Abstract
Tissue microarray analysis confirmed higher dimethylarginine dimethylaminohydrolase-1 (DDAH1) expression in prostate cancer (PCa) compared to benign and normal prostate tissues. DDAH1 regulates nitric oxide (NO) production by degrading endogenous nitric oxide synthase (NOS) inhibitor, asymmetric dimethylarginine (ADMA). This study examined whether DDAH1 has any physiological role in PCa progression. Using overexpression of DDAH1 in PCa (PC3 and LNCaP) cell lines, we found that DDAH1 promotes cell proliferation, migration and invasion by lowering ADMA levels, as well as increasing NO production. VEGF, HIF-1α and iNOS were upregulated in DDAH1 expressing cells as result of elevated NO. DDAH1 increased secretion of pro-angiogenic signals bFGF and IL-8, into conditioned media. Treatment of DDAH1-positive PCa cells with NOS inhibitors (L-NAME and 1400 W) attenuated DDAH1 activity to promote cell growth. Xenografts derived from these cells grew significantly faster (> twofold) than those derived from control cells. Proliferation rate of cells stably expressing mutant DDAH1 was same as control cells unlike wild-type DDAH1-positive PCa cells. Xenograft tumors derived from mutant-positive cells did not differ from control tumors. VEGF, HIF-1α and iNOS expression did not differ in DDAH1 mutant-positive tumors compared to control tumors, but was upregulated in wild-type DDAH1 overexpressing tumors. Furthermore, CD31 immunostaining on xenograft tissues demonstrated that DDAH1 tumors had high endothelial content than mutant DDAH1 tumors. These data suggest that DDAH1 is an important mediator of PCa progression and NO/DDAH pathway needs to be considered in developing therapeutic strategies targeted at PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In prostate cancer (PCa) progression, tumor cells require high nutrients and oxygen supply due to their uncontrolled growth. Cancerous cells encounter these limitations by altering the tumor vasculature. In solid tumors, neovasculature around the tumor regulates cancer cells growth and metastasis [1,2,3]. Tumor angiogenesis plays an important role in tumorigenicity and metastasis [4]. To alter the vasculature, cancer cells undergo changes which can rework the angiogenic regulatory pathways and contribute to the changes in expression of angiogenic factors for the development of vasculature around the tumor [5, 6]. Nitric oxide (NO), a key regulator of angiogenesis, promotes endothelial cell proliferation, migration, dissociation and degradation of extracellular matrix [7]. In cancers like gynecological, breast, neuronal, prostate, head and neck, NO production has been positively correlated with tumor grade and it has a pathological role in cancer by controlling tumor blood supply [8]. In addition to angiogenesis, NO plays important roles in cell cycle progression, metastasis and survival [9]. Physiological NO is biosynthesized by three isoforms of nitric oxide synthases (NOS) (neuronal NOS, endothelial NOS and inducible NOS) from l-arginine, oxygen and NADPH in a highly regulated manner [8]. In epithelial cancers like colon, prostate, breast, bladder and skin cancers, iNOS expression is positively correlated with the aggressiveness of cancer. Other NOS isoforms are also detected in different cancers [10]. However, excessive generation of NO (primarily driven by iNOS) could play a major role in many diseases like idiopathic pulmonary fibrosis (IPF), sepsis, migraine headaches and cancer [11]. Therefore, any alterations in NO production may play an important role in regulation of angiogenesis as a result in tumor progression. NOS activity is controlled by endogenous NOS inhibitors including asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and L-monomethylarginine (L-NMMA).
ADMA is released from the methylated arginines of proteins during proteolysis and autophagy. It is metabolized by the enzyme dimethylarginine dimethylaminohydrolase (DDAH). Overexpression of DDAH reduces tissue ADMA levels and enhances angiogenesis [12]. DDAH is a cysteine hydrolase enzyme that is expressed in all nucleated mammalian cells in two isoforms DDAH1 and DDAH2. DDAH1 is widely expressed in liver, kidney, pancreas and forebrain at the sites of nNOS expression. DDAH2 is predominantly expressed in vascular endothelium, where eNOS is expressed. About 80% of endogenous ADMA is metabolized mainly by the DDAH1 isoform [13]. Notably, DDAH1 overexpression has been detected in a series of human tumors such as melanoma, hepatocellular carcinoma, glioblastoma and prostate cancer [14,15,16,17]. It has been reported that DDAH1 is involved in cerebral tumor growth and the development of tumor vasculature [14]. Overexpression of DDAH1 in a glioma cell line leads to increase NO synthesis and increased production of vascular endothelial cell growth factor (VEGF) promoting angiogenesis. Tumors derived from these cells grow almost twice faster than controls, highlighting the importance of DDAH1 in glioblastoma. Expression of both isoforms has been detected in prostate tissue [18]. Importantly, using L-NAME, a direct NOS inhibitor not degraded by DDAH, Vanella et al. reported importance of targeting DDAH to better control NO biosynthesis and inhibit angiogenesis [6].
Previously, we have reported two protein-profiling studies on biopsies and prostatectomy tissues identifying differentially expressed proteins in PCa. Both studies have identified DDAH1 as being overexpressed in PCa compared to benign prostate epithelium (BPH) [19]. To the best of our knowledge, no reports have shown association between DDAH1 expression in PCa progression and its correlation with malignancy. As the precise molecular mechanisms DDAH1 follows in PCa progression is not investigated so far, the main objective of the present study is the functional characterization of DDAH1 alterations and its hydrolase activity on endogenous NOS inhibitors associated to PCa. The effect of DDAH1 expression and its hydrolase activity on different cellular events using both hormone-dependent and hormone-independent PCa cell lines in in vitro and in vivo xenografts were examined to determine the role of DDAH1 in PCa.
Materials
All chemicals and antibodies used in this study were purchased from Sigma-Aldrich (Missouri, USA) and Cell Signalling Technologies (CST, USA), respectively, unless otherwise specified.
Cell culture
Human prostate cancer cell lines (LNCaP and PC3), HAEC and BOSC23 cells were obtained from ATCC (Manassas, USA). All cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 0.1% streptomycin–penicillin, 1% nonessential amino acids and 1% sodium pyruvate except HAEC which was grown in EBM-2 (Lonza) containing EGM-2 (Lonza) media. To avoid mycoplasma contamination in cell culture, cells were regularly tested for mycoplasma using gene-specific primers in RT-PCR.
Immunohistochemistry
Human prostate cancer tumor microarrays (TMA) were purchased from Abcam, USA. TMAs were printed with 96 specimens from 96 patients (benign hyperplasia: 46, normal prostate: 2 and malignant: 48) with progressive Gleason score and TNM stages in duplicates. TMA slides were deparaffinized using xylene (2 × 10 min) and a series of decreasing ethanol concentrations according to the standard protocol. To block endogenous peroxidase activity, 3% peroxide quenching solution was added and incubated for 15 min. For antigen retrieval, slides were cooked in 10 mM citrate buffer (pH 6.0) using a microwave oven for 20 min, at 700 W. Slides were allowed to cool down to RT in citrate buffer and washed with deionized water followed by PBS buffer (pH 7.4, 3 × 10 min). TMAs were blocked with blocking buffer (10% NHS in PBST) for 2 h to avoid nonspecific binding of antibodies. After washing twice in PBS, the slides were incubated overnight with rabbit anti-DDAH1 monoclonal antibody (1:500) at 4 °C. After washing in PBST next day, the slides were incubated with biotinylated anti-rabbit secondary antibody for 2 h at RT followed by washing in PBS (2 × 5 min). For detection, the slides were incubated with ABC reagent (Vector laboratories, USA) for 2 h. After washing excess reagent with PBS, the DDAH1 expression was visualized by 0.1% DAB (Vector laboratories, USA) reagent containing 0.01% H2O2. DAB staining was terminated by washing with MQ water. Further TMAs were counterstained with hematoxylin stain. TMAs were dehydrated using ethanol, and tissues on slides were mounted using DPX mounting media (Himedia, India). Images were obtained using phase contrast microscope (Olympus Xi72, Japan).
Cloning for DDAH1 overexpression and site-directed mutagenesis
Wild-type and mutant DDAH1 protein expressing recombinant vectors were generated by cloning the coding region of the human DDAH1 (accession number NM_012137.3). The detailed procedures are provided as supplementary information.
Virus production and infection of target cells
BOSC23 packaging cells were transfected with either pMSCV or pMSCV-DDAH1 wild-type or mutant with pCL-Ampho packaging vector using lipofectamine 3000 (Invitrogen, USA). The transfection mixture was prepared by mixing 5 μg of plasmid DNA and 5 μl of lipofectamine 3000. The mixture was added drop wise into the cell culture medium. After 12 h post-transfection, medium was replaced with fresh growth medium. The media supernatant containing virus was collected at every 24 h. The cells were allowed to grow for next 72 h in fresh medium for another round of virus collection. The virus-containing media were filtered using 0.45-μm sterile filtered directly on the target cells (LNCaP and PC3 cells) at around 50% of confluence. Infection cycles were repeated twice for every 12 h. Infected target cells were grown in growth medium for 24 h, and recombinant cells were selected by adding puromycin (2 μg/ml). The cells were grown in selection media until all cells died in control dishes. The cells grown as colonies with resistance to puromycin were propagated further and verified for overexpression of wt and mutant DDAH1 in both LNCaP and PC3 cells.
Proliferation assay
To determine the effect of DDAH1 on PCa cells proliferation, definite count of LNCaP and PC3 cells stably expressing DDAH1 and control cells were grown under standard growth conditions (37 °C with 5% CO2 supply). At each designated time point as indicated, cells were harvested by trypsinization followed by centrifugation at 2000 rpm for 2 min. The pellet was resuspended in 0.5 ml of media, and 10 µl of cell suspension was mixed with trypan blue (1:1 V/V) before counting viable cells directly using an automated cell counter (Life Technologies, USA). The total number of viable cells was plotted against time in hours cultivated for cell growth.
Cell-based citrulline assay
The DDAH1 enzyme activity in PCa cells was determined by estimating citrulline produced from enzyme–substrate ADMA. The amount of citrulline generated from ADMA in DDAH1 enzyme reaction was determined according to Knipp et al. [20].
Measurement of NO in PCa cells
The 4, 5-diaminofluorescein (DAF-FM DA) reagent was used for the measurement of nitric oxide qualitatively. It is non-fluorescent, cell permeant and passively diffuses across cellular membranes. After dye uptake, it is deacetylated by intracellular esterases and becomes fluorescent upon reacting with NO. To evaluate the regulation of DDAH1 on NO synthesis, DAF-FM DA staining was performed according to the manufacturer protocol. Briefly, 35,000 cells per well were seeded in 12-well plate and grown for 24 h. The culture media was discarded, and cells were washed with Hank’s balanced salt solution (HBSS). The buffer was replaced with HBSS containing 2.5 μm DAF-FM DA reagent. The plates were incubated for 20 min in the dark and washed thoroughly twice with HBSS to remove excess stain. The plates were observed under fluorescence microscope (Olympus IX71), and images were captured to determine the amount of NO produced. As DAF-FMDA is pH sensitive, we have also determined NO levels using alternative fluorometric assay by measuring NO2 −/NO3 − from culture medium which is directly proportional to NO production from the cells. The assay is performed according to the manufacturer’s protocol (nitrate/nitrite fluorometric assay kit, Cayman chemical company, USA).
Reverse transcriptase polymerase chain reaction (RT-PCR) and western blotting
For RT-PCR, total cellular RNA was extracted by using Trizol method. cDNA synthesis followed by PCR using gene-specific primers was performed. For western blotting, the protein lysates were prepared by lysing LNCaP and PC3 cell pellets directly in M-PER buffer (Thermo, USA). The supernatants were cleared by centrifugation at 13,000 rpm for 10 min at 4 °C. For detailed protocols, supplementary information can be referred.
Estimation of IL-8, bFGF and ADMA using ELISA
The angiogenic factors and ADMA in culture medium collected from LNCaP and PC3 cells stably expressing DDAH1 or with DDAH1 downregulated by siRNA and respective control cells were estimated by using standard kits (basic FGF: R&D systems, IL8: BioLegend, and ADMA: Cloud-Clone Corp). The media supernatants from experimental cells were collected at designated time points and analyzed using the kits procured as mentioned above, according to manufacturer’s protocol. To estimate intracellular ADMA, cells were lysed in lysis buffer same as for citrulline assay. For normalization, total protein in samples was estimated by Bradford reagent.
Cell migration and invasion assay
To determine the effect of DDAH1 on invasion and migration, these assays were performed using Boyden transwell chambers (Corning). LNCaP and PC3 cells stably expressing DDAH1 or with DDAH1 downregulated by siRNA and respective control cells were collected in migration buffer (serum-free RPMI-1640, 2 mM CaCl2, 1 mM MgCl2, 0.2 mM MnCl2 and 0.5% BSA). For invasion assay, the cells were added onto the membrane (coated with 100 µL of Matrigel matrix, 200–300 µg/mL) of assay plates. The upper chamber inserts were placed in reservoir chamber filled with migration buffer. After 24 h, cells that pass through the membrane were fixed with 4% PFA and stained with 0.5% crystal violet. For migration assay, the same protocol was followed except that uncoated Boyden chambers were used. The migrated and invaded cells were observed under optical microscope. Each image has been captured with non-overlapping areas, and numbers of migrated and invaded cells were counted in each image.
Xenograft tumor models
To analyze the effects of DDAH1 overexpression on tumor cells growth in vivo, subcutaneous tumor xenograft model has been applied. All animal experiments were performed according to the guidelines and requirements of institutional animal ethical committee (IAEC; protocol number: IICT/03/2017). The experiments were performed in three groups each consisting six male nude mice aged between 4 and 6 weeks (Vivo biotech, India). The three groups were designated as pMSCV-Empty, pMSCV-DDAH1 and pMSCV-mutDDAH1. PC3 cells stably expressing either DDAH1 or mutant DDAH1 and vector control were pelleted and resuspended in sterile DPBS. The respective cell suspension (1 × 107 cells in 100 μl) was mixed with equal amount of Matrigel (1:1) per mouse and injected subcutaneously into the right flank of nude mice to initiate the study. Tumor diameters were measured twice weekly using digital vernier calipers, and volumes were calculated using the equation a (b 2)/2 [21], where a and b represent the length and width of the tumor, respectively. After tumors reached 800 mm3 in 4 weeks of time, mice were sacrificed by excessive dose of CO2 inhalation and observed for any gross pathological changes in the internal organs. The excised primary tumors were processed and fixed in formalin. Fixed and paraffin embedded xenograft tissues were cut at 5 μm thickness, stained with hematoxylin and eosin following standard procedure and examined under light microscope (IX71, Olympus, Japan).
Statistical analysis
The data are presented as mean ± SD for three individual experiments. One-way variance analysis and Student’s t test were used where appropriate; p ≤ 0.05 was considered as significant.
Results
DDAH1 is highly expressed in prostate cancer tissue
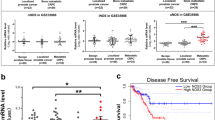
Our proteomics data reported previously identified DDAH1 overexpression in PCa. Hence, in the present study we determined the tissue-specific expression of DDAH1 using immunohistochemistry on PCa TMAs. TMAs stained with DDAH1 and counterstained with hematoxylin were examined by pathologist to evaluate DDAH1 expression. The expression of DDAH1 based on immunostaining was scored as weak (+), predominant (++) and strong (+++) staining. Immunohistochemistry for DDAH1 showed weak (+) cytoplasmic staining in non-neoplastic tissues BPH, unaffected secretory epithelia (BPE) and normal prostate compared to all tumor tissues on TMA slides investigated. Remarkably, in BPH samples only hyperplastic epithelium revealed weak (+) staining for DDAH1, whereas tumor cells showed predominant (++) to intensive (+++) staining. DDAH1 staining score for individual tissue cores printed on TMA is provided in Supplementary Table 2. The mean relative expression of DDAH1 in PCa tissues was significantly high compared to BPH tissue sections (p < 0.0001) (Fig. 1a). This result confirms that DDAH1 is overexpressed in malignant PCa, but not in BPH and normal prostate tissue.
Altered expression of DDAH1 in PCa. a Immunostaining with rabbit anti-DDAH1 [1:500] and universal immunoperoxidase detection system for DDAH1 (brown color) on tissue microarrays printed with hyperplasia, malignant and normal prostate tissues. Mean DDAH1 expression scores (weak to strong staining) in hyperplasia and cancer tissues indicate significant overexpression of DDAH1 in PCa. b Alteration of DDAH1 expression in LNCaP and PC3 cell lines. LNCaP and PC3 cells were transduced with either pMSCV or pMSCV-DDAH1 recombinant vector for overexpression of DDAH1. Stable overexpression of DDAH1 in PCa cell lines was confirmed (upper panel). PCa cells were transfected with either scrambled siRNA (siRNA-Scr) or DDAH1-specific siRNA (siRNA-DDAH1). Downregulation of DDAH1 in both PC3 and LNCaP cells was confirmed. Only representative images are shown here. c, d and e Overexpression of DDAH1 enhanced proliferation rate of PCa cells. Contrarily, with DDAH1 downregulation, the proliferation rate of both PCa cell lines was significantly inhibited. Data presented are the mean of ± SD of at least independent three experiments, ***p value ≤ 0.001
Altered expression of DDAH1 in PCa cell lines affects cell proliferation
To understand the role of DDAH1 on PCa progression, DDAH1-producing constructs were generated and transferred into PC3 and LNCaP cells by retroviral transduction. The DDAH1-transduced LNCaP and PC3 cells showed clear overexpression of DDAH1 mRNA and protein (Fig. 1b). Transfection of gene-specific siRNA for DDAH1 confirmed downregulation of DDAH1 compared to scrambled siRNA-transfected PCa cells (Fig. 1b). First, we studied whether altered expression of DDAH1 affects the proliferation rate of PCa cell lines. Cell proliferation assays showed significantly increased proliferation of both PC3 and LNCaP cells with exogenous DDAH1 expression (Fig. 1c, d). The proliferation rates of these cells are 28 and 50% higher in LNCaP and PC3 cells, respectively. Downregulation of DDAH1 by siRNA interference led to reduced proliferation in both LNCaP and PC3 cells (Fig. 1e, p ≤ 0.0005).
Dysregulation of DDAH1 activity effects ADMA and NO levels in PCa cells
The specific activity of DDAH1 was determined by measuring the amount of citrulline (μM/min) released. Based on the citrulline released, we observed that DDAH1 activity is increased due to overexpression, whereas downregulation led to decreased enzyme activity in both the cell types (Fig. 2a). The observed changes in DDAH1 activity are more prominent in PC3 cells compared to LNCaP cells. Further, in DDAH1-positive PCa cells, ADMA levels are significantly low compared to control cells (p ≤ 0.05). In PCa cells, in which DDAH1 is depleted by siRNA, higher intracellular ADMA is accumulated (p ≤ 0.05) (Fig. 2b). Since ADMA is an endogenous inhibitor for all three isoforms of NOS, we measured the production of NO qualitatively and quantitatively. With DDAH1 overexpression, we observed an increased intensity of DAF-FM DA, fluorescence indicating higher NO in LNCaP and PC3 cells, whereas downregulation led to low NO levels correlating with accumulated ADMA (Supplementary Figure 2A). As fluorescence methods are pH sensitive, NO levels were estimated quantitatively using NO3 −/NO2 − fluorometric assay. In line with fluorescence results, we observed fourfold higher NO production with DDAH1 expression (p ≤ 0.05) and lower NO levels due to DDAH1 downregulation (p ≤ 0.01) (Fig. 2c).
a Altered DDAH1 activity in PCa cells. Cell-based citrulline assay was performed to determine DDAH1 activity in cell lysates. In DDAH1-transduced PCa cell lines, DDAH1 activity was significantly increased compared to control cells. In PCa cells transfected with DDAH1-specific siRNA, DDAH1 activity was decreased compared to cells transfected with scrambled siRNA control. b In PCa cells with DDAH1 overexpression, ADMA levels are significantly decreased compared to control cells. In agreement, downregulation of DDAH1 by siRNA led to accumulation of intracellular ADMA. c Due to elevated DDAH1 activity and decreased ADMA levels, nitric oxide (NO) production by NOS enzymes was increased in PCa cells. Downregulation of DDAH1 in PCa cells inhibited NO production because higher ADMA levels are inhibiting NOS enzymes. Data presented are the mean of ± SD of at least independent three experiments and statistical significance *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
Altered DDAH1 activity controls expression of NO regulatory genes in PCa cells
The production of NO within the tumor microenvironment promotes tumor growth mainly by stimulating angiogenesis. Tumor angiogenesis is a multistage process regulated by the expression of different pro-angiogenesis factors such as VEGF, HIF-1α, integrins and basic fibroblast growth factor (bFGF). From the RT-PCR and western blot results, it is clear that the overexpression of DDAH1 in PC3 and LNCaP cells induced expression of NO-regulated genes VEGF, c-Myc, HIF-1α and iNOS. Downregulation of DDAH1 negatively regulated RNA and protein-level expression of the same target genes in LNCaP and PC3 cells (Supplementary Figure 1 and Fig. 3a). As we observed expression of the angiogenic factors VEGF and HIF-1α is associated with DDAH1 activity, angiogenic potential of the DDAH1 in PCa cells was investigated. Pro-angiogenic signals such as VEGF, platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF) and IL-8 promote angiogenesis. In search of these factors secreted by DDAH1 expression in PCa cells, we observed that the overexpression of DDAH1 increased release of bFGF and IL-8 in both PCa cells (Fig. 3c, d, p ≤ 0.01). In support of this observation, with downregulation of DDAH1 we found low levels of IL-8 and bFGF in CM compared to control PCa cells with DDAH1 expression (Fig. 3b, c, p ≤ 0.05).
DDAH1 regulates expression of NO downstream genes. a Dysregulation of DDAH1 in PCa cells regulates the NO production and NO regulatory genes. With DDAH1 overexpression or downregulation, VEGF, HIF-1α, iNOS and c-MYC protein levels in both PC3 and LNCaP cells were measured by western blotting. The DDAH1 expression is directly correlated with expression of measured target proteins. b and c The angiogenic factors like bFGF and IL-8 are elevated in conditioned media collected from DDAH1-positive PC3 and LNCaP cells, whereas downregulation attenuated secretion of these factors into conditioned media. Data presented are the mean of ± SD of at least independent three experiments and statistical significance *p ≤ 0.05, **p ≤ 0.01
DDAH1 promotes migration and invasion of LNCaP and PC3 cells
Once pro-angiogenic factors such as bFGF, VEGF, PDGF, and EGF activate their specific receptors, tumor cells release proteases to degrade the basement membrane for cell migration [22]. Therefore, to understand the role of DDAH1 in PCa metastasis, we performed migration and Matrigel invasion assays using Boyden chambers. Haptotactic cell migration assays demonstrated that overexpression of DDAH1 in LNCaP and PC3 cells promotes cell migration. Simultaneously, the observed effect on cell migration was abolished upon downregulation of DDAH1 by siRNA (Fig. 4a). Additionally, results from Matrigel invasion assays validate that DDAH1 promotes invasion of LNCaP and PC3 cells, whereas its downregulation significantly decreases their invasive potential (Fig. 4a). Number of migrated and invaded cells in respective assays confirms that DDAH1 has significantly affected PCa cells migration and invasion (p ≤ 0.005; Fig. 4b, c). NO induces the tyrosine phosphorylation of FAK which is normally activated by integrins and growth factors [23]. FAK intimately regulates the cell motility process mainly through adhesion, spreading, migration and survival. The role of active FAK in cell motility is mediated by recruiting active SRC at Y397 position of FAK and phosphorylation of its downstream substrates [24]. To investigate whether the activation of key target proteins is involved in DDAH1-induced migration and invasion, we measured the activation of FAK and SRC kinases. We observed significantly increased phosphorylation of FAK and SRC in DDAH1 overexpressing LNCaP and PC3 cells. Concurrently, upon downregulation of DDAH1, we observed a significant reduction in the phosphorylation of FAK and SRC leading to their inactivation in PCa cells (Fig. 4d).
DDAH1 promotes PCa cells migration and invasion in in vitro. a. In PC3 and LNCaP cells, DDAH1 expression was either overexpressed or downregulated and the migration and invasion potential of these cells were measured. b and c The % of PCa cells migrated or invaded was increased due to high DDAH1 expression, whereas its knock down by siRNA minimizes migration and invasion potential of both the cells. Data presented are the mean of ± SD of at least independent three experiments and statistical significance *p ≤ 0.05, *p ≤ 0.01, ***p ≤ 0.001. d. With DDAH1 overexpression or downregulation, activation of FAK and SRC by phosphorylation (pFAK (Y397) and pSRC(Y416)) involved in migration and invasion of PC3 and LNCaP cells was determined by western blotting. Only representative blots were presented here
DDAH1 regulates PCa cells growth through NO
From the above results, DDAH1 is considered to be controlling PCa cells proliferation, migration and invasion. Hence, it is necessary to disseminate the regulatory mechanisms of DDAH1 involved in PCa cells growth. Since, DDAH1 indirectly regulates NO production in tumor cells, inhibition of NOS using L-NAME and 1400 W attenuated DDAH1 effect on PCa cells (Fig. 5a, b). Besides, these results are confirmed by reduced NO levels in DDAH1-transduced PCa cells due to the complete inhibition of NOS enzymes (Fig. 5c and Supplementary Figure 2B). In DDAH1-positive cells, inhibition of NOS by L-NAME and 1400 W reversed higher expression of VEGF, HIF-1α and iNOS to basal levels (Fig. 5d). This may be due to the fact that DDAH1 function in PCa cells is relying on NO produced by iNOS. These results together indicate that DDAH1 activity may be associated with regulation of proliferation, migration and invasion through NO production which directs overexpression of its target genes and thus influences PCa cells growth and survival.
NOS inhibitors attenuate proliferation of PCa cells with DDAH1 overexpression. PC3 (a) and LNCaP (b) cells with DDAH1 overexpression were treated with L-NAME and 1400 W, and cell proliferation was determined. Inhibition of NOS enzymes diminished DDAH1-positive effect on PC3 and LNCaP cells proliferation. c Inhibition of NOS enzymes in PC3 and LNCaP cells with DDAH1 overexpression reduced higher level of NO production. Data presented are the mean of ± SD of at least independent three experiments and statistical significance *p ≤ 0.05, **p ≤ 0.01. d NOS inhibition reverted overexpression of NO regulatory genes VEGF, HIF-1α and iNOS in PC3 and LNCaP cells transduced for forced DDAH1 expression. Only representative western blots from PC3 and LNCaP cell lysates are shown here
DDAH1-mediated increase in PCa cells growth is reliant solely upon its hydrolase activity
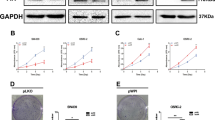
Since DDAH1 is an enzyme, the PCa cells growth could be promoted either by the hydrolase activity of the enzyme or by the protein itself. To better understand this, we generated PC3 and LNCaP cell lines stably expressing an active site (Cys–His–Glu) mutant DDAH1. The replacement of active site cysteine with alanine (C274A) inactivated the enzyme in metabolizing ADMA in PCa cells. Stable overexpression of mutant DDAH1 (pMSCV-mutDDAH1) was confirmed by western blot analysis (Fig. 6f). The mutant DDAH1 overexpressing LNCaP and PC3 cells showed significantly lower DDAH1 enzymatic activity than cells with wild-type DDAH1 overexpression (p ≤ 0.05; Fig. 6a). Moreover, the growth rate of the DDAH1 mutant and control cells did not change significantly, whereas active DDAH1-positive cells displayed increased rate of proliferation (Fig. 6b, c, p ≤ 0.05). Further, higher ADMA levels and decreased NOS activity in cells with mutant DDAH1 expression compared to wild-type DDAH1-positive cells manifest that the hydrolase activity mediates PCa cells growth (Fig. 6d, e, p ≤ 0.02). VEGF expression in mutant DDAH1-positive LNCaP and PC3 cells did not differ compared to control cells unlike its overexpression in cells with wild-type DDAH1 (Fig. 6f). These results together suggest that the enzyme hydrolase activity of DDAH1 interferes with ADMA and NO levels in tumor cells and thus influences cell growth.
DDAH1 hydrolase enzyme activity is associated with PCa cells growth. a PC3 and LNCaP cells were engineered to stably express active site mutant DDAH1 (C274A). In mutant DDAH1-positive cells, enzyme activity was significantly less compared to wild-type DDAH1 expressing cells and comparable to control cells. b and c Overexpression of mutant DDAH1 did not show any effect on proliferation of both PC3 and LNCaP cells unlike wild-type DDAH1. d In PCa cells with mutant DDAH1 overexpression, ADMA levels were not significantly decreased compared to control cells not alike in wild-type DDAH1-positive cells. e In agreement with ADMA levels, like control cells no significant change in NO production was observed with mutant DDAH1 overexpression, whereas NO levels are elevated in PCa cells with wild-type DDAH1 compared to control cells. f Because of inactive enzyme activity in mutant DDAH1-positive PCa cells, VEGF expression was significantly less compared to cells with wild-type DDAH1. Representative blots are presented here. Data presented are the mean of ± SD of at least independent three experiments and statistical significance *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001
DDAH1 promotes PCa growth in vivo
To determine DDAH1 role in PCa growth, the present study further examined the effects of DDAH1 on PCa growth by establishing PC3 xenograft nude mouse models. Mice subcutaneously injected with PC3 cells which were transduced for DDAH1 overexpression exhibited significantly larger tumors as compared with control group. The control group mice were injected with PC3 cells which were transduced for control empty vector. Conversely, mice injected with PC3 cells overexpressing mutant DDAH1 exhibited significantly smaller tumors as compared with mice injected with PC3 cells which were transduced for wild-type DDAH1 (Fig. 7a). Once tumor growth was initiated, the sizes of all tumors in xenograft mice were recorded up to 24 days post-injection. The tumor volumes and weights clearly suggest that the tumors with wild-type DDAH1 grow at significantly faster rates compared to control cells and cells with mutant DDAH1 expression (pMSCV-Empty: 428 ± 77 mm3; 280.12 ± 28.13 mg, pMSCV-DDAH1: 707 ± 52 mm3; 548 ± 56.44 mg, pMSCV-mutDDAH1: 533 ± 72 mm3; 310 ± 25.19 mg) (Fig. 7b, c). Histology of xenograft sections from H & E staining observed that all tumors from three groups grew in a nodular shape. Cells from pMSCV-Empty and pMSCV-mutDDAH1(C274A) tumors are restricted to subcutaneous region, but cells from pMSCV-DDAH1 tumors not only restricted to subcutaneous region, but also invaded into muscular region. Few mitotic figures appeared in cells from pMSCV-Empty and pMSCV-mutDDAH1(C274A) tumors, but many mitotic figures appeared from cells of pMSCV-DDAH1 tumor. Necrosis at peripheral and central areas in xenograft tumors around proliferation sites was observed in pMSCV-DDAH1 compared to pMSCV-Empty and pMSCV-mutDDAH1 xenografts (Fig. 7d).
DDAH1 expression positively correlated with tumor growth in in vivo. a The representative images from mice bearing tumors from PC3 cells transduced for stable overexpression of either DDAH1 or mutant DDAH1 and control cells with empty vector. b and c In vivo growth rate and weights of tumor xenografts in nude mice. Data presented are the mean of ± SD of six mice from each group and statistical significance **p ≤ 0.01, NS = No significance. d Histology of xenograft tumor sections was analyzed by H & E staining. To evaluate expression of DDAH1 and NO-regulated genes involved in angiogenesis, immunohistochemistry was performed. Arrows represent the expression of representative gene. e Representative images of xenograft tumor sections were stained for the endothelial marker CD31 (red color) detected using an Alexa-555-conjugated secondary antibody that fluoresces red and DAPI for nuclear stain
Immunohistochemical analysis was used to stain DDAH1 to confirm its overexpression in transduced cells injected and VEGF, HIF-1α and iNOS to correlate in vitro result. Immunostaining for DDAH1 confirms the overexpression of wild-type and mutant DDAH1 in tumor xenograft. There were more cells stained positive for VEGF, HIF-1α and iNOS with DDAH1 overexpression, as compared to control tumors. In tumors overexpressing mutant DDAH1, tumor cells showed a weak staining for VEGF, HIF-1α and almost no staining for iNOS like in control tumors (Fig. 7d). These results are very well correlating with overexpression of these targets in vitro analysis. Further, we stained CD31 endothelial marker in tumor xenograft tissues to detect tumor microvessels. There were many CD31 stained vessels in tumors with DDAH1 overexpression, as compared with the control tumors. It was observed that in tumors overexpressing mutant DDAH1, very few CD31 stained vessels like control tumors as compared with the DDAH1 overexpressing tumors (Fig. 7e and Supplementary Figure 4).
Discussion
Proteomic analysis of PCa tissue samples identified significant overexpression of DDAH1 in cancer compared to the benign and normal prostate tissues [19]. Moreover, DDAH1 overexpression in different cancer types has been reported [17]. Our study using TMAs immunostaining found that DDAH1 overexpressed in PCa, but not in BPH and normal prostate. Immunostaining for DDAH1 was restricted to cytoplasm in tumor cells. The degree of DDAH1 expression was very well correlated with aggressiveness of PCa suggesting its role in disease progression. To the best of our knowledge, this is the first report establishing association between DDAH1 expression and PCa. DDAH1 metabolizes ADMA, which is an endogenous inhibitor of NOS implicated in pathophysiology of different diseases including cancer [11]. However, understanding the physiological role of DDAH1 in PCa may provide basis for its role in disease pathophysiology and highlights its potential to inhibit its enzyme activity in cancer cells. In this study, LNCaP and PC3 cells were engineered to stably express either active DDAH1 or an active site mutant of DDAH1 and depleted for DDAH1 by siRNA in order to establish whether DDAH1 activity mediates tumor growth and angiogenesis.
In vitro characterization of LNCaP and PC3 cell clones overexpressing DDAH1 demonstrated that DDAH1 promotes cell growth. Altered expression of DDAH1 in PCa cells evidently regulates the NO synthesis through ADMA metabolism. Higher activity of DDAH1 led to reduced ADMA and elevated NO levels in PCa cells or vice versa with lower DDAH1 activity by siRNA. Previously, it has been reported that DDAH1 overexpression in rat C6 glioma [14] and endothelial cells [25, 26] result in an increase in VEGF expression in in vitro and in vivo. In various cancers, NO directly regulates expression of VEGF, HIF-1α, c-Myc and iNOS [27, 28]. Likewise, our studies also found that there was a significant increase in VEGF, HIF-1α, c-Myc and iNOS expression, downstream of an increase in NO production. HIF-1α and c-Myc are involved in the broad range of cellular activities like transactivation of glycolytic enzymes and mitochondrial biogenesis genes, respectively, which lead to boosting cellular energetic metabolism for cell proliferation [29]. Therefore, it is intriguing to speculate that the observed increase in NO level is due to the active DDAH1 in its overexpressing PCa cell lines. In tumor cells, NO induces HIF-1α expression through MAPK and PI3K under normoxic conditions and also stabilizes HIF-1α by inhibiting prolyl hydroxylases [30]. Furthermore, activated HIF-1α acts as a transcription factor and induces the expression of iNOS and VEGF [31]. Our results also show that VEGF and iNOS are upregulated in DDAH1-positive cells possibly explaining the observed repression and/or stabilization of HIF-1α. In tumor cells, elevated NO levels contribute to tumor angiogenesis by upregulating VEGF and VEGF-induced neovascularization. VEGF is identified as potent tumor angiogenic factor in many cancers [11, 32]. As NO-associated gene expression is linked to angiogenesis, from these results, we note that DDAH1 expression is involved in tumor angiogenesis. Most importantly, tumor cells regulate angiogenesis in hypoxia through different mechanisms, by altering angiogenic factors to induce neovascularisation. The overexpression of pro-angiogenic factors like VEGF induces several genes such as bFGF, IL8, TNF involved in the promotion of tumor angiogenesis [32, 33]. In our findings, we observed elevated levels of secreted bFGF and IL8 in culture media from PCa cells with DDAH1 overexpression. Accordingly, its downregulation attenuated their expression and release by cancer cells. Therefore, we assume that DDAH1 controls NO production in tumor cells indirectly by metabolizing ADMA, which in turn induces pro-angiogenic signals which promote angiogenesis. The proliferation of DDAH1 expressing cells treated with NOS inhibitors (L-NAME and 1400 W) was significantly slower than the untreated and control cells without forced DDAH1 expression. It should be noted that the NOS enzymes are more affectively inhibited in control cells due to normal levels of ADMA, thereby inhibiting cell proliferation. We also found that increase in NO production and its downstream effect on overexpression of VEGF, HIF-1α and iNOS in DDAH1-positive PCa cells are restored upon inhibition of NOS enzymes. These results suggest that the DDAH1 hydrolase activity in metabolizing ADMA consequently increasing NO production conveys higher proliferation and angiogenesis of PCa cells.
In the process of angiogenesis, cancer cells migrate and invade into the neighboring tissues and organs through surrounding blood vessels. From cell migration and invasion assays, we found that overexpression of DDAH1 in LNCaP and PC3 cells promotes cell migration and invasion in vitro. Consistently, downregulation of DDAH1 led to decreased migration and invasion potential of PCa cells. Overexpression of DDAH1 in trophoblast cells results in decreased ADMA and increased NO production. The motility and invasion of human trophoblast cells are increased by DDAH1 overexpression in response to hepatocyte growth factor (HGF) stimulation [34]. The expression, activation of several genes and integrins, is very important in cancer cell metastasis. NO stimulates phosphorylation of FAK, i.e., commonly activated by integrins and growth factors [23]. Usually, integrins are involved in tumor cells migration and invasion [35]. The integrins upon binding to the specific ligands (extracellular matrix proteins) activate signaling pathways involved in tumor cell metastasis [36]. In PCa, ligation of integrins with respective ligands activates FAK which interacts and activates PI3 kinase for metastasis. Alternatively, elevated NO induces activation of FAK by autophosphorylation on Y397 providing substrate for activated SRC (pSRC-Tyr 416) for additional phosphorylation leading to its full activation [37]. Activated FAK and SRC kinases phosphorylate many FAK-associated SRC substrates including Crk-associated substrate (CAS), paxillin and p190RhoGAP playing a central role in the reorganization of the actin in cytoskeleton and migration [38]. The results obtained in the present study show the involvement of activated FAK and SRC kinase in DDAH1-mediated cell invasion and migration through ADMA metabolism may play a role in PCa metastasis.
Further, LNCaP and PC3 cells were engineered to stably express an active site mutant of DDAH1 in order to establish whether the above results highlighting DDAH1 mediated increase in tumor cells growth and angiogenesis is reliant solely upon its hydrolase activity. In vitro characterization of the PCa cells expressing mutant DDAH1 demonstrated that significantly less enzyme activity with increased ADMA levels than wild-type DDAH1 overexpressing cells. This confirmed that replacement of the active site cysteine with alanine led to inactivation of DDAH1. It should be noted that the observed basal enzyme activity in mutant DDAH1-positive cells is due to endogenous DDAH activity [13]. The proliferation rate of mutant DDAH1-positive cells is similar to control cells and significantly lower than the cells with wild-type DDAH1 overexpression suggesting DDAH1 hydrolase activity conveys higher proliferation of PCa cells. Here, we show that the increase in NO production and overexpression of VEGF expression, NO downstream is attenuated in the mutant DDAH1 overexpressing LNCaP and PC3 cells. Therefore, the implications are that DDAH1 hydrolase activity in regulation of VEGF expression is NO dependent and thereby involved in tumor cell proliferation and angiogenesis.
Investigation of DDAH1 role in PCa progression using PCa cell lines in in vitro expressing a defined phenotypic change gives an opportunity to examine its role in tumor development in in vivo. In in vivo experiments, the growth rate of DDAH1 expressing xenografts in nude mice is significantly higher than the control tumors, suggesting that expression of the DDAH1 conveys growth advantage to these tumors over those derived from the empty vector transfected cells. Interestingly, the xenografts with mutant DDAH1 expressing cells grew slower with intermediate tumor size between control and wild-type overexpressing xenografts after 24 days. Initially, the mutant DDAH1 xenografts grew at the same rate as control tumors. Taken together, these results are consistent as seen in in vitro, suggesting that the active DDAH1 impart benefit for growth of these tumors in vivo. VEGF, HIF1-α and iNOS expression was significantly increased by wild-type DDAH1 overexpressing tumors like in control tumors, and was low in mutant DDAH1 overexpressing tumors. Consequently, expression of angiogenic factors downstream of DDAH1 in PCa cells in vivo is NO dependent. Boult et al. [39] reported that DDAH1-mediated mechanisms were involved in tumor progression and angiogenesis in the glioblastoma. In solid tumors, angiogenesis process is crucial for their advancement and increased micro-vessel density (MVD), an indicator of aggressiveness and metastatic potential [33]. Expression of endothelial markers like CD31, CD34 and von Willebrand factor (vWF) assess whether the MVD is increased in tumors due to angiogenesis [40]. CD31 staining of xenografts from this study revealed that wild-type DDAH1 tumor tissue sections had more vasculature and higher endothelial cell content compared to control tumor sections. The mutant DDAH1 overexpressing sections stained very weak for CD31, same as control tumor tissue sections showing less endothelial content than wild-type DDAH1 tissues. The data obtained show that the DDAH1 overexpression increases PCa cells proliferation, survival and angiogenesis around the tumor and involved in the tumor progression. These findings reveal that DDAH1 by regulating NO and its downstream VEGF cascade is involved in tumor phenotype described in this study. In cancer therapeutics, targeting angiogenesis is attracting substantial attention. There are multiple clinical trials of anti-angiogenic agents in PCa are underway. Some of these are in development phase, and some are discontinued due to their futility and/or toxicity [41,42,43]. Hence, role of multiple targets involved in tumor angiogenesis needs to be discovered for new therapeutics against PCa. Development of inhibitors against multiple targets to control tumor NO production and subsequently angiogenesis and cancer progression improve clinical outcome in PCa patients. Along these lines, the results from our study further elucidate the role of ADMA/DDAH pathway in PCa and suggest a novel role for DDAH1-targeted therapy to improve outcome in PCa patients.
In summary, we have demonstrated that the overexpression of enzymatically active DDAH1 confers prostate cancer cells growth in in vitro and in vivo. Also, this study shows that the elevated DDAH1 results in enhanced NO production and its downstream VEGF and HIF1 expression due to reduced tumor ADMA. Further, the increase in in vivo tumor growth observed is due to the enzymatic activity of DDAH1. Recent studies show growing body of evidence, suggesting that DDAH enzymes may have different roles in pathophysiology and tumor progression. We also speculate that, apart from its enzymatic activity, DDAH1 protein itself might regulate other mechanisms and those mechanisms have to be analyzed further. Thus, our study reiterates that DDAH1 is an important regulator of cancer progression and provides a rationale to develop small molecule inhibitors against DDAH1 for targeting ADMA/DDAH pathway for the treatment of cancer. Our study suggests an additional DDAH1-targeted therapy to improve therapeutic strategies for PCa treatment.
Abbreviations
- PCa:
-
Prostate cancer
- DDAH1:
-
Dimethylarginine dimethylaminohydrolase-1
- ADMA:
-
Asymmetric dimethylarginine
- NO:
-
Nitric oxide
References
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64(2):327–336
Fidler IJ, Ellis LM (1994) The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell 79(2):1825–188
Folkman J (1995) Seminars in medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333(26):1757–1763. https://doi.org/10.1056/NEJM199512283332608
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. Cell 100(1):57–70
Chang C-F, Diers AR, Hogg N (2015) Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic Biol Med 79:324–336. https://doi.org/10.1016/j.freeradbiomed.2014.11.012
Sorrenti V (2011) The DDAH/NOS pathway in human prostatic cancer cell lines: antiangiogenic effect of L-NAME. Int J Oncol 39:1303–1310. https://doi.org/10.3892/ijo.2011.1107
Ziche M (1994) Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance. J Clin Invest 94:2036–2044
Janakiram NB, Rao CV (2012) iNOS-selective inhibitors for cancer prevention: promise and progress. Future Med Chem 4(17):2193–2204. https://doi.org/10.4155/fmc.12.168
Muntane J, la Mata MD (2010) Nitric oxide and cancer. World J Hepatol 2(9):337–344. https://doi.org/10.4254/wjh.v2.i9.337
Vannini F, Kashfi K, Nath N (2015) The dual role of iNOS in cancer. Redox Biol 6:334–343
Leiper J, Nandi M (2011) The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat Rev Drug Discov 10(4):277–291. https://doi.org/10.1038/nrd3358
Jacobi J, Sydow K, von Degenfeld G, Zhang Y, Dayoub H, Wang B, Patterson AJ, Kimoto M, Blau HM, Cooke JP (2005) Overexpression of dimethylarginine dimethylaminohydrolase reduces tissue asymmetric dimethylarginine levels and enhances angiogenesis. Circulation 111(11):1431–1438. https://doi.org/10.1161/01.CIR.0000158487.80483.09
Palm F, Onozato ML, Luo Z, Wilcox CS (2007) Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 293(6):H3227–H3245. https://doi.org/10.1152/ajpheart.00998.2007
Kostourou V, Robinson SP, Cartwright JE, Whitley GS (2002) Dimethylarginine dimethylaminohydrolase I enhances tumour growth and angiogenesis. Br J Cancer 87(6):673–680. https://doi.org/10.1038/sj.bjc.6600518
Wang Y, Hu S, Gabisi AM Jr, Er JA, Pope A, Burstein G, Schardon CL, Cardounel AJ, Ekmekcioglu S, Fast W (2014) Developing an irreversible inhibitor of human DDAH-1, an enzyme upregulated in melanoma. Chem Med Chem 9(4):792–797. https://doi.org/10.1002/cmdc.201300557
Ummanni R, Junker H, Zimmermann U, Venz S, Teller S, Giebel J, Scharf C, Woenckhaus C, Dombrowski F, Walther R (2008) Prohibitin identified by proteomic analysis of prostate biopsies distinguishes hyperplasia and cancer. Cancer Lett 266(2):171–185
Buijs N, Oosterink JE, Jessup M, Schierbeek H, Stolz DB, Houdijk AP, Geller DA, van Leeuwen PA (2017) A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: dimethylarginine dimethylaminohydrolase 1. Angiogenesis. https://doi.org/10.1007/s10456-017-9567-4
Tran CT, Fox MF, Vallance P, Leiper JM (2000) Chromosomal localization, gene structure, and expression pattern of DDAH1: comparison with DDAH2 and implications for evolutionary origins. Genomics 68(1):101–105. https://doi.org/10.1006/geno.2000.6262
Ummanni R, Mundt F, Pospisil H, Venz S, Scharf C, Barett C, Falth M, Kollermann J, Walther R, Schlomm T, Sauter G, Bokemeyer C, Sultmann H, Schuppert A, Brummendorf TH, Balabanov S (2011) Identification of clinically relevant protein targets in prostate cancer with 2D-DIGE coupled mass spectrometry and systems biology network platform. PLoS One 6(2):e16833. https://doi.org/10.1371/journal.pone.0016833
Knipp M, Vasak M (2000) A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem 286(2):257–264. https://doi.org/10.1006/abio.2000.4805
Ware JL, DeLong ER (1985) Influence of tumour size on human prostate tumour metastasis in athymic nude mice. Br J Cancer 51(3):419–423
Guan X (2015) Cancer metastases: challenges and opportunities. Acta Pharm Sin B 5(5):402–418. https://doi.org/10.1016/j.apsb.2015.07.005
Monteiro HP, Gruia-Gray J, Peranovich TMS, De Oliveira LCB, Stern A (1999) Nitric oxide stimulates tyrosine phosphorylation of focal adhesion kinase, SRC kinase, and mitogen-activated protein kinases in murine fibroblasts. Free Radic Biol Med 28(2):174–182
Figel S (2011) Focal adhesion kinase controls prostate cancer progression via intrinsic kinase and scaffolding functions. Anti-Cancer Agents Med Chem 11:607–616
Smith CL, Birdsey GM, Anthony S, Arrigoni FI, Leiper JM, Vallance P (2003) Dimethylarginine dimethylaminohydrolase activity modulates ADMA levels, VEGF expression, and cell phenotype. Biochem Biophys Res Commun 308(4):984–989
Hasegawa K, Wakino S, Tanaka T, Kimoto M, Tatematsu S, Kanda T, Yoshioka K, Homma K, Sugano N, Kurabayashi M, Saruta T, Hayashi K (2006) Dimethylarginine dimethylaminohydrolase 2 increases vascular endothelial growth factor expression through Sp1 transcription factor in endothelial cells. Arterioscler Thromb Vasc Biol 26(7):1488–1494. https://doi.org/10.1161/01.ATV.0000219615.88323.b4
Fukumura D, Kashiwagi S, Jain RK (2006) The role of nitric oxide in tumour progression. Nat Rev Cancer 6(7):521–534. https://doi.org/10.1038/nrc1910
Du Q, Zhang X, Liu Q, Zhang X, Bartels CE, Geller DA (2013) Nitric oxide production upregulates Wnt/beta-catenin signaling by inhibiting Dickkopf-1. Cancer Res 73(21):6526–6537. https://doi.org/10.1158/0008-5472.CAN-13-1620
Chang CF, Diers AR, Hogg N (2015) Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic Biol Med 79:324–336. https://doi.org/10.1016/j.freeradbiomed.2014.11.012
Faton (2002) Role of nitric oxide in the regulation hif1a during hypoxia. Am J Physiol Cell Physiol 283:C178–C186
Semenza GL (2010) HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20(1):51–56. https://doi.org/10.1016/j.gde.2009.10.009
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69(Suppl 3):4–10. https://doi.org/10.1159/000088478
Sooriakumaran P, Kaba R (2005) Angiogenesis and the tumour hypoxia response in prostate cancer: a review. Int J Surg 3(1):61–67. https://doi.org/10.1016/j.ijsu.2005.03.013
Ayling LJ, Whitley GS, Aplin JD, Cartwright JE (2006) Dimethylarginine dimethylaminohydrolase (DDAH) regulates trophoblast invasion and motility through effects on nitric oxide. Hum Reprod 21(10):2530–2537. https://doi.org/10.1093/humrep/del111
Varner JA, Cheresh DA (1996) Tumor angiogenesis and the role of vascular cell integrin alphavbeta3. Important Adv Oncol 69–87
Figel S, Gelman IH (2011) Focal adhesion kinase controls prostate cancer progression via intrinsic kinase and scaffolding functions. Anticancer Agents Med Chem 11(7):607–616
Zhao X, Guan JL (2011) Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 63(8):610–615. https://doi.org/10.1016/j.addr.2010.11.001
Guarino M (2010) Src signaling in cancer invasion. J Cell Physiol 223(1):14–26. https://doi.org/10.1002/jcp.22011
Boult JK, Walker-Samuel S, Jamin Y, Leiper JM, Whitley GS, Robinson SP (2011) Active site mutant dimethylarginine dimethylaminohydrolase 1 expression confers an intermediate tumour phenotype in C6 gliomas. J Pathol 225(3):344–352. https://doi.org/10.1002/path.2904
Trojan L, Thomas D, Friedrich D, Grobholz R, Knoll T, Alken P, Michel MS (2004) Expression of different vascular endothelial markers in prostate cancer and BPH tissue: an immunohistochemical and clinical evaluation. Anticancer Res 24(3a):1651–1656
Kluetz PG, Figg WD, Dahut WL (2010) Angiogenesis inhibitors in the treatment of prostate cancer. Expert Opin Pharmacother 11(2):233–247
Mukherji D, Temraz S, Wehbe D, Shamseddine A (2013) Angiogenesis and anti-angiogenic therapy in prostate cancer. Crit Rev Oncol Hematol 87(2):122–131
Fu W, Madan E, Yee M, Zhang H (2012) Progress of molecular targeted therapies for prostate cancers. Biochim Biophys Acta (BBA) Rev Cancer 1825(2):140–152
Acknowledgements
This work is supported by SMILE (CSC-0111) project supported by Council for Scientific and Industrial Research (CSIR) under 12th five-year plan during 2012 to 2017. KKR acknowledge UGC for CSIR-UGC fellowship for graduate students.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reddy, K.R.K., Dasari, C., Duscharla, D. et al. Dimethylarginine dimethylaminohydrolase-1 (DDAH1) is frequently upregulated in prostate cancer, and its overexpression conveys tumor growth and angiogenesis by metabolizing asymmetric dimethylarginine (ADMA). Angiogenesis 21, 79–94 (2018). https://doi.org/10.1007/s10456-017-9587-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9587-0