage therapy could be attractive in this context. After submerging juvenile Solea senegalensis in a phage bath for 6 h, the growth of A. salmonicida was inhibited. After 72 h, none of the fish had died, while a mortality rate of 36% was reported when fish were exposed only to the pathogen without phages (Silva et al. 2016).
On a less positive note, a 2007 study from the UK on Atlantic salmon and rainbow trout (Verner-Jeffreys et al. 2007) showed that phage administration by the intraperitoneal route or by oral infeed against did not offer protection for fish against A. salmonicida subsp. salmonicida. Because promising results in vitro do not seem to be always reflected in in vivo challenges (Tsonos et al. 2014), more research studies are clearly needed on the use of phages to treat/prevent fish infections by A. salmonicida subsp. salmonicida.
6 Keep Going Until Efficient Phagotherapy
With the emergence of multidrug-resistant bacterial strains, we can observe the return in force of the phagotherapy. The natural, specific, and quick way that phages eliminate bacteria suggest the possibility of creating an alternative or complement treatment that is effective and simple against pathogenic bacteria in aquaculture (Dy et al. 2018), as already done in other fields. On the other hand, several parameters have yet to be optimized, such as isolation of hyper-efficient phages accompanied with a well genomic and phenotypic characterization. Understanding the host range and ensuring that the phages will not transfer unwanted genes to the bacterial community is a priority. It will also be of prime importance to increase our understanding about the mechanisms used by pathogenic bacteria to protect themselves against phages. In this sense, it will be crucial to avoid repeating the same mistakes made with antibiotics. Finally, the increase of in vivo experiments could allow us to determine the best route of administration in an aquaculture context and to confirm the feasibility of this approach.
This chapter shows that the commercial use of phages against fish diseases has still some hurdles to clear. However, the fact that phage-based products are already marketed might help to pave the way for more similar products in aquaculture and specifically against furunculosis. It is hoped that the BAFADOR® product offered against P. fluorescens and A. hydrophila to protect and cure farmed eel is just the beginning.
In parallel, other alternative treatments to antibiotics should be deployed to avoid relying on a single therapeutic strategy. For example, improving the composition and methods of vaccination in aquaculture is a topic of research for many groups (Gudding et al. 1999; Hastein et al. 2005; Kashulin et al. 2017). It has been also demonstrated that phages are more effective when used with antibiotics, regardless of the antibiotic resistance state of the bacterium (Comeau et al. 2007). This phenomenon, called the phage-antibiotic synergy, suggested that other molecules or combined treatments can also be synergistic with phages. For example, carvacrol, an essential oil, combined with pneumococcal phage lysozyme improves their lytic activity (Díez-Martínez et al. 2013). The use of probiotics is also a popular approach (see chapter “Host-Microbiota Interactions and their Importance in Promoting Growth and Resistance to Opportunistic Diseases in Salmonids”) in aquaculture (Das et al. 2008; Hai 2015) that could be combined with phagotherapy.
Governmental approval will also likely be needed for these phage-based products. The acceptance of the phage technology and its adoption by the fish farmers and consumers may dictate its commercial success. If fish consumers and producers are willing to accept phage treatments once proven to be effective, reproducible, and safe, then different governmental agencies will be more prone to approve the use of these products.
References
Abedon ST (2014) Phage therapy: eco-physiological pharmacology. Scientifica (Cairo) 2014:581639. https://doi.org/10.1155/2014/581639
Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM (2011) Phage treatment of human infections. Bacteriophage 1:66–85. https://doi.org/10.4161/bact.1.2.15845
Abuladze T et al (2008) Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl Environ Microbiol 74:6230–6238. https://doi.org/10.1128/AEM.01465-08
Atad I, Zvuloni A, Loya Y, Rosenberg E (2012) Phage therapy of the white plague-like disease of Favia favus in the Red Sea. Coral Reefs 31:665–670. https://doi.org/10.1007/s00338-012-0900-5
Bai J, Kim Y-T, Ryu S, Lee J-H (2016) Biocontrol and rapid detection of food-borne pathogens using bacteriophages and endolysins. Front Microbiol 7:474. https://doi.org/10.3389/fmicb.2016.00474
Barbu EM, Cady KC, Hubby B (2016) Phage therapy in the era of synthetic biology. Cold Spring Harb Perspect Biol 8:a023879. https://doi.org/10.1101/cshperspect.a023879
Barrangou R et al (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712. https://doi.org/10.1126/science.1138140
Bartkova S, Leekitcharoenphon P, Aarestrup FM, Dalsgaard I (2017) Epidemiology of Danish Aeromonas salmonicida subsp. salmonicida in fish farms using whole genome sequencing. Front Microbiol 8:2411. https://doi.org/10.3389/fmicb.2017.02411
Bergh O, Børsheim KY, Bratbak G, Heldal M (1989) High abundance of viruses found in aquatic environments. Nature 340:467–468. https://doi.org/10.1038/340467a0
Bourdin G et al (2014) Amplification and purification of T4-Like Escherichia coli phages for phage therapy: from laboratory to pilot scale. Appl Environ Microbiol 80:1469–1476. https://doi.org/10.1128/AEM.03357-13
Bratbak G, Heldal M, Norland S, Thingstad TF (1990) Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol 56:1400–1405
Brown R, Lengeling A, Wang B (2017) Phage engineering: how advances in molecular biology and synthetic biology are being utilized to enhance the therapeutic potential of bacteriophages. Quant Biol 5:42–54. https://doi.org/10.1007/s40484-017-0094-5
Brüssow H, Canchaya C, Hardt W-D (2004) Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev 68:560–602. https://doi.org/10.1128/MMBR.68.3.560-602.2004
Casjens S (2003) Prophages and bacterial genomics: what have we learned so far? Mol Microbiol 49:277–300. https://doi.org/10.1046/j.1365-2958.2003.03580.x
Castillo D et al (2012) Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J Fish Dis 35:193–201. https://doi.org/10.1111/j.1365-2761.2011.01336.x
Chan KG (2016) Whole-genome sequencing in the prediction of antimicrobial resistance. Expert Rev Anti-Infect Ther 14:617–619. https://doi.org/10.1080/14787210.2016.1193005
Chan BK, Abedon ST (2012) Phage therapy pharmacology. Phage cocktails. Adv Appl Microbiol 78:1–23. https://doi.org/10.1016/B978-0-12-394805-2.00001-4
Chan BK, Abedon ST, Loc-Carrillo C (2013) Phage cocktails and the future of phage therapy. Future Microbiol 8:769–783
Chanishvili N (2012) Chapter 1—Phage therapy—history from Twort and d’Herelle through soviet experience to current approaches. In: Łobocka M, Szybalski WT (eds) Bacteriophages, part B, Advances in virus research, vol 83. Academic, Amsterdam, pp 3–40. https://doi.org/10.1016/B978-0-12-394438-2.00001-3
Chen L et al (2018) In vitro design and evaluation of phage cocktails against Aeromonas salmonicida. Front Microbiol 9:1476. https://doi.org/10.3389/fmicb.2018.01476
Cho MK, Magnus D, Caplan AL, McGee D (1999) Ethical considerations in synthesizing a minimal genome. Science 286:2087–2090. https://doi.org/10.1126/science.286.5447.2087
Christiansen RH, Dalsgaard I, Middelboe M, Lauritsen AH, Madsen L (2014) Detection and quantification of Flavobacterium psychrophilum-specific bacteriophages in vivo in rainbow trout upon oral administration: implications for disease control in aquaculture. Appl Environ Microbiol 80:7683–7693. https://doi.org/10.1128/AEM.02386-14
Cisek AA, Dąbrowska I, Gregorczyk KP, Wyżewski Z (2017) Phage therapy in bacterial infections treatment: one hundred years after the discovery of bacteriophages. Curr Microbiol 74:277–283. https://doi.org/10.1007/s00284-016-1166-x
Comeau AM, Tétart F, Trojet SN, Prère MF, Krisch HM (2007) Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2:e799. https://doi.org/10.1371/journal.pone.0000799
Cuenco ML, Stickney RR, Grant WE (1985) Fish bioenergetics and growth in aquaculture ponds: III. Effects of intraspecific competition, stocking rate, stocking size and feeding rate on fish productivity. Ecol Model 28:73–95. https://doi.org/10.1016/0304-3800(85)90014-6
d’Herelle F (1917) Sur un microbe invisible antagoniste des bacilles dysentériques. C R Acad Sci 165:373–375
Dallaire-Dufresne S, Tanaka KH, Trudel MV, Lafaille A, Charette SJ (2014) Virulence, genomic features, and plasticity of Aeromonas salmonicida subsp. salmonicida, the causative agent of fish furunculosis. Vet Microbiol 169:1–7. https://doi.org/10.1016/j.vetmic.2013.06.025
Das S, Ward LR, Burke C (2008) Prospects of using marine actinobacteria as probiotics in aquaculture. Appl Microbiol Biotechnol 81:419–429. https://doi.org/10.1007/s00253-008-1731-8
De Paepe M et al (2014) Temperate phages acquire DNA from defective prophages by relaxed homologous recombination: the role of Rad52-Like recombinases. PLoS Genet 10:e1004181. https://doi.org/10.1371/journal.pgen.1004181
Díez-Martínez R et al (2013) Improving the lethal effect of Cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob Agents Chemother 57:5355–5365. https://doi.org/10.1128/AAC.01372-13
Doron S et al (2018) Systematic discovery of antiphage defense systems in the microbial pangenome. Science 359:eaar4120. https://doi.org/10.1126/science.aar4120
Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B, Delattre A-S, Lavigne R (2012) Learning from bacteriophages—advantages and limitations of phage and phage-encoded protein applications. Curr Protein Pept Sci 13:699–722
Dufour N, Henry M, Ricard J-D, Debarbieux L (2016) Commentary: morphologically distinct Escherichia coli bacteriophages differ in their efficacy and ability to stimulate cytokine release in vitro. Front Microbiol 7:1029
Dy RL, Rigano LA, Fineran PC (2018) Phage-based biocontrol strategies and their application in agriculture and aquaculture. Biochem Soc Trans 46:1605–1613. https://doi.org/10.1042/BST20180178
Fiers W et al (1976) Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature 260:500–507. https://doi.org/10.1038/260500a0
Girons IS et al (2000) The LE1 bacteriophage replicates as a plasmid within Leptospira biflexa: construction of an L. biflexa-Escherichia coli shuttle vector. J Bacteriol 182:5700–5705. https://doi.org/10.1128/JB.182.20.5700-5705.2000
Golais F, Hollý J, Vítkovská J (2013) Coevolution of bacteria and their viruses. Folia Microbiol (Praha) 58:177–186. https://doi.org/10.1007/s12223-012-0195-5
Gon Choudhury T, Tharabenahalli Nagaraju V, Gita S, Paria A, Parhi J (2017) Advances in bacteriophage research for bacterial disease control in aquaculture. Rev Fish Sci Aquac 25:113–125. https://doi.org/10.1080/23308249.2016.1241977
Gudding R, Lillehaug A, Evensen (1999) Recent developments in fish vaccinology. Vet Immunol Immunopathol 72:203–212. https://doi.org/10.1016/S0165-2427(99)00133-6
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119:917–935. https://doi.org/10.1111/jam.12886
Hargreaves KR, Clokie MRJ (2014) Clostridium difficile phages: still difficult? Front Microbiol 5. https://doi.org/10.3389/fmicb.2014.00184
Hastein T, Gudding R, Evensen O (2005) Bacterial vaccines for fish—an update of the current situation worldwide. Dev Biol 121:55–74
Hatfull GF, Hendrix RW (2011) Bacteriophages and their genomes. Curr Opin Virol 1:298–303. https://doi.org/10.1016/j.coviro.2011.06.009
Hossain MJ, Rahman KS, Terhune JS, Liles MR (2012) An outer membrane porin protein modulates phage susceptibility in Edwardsiella ictaluri. Microbiology 158:474–487. https://doi.org/10.1099/mic.0.054866-0
Hyman P, Abedon ST (2010) Bacteriophage host range and bacterial resistance. Adv Appl Microbiol 70:217–248. https://doi.org/10.1016/S0065-2164(10)70007-1
Imbeault S, Parent S, Lagacé M, Uhland CF, Blais JF (2006) Using bacteriophages to prevent furunculosis caused by Aeromonas salmonicida in farmed brook trout. J Aquat Anim Health 18:203–214. https://doi.org/10.1577/H06-019.1
Jinek M et al (2012) A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337:816–821. https://doi.org/10.1126/science.1225829
Jun JW et al (2013) Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture 416–417:289–295. https://doi.org/10.1016/j.aquaculture.2013.09.045
Kalatzis GP, Castillo D, Katharios P, Middelboe M (2018) Bacteriophage interactions with marine pathogenic vibrios: implications for phage therapy. Antibiotics 7:E15. https://doi.org/10.3390/antibiotics7010015
Kashulin A, Seredkina N, Sørum H (2017) Cold-water vibriosis. The current status of knowledge. J Fish Dis 40:119–126. https://doi.org/10.1111/jfd.12465
Khan Mirzaei M et al (2016) Morphologically distinct Escherichia coli bacteriophages differ in their efficacy and ability to stimulate cytokine release in vitro. Front Microbiol 7:437
Kilcher S, Loessner MJ (2018) Engineering bacteriophages as versatile biologics. Trends Microbiol. https://doi.org/10.1016/j.tim.2018.09.006
Kilcher S, Studer P, Muessner C, Klumpp J, Loessner MJ (2018) Cross-genus rebooting of custom-made, synthetic bacteriophage genomes in L-form bacteria. Proc Natl Acad Sci 115:567–572. https://doi.org/10.1073/pnas.1714658115
Kim JH et al (2015) Biological control of Aeromonas salmonicida subsp. salmonicida infection in rainbow trout (Oncorhynchus mykiss) using Aeromonas phage PAS-1. Transbound Emerg Dis 62:81–86. https://doi.org/10.1111/tbed.12088
Klumpp J, Loessner M (2013) New research on bacteriophages and food safety. In: Sofos J (ed) Advances in microbial food safety. Woodhead Publishing Series in Food Science, Technology and Nutrition Elsevier Science, Cambridge, p 321
Kronheim S et al (2018) A chemical defence against phage infection. Nature 564:283–286. https://doi.org/10.1038/s41586-018-0767-x
Laanto E, Bamford JKH, Ravantti JJ, Sundberg LR (2015) The use of phage FCL-2 as an alternative to chemotherapy against columnaris disease in aquaculture. Front Microbiol 6:829. https://doi.org/10.3389/fmicb.2015.00829
Labrie SJ, Samson JE, Moineau S (2010) Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327. https://doi.org/10.1038/nrmicro2315
Lemay ML, Tremblay DM, Moineau S (2017) Genome engineering of virulent lactococcal phages using CRISPR-Cas9. ACS Synth Biol 6:1351–1358. https://doi.org/10.1021/acssynbio.6b00388
Lipinski T, Gamian A, Zuziak E, Korzeniowska-Kowal A, Gorski A (2016) Purified bacteriophage, its preparation and application. http://www.google.com/patents/US9255251
Lu TK, Koeris MS (2011) The next generation of bacteriophage therapy. Curr Opin Microbiol 14:524–531. https://doi.org/10.1016/j.mib.2011.07.028
Mahichi F, Synnott AJ, Yamamichi K, Osada T, Tanji Y (2009) Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol Lett 295:211–217. https://doi.org/10.1111/j.1574-6968.2009.01588.x
Marraffini LA, Sontheimer EJ (2008) CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322:1843–1845. 322/5909/1843 [pii]. https://doi.org/10.1126/science.1165771
Martel B, Moineau S (2014) CRISPR-Cas: an efficient tool for genome engineering of virulent bacteriophages. Nucleic Acids Res 42:9504–9513. https://doi.org/10.1093/nar/gku628
Mateus L et al (2014) Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture 424–425:167–173. https://doi.org/10.1016/j.aquaculture.2014.01.001
McKinley EB (1923) The bacteriophage in the treatment of infections. Arch Intern Med 32:899–910. https://doi.org/10.1001/archinte.1923.00110240092005
Monteiro R, Pires DP, Costa AR, Azeredo J (2018) Phage therapy: going temperate? Trends Microbiol. https://doi.org/10.1016/J.TIM.2018.10.008
Moreirinha C et al (2018) Protein expression modifications in phage-resistant mutants of Aeromonas salmonicida after AS-A phage treatment. Antibiotics 7:21. https://doi.org/10.3390/antibiotics7010021
Nakai T, Park SC (2002) Bacteriophage therapy of infectious diseases in aquaculture. Res Microbiol 153:13–18. https://doi.org/10.1016/S0923-2508(01)01280-3
Nale JY et al (2016) Bacteriophage combinations significantly reduce Clostridium difficile growth in vitro and proliferation in vivo. Antimicrob Agents Chemother 60:968–981. https://doi.org/10.1128/AAC.01774-15
Nicolaou KC, Rigol S (2018) A brief history of antibiotics and select advances in their synthesis. J Antibiot (Tokyo) 71:153–184. https://doi.org/10.1038/ja.2017.62
Nishiki I, Minami T, Murakami A, Hoai TD, Fujiwara A (2018) Multilocus sequence analysis of Vibrionaceae isolated from farmed amberjack and the development of a multiplex PCR assay for the detection of pathogenic species. J Fish Dis 41:1295–1301. https://doi.org/10.1111/jfd.12823
Park SC, Nakai T (2003) Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis Aquat Org 53:33–39. https://doi.org/10.3354/dao053033
Pereira C et al (2011a) Bacteriophages with potential for inactivation of fish pathogenic bacteria: survival, host specificity and effect on bacterial community structure. Mar Drugs 9:2236–2255. https://doi.org/10.3390/md9112236
Pereira C et al (2011b) Evaluating seasonal dynamics of bacterial communities in marine fish aquaculture: a preliminary study before applying phage therapy. J Environ Monit 13:1053–1058. https://doi.org/10.1039/c0em00434k
Plunkett G, Rose DJ, Durfee TJ, Blattner FR (1999) Sequence of Shiga toxin 2 phage 933W from Escherichia coli O157:H7: Shiga toxin as a phage late-gene product. J Bacteriol 181:1767–1778
Prasad Y, Arpana KD, Sharma AK (2011) Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. J Environ Biol 32:161–168
Proctor LM, Fuhrman JA (1990) Viral mortality of marine bacteria and cyanobacteria. Nature 343:60–62. https://doi.org/10.1038/343060a0
Romero A et al (2016) The animal model determines the results of Aeromonas virulence factors. Front Microbiol 7:1574. https://doi.org/10.3389/fmicb.2016.01574
Samson JE, Magadán AH, Sabri M, Moineau S (2013) Revenge of the phages: defeating bacterial defences. Nat Rev Microbiol 11:675–687. https://doi.org/10.1038/nrmicro3096
Sanger F et al (1977) Nucleotide sequence of bacteriophage phi X174 DNA. Nature 265:687–695
Sanger F, Coulson AR, Hong GF, Hill DF, Petersen GB (1982) Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol 162:729–773. https://doi.org/10.1016/0022-2836(82)90546-0
Sarhan WA, Azzazy HME (2015) Phage approved in food, why not as a therapeutic? Expert Rev Anti-Infect Ther 13:91–101. https://doi.org/10.1586/14787210.2015.990383
Schulz P, Robak S, Dastych J, Siwicki AK (2019) Influence of bacteriophages cocktail on European eel (Anguilla anguilla) immunity and survival after experimental challenge. Fish Shellfish Immunol 84:28–37. https://doi.org/10.1016/j.fsi.2018.09.056
Silva YJ et al (2016) Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with Phage AS-A. Aquaculture 450:225–233. https://doi.org/10.1016/j.aquaculture.2015.07.025
Sime-Ngando T (2014) Environmental bacteriophages: viruses of microbes in aquatic ecosystems. Front Microbiol 5:1–14. https://doi.org/10.3389/fmicb.2014.00355
Smith HO, Welcox KW (1970) A restriction enzyme from Hemophilus influenzae. I. Purification and general properties. J Mol Biol 51:379–391. https://doi.org/10.1016/0022-2836(70)90149-X
Smith HO, Hutchison CA, Pfannkoch C, Venter JC (2003) Generating a synthetic genome by whole genome assembly: X174 bacteriophage from synthetic oligonucleotides. Proc Natl Acad Sci 100:15440–15445. https://doi.org/10.1073/pnas.2237126100
Sulakvelidze A, Alavidze Z (2001) Bacteriophage therapy. Antimicrob Agents Chemother 45:649–659. https://doi.org/10.1128/AAC.45.3.649
Summers WC (2012) The strange history of phage therapy. Bacteriophage 2:130–133. https://doi.org/10.4161/bact.20757
Suttle CA (2007) Marine viruses—major players in the global ecosystem. Nat Rev Microbiol 5:801–812. https://doi.org/10.1038/nrmicro1750
Tan D, Svenningsen SL, Middelboe M (2015) Quorum sensing determines the choice of antiphage defense strategy in Vibrio anguillarum. MBio 6:e00627. https://doi.org/10.1128/mBio.00627-15
Taylor-Brown A et al (2017) Culture-independent genomics of a novel chlamydial pathogen of fish provides new insight into host-specific adaptations utilized by these intracellular bacteria. Environ Microbiol 19:1899–1913. https://doi.org/10.1111/1462-2920.13694
Trudel MV et al (2016) Diversity of antibiotic-resistance genes in Canadian isolates of Aeromonas salmonicida subsp. salmonicida: dominance of pSN254b and discovery of pAsa8. Sci Rep 6. https://doi.org/10.1038/srep35617
Tsonos J et al (2014) Hurdles in bacteriophage therapy: deconstructing the parameters. Vet Microbiol 171:460–469. https://doi.org/10.1016/j.vetmic.2013.11.001
Twort FW (1915) An investigation on the nature of ultra-microscopic viruses. Lancet 186:1241–1243
Verner-Jeffreys DW et al (2007) Furunculosis in Atlantic salmon (Salmo salar L.) is not readily controllable by bacteriophage therapy. Aquaculture 270:475–484. https://doi.org/10.1016/j.aquaculture.2007.05.023
Vincent AT et al (2014) Detection of variants of the pRAS3, pAB5S9, and pSN254 plasmids in Aeromonas salmonicida subsp. salmonicida: multidrug-resistance, interspecies exchanges, and plasmid reshaping. Antimicrob Agents Chemother 58:7367–7374. https://doi.org/10.1128/AAC.03730-14
Vincent AT, Emond-Rheault J-G et al (2016a) Antibiotic resistance due to an unusual ColE1-type replicon plasmid in Aeromonas salmonicida. Microbiology 162:942–953. https://doi.org/10.1099/mic.0.000286
Vincent AT, Trudel MV et al (2016b) Increasing genomic diversity and evidence of constrained lifestyle evolution due to insertion sequences in Aeromonas salmonicida. BMC Genomics 17:44. https://doi.org/10.1186/s12864-016-2381-3
Vincent AT, Paquet VE, Bernatchez A, Tremblay DM, Moineau S, Charette SJ (2017a) Characterization and diversity of phages infecting Aeromonas salmonicida subsp. salmonicida. Sci Rep 7(1). https://doi.org/10.1038/s41598-017-07401-7
Vincent AT, Rouleau FD, Moineau S, Charette SJ (2017b) Study of mesophilic Aeromonas salmonicida A527 strain sheds light on the species’ lifestyles and taxonomic dilemma. FEMS Microbiol Lett 364:fnx239. https://doi.org/10.1093/femsle/fnx239
Vincent AT et al (2019) Investigation of the virulence and genomics of Aeromonas salmonicida strains isolated from human patients. Infect Genet Evol 68:1–9. https://doi.org/10.1016/J.MEEGID.2018.11.019
Wang Y et al (2017) Bacteriophage therapy for the control of Vibrio harveyi in greenlip abalone (Haliotis laevigata). Aquaculture 473:251–258. https://doi.org/10.1016/j.aquaculture.2017.01.003
Weiss B, Richardson CC (1967) Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A 57:1021–1028. https://doi.org/10.1073/pnas.57.4.1021
Wright A, Hawkins CH, Änggård EE, Harper DR (2009) A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357. https://doi.org/10.1111/j.1749-4486.2009.01973.x
Wright PM, Seiple IB, Myers AG (2014) The evolving role of chemical synthesis in antibacterial drug discovery. Angew Chem Int Ed Engl 53:8840–8869. https://doi.org/10.1002/anie.201310843
Yoichi M, Abe M, Miyanaga K, Unno H, Tanji Y (2005) Alteration of tail fiber protein gp38 enables T2 phage to infect Escherichia coli O157:H7. J Biotechnol 115:101–107. https://doi.org/10.1016/j.jbiotec.2004.08.003
Zhu W et al (2015) Identification of three extra-chromosomal replicons in Leptospira pathogenic strain and development of new shuttle vectors. BMC Genomics 16:90. https://doi.org/10.1186/s12864-015-1321-y
Zschach H et al (2015) What can we learn from a metagenomic analysis of a Georgian bacteriophage cocktail? Viruses 7:6570–6589. https://doi.org/10.3390/v7122958
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vincent, A.T., Paquet, V.E., Moineau, S., Charette, S.J. (2019). Would Bacteriophages Be a New Old Complement to Antibiotics in Aquaculture?. In: Derome, N. (eds) Microbial Communities in Aquaculture Ecosystems. Springer, Cham. https://doi.org/10.1007/978-3-030-16190-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-16190-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16189-7
Online ISBN: 978-3-030-16190-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)