Abstract
Endoplasmic reticulum (ER) stress has been identified as a primary factor involved in brain ischemia-reperfusion injury progression. p21-activated kinase 2 (Pak2) is a novel ER function regulator. The aim of our study is to explore the influence of Pak2 on ER stress and determine whether melatonin attenuates ER stress-mediated cell death by modulating Pak2 expression in vitro using N2a cells. The results of our study demonstrated that hypoxia-reoxygenation (HR) injury repressed the levels of Pak2, an effect that was accompanied by activation of ER stress. In addition, decreased Pak2 was associated with oxidative stress, calcium overload, and caspase-12-mediated apoptosis activation in HR-treated N2a cells. Interestingly, melatonin treatment reversed the decreased Pak2 expression under HR stress. Knockdown of Pak2 abolished the protective effects of melatonin on ER stress, oxidative stress, and caspase-12-related N2a cells death. Additionally, we found that Pak2 was regulated by melatonin via the AMPK pathway; inhibition of AMPK prevented melatonin-mediated Pak2 upregulation, a result that was accompanied by an increase in N2a cell death. Altogether, these results identify the AMPK-Pak2 axis as a new signaling pathway responsible for ER stress and N2a cell viability under HR injury. Modulation of the AMPK-Pak2 cascade via supplementation of melatonin might be considered an effective approach to attenuate reperfusion-mediated N2a cell damage via repression of ER stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a cerebrovascular event characterized by severe cerebral ischemia and is the fifth leading cause of death and the primary contributor of adult disability in the USA. Although restoration of blood flow and reoxygenation during reperfusion therapy is crucial for rescuing stroke victims, paradoxically, this rescue initiates a cascade of events that may lead to additional cell injury, known as ischemia-reperfusion (IR) injury, which frequently exceeds the original ischemic insult (Lan et al. 2019; Wei et al. 2019). During IR injury, there is a cerebral cellular energy crisis caused by oxidative stress through collapse of mitochondrial oxidative phosphorylation and glycolysis (Abeysuriya et al. 2018; Boga et al. 2018). Insufficient cellular ATP content and mitochondrial dysfunction interrupt a wide range of indispensable ATP-dependent processes, including ion balance across neuronal membranes (Brazao et al. 2018; Hao et al. 2018). The mechanism underlying cerebral IR injury is complex, and thus, there is no effective drug to attenuate IR injury.
Melatonin, a hormone generated and released by the pineal gland, affects biological clock homeostasis. Several previous studies have reported the beneficial effects of melatonin on the human body (Angelova et al. 2018; Erland et al. 2018; Erland et al. 2018). For example, melatonin attenuates posttraumatic cardiac dysfunction in rats by inhibiting Drp1-related mitochondrial fission (Ding et al. 2018). In addition, melatonin retards the progression of fatty liver disease via repressing the NR4A1/DNA-PKcs/p53 pathway (Zhou et al. 2018). Melatonin prevents oxidative stress-induced endothelial cell pyroptosis via regulation of the long noncoding RNA MEG/miR-223/NLRP3 axis (Zhang et al. 2018). Moreover, melatonin enhances apoptosis in tongue squamous cell carcinoma in a manner dependent on MT2-TFE3-dependent autophagy (Fan et al. 2018). In the cerebrovascular system, careful studies from several laboratories have demonstrated the protective effects exerted by melatonin on reperfusion-induced brain injury. Melatonin improves OPA1-related mitochondrial fusion, inhibits mPTP opening-mediated cell death (Fan et al. 2018), reverses the thioredoxin antioxidative system (Galano and Reiter 2018), represses mitochondrial fission (Zhou et al. 2017, 2018), and improves autophagy activity (Zhu et al. 2018). This evidence indicates that melatonin may have protective effects in brain IR injury.
Endoplasmic reticulum (ER) stress has been acknowledged as a primary pathogenesis of brain IR injury. At the molecular level, ER orchestrates protein generation, and ER stress leads to the unfolded protein response (UPR). Inhibition of the UPR is beneficial for brain tissue because it allows for continued protein translation. Recent studies have indicated that early neural damage caused by cerebral IR injury may be related to ER stress, intracellular Ca2+ overload, free radical overproduction, and the inflammatory response. Subsequently, the middle stages of cerebral IR injury may primarily be associated with neuronal apoptosis via ER-induced apoptosis or necrosis. Although several studies have found that melatonin has the ability to affect ER stress in brain IR injury, the mechanism by which melatonin regulates ER stress remains unknown.
p21-activated kinase 2 (Pak2) is a novel stress-responsive kinase localized in ER membranes. A recent study identified Pak2 as a novel therapeutic target for ER stress response (Binder et al. 2019). In addition, in pancreatic acinar cells, Pak2 activation is required for Na+-K+ ATPase activation and pancreatic fluid secretion (Ramos-Alvarez et al. 2018). Pak2 also modulates cancer migration (Matsunuma et al. 2018) and endothelial cell death (Chelvanambi et al. 2018). However, no studies have explored the influence of Pak2 on brain IR injury. In addition, whether Pak2 is involved in melatonin-mediated ER protection remains unknown. Accordingly, the aim of our study is to explore the changes in Pak2 in brain IR injury and determine whether melatonin protects neuronal viability against IR injury by repressing ER stress in a manner dependent on Pak2 upregulation in vitro.
Methods
Cell culture and treatment
Cell studies were performed using N2a cells (ATCC ® Number: ATCC ® CCL-131™) and mouse CATH.a cells (ATCC® CRL-11179™). The hypoxia-reoxygenation model was established based on a previous study (Zhou et al. 2018). In brief, N2a cells were cultured in DMEM with 20% FBS (Zhou et al. 2017). Then, N2a cells were cultured in DMEM in a hypoxia chamber containing 5% CO2 and 95% N2. One hour later, the medium was replaced with fresh DMEM with 20% FBS, and cells were moved to a 5% CO2 incubator to induce reoxygenation injury for 2 h. Melatonin (1–20 μm) was added to the medium 2 h before HR injury. To prevent melatonin-mediated AMPK activation, Compound C (cc, 5 μM, Selleck Chemicals) was added 45 min before melatonin treatment (Hardeland 2018).
MTT and LDH release assays
MTT assay was conducted in 96-well plates. Then, after HR injury, cells were incubated with MTT solution (Sigma-Aldrich) at 37 °C for 4 h. Then, the medium was removed, and 100 μl DMSO was added to each well. The absorbance was measured at a wavelength of 570 nm (Park et al. 2018). The data are expressed as the ratio of the optical density (OD) value of the treated group to the OD of the control group. LDH is released into the medium when cellular membranes rupture. To evaluate the LDH level in the medium, an LDH Release Detection kit (Beyotime Institute of Biotechnology) was used according to the manufacturer’s protocol (Kazakov et al. 2018).
ELISA
GSH, GPx, and SOD are important antioxidants that scavenge free radicals and, therefore, suppress the extent of oxidative stress and reduce the oxidative stress injury of N2a exposed to HR injury (Jin et al. 2018). The levels of SOD, GPx, and GSH were measured using commercial kits (Beyotime Institute of Biotechnology, China) following the manufacturer’s instructions (Li et al. 2018). To analyze changes in caspase-3 and caspase-12, caspase-3/-12 activity kits (Caspase-3 Assay Kit, Abcam, #ab39401; Caspase-12 Assay Kit, Abcam, #ab65664) were used according to the manufacturer’s protocol. The wavelength at 400 nm was recorded via a microplate reader to reflect the caspase-3 and caspase-12 activities (Shi et al. 2018).
Detection of calcium concentration
Intracellular calcium was measured using the calcium-dependent fluorescent dye Fura-2. After treatment, cells were treated with Fura-2 (2 μM) for 30 min at 37 °C. Subsequently, PBS was used to wash the remaining probe and images were captured using an inverted microscope (BX51; Olympus Corporation, Tokyo, Japan). Pictures were analyzed with Image-Pro Plus 4.5 software (Media Cybernetics, Inc., Rockville, MD, USA). In brief, the fluorescence pictures (green fluorescence) were converted to the grayscale pictures with the help of Image-Pro Plus 4.5 software (Jeelani et al. 2018). Then, green fluorescence intensity was recorded as the grayscale intensity. Subsequently, relative grayscale intensity was expressed as a ratio to that of control group (Faughnan et al. 2019).
Immunostaining
The samples were first washed with cold phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 30 min at room temperature, and then permeabilized in 0.1% Triton X-100 for 10 min at 4 °C. Subsequently, 10% goat serum albumin (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to block the samples for 1 h at room temperature (Fukumoto et al. 2018). Subsequently, samples were incubated with primary antibodies overnight at 4 °C. After three rinses in PBS, secondary antibodies (Alexa Fluor 488 donkey anti-rabbit secondary antibodies (1:1000; cat. no. A-21206; Invitrogen; Thermo Fisher Scientific, Inc.) were added to the samples for 1 h at room temperature. The following primary antibodies were used in the present study: p-AMPK (1:1000, Abcam, #ab23875) and Pak2 (1:1000; Abcam; #ab76239). Images were observed with an inverted microscope (magnification × 40; BX51; Olympus Corporation, Tokyo, Japan). Image-Pro Plus 4.5 software (Media Cybernetics, Inc., Rockville, MD, USA) was used to quantify the immunofluorescence according to a previous study (Zhou et al. 2018). In brief, fluorescence pictures (red and green fluorescence) were converted to the grayscale pictures with the help of Image-Pro Plus 4.5 software. Then, red/green fluorescence intensities were separately recorded as the grayscale intensity. Subsequently, relative grayscale intensity was expressed as a ratio to that of control group. At least 50 cells in each group were observed in the immunofluorescence assay.
Transfection with small interfering RNA
In the present study, to inhibit Pak2 expression, small interference (si)RNA against Pak2 was used. The siRNA was designed and purchased from Yangzhou Ruibo Biotech Co., Ltd. (Yangzhou, China) (Abukar et al. 2018). To transfect the siRNA, Opti-Minimal Essential Medium (Invitrogen; Thermo Fisher Scientific, Inc.) was incubated with nasal epithelial cell (1 × 106) for at least 24 h. Subsequently, Lipofectamine® 2000 transfection reagent (Thermo Fisher Scientific, Inc.) was used according to the manufacturer’s protocol to perform siRNA transfection (70 nM/well of siRNA) (Serrato et al. 2018). Following transfection for 36–48 h, cells were lysed, and the proteins were isolated to measure the Pak2 expression via western blotting.
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining
A TUNEL assay was performed using a one-step TUNEL kit (Beyotime Institute of Biotechnology, Haimen, China) according to the manufacturer’s instructions. TUNEL staining was performed with fluorescein-dUTP (Invitrogen; Thermo Fisher Scientific, Inc.) to stain apoptotic cell nuclei, and DAPI (5 mg/ml) was used to stain all cell nuclei at room temperature for 3 min (Davidson et al. 2018). Cells in which the nucleus was stained with fluorescein-dUTP were defined as TUNEL positive. The slides were then imaged under an inverted microscope (BX51; Olympus Corporation, Tokyo, Japan) (Schindler et al. 2018).
RNA extraction and qPCR analysis
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to isolate total RNA from cells. Subsequently, the Reverse Transcription kit (Kaneka Eurogentec S.A., Seraing, Belgium) was applied to transcribe RNA (1 μg in each group) into cDNA at room temperature (~ 25 °C) for 30 min. The qPCR was performed with primers and matched probes from the Universal Fluorescence-labeled Probe Library (Roche Applied Science) using SYBR™ Green PCR Master Mix (Thermo Fisher Scientific, Inc.) (Quintela et al. 2018). The primers used in the present study were as follows: CHOP, forward primer 5′-CATGGACGAGCTGGCCTTC-3′, reverse primer 5′-ATCCTGTAGTGATGTATCAGG-3′; PERK, forward primer 5′-TGTCCAGTCCGTAACTGAC-3′, reverse primer 5′-TTCGATACCTGACTTAC-3′; GRP78, forward primer 5′-GCTACTTGTGAGGTCGATTC-3′, reverse primer 5′-GCCGTATACCGTGGTATGTCTG-3′; and GAPDH, forward primer 5′-GCTACAGCTTCACCACCACA-3′ and forward primer 5′-GCCATCTCT TGCTCGAAGTC-3′). The cycling conditions were as follows: 95 °C for 8 min, followed by 35 cycles of 95 °C for 10 s and 72 °C for 12 s, for telomere PCR. The mRNA ratio of the target genes to GAPDH was calculated using the 2−ΔΔCt method (Moore et al. 2018).
Flow cytometric analysis of ROS
To observe the ROS levels, flow cytometric analysis was used. In brief, cells (1 × 106) were washed with PBS, and MitoSOX red mitochondrial superoxide indicator (Molecular Probes, USA) (5 mg/ml; dihydroethidium; Molecular Probes; Thermo Fisher Scientific, Inc.) was incubated with the cells for ~ 30 min at 37 °C in the dark. Subsequently, the cells were washed with PBS to remove the ROS probe. Following resuspension in PBS, the cells were immediately analyzed using a flow cytometer (Sysmex Partec GmbH, Görlitz, Germany). The quantification of cellular ROS was performed per 10,000 cells in each group, and the data were analyzed with Flowmax software (Sysmex Partec, Version 2.3, Germany) (Mehra et al. 2018).
Western blotting and antibodies
Cells were lysed in Laemmli Sample Buffer (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were isolated and concentrations were determined using the Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol (Korbel et al. 2018). Total protein (40–60 μg) was separated by 12–15% SDS-PAGE. Following electrophoresis, the proteins were transferred to a polyvinylidene fluoride membrane (Roche Applied Science, Penzberg, Germany). Bands were detected using an enhanced chemiluminescence substrate (Applygen Technologies, Inc., Beijing, China). Membranes were blocked with 5% nonfat dried milk in Tris-buffered saline containing 0.05% Tween-20 (TBST) for 2 h at room temperature. Band intensities were normalized to the respective internal standard signal intensity (GAPDH; 1:2000; cat. no. ab9485; both Abcam). The experiment was repeated three times. The primary antibodies used in the study were as follows: CHOP (1:1000, Abcam, #ab11419), PERK (1:1000, Abcam, #ab65142), GRP78 (1:1000, Abcam, #ab21684), AMPK (1:1000, Abcam, #ab131512), p-AMPK (1:1000, Abcam, #ab23875), Pak2 (1:1000; Abcam; #ab76239), Caspase-3 (1:1000, Abcam, #ab32351), Caspase-12 (1:1000, Abcam, #ab2484), and PARP (1:1000, Abcam, #ab32064). The second antibodies used in the present study were as follows: horseradish peroxidase-conjugated secondary antibodies (1:2000; cat. nos. 7076 and 7074; Cell Signaling Technology, Inc.) for 1 h at room temperature. Band intensities were normalized to the respective internal standard signal intensity (GAPDH) using Quantity One Software (version 4.6.2; Bio-Rad Laboratories, Inc.) (Zhang et al. 2018).
Data analysis
The results of our study were obtained from three independent experiments, and the data are presented as the mean ± SEM. Statistical analyses were conducted using one-way ANOVA followed by Tukey’s test to compare variable groups using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Pak2 is downregulated in response to hypoxia-reoxygenation (HR) injury
In the present study, hypoxia-reoxygenation (HR) injury was used to mimic neuronal ischemia-reperfusion (IR) injury in vitro. Then, western blotting was conducted to analyze the expression of Pak2 in the presence of melatonin. As shown in Fig. 1a, b, compared with the control group, HR injury significantly reduced the expression of Pak2 in N2a cells, whereas melatonin dose-dependently reversed the levels of Pak2 in the setting of HR injury. Notably, under normal conditions, melatonin had no influence on Pak2 expression (Fig. 1c, d). Subsequently, an MTT assay was conducted to observe cell death. As shown in Fig. 1e, compared with the control group, HR injury reduced cell viability, and this effect was reversed by melatonin treatment in a dose-dependent manner (Fig. 1e). Therefore, the above information indicated that both N2a cells viability and Pak2 expression were suppressed by HR injury.
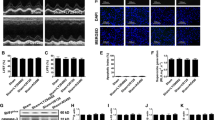
Melatonin improves Pak2 expression under hypoxia-reoxygenation (HR) injury. a, b Proteins were isolated from N2a cells, and then, western blotting was used to detect the expression Pak2 in response to HR injury. Melatonin was added to the medium of N2a cells to attenuate HR injury. c, d Under normal conditions, the role of melatonin on Pak2 was determined via western blotting. e An MTT assay was used to determine N2a cell viability under HR injury. Melatonin was added to the medium of N2a cells to attenuate HR injury. f, g N2a cells were transfected with siRNA against Pak2, and then, the knockdown efficiency was confirmed via western blotting. h, i CATH.a cell was transfected with siRNA against Pak2, and the expression of Pak was determined via western blotting. j An LDH release assay was used to detect the cell death in response to HR injury and Pak2 knockdown. k Caspase-3 activity was monitored via ELISA, and activation of caspase-3 was evaluated. N2a cells were transfected with Pak2 siRNA to repress melatonin-mediated Pak2 upregulation. l LDH release assay in CATH.a cells. m Caspase-3 activity was evaluated using ELISA in CATH.a cells. *P < 0.05 vs. control group, #P < 0.05 vs. HR group, @P < 0.05 vs. HR + melatonin group
To verify the causal role of Pak2 in HR-mediated N2a cells stress, melatonin-treated cells were transfected with siRNA against Pak2. In addition, the maximal protective action of melatonin is at 10 μM, and thus, this concentration was used in the following studies. As shown in Fig. 1f, g, compared with the control group, HR injury repressed Pak2 expression. Interestingly, melatonin treatment reversed the decrease in the Pak2 level, and this effect was negated by transfection with Pak2 siRNA in N2a cells. Similar results were also observed in CATH.a cell, a catecholaminergic cell line of neuronal origin (Fig. 1h, i). Moreover, an LDH release assay was used to analyze the cell death in response to Pak2 knockdown. As shown in Fig. 1j, HR-mediated LDH release was inhibited by melatonin treatment, whereas Pak2 knockdown reinduced LDH release in melatonin-treated N2a cells, indicating that melatonin-mediated neuronal protection is dependent on Pak2. Similar results were obtained by analyzing caspase-3 activity (Fig. 1k). Similar results were also noted in CATH.a cell (Fig. 1l, m). Therefore, the above information indicated that Pak2 was repressed by HR injury and melatonin-mediated Pak2 upregulation promoted N2a cell survival.
Melatonin attenuates ER stress via reversing Pak2 expression
Recent studies have identified Pak2 as a novel mediator of ER stress in myocardial injury. In the present study, we asked whether ER stress is also modulated by melatonin via Pak2 in N2a cells. First, western blotting was used to observe ER stress markers in vitro. As shown in Fig. 2a–d, compared with the control group, the levels of CHOP, PERK, and GRP78 were rapidly upregulated in response to HR injury, indicative of ER stress activation in HR-treated N2a cells. Interestingly, melatonin treatment attenuated the expression of CHOP, PERK, and GRP78, and this effect was negated after Pak2 was knocked down. This result was further supported via qPCR. As shown in Fig. 2e–g, compared with the control group, HR injury elevated the transcription of CHOP, PERK, and GRP78. Interestingly, melatonin treatment inhibited the transcription of CHOP, PERK, and GRP78, and this effect was negated by Pak2 knockdown. Altogether, the above information indicated that ER stress is activated by HR injury and was inhibited by melatonin via upregulation of Pak2 expression.
ER stress is modulated by Pak2 in N2a cells. a, d Proteins were isolated from N2a cells, and then, western blotting was used to detect the expression of ER stress markers in response to HR injury. N2a cells were transfected with siRNA against Pak2 in the presence of melatonin treatment. e, g RNA was isolated from N2a cells, and then, qPCR was employed to analyze the transcription of CHOP, PERK, and CHOP. N2a cells were transfected with siRNA against Pak2 in the presence of melatonin treatment. *P < 0.05 vs. control group, #P < 0.05 vs. HR group, @P < 0.05 vs. HR + melatonin group
Melatonin modulates calcium balance and oxidative stress via Pak2
Oxidative stress and calcium balance have been found to be regulated by ER stress according to previous studies (Nawaz et al. 2018; Schoenfeld et al. 2018). In the present study, we asked whether melatonin modulated the calcium balance and oxidative injury in HR-treated N2a cells via Pak2. First, ROS levels were detected via flow cytometry. As shown in Fig. 3a, b, compared with the control group, HR injury elevated ROS production in N2a cells, whereas melatonin treatment alleviated ROS overload. However, the antioxidative property of melatonin was dependent on Pak2 because knockdown of Pak2 abolished the inhibitory effect of melatonin on ROS overproduction. This finding was further supported via ELISA to analyze the cellular antioxidant content in the setting of cardiac reperfusion injury (Zhou et al. 2018). As shown in Fig. 3c–f, compared with the control group, HR injury reduced the levels of GSH, SOD, and GPX, an effect that was accompanied by an increase in the levels of MDA. Interestingly, melatonin treatment reversed the GSH/SOD/GPX content and reduced the levels of MDA in HR-treated N2a cells. Interestingly, the regulatory effects of melatonin on the cellular antioxidants system were counteracted in Pak2 knockdown N2a cells, indicating that the antioxidative action of melatonin was handled by Pak2.
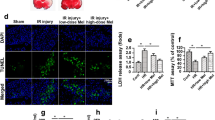
Melatonin modulates calcium balance and oxidative stress in HR-treated N2a cells via Pak2. a, b An ROS probe was used to analyze the production of ROS. Flow cytometry was used to analyze ROS overproduction in N2a cells. N2a cells were transfected with siRNA against Pak2 in the presence of melatonin treatment. c–f ELISA for cellular antioxidants. N2a cells were transfected with siRNA against Pak2 in the presence of melatonin treatment. g, h The calcium probe Fura-2 was used to stain the calcium in N2a cells, and then, calcium overload was determined via immunofluorescence. *P < 0.05 vs. control group, #P < 0.05 vs. HR group, @P < 0.05 vs. HR + melatonin group
In addition to N2a cell oxidative stress, calcium overload was observed using a calcium probe. As shown in Fig. 3g, h, compared with the control group, HR injury elevated the calcium content, and this effect was repressed by melatonin. Interestingly, silencing of Pak2 reactivated calcium overloading in melatonin-treated N2a cells, indicating that HR-mediated calcium imbalance, could be corrected by melatonin via Pak2 upregulation. Altogether, these results indicate that oxidative stress and calcium balance are handled by melatonin via Pak2.
ER-related caspase-12-mediated apoptosis is modulated by melatonin via Pak2
Excessive ER stress promotes activation of caspase-12 that enhances caspase-3 activation, which in turn initiates the apoptosis pathway in N2a cells. As shown in Fig. 4a, compared with the control group, caspase-12 activity was rapidly increased in N2a cells subjected to HR injury. Interestingly, melatonin treatment inhibited the activation of caspase-12, and this effect was nullified by Pak2 knockdown. This finding was further supported by western blotting. As shown in Fig. 4b–e, compared with the control group, HR injury elevated the expression of caspase-12, an effect that was accompanied by an increase in the expression of cleaved caspase-3. In addition, the expression of PARP, a substrate of caspase-3, was also augmented in response to HR injury, indicative of caspase-3 activation in HR-treated N2a cells. Interestingly, melatonin treatment repressed caspase-12 expression, followed by a drop in cleaved caspase-3 and PARP expression. However, silencing of Pak2 abolished the regulatory effects of melatonin on caspase-12, capsase-3, and PARP, indicating that the ER-related caspase-12-mediated apoptosis pathway was modulated by melatonin through a mechanism involving upregulation of Pak2 expression.
Caspase-12-related apoptosis is inhibited by melatonin through upregulation of Pak2. a An ELISA was used to detect the activity of caspase-12 in N2a cells. N2a cells were transfected with siRNA against Pak2 in the presence of melatonin treatment. b–e Proteins were isolated from N2a cells, and then, western blotting was used to detect the expression of caspase-12, caspase-3, and PARP. N2a cells were transfected with siRNA against Pak2 in the presence of melatonin treatment. f, g A TUNEL assay was used verify the cell death induced by Pak2 knockdown. The number of TUNEL-positive cells was recorded in different groups. N2a cells were transfected with siRNA against Pak2 in the presence of melatonin treatment. *P < 0.05 vs. control group, #P < 0.05 vs. HR group, @P < 0.05 vs. HR + melatonin group
This result was further supported by a TUNEL assay. As shown in Fig. 4f, g, compared with the control group, the number of TUNEL-positive cells rapidly increased in HR-treated N2a cells and was reduced to near-normal levels with melatonin treatment. Interestingly, silencing of Pak2 elevated the ratio of TUNEL-positive cells despite treatment with melatonin. Therefore, the above data highlight that melatonin-mediated Pak2 activation is critical for N2a cells survival under HR-induced ER apoptosis.
Melatonin upregulates Pak2 expression via the AMPK pathway
Next, experiments were performed to analyze the mechanism by which melatonin augments Pak2 expression in HR-treated N2a cells. Notably, the AMPK pathway has been found to be the upstream inhibitor of ER stress in several disease models (Korbel et al. 2018; Montoya-Zegarra et al. 2018). Accordingly, we asked whether melatonin inhibited ER stress and increased Pak2 expression via the AMPK pathway. First, western blotting was used to verify alterations in the AMPK pathway. As shown in Fig. 5a–c, compared with the control group, the AMPK pathway was inactivated by HR injury, as indicated by decreased p-AMPK expression in HR-treated N2a cells. Interestingly, melatonin treatment reversed the expression of p-AMPK, indicative of activation of the AMPK pathway in response to melatonin treatment. To confirm whether AMPK pathway activation contributed to the melatonin-mediated Pak2 upregulation, an AMPK inhibitor was added to melatonin-treated N2a cells. As shown in Fig. 5a–c, compared with the melatonin group, application of Compound C abolished the promotive effect of melatonin on the AMPK pathway. Interestingly, inhibition of the AMPK pathway also repressed melatonin-mediated Pak2 upregulation. This finding was further supported by immunofluorescence staining (Fig. 5d).
Melatonin regulates Pak2 expression via the AMPK pathway. a–c Proteins were isolated from N2a cells, and then, western blotting was used to detect the expression p-AMPK and Pak2 in response to HR injury. Compound C was added to the medium to prevent melatonin-mediated AMPK activation. d Immunofluorescence assay for p-AMPK and Pak2. e ELISA for caspase-12 activity. Melatonin-treated N2a cells were cultured with Compound C to inhibit AMPK activation. f–h ELISA for cellular antioxidants. Changes in cellular antioxidants were determined to analyze redox balance in N2a cells treated with melatonin and/or AMPK inhibition. *P < 0.05 vs. control group, #P < 0.05 vs. HR group, @P < 0.05 vs. HR + melatonin group
Last, experiments were performed to verify whether the AMPK pathway was also involved in HR-mediated N2a cells death. Caspase-12 activity was determined via ELISA. Compared with the control group, HR injury elevated caspase-12 activity (Fig. 5e), and this effect was reversed by melatonin treatment. Interestingly, treatment with Compound C reactivated caspase-12 in melatonin-treated N2a cells, indicating that caspase-12-related N2a cells death, was modulated by the AMPK pathway. In addition, oxidative stress parameters were detected in cells treated with Compound C. As shown in Fig. 5f–h, compared with the HR group, melatonin reversed the levels of GSH, GPX, and SOD in N2a cells, and this effect was negated by Compound C treatment. Therefore, this information indicates that the AMPK pathway is activated by melatonin and contributes to Pak2 upregulation and N2a cell survival under HR injury.
Discussion
The primary finding in the present study is that we identify Pak2 as a novel mediator of ER stress in N2a cell reperfusion injury in vitro. In response to hypoxia-reoxygenation (HR) injury, the expression of Pak2 was rapidly downregulated in N2a cells. Interestingly, melatonin treatment reversed the levels of Pak2 in N2a cells and increased Pak2 expression attenuated ER stress, inhibited oxidative injury, and corrected calcium overload induced by HR injury. In addition, Pak2 was required for melatonin-mediated N2a cells survival by preventing caspase-12 activation. Moreover, we demonstrated that melatonin treatment modulated Pak2 expression through the AMPK pathway; inhibition of the AMPK pathway inhibited melatonin-mediated Pak2 upregulation and promoted N2a cells death. To the best of our knowledge, this is the first investigation illustrating the neuronal protective effects and potential mechanisms of melatonin and Pak2 in the context of brain IR injury in vitro (Deussen 2018; Na et al. 2018).
The endoplasmic reticulum (ER), a multifunctional cellular organelle, affects several cellular physiological processes, including but not limited to protein biosynthesis (Zhou et al. 2019), lipid metabolism, calcium homeostasis (Zhou et al. 2018), oxidative modulation, and apoptosis activation (Ikeda et al. 2019; Medinas et al. 2019). After exposure to stress conditions, such as hypoxia, inflammation, and irradiation, ER stress is activated, evidenced by the accumulation of unfolded/misfolded proteins, cytoplasmic calcium overload, and ROS outburst (Kim et al. 2018; Yang et al. 2019). At the molecular level, there are three signaling branches of the ER stress pathway, and they are transcription factor-6 (ATF-6), inositol requiring enzyme 1 (IRE1), and PERK (Liao et al. 2018; Shah et al. 2019). In the present study, we found that PAMPK was significantly increased in response to HR injury and was inhibited by melatonin. This alteration was followed by augmentation of caspase-12 and CHOP expression. At the molecular level, increased CHOP promotes the cleavage of caspase-12, and the latter promotes the activation of caspase-3. Interestingly, melatonin treatment inhibited the HR-mediated caspase-12 upregulation and CHOP activation, consequently contributing to downregulation of caspase-3. This result demonstrates that ER stress and ER-related apoptosis are modulated by melatonin in HR-treated N2a cell damage.
Pak2, a member of the p21-activated kinase family of serine/threonine kinases, was initially identified as a binding partner of the Rho GTPases, Cdc42, and RacI. Pak2 plays a critical role in several cellular functions, such as migration, proliferation, and apoptosis. Several studies have reported the protective actions of Pak2 under various stress conditions. For example, in pancreatic acinar cells, ATP production is affected by Pak2 (Ramos-Alvarez et al. 2018). In claudin-low breast cancer, Pak2 affects cell migration and epithelial-to-mesenchymal transition (Matsunuma et al. 2018). In childhood acute lymphoblastic leukemia (Botker et al. 2018; Reddy et al. 2018) and HER2-positive breast cancer (Montoya-Zegarra et al. 2018), regulation of Pak2 has been demonstrated as a therapeutic strategy. In addition, Pak2 inhibits cancer cell proliferation (Fukumoto et al. 2018; Gianni-Barrera et al. 2018). Importantly, a recent study found that Pak2 plays a regulatory role in modulating ER stress in cardiomyocytes under tunicamycin stress and/or pressure overload (Binder et al. 2019; Gonzalez et al. 2018). This finding was validated in our current study. We found that Pak2 expression was rapidly downregulated in HR-treated N2a cells and this process could be reversed by melatonin. Notably, decreased Pak2 was associated with ER stress activation, caspase-12 upregulation and apoptosis initiation. In addition, Pak2 knockdown promoted oxidative stress and calcium imbalance. These results provide evidence to support the functional role of Pak2 in modulating ER stress and redox balance. Based on our results, Pak2 could be considered a new ER stress regulator in cerebrovascular system. More studies are required to verify the role of Pak2 in oxidative stress and calcium overloading in cerebrovascular system.
Further, we found that Pak2 expression was affected by the AMPK pathway. In the setting of HR injury, the AMPK pathway was inactivated, and this effect was reversed by melatonin, as indicated by the increase in p-AMPK. Interestingly, inhibition of the AMPK pathway abolished Pak2 upregulation, a result that was followed by an elevation in N2a cells death. This finding was consistent with the previous findings (Giatsidis et al. 2018). For example, in neuronal glucose uptake and insulin sensitivity, AMPK inhibitor treatment leads to a reduction in Pak2 activity, along with a marked decrease in glucose uptake in N2a cells (Gonzalez et al. 2018). A recent study further illustrated that Pak2 appears to be one of the downstream substrates of AMPK in human cancer cells and/or adult stem cells (Mehra et al. 2018). In addition, AMPK-modulated Pak2 expression also participates in the phagocytic ability of macrophages and neutrophils (Li et al. 2018). These results, combined with our data, reveal a novel function for AMPK in enhancing Pak2 activity and subsequently attenuating ER stress as well as neuronal death.
Overall, our study reports a novel regulator of ER stress and HR-mediated N2a cell death. Pak2 was inhibited by HR injury and contributed to ER stress activation, oxidative stress, and calcium overload. Melatonin treatment reversed Pak2 expression by activating the AMPK pathway, ultimately attenuating ER stress and caspase-12-mediated N2a cell death. However, N2a cell line is a murine neuroblastoma, and thus, a neuronal model using animal experiments is necessary in the future to support our findings.
References
Abeysuriya RG, Lockley SW, Robinson PA, Postnova S (2018) A unified model of melatonin, 6-sulfatoxymelatonin, and sleep dynamics. J Pineal Res 64:e12474. https://doi.org/10.1111/jpi.12474
Abukar Y, Ramchandra R, Hood SG, McKinley MJ, Booth LC, Yao ST, May CN (2018) Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res Cardiol 113:35. https://doi.org/10.1007/s00395-018-0695-9
Angelova PR, Barilani M, Lovejoy C, Dossena M, Viganò M, Seresini A, Piga D, Gandhi S, Pezzoli G, Abramov AY, Lazzari L (2018) Mitochondrial dysfunction in parkinsonian mesenchymal stem cells impairs differentiation. Redox Biol 14:474–484. https://doi.org/10.1016/j.redox.2017.10.016
Binder P, Wang S, Radu M, Zin M, Collins L, Khan S, Li Y, Sekeres K, Humphreys N, Swanton E, Reid A, Pu F, Oceandy D, Guan K, Hille SS, Frey N, Müller OJ, Cartwright EJ, Chernoff J, Wang X, Liu W (2019) Pak2 as a novel therapeutic target for Cardioprotective endoplasmic reticulum stress response. Circ Res 124:696–711. https://doi.org/10.1161/CIRCRESAHA.118.312829
Boga JA, Caballero B, Potes Y, Perez-Martinez Z, Reiter RJ, Vega-Naredo I, Coto-Montes A (2018) Therapeutic potential of melatonin related to its role as an autophagy regulator: a review. J Pineal Res 66:e12534. https://doi.org/10.1111/jpi.12534
Botker HE et al (2018) Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol 113:39. https://doi.org/10.1007/s00395-018-0696-8
Brazao V, Colato RP, Santello FH, Vale GTD, Gonzaga NA, Tirapelli CR, JCD P Jr (2018) Effects of melatonin on thymic and oxidative stress dysfunctions during Trypanosoma cruzi infection. J Pineal Res 65:e12510. https://doi.org/10.1111/jpi.12510
Chelvanambi S, Bogatcheva NV, Bednorz M, Agarwal S, Maier B, Alves NJ, Li W, Syed F, Saber MM, Dahl N, Lu H, Day RB, Smith P, Jolicoeur P, Yu Q, Dhillon NK, Weissmann N, Twigg III HL, Clauss M (2018) HIV-Nef protein persists in the lungs of Aviremic HIV patients and induces endothelial cell death. Am J Respir Cell Mol Biol 60:357–366. https://doi.org/10.1165/rcmb.2018-0089OC
Davidson SM, Arjun S, Basalay MV, Bell RM, Bromage DI, Bøtker HE, Carr RD, Cunningham J, Ghosh AK, Heusch G, Ibanez B, Kleinbongard P, Lecour S, Maddock H, Ovize M, Walker M, Wiart M, Yellon DM (2018) The 10th biennial hatter cardiovascular institute workshop: cellular protection-evaluating new directions in the setting of myocardial infarction, ischaemic stroke, and cardio-oncology. Basic Res Cardiol 113:43. https://doi.org/10.1007/s00395-018-0704-z
Deussen A (2018) Mechanisms underlying coronary autoregulation continue to await clarification. Basic Res Cardiol 113:34. https://doi.org/10.1007/s00395-018-0693-y
Ding M, Ning J, Feng N, Li Z, Liu Z, Wang Y, Wang Y, Li X, Huo C, Jia X, Xu R, Fu F, Wang X, Pei J (2018) Dynamin-related protein 1-mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction inrats and the protective effect of melatonin. J Pineal Res 64:e12447. https://doi.org/10.1111/jpi.12447
Erland LAE, Shukla MR, Singh AS, Murch SJ, Saxena PK (2018) Melatonin and serotonin: mediators in thesymphony of plant morphogenesis. J Pineal Res 64:e12452. https://doi.org/10.1111/jpi.12452
Erland LAE, Yasunaga A, Li ITS, Murch SJ, Saxena PK (2018) Direct visualization of location and uptake of applied melatonin and serotonin in living tissues and their redistribution in plants in response to thermal stress. J Pineal Res 66:e12527. https://doi.org/10.1111/jpi.12527
Fan T, Pi H, Li M, Ren Z, He Z, Zhu F, Tian L, Tu M, Xie J, Liu M, Li Y, Tan M, Li G, Qing W, Reiter RJ, Yu Z,Wu H, Zhou Z (2018) Inhibiting MT2-TFE3-dependent autophagy enhances melatonin-induced apoptosis intongue squamous cell carcinoma. J Pineal Res 64:e12457. https://doi.org/10.1111/jpi.12457
Faughnan ME, Gossage JR, Chakinala MM, Oh SP, Kasthuri R, Hughes CCW, McWilliams JP, Parambil JG, Vozoris N, Donaldson J, Paul G, Berry P, Sprecher DL (2019) Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia. Angiogenesis 22:145–155. https://doi.org/10.1007/s10456-018-9646-1
Fukumoto M, Kondo K, Uni K, Ishiguro T, Hayashi M, Ueda S, Mori I, Niimi K, Tashiro F, Miyazaki S, Miyazaki JI, Inagaki S, Furuyama T (2018) Tip-cell behavior is regulated by transcription factor FoxO1 under hypoxic conditions in developing mouse retinas. Angiogenesis 21:203–214. https://doi.org/10.1007/s10456-017-9588-z
Galano A, Reiter RJ (2018) Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J Pineal Res 65:e12514. https://doi.org/10.1111/jpi.12514
Gianni-Barrera R, Butschkau A, Uccelli A, Certelli A, Valente P, Bartolomeo M, Groppa E, Burger MG, Hlushchuk R, Heberer M, Schaefer DJ, Gürke L, Djonov V, Vollmar B, Banfi A (2018) PDGF-BB regulates splitting angiogenesis in skeletal muscle by limiting VEGF-induced endothelial proliferation. Angiogenesis 21:883–900. https://doi.org/10.1007/s10456-018-9634-5
Giatsidis G, Cheng L, Haddad A, Ji K, Succar J, Lancerotto L, Lujan-Hernandez J, Fiorina P, Matsumine H, Orgill DP (2018) Noninvasive induction of angiogenesis in tissues by external suction: sequential optimization for use in reconstructive surgery. Angiogenesis 21:61–78. https://doi.org/10.1007/s10456-017-9586-1
Gonzalez NR, Liou R, Kurth F, Jiang H, Saver J (2018) Antiangiogenesis and medical therapy failure in intracranial atherosclerosis. Angiogenesis 21:23–35. https://doi.org/10.1007/s10456-017-9578-1
Hao L, Sun Q, Zhong W, Zhang W, Sun X, Zhou Z (2018) Mitochondria-targeted ubiquinone (MitoQ) enhances acetaldehyde clearance by reversing alcohol-induced posttranslational modification of aldehyde dehydrogenase 2: a molecular mechanism of protection against alcoholic liver disease. Redox Biol 14:626–636. https://doi.org/10.1016/j.redox.2017.11.005
Hardeland R (2018) Melatonin and inflammation-story of a double-edged blade. J Pineal Res 65:e12525. https://doi.org/10.1111/jpi.12525
Ikeda T, Kobayashi S, Morimoto C (2019) Effects of repetitive transcranial magnetic stimulation on ER stress-related genes and glutamate, gamma-aminobutyric acid and glycine transporter genes in mouse brain. Biochem Biophys Rep 17:10–16. https://doi.org/10.1016/j.bbrep.2018.10.015
Jeelani R, Maitra D, Chatzicharalampous C, Najeemuddin S, Morris RT, Abu-Soud HM (2018) Melatonin prevents hypochlorous acid-mediated cyanocobalamin destruction and cyanogen chloride generation. J Pineal Res 64:e12463. https://doi.org/10.1111/jpi.12463
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587. https://doi.org/10.1016/j.redox.2017.11.004
Kazakov A, Hall RA, Werner C, Meier T, Trouvain A, Rodionycheva S, Nickel A, Lammert F, Maack C, Böhm M, Laufs U (2018) Raf kinase inhibitor protein mediates myocardial fibrosis under conditions of enhanced myocardial oxidative stress. Basic Res Cardiol 113:42. https://doi.org/10.1007/s00395-018-0700-3
Kim SH, Kwon DY, Kwak JH, Lee S, Lee YH, Yun J, Son T, Jung YS (2018) Tunicamycin-induced ER stress is accompanied with oxidative stress via abrogation of sulfur amino acids metabolism in the liver. Int J Mol Sci 19. https://doi.org/10.3390/ijms19124114
Korbel C, Gerstner MD, Menger MD, Laschke MW (2018) Notch signaling controls sprouting angiogenesis of endometriotic lesions. Angiogenesis 21:37–46. https://doi.org/10.1007/s10456-017-9580-7
Lan S, Liu J, Luo X, Bi C (2019) Effects of melatonin on acute brain reperfusion stress: role of hippo signaling pathway and MFN2-related mitochondrial protection. Cell Stress Chaperones 24:235–245. https://doi.org/10.1007/s12192-018-00960-2
Li J, Cai SX, He Q, Zhang H, Friedberg D, Wang F, Redington AN (2018) Intravenous miR-144 reduces left ventricular remodeling after myocardial infarction. Basic Res Cardiol 113:36. https://doi.org/10.1007/s00395-018-0694-x
Li R, Xin T, Li D, Wang C, Zhu H, Zhou H (2018) Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol 18:229–243. https://doi.org/10.1016/j.redox.2018.07.011
Liao W, Zheng Y, Fang W, Liao S, Xiong Y, Li Y, Xiao S, Zhang X, Liu J (2018) Dual specificity phosphatase 6protects neural stem cells from beta-amyloid-induced cytotoxicity through ERK1/2 inactivation. Biomolecules 8:e181. https://doi.org/10.3390/biom8040181
Matsunuma R, Chan DW, Kim BJ, Singh P, Han A, Saltzman AB, Cheng C, Lei JT, Wang J, Roberto da Silva L, Sahin E, Leng M, Fan C, Perou CM, Malovannaya A, Ellis MJ (2018) DPYSL3 modulates mitosis, migration, and epithelial-to-mesenchymal transition in claudin-low breast cancer. Proc Natl Acad Sci U S A 115:E11978–E11987. https://doi.org/10.1073/pnas.1810598115
Medinas DB, Cabral-Miranda F, Hetz C (2019) ER stress links aging to sporadic ALS. Aging (Albany NY) 11:5–6. https://doi.org/10.18632/aging.101705
Mehra P, Guo Y, Nong Y, Lorkiewicz P, Nasr M, Li Q, Muthusamy S, Bradley JA, Bhatnagar A, Wysoczynski M, Bolli R, Hill BG (2018) Cardiac mesenchymal cells from diabetic mice are ineffective for cell therapy-mediated myocardial repair. Basic Res Cardiol 113:46. https://doi.org/10.1007/s00395-018-0703-0
Montoya-Zegarra JA, Russo E, Runge P, Jadhav M, Willrodt AH, Stoma S, Nørrelykke SF, Detmar M, Halin C (2018) AutoTube: a novel software for the automated morphometric analysis of vascular networks in tissues. Angiogenesis. https://doi.org/10.1007/s10456-018-9652-3
Moore JBT et al (2018) Epigenetically modified cardiac mesenchymal stromal cells limit myocardial fibrosis and promote functional recovery in a model of chronic ischemic cardiomyopathy. Basic Res Cardiol 114:3. https://doi.org/10.1007/s00395-018-0710-1
Na HJ, Yeum CE, Kim HS, Lee J, Kim JY, Cho YS (2018) TSPYL5-mediated inhibition of p53 promotes human endothelial cell function. Angiogenesis. https://doi.org/10.1007/s10456-018-9656-z
Nawaz IM, Chiodelli P, Rezzola S, Paganini G, Corsini M, Lodola A, di Ianni A, Mor M, Presta M (2018) N-tert-butyloxycarbonyl-Phe-Leu-Phe-Leu-Phe (BOC2) inhibits the angiogenic activity of heparin-binding growth factors. Angiogenesis 21:47–59. https://doi.org/10.1007/s10456-017-9581-6
Park HJ, Park JY, Kim JW, Yang SG, Jung JM, Kim MJ, Kang MJ, Cho YH, Wee G, Yang HY, Song BS, Kim SU, Koo DB (2018) Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J Pineal Res 64:e12458. https://doi.org/10.1111/jpi.12458
Quintela T, Gonçalves I, Silva M, Duarte AC, Guedes P, Andrade K, Freitas F, Talhada D, Albuquerque T, Tavares S, Passarinha LA, Cipolla-Neto J, Santos CRA (2018) Choroid plexus is an additional source of melatonin in the brain. J Pineal Res 65:e12528. https://doi.org/10.1111/jpi.12528
Ramos-Alvarez I, Lee L, Jensen RT (2018) Cyclic AMP-dependent protein kinase a and EPAC mediate VIP and secretin stimulation of PAK4 and activation of Na+, K+-ATPase in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol 316:G263–G277. https://doi.org/10.1152/ajpgi.00275.2018
Reddy KRK, Dasari C, Duscharla D, Supriya B, Ram NS, Surekha MV, Kumar JM, Ummanni R (2018) Dimethylarginine dimethylaminohydrolase-1 (DDAH1) is frequently upregulated in prostate cancer, and its overexpression conveys tumor growth and angiogenesis by metabolizing asymmetric dimethylarginine (ADMA). Angiogenesis 21:79–94. https://doi.org/10.1007/s10456-017-9587-0
Schindler L, Dickerhof N, Hampton MB, Bernhagen J (2018) Post-translational regulation of macrophage migration inhibitory factor: basis for functional fine-tuning. Redox Biol 15:135–142. https://doi.org/10.1016/j.redox.2017.11.028
Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Bradley MD, Wagner BA, Buettner GR, Monga V, Milhem M, Spitz DR, Allen BG (2018) Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol 14:417–422. https://doi.org/10.1016/j.redox.2017.09.012
Serrato AJ, Romero-Puertas MC, Lazaro-Payo A, Sahrawy M (2018) Regulation by S-nitrosylation of the Calvin-Benson cycle fructose-1,6-bisphosphatase in Pisum sativum. Redox Biol 14:409–416. https://doi.org/10.1016/j.redox.2017.10.008
Shah SS, Rodriguez G, Musick A, Walters W, de Cordoba N, Barbarite E, Marlow M, Marples B, Prince J,Komotar R, Vanni S, Graham R (2019) Targeting glioblastoma stem cells with 2-deoxy-D-glucose (2-DG) potentiates radiation-induced unfolded protein response (UPR). Cancers (Basel) 11:e159. https://doi.org/10.3390/cancers11020159
Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H (2018) Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol 14:59–71. https://doi.org/10.1016/j.redox.2017.08.013
Wei N, Pu Y, Yang Z, Pan Y, Liu L (2019) Therapeutic effects of melatonin on cerebral ischemia reperfusion injury: role of yap-OPA1 signaling pathway and mitochondrial fusion. Biomed Pharmacother 110:203–212. https://doi.org/10.1016/j.biopha.2018.11.060
Yang J, Kim KS, Iyirhiaro GO, Marcogliese PC, Callaghan SM, Qu D, Kim WJ, Slack RS, Park DS (2019) DJ-1 modulates the unfolded protein response and cell death via upregulation of ATF4 following ER stress. Cell Death Dis 10:135. https://doi.org/10.1038/s41419-019-1354-2
Zhang H, Jin B, Faber JE (2018) Mouse models of Alzheimer's disease cause rarefaction of pial collaterals and increased severity of ischemic stroke. Angiogenesis. https://doi.org/10.1007/s10456-018-9655-0
Zhang Y, Liu X, Bai X, Lin Y, Li Z, Fu J, Li M, Zhao T, Yang H, Xu R, Li J, Ju J, Cai B, Xu C, Yang B (2018) Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J Pineal Res 64:e12449. https://doi.org/10.1111/jpi.12449
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017) Melatonin suppressesplatelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res 63:e12438. https://doi.org/10.1111/jpi.12438
Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y (2018) Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J Pineal Res 65:e12503. https://doi.org/10.1111/jpi.12503
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018) Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res 64. https://doi.org/10.1111/jpi.12471
Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y (2018) BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis 21:599–615. https://doi.org/10.1007/s10456-018-9611-z
Zhou H, Wang J, Hu S, Zhu H, Toanc S, Ren J (2019) BI1 alleviates cardiac microvascular ischemia-reperfusion injury via modifying mitochondrial fission and inhibiting XO/ROS/F-actin pathways. J Cell Physiol 234:5056–5069. https://doi.org/10.1002/jcp.27308
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J (2018) Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 15:335–346. https://doi.org/10.1016/j.redox.2017.12.019
Zhou H, Wang J, Zhu P, Zhu H, Toan S, Hu S, Ren J, Chen Y (2018) NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2alpha. Basic Res Cardiol 113:23. https://doi.org/10.1007/s00395-018-0682-1
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y (2017) Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 13:498–507. https://doi.org/10.1016/j.redox.2017.07.007
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ 25:1080–1093. https://doi.org/10.1038/s41418-018-0086-7
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Zhou H, Chen Y (2018) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23:101–113. https://doi.org/10.1007/s12192-017-0827-4
Acknowledgments
The authors are grateful to the Institute of Basic Medicine Science of the Shanghai Pudong Hospital, Shanghai Fu Dan University School of Medicine for assistance.
Funding
This study was supported by the main subject of the discipline construction of the Pudong New Area Health and Family Planning Commission (PWZzk2017-16) and Pudong New Area Health and Family Planning Commission Leading Talent Development Program (PWRL2017-03).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xing, J., Xu, H., Liu, C. et al. Melatonin ameliorates endoplasmic reticulum stress in N2a neuroblastoma cell hypoxia-reoxygenation injury by activating the AMPK-Pak2 pathway. Cell Stress and Chaperones 24, 621–633 (2019). https://doi.org/10.1007/s12192-019-00994-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-019-00994-0