Abstract
Mitochondrial fission and mitophagy are considered key processes involved in the pathogenesis of cardiac microvascular ischemia reperfusion (IR) injury although the upstream regulatory mechanism for fission and mitophagy still remains unclear. Herein, we reported that NR4A1 was significantly upregulated following cardiac microvascular IR injury, and its level was positively correlated with microvascular collapse, endothelial cellular apoptosis and mitochondrial damage. However, NR4A1-knockout mice exhibited resistance against the acute microvascular injury and mitochondrial dysfunction compared with the wild-type mice. Functional studies illustrated that IR injury increased NR4A1 expression, which activated serine/threonine kinase casein kinase2 α (CK2α). CK2α promoted phosphorylation of mitochondrial fission factor (Mff) and FUN14 domain-containing 1 (FUNDC1). Phosphorylated activation of Mff enhanced the cytoplasmic translocation of Drp1 to the mitochondria, leading to fatal mitochondrial fission. Excessive fission disrupted mitochondrial function and structure, ultimately triggering mitochondrial apoptosis. In addition, phosphorylated inactivation of FUNDC1 failed to launch the protective mitophagy process, resulting in the accumulation of damaged mitochondria and endothelial apoptosis. By facilitating Mff-mediated mitochondrial fission and FUNDC1-required mitophagy, NR4A1 disturbed mitochondrial homeostasis, enhanced endothelial apoptosis and provoked microvascular dysfunction. In summary, our data illustrated that NR4A1 serves as a novel culprit factor in cardiac microvascular IR injury that operates through synchronous elevation of fission and suppression of mitophagy. Novel therapeutic strategies targeting the balance among NR4A1, fission and mitophagy might provide survival advantage to microvasculature following IR stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In patients with acute myocardial infarction, although a timely reperfusion strategy could open up the occluded coronary arteries, it unfortunately provokes ischemia reperfusion (IR) injury to the heart [18]. Notably, endothelial dysfunction has been acknowledged to play an unfavorable role in the development of cardiac IR injury [24, 31]. At the cellular and molecular levels, defects in vasodilatation interrupt the blood flow to myocardium. Besides, endothelial hyperpermeability and junctional loss derived from endothelial death propagate excessive leukocyte adhesion and micro-thrombus formation [16]. Per our clinical observations, microvascular IR injury occurs in approximately 10–50% of patients during and after reperfusion [6, 7]. Considering the essential role of microcirculation in the exchange of matter, oxygen and energy between blood and cardiomyocytes, reperfusion injury to the microvascular bed post-ischemia can impose additional myocardial damage and increase the 30-day mortality rate in patients with reperfusion injury [12]. Therefore, understanding the cellular and molecular mechanisms of microvascular IR injury may pave the road to new treatment modalities, which are pertinent for the management of cardiac IR injury in clinical practice.
We have conducted a number of studies focusing on the role of mitochondrial homeostasis in microvascular IR injury. Our data confirmed that both mitophagy [53, 61, 62] and mitochondrial fission [51, 60] are vital for the maintenance of microvascular integrity and micro-endothelial viability [21]. Excessive mitochondrial fission provokes superfluous mitochondrial fragmentation, leading to energy disorder and apoptotic events [19, 54]. In contrast, mitophagy could sweep injured mitochondrial debris and thus render the IR-damaged microvasculature less sensitive [47]. However, the upstream regulatory mechanism for mitophagy and mitochondrial fission remains unclear. Our recent work using chronic high fat-induced hepatic injury demonstrated that nuclear receptor subfamily 4 group A member 1 (NR4A1) possesses the ability to regulate fission and mitophagy in a synchronized fashion [50]. NR4A1 promotes dynamic-related protein 1 (Drp1) activation and limits BCL2 interacting protein 3 (Bnip3) transcription, leading to excessive Drp1-associated fission and defective Bnip3-required mitophagy. Through modulation of fission and mitophagy, NR4A1 aggravates hepatic apoptosis and liver dysfunction during the development of hepatic steatosis and chronic metabolic injury. Notably, careful scrutiny from other laboratories has also confirmed a role for NR4A1 in heart failure [25] and oxidative stress-induced cardiac death [49]. Based on these findings, we speculated that NR4A1 may be involved in microvascular IR injury via the regulation of mitochondrial fission and mitophagy.
Mitochondrial fission and fragmentation are commonly observed in cells with cardiac IR injury. During mitochondrial fission, Drp1 is a critical executor that can assemble into a ring structure along the mitochondrial tubules, leading to the division of mitochondrial cells into daughter mitochondria [56]. Remarkably, Drp1 recruitment to the mitochondria requires its corresponding receptors, which are located on the mitochondrial outer membrane. Our previous studies have demonstrated that mitochondrial fission factor (Mff), the Drp1 receptor, is increased in response to IR injury [19, 51]. This study was designed to determine the role of NR4A1 in Mff-related mitochondrial fission. Given the essential role of mitophagy as a housekeeper of mitochondrial homeostasis apart from mitochondrial fission, cells utilize mitophagy to remove defective or divided mitochondria, in an effort to maintain mitochondrial quantity and quality [10, 13]. Mitophagy is regulated by several receptors, including Parkin [60], FUN14 domain-containing 1 (FUNDC1) [61] and Bnip3 [19]. However, the precise role of FUNDC1-mediated mitophagy in microvascular IR injury is not completely understood.
Mff is activated via post-transcriptional phosphorylation [41], whereas phosphorylated FUNDC1 is the inactive form [48]. This information illustrates that phosphorylation modification is the regulatory mechanism for both Mff and FUNDC1. Interestingly, NR4A1 has been associated with the activation of casein kinase2 α (CK2α) [43], a messenger-independent protein serine/threonine kinase [27]. To date, more than 300 substrates of CK2α have been identified, including FUNDC1 [28]. Thus, it is plausible to speculate that NR4A1 governs the regulation of Mff and FUNDC1 phosphorylation through CK2α. In this study using loss- and gain-of-function for NR4A1 both in vivo and in vitro, we reported that elevated NR4A1 was likely a culprit factor for the development of cardiac microvasculature IR injury through CK2α-mediated suppression of FUNDC1-mediated mitophagy and stimulation of Mff-mediated mitochondrial fission.
Materials and methods
Cardiac ischemia reperfusion injury (IR injury) model in vivo
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals established by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996) and were approved by the Institutional Animal Care and Use Committees at the PLA General Hospital and the University of Wyoming. NR4A1 knockout (NR4A1-KO) mice were generated according to our previous study [50]. Then, adult wild-type (WT) and NR4A1-KO mice (12 week-old, male) underwent cardiac IR injury. The model was performed in vivo via an 8.0 surgical suture ligation of the left anterior descending coronary artery for approximately 45 min to induce ischemia damage. Then, the slipknot was loosened for approximately 0–24 h to induce the reperfusion injury. After the cardiac IR injury, blood was collected, and CK-MB, troponin T and LDH were analyzed via ELISA according to our previous study [51].
Gelatin-ink staining and echocardiography
Gelatin-ink staining was used to evaluate the structural patency of microvasculature according to our previous study [51]. First, immediately following the completion of IR injury, gelatin-ink (3% gelatin and ink) was injected into the heart via the jugular vein at 30 °C. Then, hearts were cut and maintained at 4 °C for 1 h. Finally, after 4% paraformaldehyde fixation, cryosectioning was carried out and samples were observed under a microscopy. Echocardiography was performed in mice after reperfusion according to our previous study [51]. An echocardiogram (14.0 MHz, Sequoia C512; Acuson, Germany) was used to detect both two-dimensional and M-mode images.
Hypoxia reoxygenation injury (HR injury) model in vitro
Cardiac microvascular endothelial cells (CMECs) were isolated from WT and NR4A1 knockout (NR4A1-KO) mice according to our previous study [51, 56], and CD31 staining and Dil-acetylated low-density lipoprotein intake assay was conducted to identify the endothelial cells. Subsequently, these CMECs were used to induce hypoxia reoxygenation injury model in vitro. Hypoxia preconditioning was performed in a tri-gas incubator with an N2 concentration of 95% and a CO2 concentration of 5% for 45 min. Then, the cells were grown under normal culture conditions for 6 h to induce the reoxygenation injury.
Western blot
Samples were lysed in ice-cold RIPA buffer supplemented with a protease cocktail. Then, the samples were centrifuged at 14,000 rpm for 20 min at 4 °C. Equal amounts of protein were separated via SDS-PAGE and then transferred to PVDF membranes (Millipore, Billerica, MA, USA). Next, 5% bovine serum albumin was used to block the samples for 1 h at room temperature [34]. Then, the membranes were incubated overnight at 4 °C with the following primary antibodies: Bcl2 (1:1000, Cell Signaling Technology, #3498), Bax (1:1000, Cell Signaling Technology, #2772), caspase9 (1:1000, Cell Signaling Technology, #9504), procaspase3 (1:1000, Abcam, #ab13847), cleaved caspase3 (1:1000, Abcam, #ab49822), c-IAP (1:1000, Cell Signaling Technology, #4952), survivin (1:1000, Cell Signaling Technology, #2808), Drp1 (1:1000, Abcam, #ab56788), Fis1 (1:1000, Abcam, #ab71498), Mid49 (1:1000, Bioss, bs-12633R), Mid51 (1:1000, Bioss, bs-12634R), Opa1 (1:1000, Abcam, #ab42364), Mfn1 (1:1000, Abcam, #ab57602), Mff (1:1000, Cell Signaling Technology, #86668), p-Mff (1:1000, Cell Signaling Technology, #49281), LC3I/II (1:1000, Cell Signaling Technology, #12741), LC3II (1:1000, Cell Signaling Technology, #3868), Tom20 (1:1000, Cell Signaling Technology, #42406), Cyt-c (1:1000; Abcam; #ab90529), p-eNOS (Ser1117) (1:1000, Abcam, #ab184154), ET-1 (1:1000, Abcam, #ab2786), IL-8 (1:1000, Abcam, #ab7747), MMP9 (1:1000, Abcam, #ab38898), MIP1α (1:1000, Abcam, #ab71152), Tim23 (1:1000, Santa Cruz Biotechnology, #sc-13298), p62 (1:1000, Cell Signaling Technology, #5114), NR4A1 (1:1000, Abcam, #ab109180), CK2α (1:1000, Cell Signaling Technology, #2656). The anti-p-FUNDC1 (ser13) (1:500) and anti-FUNDC1 (1:1000) poly-clonal antibodies were produced by immunizing rabbits with synthesized and purified phosphorylated and nonphosphorylated peptides from FUNDC1 (Abgent, SuZhou, China) according to our previous study [53]. Then, the membranes were incubated with secondary antibodies at room temperature for 1 h. Band intensities were quantified using Image-Pro Plus 6.0 software [20].
MTT, caspase3/9 activities and TUNEL assays
The MTT assay was used to detect cellular viability. In brief, MTT solution (5 mg/ml in approximately 20 μl) was added to the medium for 4 h. Then, the supernatant was discarded, and 100 μl of DMSO was supplied to the culture for 30 min. Then, the optical density value of 490 nm (OD) was measured [36]. The caspase3/9 activities and TUNEL assays were conducted to detect cellular apoptosis according to our previous study [53]. The level of caspase3/9 activities was expressed as a percentage of the control group. The TUNEL-positive cells were imaged and quantitated percentage-wise [57].
Immunofluorescence and immunohistochemistry
Tissues and cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 10 min. Then, 5% normal goat serum was used to block the samples for 1 h at room temperature. Then, the samples were incubated with primary antibodies overnight at 4 °C. After extensive washing, the samples were observed under an Axio Observer Z1 microscope [5]. The primary antibodies used in the present study were as follows: p-eNOS (Ser1117) (1:1000, Abcam, #ab184154), NR4A1 (1:500, Abcam, #ab109180), Drp1 (1:1000, Abcam, #ab56788), VCAM1 (1:1000, Abcam, #ab134047), VE-cadherin (1:1000, Abcam, #ab205336), Gr1 (1:1000, Abcam, #ab25377), troponin T (1:1000, Abcam, #ab8295), ICAM1 (1:1000, Abcam, #ab119871), Tom20, (mitochondrial antibody, 1:500, Abcam, #ab186734) and Lamp1 (lysosome antibody, 1:500, Abcam, #ab24170). The mitochondrial and lysosome antibodies were used to mark mitochondria and lysosomes, respectively [40].
EM analysis of microvasculature
The heart microvascular ultrastructure was evaluated using electron photomicrographs (EM). Heart samples were fixed in 5% glutaraldehyde and 4% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) with 0.05% CaCl2 for 24 h. After washing in 0.1 M sodium cacodylate buffer, tissues were post-fixed in 1% OsO4 and 0.1 M cacodylate buffer overnight, dehydrated and embedded in Embed-812 resin [17]. The sections were stained with 2% uranyl acetate followed by 0.4% lead citrate and viewed with a Philips 400 electron microscope (electron microscopy sciences) [42].
Transwell assay
The transwell assay was used to evaluate the migration of endothelial cells. First, endothelial cells were cultured under HR injury; then, these cells were digested and added to a 24-well transwell plate for 24 h under normal culture conditions [58]. Subsequently, extra liquids in the upper and lower chambers were discarded. After wiping the cells in the upper chamber, cells in the lower chamber were fixed via pre-chilled formalin and stained with 1% crystal violet for 10 min. Finally, photos were taken under a microscope, and the observation results were recorded [52].
Detection of CMEC permeability and transendothelial electrical resistance (TER)
A FITC-dextran clearance assay was performed to monitor changes in CMEC permeability per our previous report [56]. After HR treatment, CMECs were incubated with FITC-dextran (final concentration: 1 mg/ml), and were allowed to permeate through the cell monolayer. Two hours later, the FITC content remaining in the plate was measured using a fluorescent plate reader (Bio-Rad, USA) to detect the extent of permeability. TER is a measure of ionic conductance of endothelial cells and is used to assess junctional function. TER decreases when endothelial cells retract or lose adhesion. Using an in vitro Vascular Permeability Assay Kit (ECM640, Millipore, USA), CMECs were seeded onto collagen-coated inserts at a density of 100,000 cells/insert. After reaching confluence, an electrical endothelial resistance system (Millipore, USA) was used to measure TER per our previous description [48].
Mitochondrial membrane potential (ΔΨ m), mPTP opening and ATP production
Mitochondrial transmembrane potential was observed using a JC-1 Kit (Beyotime, China). Images were captured using a fluorescence microscope (OLYMPUS DX51; Olympus, Tokyo, Japan) and quantified with Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA) to obtain the mean densities of the regions of interest, which were normalized to those in the control group. The mPTP opening was visualized as a rapid dissipation of tetramethylrhodamine ethyl ester fluorescence. The arbitrary mPTP opening time was determined as the time when the tetramethylrhodamine ethyl ester fluorescence intensity decreased by half between the initial and residual fluorescence intensities according to a previous study [61]. Cellular ATP levels were measured using a firefly luciferase-based ATP assay kit (Beyotime) based on a fluorescence technique (Genmed Scientifics Inc.) according to the protocol [14].
Oxygen consumption rate (OCR) and ROS detection
The cellular oxygen consumption rate (OCR) was evaluated using an XFe96 extracellular flux analyzer (Agilent Technologies) as we previously reported [50, 59]. Intracellular ROS and superoxide were measured using 2′,7′-dichlorofluorescein-diacetate (10 μM, DCFH-DA, Beyotime Institute of Biotechnology, Jiangsu, China). The positive and negative control groups for ROS staining were set via administration of rotenone (Sigma, cat.no.83-79-4) and mitoquinone (MitoQ, MedKoo Biosciences, Inc., cat.no.317102), respectively. MitoQ (2 μM) was added in medium for 30 min prior to HR treatment. Rotenone (1 μM) was applied into CMEC for 30 min. Then, fluorescence microscopy (Olympus) and flow cytometry was used to examine the ROS concentration [63].
siRNA transfection
Transient knockdown assays were performed using DharrmaFECT 1 (Dharmacon, Lafayette, CO, USA), according to the manufacturer’s manual. The siRNA against FUNDC1 was purchased from Santa Cruz Biotechnology as our previous study reported [61]. Cells were harvested 96 h after transfection and used for further analysis.
Mff mutant vector construction and CK2α overexpression
Plasmid transfection was performed using Turbofect (Thermo Scientific, MA, USA) or Attractence transfection reagent (QIAGEN, Valencia, CA, USA). The serine at site 146 of the constitutively active form of Mff (c.a.Mff) was replaced with aspartic acid (mimicking the phosphorylation at site146). For lentiviral packaging, the PCR-amplified mutant Mff fragments were cloned into pCMV vectors. Then, the above vectors were triple-transfected into 293T cells using Lipofectamine 2000 [3]. After transfection for 48 h, the supernatant fraction containing lentiviral particles was collected. Following amplification, the supernatant was acquired and filtered and then applied to infect CMECs as we previously described [19].
For overexpression of CK2α, the pDC316-mCMV-CK2α plasmid, purchased from Vigene Bioscience, was transfected with the framework plasmid (1:1) into 293T cells. After transfection for 48 h, the viral supernatant was collected and identified by PCR. Following amplification, the supernatant was acquired again and filtered through a 0.45-μm filter to obtain the Ad-CK2α which was used to transfect CMECs to overexpress CK2α.
Statistical analysis
Data are expressed as the mean ± SEM. For comparisons among more groups, one-way ANOVA was used, and statistical significance was considered at p < 0.05.
Results
NR4A1 is upregulated after cardiac IR injury and contributes to microvascular damage
First, qPCR and western blots were used to quantify NR4A1 alterations in the context of IR injury. Compared with that in the non-infarcted zone, NR4A1 was significantly increased in the infarct area both at transcription (Supplemental Fig. 1A) and proteins expression levels (Fig. 1a, b) in a time-dependent manner. In addition, NR4A1 expression was the highest after 6 h of reperfusion. Therefore, reperfusion for 6 h was used in the subsequent experiments. Similar results were obtained in cardiac microvascular endothelial cells (CMECs) with hypoxia reoxygenation (HR) injury in vitro. Those cells, characterized by CD31 staining and Dil-acetylated LDL intake assay (Supplemental Fig. 1B), expressed higher NR4A1 levels in response to the HR injury (Supplemental Fig. C, D), reconfirming that IR injury upregulated NR4A1 expression in cardiac endothelial system.
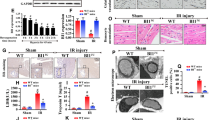
NR4A1 was upregulated in response to cardiac IR injury and contributed to the microvascular damage. a, b After IR injury, proteins were isolated and western blotting was carried out to analyze the levels of NR4A1 before and after reperfusion. #P < 0.05 vs. 0-h reperfusion. c, d Level of NR4A1 was verified using Western blots in NR4A1 knockout (NR4A1KO) mice. e, f Immunohistochemistry assay of NR4A1 in WT and NR4A1KO mice. The enlarged box in each panel denotes amplified cardiac microvessels. g After IR injury, heart tissues were isolated and the electron microscopy was used to evaluate the ultra-structural changes in microvascular under IR injury. Red arrows indicate the swollen endothelial nuclear and the yellow arrows represent damaged microvascular wall. h, i After IR injury, the gelatin-ink was injected into the hearts and the microvascular perfusion defect was detected via gelatin-ink staining. j Hematoxylin and eosin staining for red blood cell morphology in different groups. k, l TUNEL assay was used to tag the apoptotic endothelial cells and the cellular apoptotic rate was counted. #P < 0.05 vs. sham group; *P < 0.05 vs. WT + IR group
Next, to explore the detailed role of NR4A1 in cardiac microvascular IR injury, NR4A1-KO mice were used. Western blots (Fig. 1c, d) and immunohistochemistry (Fig. 1e, f) of NR4A1 demonstrated that NR4A1 was significantly increased in cardiac vessels, and these changes were rescued in NR4A1-KO mice. Following deletion of NR4A1, cardiac injury markers such as CK-MB, Troponin T and LDH were significantly reduced (Supplemental Fig. 2A–C). Moreover, loss of NR4A1 also sustained cardiac function in the context of IR injury (Supplemental Fig. 2D–F).
To observe microvascular structural changes with NR4A1 ablation, electron microscopy (EM) was used. Irregular endothelial swelling and luminal stenosis were identified in cardiac microvessels following IR injury in WT mice but not in NR4A1-KO mice (Fig. 1g). Furthermore, to observe microvascular reperfusion defects, we used gelatin-ink to fill the vessels. IR injury seriously reduced the density of gelatin-ink, indicative of microvascular blockage (Fig. 1h, i). However, NR4A1-KO helps to keep the vessels open. These data illustrated that NR4A1 activation may account for the microvascular defect. As the consequence of microvascular blockade, red blood cells went through morphological changes to transform from “parachute” or “arrow” to “swollen” or “massed” shapes as evidenced by H&E staining (Fig. 1j). This may be resulted from the stoppage of turbulent blood flow or a secondary effect due to capillary blockage by other cells, such as leukocytes. However, red blood cells in NR4A1-KO mice exhibited regular shapes. Finally, the TUNEL assay was carried out to measure endothelial damage in response to IR injury. The number of TUNEL-positive endothelial cells was increased after IR injury but decreased in NR4A1-KO mice (Fig. 1k, l). This notion was further supported by the observation in vitro that loss of NR4A1 reversed endothelial viability and reduced CMEC apoptosis, as assessed by MTT assay (Supplemental Fig. 3A) and caspase-3 activity (Supplemental Fig. 3B), respectively.
Loss of NR4A1 reduces the microvascular hyperpermeability
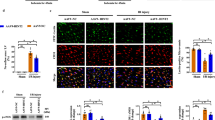
The microvascular hyperpermeability is detectable early in the course of IR progression and is vital for the IR-mediated myocardial inflammation response and micro-thrombus formation, which participates in vascular clotting and lumen loss. As shown in Fig. 2a, b, IR injury induced the downregulation of VE-cadherin, a junctional protein that sustains microvascular permeability, and this effect was reversed by NR4A1 deletion. Furthermore, through immunohistochemistry analysis of plasma albumin, we demonstrated that IR injury induced more plasma albumin leakage from the vessel into myocardial tissue, and that NR4A1 deletion weakened the diffusion of the plasma albumin into the outer surface of the vessel wall (Fig. 2c, d). The increase of endothelial permeability was followed by more Gr-1+ neutrophils migration into the myocardial tissue; the effect of which was inhibited by NR4A1 deletion (Fig. 2e, f). This information indicated that IR evoked the microvascular hyperpermeability via upregulating NR4A1.
NR4A1 regulated microvascular permeability and inflammation response under IR injury. a, b The endothelial barrier integrity was assessed via co-staining of vascular endothelial cadherin (VE-cadherin) and CD31. Discontinuous expression of VE-cadherin could be observed in the IR injury group indicative of the broken endothelial barrier. However, loss of NR4A1 could reverse the continuous linear of VE-cadherin fluorescence. c, d The leakage of plasma albumin out of the surface of the vessel wall into interstitial spaces suggested the increased microvascular permeability after IR injury. e, f Representative images of the accumulation of Gr1+ cell in myocardial tissue. g, i The transcriptional alteration of inflammation factors IL-6, TNFα, MCP1. j–m The protein expression of IL-8, MMP9 and MIP1α. #P < 0.05 vs. sham group; *P < 0.05 vs. WT + IR group
Furthermore, accumulated Gr-1+ neutrophils in myocardial tissue were closely accompanied with elevated inflammatory response in reperfused hearts using western blot and qPCR assay. Levels of interleukin 6 (IL-6), monocyte chemotactic protein 1 (MCP1), and tumor necrosis factor α (TNFα) transcription (Fig. 2g–i) were increased after IR injury. Besides, protein expression of interleukin 8 (IL-8), matrix metalloproteinase 9 (MMP9), and macrophage inflammatory protein 1α (MIP1α) were also elevated in myocardial tissues in response to IR injury (Fig. 2j–m). However, NR4A1 deletion prevented IR-induced rises in the protein expression and transcription of inflammation factors.
NR4A1 regulates the microvascular vasodilation and barrier function
The function of microcirculation is to regulate blood flow via vasodilatation [16]. However, we found that IR injury overtly reduced the p-eNOS content, as determined by immunofluorescence (Fig. 3a, b) and western blots (Fig. 3c, d). In contrast, ET-1, a vasoconstrictor, was largely increased, as evidenced using Western blot analysis (Fig. 3d, e) and qPCR (Supplemental Fig. 4A). Notably, once NR4A1 was deleted in the heart, p-eNOS was increased, whereas ET-1 was decreased.
NR4A1 negatively controlled endothelial barrier function and microvascular vasodilation. a, b Immunohistochemistry of phosphoendothelial nitric oxide synthase (p-eNOS) expression. c, e The expression of p-eNOS and ET-1 in reperfusion hearts with NR4A1 deletion or not. f, g The change of intercellular adhesion molecule-1 (ICAM1) expression in response to IR with or without NR4A1. h, i Vascular cell adhesion molecule-1 (VCAM1) content was measured via immunohistochemistry. j, k Endothelial migration was evaluated via transwell assay. #P < 0.05 vs. sham group or control group; *P < 0.05 vs. WT + IR group or WT-cell + HR group
Apart from vasodilatation, damage to microvessel integrity and barrier function is considered the key step in microcirculatory dysfunction. However, IR injury upregulated the levels of intercellular adhesion molecule-1 (ICAM1) (Fig. 3f, g) and vascular cell adhesion molecule-1 (VCAM1) (Fig. 3h, i) on the microvascular surface, favoring a significant increase for the risk of potential micro-thrombus formation. However, NR4A1 deficiency considerably abated such changes. Subsequently, endothelial barrier function and integrity were examined in CMECs isolated from WT mice and NR4A1-KO mice via FITC-dextran clearance and transendothelial electrical resistance (TER) assay. FITC-dextran was applied on top of the inserts and allowed to permeate through cell monolayers. The decreased endothelial barrier function resulted in the retention of more FITC-dextran. Thus, FITC content remaining in the plate indicates the extent of CMEC barrier dysfunction. Regarding TER assay, TER value increases when endothelial cells adhere and spread out and decreases when endothelial cells retract or lose adhesion, reflecting endothelial barrier integrity. HR injury increased the remained FITC content (Supplemental Fig. 4B) and reduced the TER value in vitro (Supplemental Fig. 4C), indicative of the damage to CMEC integrity and barrier function; this effect of which was inhibited by NR4A1 deletion.
In addition to CMEC integrity, endothelial migration is vital for revascularization in the infarcted heart. A transwell assay showed that HR repressed the migratory responses of CMECs, and these changes were improved in NR4A1-deleted cells (Fig. 3j, k).
NR4A1 enhances mitochondrial fission through induction of Mff post-transcription phosphorylation
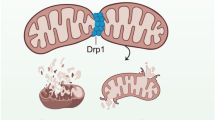
Mitochondrial dysfunction is definitively involved in the pathogenesis of cardiac IR injury [30]. Notably, mitochondrial fission is the regulatory factor underlying mitochondrial homeostasis. Thus, we observed the morphological changes of mitochondrion under HR treatment. In control cells, the mitochondria were funicular, and their average length was 8.4 ± 0.7 mm (Fig. 4a–c). However, HR forced the mitochondria to divide into several fragments, and their average length was reduced to 2.6 ± 0.5 mm. However, NR4A1-deleted cells showed predominantly elongated forms of mitochondria despite treatment with HR. Given that Drp1 translocation onto the surface of mitochondria is the prerequisite for mitochondrial fission, immunofluorescence was used to observe the co-location of Drp1 and mitochondria. As shown in Fig. 4b, compared to control group, HR injury caused more fragmented mitochondria labeled by Drp1. However, loss of NR4A1 sustained mitochondrial network and blocked the Drp1 translocation to mitochondria.
NR4A1 activated harmful mitochondrial fission via Mff in response to microvascular IR injury. a, c Mitochondria of CMECs are labeled with anti-Tom20 antibody to determine the number of cells with mitochondria fragmentation. The boxed area under each micrograph is enlarged to determine mitochondria fragmentation. b Immunofluorescence assay of mitochondrial fission was detected via co-staining of Drp1 and mitochondria. Drp1 is prone to interact with fragmented mitochondria. d–k Western blots was used to analyze the regulatory factor related to mitochondrial fission. l, m NR4A1 promoted the Mff phosphorylation. The Mff mutant is the constitutively active form of Mff (c.a.Mff) whose serine at site 146 was replaced with aspartic acid (mimicking the phosphorylation at site 146). After transfection with c.a.Mff, the phosphorylation of Mff was increased despite deletion of NR4A1. n MTT assay was used to observe the endothelial viability after c.a.Mff transfection. o, p Regaining of phosphorylated Mff re-evoked mitochondrial fission in NR4A1-deleted cells. #P < 0.05 vs. control group; *P < 0.05 vs. WT-cell + HR group; @P < 0.05 vs. NR4A1KO-cell + HR group
Subsequently, western blots were used to detect the molecular mechanism underlying HR-related mitochondrial fission. As shown in Fig. 4d–k, HR promoted Drp1 migration to mitochondria and therefore reduced the levels of cytoplasmic Drp1. Drp1 translocation to the mitochondria is required for its corresponding receptor Mff, whose activity is regulated via phosphorylation [51]. Interestingly, we demonstrated that Mff phosphorylation was significantly increased in response to HR treatment. In contrast, NR4A1 deletion suppressed Mff phosphorylated activation and therefore alleviated the contents of mitochondrial Drp1. Notably, we also detected proteins related to mitochondrial fusion, a process opposite to mitochondrial fission. Fusion-related proteins, such as Opa1 and Mfn1, were reduced after HR treatment, but this reduction was ameliorated in NR4A1-deleted cells.
To discern whether Mff is indispensable for NR4A1-activated mitochondrial fission, we transfected the Mff mutant into NR4A1-deleted cells. The Mff mutant is the constitutively active form of Mff (c.a.Mff) whose serine at site 146 was replaced with aspartic acid (mimicking the phosphorylation at site 146). After transfection with c.a.Mff, the phosphorylation of Mff was re-increased in NR4A1-deleted cells (Fig. 4l, m). Interestingly, regaining of p-Mff in NR4A1-deleted cells, the cell viability was significantly reduced via MTT assay (Fig. 4n). Subsequently, mitochondrial length was evaluated again. Compared to NR4A1-deleted cells, Mff mutant transfection re-induced mitochondrial division into several debris fragments, whose average length was approximately 2.1 ± 0.5 mm (Fig. 4o, p). Therefore, this result confirmed our hypothesis that NR4A1 governs mitochondrial fission via Mff in the IR injury setting.
Excessive mitochondrial fission promotes CMEC apoptosis
To explore the consequences of mitochondrial fission, we focused on apoptosis, especially mitochondrial apoptosis. First, the mitochondrial electrochemical gradient (ΔΨm), which was measured using JC-1, showed that HR injury impaired the ΔΨm (Fig. 5a, b). However, NR4A1 deletion reversed the stability of ΔΨm. Moreover, HR drove CMECs to produce excessive ROS as demonstrated via DCFH-DA staining (Fig. 5c, d). However, NR4A1 deficiency reduced ROS levels under HR stimulation. Additionally, the mPTP opening rate was also increased after HR injury but was decreased to normal levels with NR4A1 deficiency (Fig. 5e, f). Because of the ΔΨm dissipation, ROS eruption and long-lasting mPTP opening, the pro-apoptotic factor cyt-c was inevitably released from the mitochondria into the cytoplasm, as shown via western blot (Fig. 5g–m). However, NR4A1 deletion limited cyt-c leakage. Released cyt-c triggers apoptosome formation (a complex comprising Apaf-1 cyt-c, dATP and procaspase-9) and caspase-9 activation that in turn activates pro-caspase-3 to form the death effector cleaved caspase-3 [44, 57]. Through western blots analysis, we found that caspase-3, caspase-9 and Bax (Fig. 5g–m), were increased in HR-treated cells but reduced in cells with NR4A1 silencing (Fig. 5g–m). In contrast, anti-apoptotic protein, such as Bcl2, was reduced after HR attack but returned to normal levels after NR4A1 deficiency (Fig. 5g–m). Notably, these protective effects of NR4A1 deficiency were nullified by c.a.Mff transfection.
Mff-required mitochondrial fission was required for NR4A1-mediated mitochondrial damage. a, b Mitochondrial potential was observed via JC-1 staining. Red inflorescence means the healthy mitochondria, whereas green inflorescence indicates the collapsed mitochondrial potential. c, d Cellular ROS was detected via DCFH-DA. Rotenone (1 μM) was added into the medium and used as the positive control group. Mitoquinone (MitoQ, 2 μM) was applied 30 min before HR injury and used as the negative control group. e, f The mPTP opening time was recorded and the mPTP opening rate was normalized to control group. g–m Western blots was used to analyze the mitochondrial apoptotic proteins with or without NR4A1 deletion. n ATP production was detected via ELISA assay. o–q The oxygen consumption rate (OCR) assay was used to observe cellular respiratory function with Mff inhibition or activation. #P < 0.05 vs. control group; *P < 0.05 vs. WT-cell + HR group; @ P < 0.05 vs. NR4A1KO-cell + HR group
Apart from the amplification of apoptotic signaling, HR reduced cellular ATP production (Fig. 5n) and impaired the endothelial oxygen consumption rate (OCR) (Fig. 5o–q), which was improved in NR4A1-deleted cells in a Mff-dependent manner.
NR4A1 represses FUNDC1-required mitophagy via FUNDC1
Apart from mitochondrial fission, mitophagy is another catabolic process involving the degradation of unnecessary or dysfunctional mitochondria by lysosomes [17, 37]. In the current study, HR injury significantly reduced LC3II/LC3I ratio (Fig. 6a–g), indicative of mitophagic flux stop. However, NR4A1 deficiency reversed the LC3II/LC3I rate. Furthermore, we isolated mitochondria and observed changes in mitochondrial LC3II. HR stimulation reduced mitochondrial LC3II, which was reversed by NR4A1 deletion (Fig. 6a–g). In addition, Tom20 (mitochondrial outer membrane marker) and Tim23 (inner membrane marker) were correspondingly increased in response to HR stress and decreased in NR4A1-deleted cells (Fig. 6a–g). Additionally, p62 degradation was inhibited by HR treatment but was enhanced in NR4A1-deleted cells (Fig. 6a–g). These data indicated that HR inhibited mitophagy activity, which was reversed by NR4A1 deletion.
NR4A1 governed FUNDC1-required mitophagy. a–g Western blots were used to analyze the mitophagy parameters. FUNDC1 siRNA was used to silence the FUNDC1 expression. h, i Mitophagy activity was observed via co-staining mitochondria and lysosome at the same time. In response to HR injury, fragmented mitochondria cannot be contained by lysosome, indicative of mitophagy arrest. However, loss of NR4A1 promoted the fusion between mitochondria and lysosome in a FUNDC1-dependent manner. #P < 0.05 vs. control group; *P < 0.05 vs. WT-cell + HR group; @P < 0.05 vs. NR4A1KO-cell + HR group
FUNDC1 is a mitophagy receptor, the dephosphorylation of which allows enhanced recruitment of LC3II to the mitochondria [61]. Interestingly, HR increased FUNDC1 phosphorylation (Fig. 6a–g), indicative of FUNDC1 inactivation, which is in accordance with the impaired mitophagy activity. In contrast, NR4A1 deletion reduced the contents of phosphorylated FUNDC1 (Fig. 6a–g), suggestive of FUNDC1 activation. Interestingly, silencing of FUNDC1 via siRNA repressed mitophagy activity in NR4A1-deleted cells (Fig. 6a–g), suggesting that FUNDC1 is necessary for NR4A1 deletion-mediated mitophagy activation. Subsequently, mitochondria and lysosome co-staining was carried out to directly observe mitochondria and lysosome fusion. Meanwhile, to quantify mitophagy, the average mitophagy number (overlap of mitochondria and lysosome) was recorded. Compared to that in the control group, HR produced more round or fragmented mitochondria, which were not contained by lysosomes (Fig. 6h, i). In contrast, loss of NR4A1 promoted the fusion of lysosomes and mitochondrial debris, and thus, sustained the mitochondrial network (Fig. 6h, i). When FUNDC1 was knocked down, the beneficial effects of NR4A1 on mitophagy activation disappeared.
FUNDC1-required mitophagy interrupted mitochondrial fission and favored CMEC survival under HR
To verify the role of FUNDC1-required mitophagy in mitochondrial homeostasis and cellular fate under HR treatment, we first examined the alterations of Mff-induced mitochondrial fission. Similar to the above data, HR forced excessive mitochondrial fission as evidenced by augmented expression of mito-Drp1 and phosphorylated Mff (Fig. 7a–f). However, mitochondrial fission index (mito-Drp1 and p-Mff) was mostly suppressed by NR4A1 deletion, but upregulated via silencing FUNDC1 in NR4A1-deleted cells (Fig. 7a–f), indicating that FUNDC1-required mitophagy could antagonize mitochondrial fission. Besides, augmented mitochondrial fusion parameters (Mfn1 and Opa1) induced by NR4A1 deletion were obviously re-inhibited by FUNDC1 knockdown in NR4A1-deleted cells (Fig. 7a–f). Moreover, remarkable increase in mitochondrial fission elicited through FUNDC1 knockdown, further resulted in the collapse of mitochondrial potential (Fig. 7g, h) and ROS overproduction (Fig. 7i, j). More severely, mitochondrial apoptosis, characterized by cyt-c leakage from mitochondria into cytoplasm/nuclear, was blocked by NR4A1 under HR treatment but was strongly re-activated via FUNDC1 silencing in NR4A1-deleted cells (Fig. 7k, l). The caspase-9 activity also suggested that FUNDC1 deficiency was closely associated with increased caspase-9 protein activity albeit deletion of NR4A1 (Fig. 7m). Therefore, these data indicate that FUNDC1-related mitophagy is required for the mitochondrial homeostasis partly via counteracting mitochondrial fission and blocking mitochondrial apoptosis. In addition to mitochondrial function, we also examined the endothelial barrier function and migration capacity. As shown in Fig. 7n, o, HR-induced damage to endothelial barrier function was reversed via NR4A1 deletion; and this protective effect of NR4A1 deletion was nullified by FUNDC1 knockdown. With regards to endothelial migration, reduced number of migrated cells induced by HR was overtly elevated by NR4A1 deletion (Fig. 7p–q). However, FUNDC1 knockdown reduced endothelial migration despite ablation of NR4A1. Accordingly, this evidence suggested that FUNDC1-required mitophagy serves as the endogenous defender of mitochondrial homeostasis and endothelial function.
FUNDC1-required mitophagy interrupted mitochondrial fission and sustained mitochondrial homeostasis. a–f Mitochondrial fission parameters were measured via western blots with FUNDC1 silencing or not. g, h JC-1 staining was used to observe the mitochondrial potential. The ratio of red to green fluorescence was applied to quantify the mitochondrial potential. i, j The cellular ROS was measured via flow cytometry. k, l The cyt-c leakage from mitochondrial into cytoplasm/nuclear was observed via immunofluorescence assay. m Caspase-9 activity was detected with FUNDC1 deletion or not. n, o Endothelial barrier and permeability was detected via fluorescein isothiocyanate (FITC)-dextran clearance assay and Transendothelial electrical resistance (TER). p, q Transwell assay was carried out to analyze the endothelial migration. #P < 0.05 vs. control group; *P < 0.05 vs. WT-cell + HR group; @P < 0.05 vs. NR4A1KO-cell + HR group
NR4A1 modulates Mff and FUNDC1 via CK2α
Based on the above findings that Mff and FUNDC1 could be phosphorylated by NR4A1 in the HR injury setting, possible roles of NR4A1 in CK2α activation [43] was investigated. The latter is a messenger-independent protein serine/threonine kinase [27] that has the ability to induce several substrates or proteins phosphorylation. Accordingly, we speculated whether NR4A1 regulates Mff and FUNDC1 phosphorylation via CK2α. First, we demonstrated that CK2α was increased in response to IR treatment in vitro (Fig. 8a, b). However, loss of NR4A1 abated CK2α expression, suggesting CK2α was the downstream effector of NR4A1 in the context of HR injury. To elucidate whether CK2α was involved in Mff and FUNDC1 post-transcriptional modification, we re-introduced CK2α into NR4A1-deleted cells via adenovirus-based overexpression technology. The overexpression efficiency was confirmed using Western blot analysis (Fig. 8a, b). With the overexpression of CK2α, the phosphorylation levels of Mff and FUNDC1 were re-elevated in NR4A1-deleted cells (Fig. 8a–d). Collectively, the above data confirmed that Mff and FUNDC1 phosphorylation were both regulated by NR4A1/CK2α cascade in microvascular IR injury.
NR4A1 regulated Mff and FUNDC1 phosphorylation via CK2α. a–d Western blots was used to ensure the activation of CK2α under NR4A1 deletion or not. Subsequently, adenovirus-based overexpression technology was applied to increased CK2α in NR4A1-deleted cells to perform the gain-of function assay about CK2α. Then, the protein expression of p-Mff and p-FUNDC1 were detected. e, f TUNEL assay was used to label the apoptotic endothelial cells under CK2α overexpression or not. #P < 0.05 vs. control group; *P < 0.05 vs. WT-cell + HR group; @ P < 0.05 vs. NR4A1KO-cell + HR group
Finally, to explain whether CK2α is also involved in the fatal signal of NR4A1 under HR condition, TUNEL assay was used. The results displayed that NR4A1 deletion could reduce HR-mediated endothelial apoptosis (Fig. 8e, f), and this effect was dependent on CK2α, as CK2α overexpression in NR4A1-deleted cells increased the number of TUNEL-positive cells. Overall, these data concluded that CK2α was signaled by NR4A1 and contributed to Mff and FUNDC1 phosphorylation, eventually resulting in fission activation, mitophagy inhibition and endothelial apoptosis.
Discussion
Ample evidence has depicted a role for NR4A1 in the pathophysiological processes of diabetes and non-alcoholic fatty liver disease [45, 50]. However, little is known with regards to the role of NR4A1 in acute cardiac microvascular IR injury. In this study, we reported that (1) IR injury significantly upregulated NR4A1 in microcirculature; (2) upregulated NR4A1 contributed to microvascular perfusion defects, luminal stenosis, endothelial barrier damage, inflammatory cell permeation, and micro-endothelial apoptosis; (3) NR4A1 elicited mitochondrial apoptosis via upregulating harmful mitochondrial fission and downregulating pro-survival mitophagy by CK2α; (4) mechanistically, NR4A1 promoted the phosphorylation of Mff, which provoked mitochondrial fission; and (5) NR4A1 induced the inactivation of FUNDC1 phosphorylation, suppressing mitophagy activity. In summary, our observations described, for the first time, the comprehensive role of NR4A1 in cardiac microvascular IR injury involving CK2α activation, Mff-required mitochondrial fission, FUNDC1-related mitophagy and endothelial cell apoptosis.
Nuclear receptors represent a family of transcription factors [64] responsible for the regulation of many intracellular pathways, such as cancer, metabolic and proliferative diseases [1, 9, 35]. Nuclear receptors are termed orphans because their endogenous ligands have not yet been identified. The levels of the orphan receptor NR4A1 was found to be progressively increased during the development of diabetes [50]. Higher NR4A1 levels stimulate glucose production and elevate blood glucose [8]. Conversely, expression of an inhibitory mutant NR4A1 antagonizes gluconeogenic gene expression and lowers blood glucose levels in db/db mice [8]. Our recent study suggested that NR4A1 is associated with the progression of high fat-induced hepatic steatosis and injury via ablation of Bnip3-required mitophagy [50]. In this study, we found that NR4A1 is also associated with microvascular IR injury, which helps us to understand the injury mechanism underneath cardiac microvascular reperfusion insult. Therefore, our data indicate that the approach of inactivation of NR4A1 could be considered a further focal point to attenuate microvascular IR injury and increase patients’ benefits from reperfusion strategies including PCI and CABG.
Mitochondrial fission and mitophagy are vital for control of mitochondrial quantity and quality [4, 11, 38]. Fission is originally initiated to boost ATP production and meet the cellular energy demand through production of sister mitochondria [2, 15, 32]. However, excessive fission produces most non-functional and undesirable mitochondrial fragmentations. Based on our earlier findings, excessive fission is inclined to activate cellular apoptosis [51]. In the current study, we further demonstrated that excessive fission was correlated to the ROS overproduction, mPTP opening, ΔΨm collapse, caspase-9 related mitochondrial apoptosis activation, CMECs death and cardiac microvascular dysfunction. Therefore, our experiments provided sufficient evidence to identify the cardinal role of mitochondrial fission in mitochondrial damage and microcirculatory reperfusion injury, denoting the paradigm of mitochondrial fission activation and consequences under IR injury. Despite damage to mitochondrial homeostasis, cells employ mitophagy to remove defective mitochondria via mitophagy to limit the formation of mitochondrial debris [26, 33]. Unfortunately, in the context of IR injury, the protective mitophagy process was dampened, whereas harmful mitochondrial fission process was overtly activated. These findings were similar to those reported in previous studies that fission aggravates cardiac IR injury, whereas mitophagy provides a survival advantage to reperfused hearts [55, 61].
The key finding of the current study is that we identified the upstream regulatory mechanism responsible for fission and mitophagy. NR4A1 promoted fission and inhibited mitophagy via phosphorylating Mff and FUNDC1, respectively. Mff is the receptor of cytoplasmic Drp1, and phosphorylated Mff enhances the recruitment of cytoplasmic Drp1 to the mitochondria [39]. Sufficient mitochondria-located Drp1 forms ring structure surrounding mitochondria and then contracts, ultimately trigging mitochondrial fission [29]. For mitophagy, FUNDC1 is the receptor of LC3II [61]. The combination of LC3II and FUNDC1 promotes the fusion of mitochondria and lysosomes. However, phosphorylated FUNDC1 generates steric hindrance for LC3II binding [48], thus effectively inhibiting mitophagy. NR4A1 modified FUNDC1 phosphorylation at Ser13, leading to defective mitophagy, which failed to sweep unhealthy mitochondria. These findings first uncover that fission and mitophagy can be triggered by common upstream signals, which selectively close the protective mitophagy process and open the fatal fission process. Therefore, these results suggest a role for NR4A1 as a potential therapeutic target to regulate fission and mitophagy synchronously.
Functional assays illustrated that CK2α was required for NR4A1-mediated Mff and FUNDC1 phosphorylation modifications. CK2α, a constitutive Ser/Thr kinase, was found to be the upstream phosphorylated signaling molecule for more than 300 substrates [28]. Notably, ample evidence has demonstrated the role of CK2α in cellular apoptosis, especially in mitochondrial apoptosis [22, 23]. However, our present study enriched the harmful effects of CK2α on mitochondrial damage by showing that it promotes fission and limits mitophagy. Meanwhile, our data also noted for the first time that CK2α is actually involved in the pathogenesis of murine microvascular IR injury. Recent work from our laboratory demonstrated that CK2α expression was progressively increased in reperfused heart and promoted cardiomyocyte death via disrupting mitochondrial homeostasis [62]. Whether CK2α is also especially upregulated in microvascular after IR injury and accounts for endothelial reperfusion damage requires more evidence.
Collectively, our current study reported that cardiac microvascular IR injury is associated with upregulated NR4A1 expression, which leads to activated CK2α. CK2α then phosphorylates Mff and FUNDC1, leading to mitochondrial fission activation and mitophagy suppression. Excessive fission produced malignant mitochondria fragmentations, which could not be removed by mitophagy, ultimately mediating cellular death and inducing microvascular collapse. These findings identified that the NR4A1/CK2α pathways are vital for maintaining mitochondrial homeostasis via Mff-related fission and FUNDC1-required mitophagy, which opens a new window for the treatment of cardiac microvascular IR injury.
A number of limitations are noted for the present study. First and foremost, cardiomyocyte damage may be alleviated in response to NR4A1 deletion based on previous findings in vivo and in vitro [46, 49], in addition to the improved endothelial injury following cardiac IR. Thus, the reduction in microvascular perfusion may be resulted from improved cardiomyocyte survival which attenuates myocardial edema to relieve microvascular compression. In our study, microvascular endothelial apoptosis and barrier dysfunction were significantly reversed by NR4A1 deletion in vivo, along with the fact that NR4A1 deficiency was associated with mitophagy activation and mitochondrial fission inhibition in vitro. Endothelial protection seems to be predominant in NR4A1 deletion-offered microvascular benefit. However, we cannot rule out the possible contribution of cardiomyocyte survival in NR4A1 deficiency-offered benefits to reperfused microcirculation. Thereby, endothelial-specific NR4A1 deletion will be useful in defining the precise contributions of endothelial cell NR4A1 to cardiac microvascular IR injury.
Change history
15 June 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s00395-023-00994-3
References
Bahlawane C, Schmitz M, Letellier E, Arumugam K, Nicot N, Nazarov PV, Haan S (2017) Insights into ligand stimulation effects on gastro-intestinal stromal tumors signalling. Cell Signal 29:138–149. https://doi.org/10.1016/j.cellsig.2016.10.009
Banerjee K, Keasey MP, Razskazovskiy V, Visavadiya NP, Jia C, Hagg T (2017) Reduced FAK-STAT3 signaling contributes to ER stress-induced mitochondrial dysfunction and death in endothelial cells. Cell Signal 36:154–162. https://doi.org/10.1016/j.cellsig.2017.05.007
Bei Y, Xu T, Lv D, Yu P, Xu J, Che L, Das A, Tigges J, Toxavidis V, Ghiran I, Shah R, Li Y, Zhang Y, Das S, Xiao J (2017) Exercise-induced circulating extracellular vesicles protect against cardiac ischemia-reperfusion injury. Basic Res Cardiol 112:38. https://doi.org/10.1007/s00395-017-0628-z
Bhatelia K, Singh K, Prajapati P, Sripada L, Roy M, Singh R (2017) MITA modulated autophagy flux promotes cell death in breast cancer cells. Cell Signal 35:73–83. https://doi.org/10.1016/j.cellsig.2017.03.024
Brasacchio D, Alsop AE, Noori T, Lufti M, Iyer S, Simpson KJ, Bird PI, Kluck RM, Johnstone RW, Trapani JA (2017) Epigenetic control of mitochondrial cell death through PACS1-mediated regulation of BAX/BAK oligomerization. Cell Death Differ 24:961–970. https://doi.org/10.1038/cdd.2016.119
Chen WR, Chen YD, Tian F, Yang N, Cheng LQ, Hu SY, Wang J, Yang JJ, Wang SF, Gu XF (2016) Effects of liraglutide on reperfusion injury in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. https://doi.org/10.1161/CIRCIMAGING.116.005146
Chen WR, Hu SY, Chen YD, Zhang Y, Qian G, Wang J, Yang JJ, Wang ZF, Tian F, Ning QX (2015) Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 170:845–854. https://doi.org/10.1016/j.ahj.2015.07.014
Chen Y, Wu R, Chen HZ, Xiao Q, Wang WJ, He JP, Li XX, Yu XW, Li L, Wang P, Wan XC, Tian XH, Li SJ, Yu X, Wu Q (2015) Enhancement of hypothalamic STAT3 acetylation by nuclear receptor Nur77 dictates leptin sensitivity. Diabetes 64:2069–2081. https://doi.org/10.2337/db14-1206
Cui Y, Liu S, Cui W, Gao D, Zhou W, Luo P (2017) Identification of potential biomarkers and therapeutic targets for human IgA nephropathy and hypertensive nephropathy by bioinformatics analysis. Mol Med Rep 16:3087–3094. https://doi.org/10.3892/mmr.2017.6996
Das N, Mandala A, Naaz S, Giri S, Jain M, Bandyopadhyay D, Reiter RJ, Roy SS (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J Pineal Res. https://doi.org/10.1111/jpi.12404
Dong Y, Undyala VVR, Przyklenk K (2016) Inhibition of mitochondrial fission as a molecular target for cardioprotection: critical importance of the timing of treatment. Basic Res Cardiol 111:59. https://doi.org/10.1007/s00395-016-0578-x
Eitel I, de Waha S, Wohrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H (2014) Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol 64:1217–1226. https://doi.org/10.1016/j.jacc.2014.06.1194
Fuhrmann DC, Brune B (2017) Mitochondrial composition and function under the control of hypoxia. Redox Biol 12:208–215. https://doi.org/10.1016/j.redox.2017.02.012
Garcia-Nino WR, Correa F, Rodriguez-Barrena JI, Leon-Contreras JC, Buelna-Chontal M, Soria-Castro E, Hernandez-Pando R, Pedraza-Chaverri J, Zazueta C (2017) Cardioprotective kinase signaling to subsarcolemmal and interfibrillar mitochondria is mediated by caveolar structures. Basic Res Cardiol 112:15. https://doi.org/10.1007/s00395-017-0607-4
Guarini G, Kiyooka T, Ohanyan V, Pung YF, Marzilli M, Chen YR, Chen CL, Kang PT, Hardwick JP, Kolz CL, Yin L, Wilson GL, Shokolenko I, Dobson JG Jr, Fenton R, Chilian WM (2016) Impaired coronary metabolic dilation in the metabolic syndrome is linked to mitochondrial dysfunction and mitochondrial DNA damage. Basic Res Cardiol 111:29. https://doi.org/10.1007/s00395-016-0547-4
Heusch G (2016) The coronary circulation as a target of cardioprotection. Circ Res 118:1643–1658. https://doi.org/10.1161/CIRCRESAHA.116.308640
Hong H, Tao T, Chen S, Liang C, Qiu Y, Zhou Y, Zhang R (2017) MicroRNA-143 promotes cardiac ischemia-mediated mitochondrial impairment by the inhibition of protein kinase Cepsilon. Basic Res Cardiol 112:60. https://doi.org/10.1007/s00395-017-0649-7
Hu SY, Zhang Y, Zhu PJ, Zhou H, Chen YD (2017) Liraglutide directly protects cardiomyocytes against reperfusion injury possibly via modulation of intracellular calcium homeostasis. J Geriatr Cardiol 14:57–66. https://doi.org/10.11909/j.issn.1671-5411.2017.01.008
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587. https://doi.org/10.1016/j.redox.2017.11.004
Jovancevic N, Dendorfer A, Matzkies M, Kovarova M, Heckmann JC, Osterloh M, Boehm M, Weber L, Nguemo F, Semmler J, Hescheler J, Milting H, Schleicher E, Gelis L, Hatt H (2017) Medium-chain fatty acids modulate myocardial function via a cardiac odorant receptor. Basic Res Cardiol 112:13. https://doi.org/10.1007/s00395-017-0600-y
Kadlec AO, Beyer AM, Ait-Aissa K, Gutterman DD (2016) Mitochondrial signaling in the vascular endothelium: beyond reactive oxygen species. Basic Res Cardiol 111:26. https://doi.org/10.1007/s00395-016-0546-5
Kanki T, Kurihara Y, Jin X, Goda T, Ono Y, Aihara M, Hirota Y, Saigusa T, Aoki Y, Uchiumi T, Kang D (2013) Casein kinase 2 is essential for mitophagy. EMBO Rep 14:788–794. https://doi.org/10.1038/embor.2013.114
Kim GS, Jung JE, Narasimhan P, Sakata H, Yoshioka H, Song YS, Okami N, Chan PH (2012) Release of mitochondrial apoptogenic factors and cell death are mediated by CK2 and NADPH oxidase. J Cereb Blood Flow Metab 32:720–730. https://doi.org/10.1038/jcbfm.2011.176
Ku DD (1982) Coronary vascular reactivity after acute myocardial ischemia. Science 218:576–578
Lehmann LH, Jebessa ZH, Kreusser MM, Horsch A, He T, Kronlage M, Dewenter M, Sramek V, Oehl U, Krebs-Haupenthal J, von der Lieth AH, Schmidt A, Sun Q, Ritterhoff J, Finke D, Volkers M, Jungmann A, Sauer SW, Thiel C, Nickel A, Kohlhaas M, Schafer M, Sticht C, Maack C, Gretz N, Wagner M, El-Armouche A, Maier LS, Londono JEC, Meder B, Freichel M, Grone HJ, Most P, Muller OJ, Herzig S, Furlong EEM, Katus HA, Backs J (2018) A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med 24:62–72. https://doi.org/10.1038/nm.4452
Lin C, Chao H, Li Z, Xu X, Liu Y, Hou L, Liu N, Ji J (2016) Melatonin attenuates traumatic brain injury-induced inflammation: a possible role for mitophagy. J Pineal Res 61:177–186. https://doi.org/10.1111/jpi.12337
Litchfield DW (2003) Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J 369:1–15. https://doi.org/10.1042/BJ20021469
Meggio F, Pinna LA (2003) One-thousand-and-one substrates of protein kinase CK2? FASEB J 17:349–368. https://doi.org/10.1096/fj.02-0473rev
Oanh NTK, Park YY, Cho H (2017) Mitochondria elongation is mediated through SIRT1-mediated MFN1 stabilization. Cell Signal 38:67–75. https://doi.org/10.1016/j.cellsig.2017.06.019
Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ (2010) Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 121:2012–2022. https://doi.org/10.1161/CIRCULATIONAHA.109.906610
Pearson PJ, Schaff HV, Vanhoutte PM (1990) Acute impairment of endothelium-dependent relaxations to aggregating platelets following reperfusion injury in canine coronary arteries. Circ Res 67:385–393
Perdiz D, Lorin S, Leroy-Gori I, Pous C (2017) Stress-induced hyperacetylation of microtubule enhances mitochondrial fission and modulates the phosphorylation of Drp1 at (616)Ser. Cell Signal 39:32–43. https://doi.org/10.1016/j.cellsig.2017.07.020
Prieto-Dominguez N, Ordonez R, Fernandez A, Mendez-Blanco C, Baulies A, Garcia-Ruiz C, Fernandez-Checa JC, Mauriz JL, Gonzalez-Gallego J (2016) Melatonin-induced increase in sensitivity of human hepatocellular carcinoma cells to sorafenib is associated with reactive oxygen species production and mitophagy. J Pineal Res 61:396–407. https://doi.org/10.1111/jpi.12358
Pryds K, Nielsen RR, Jorsal A, Hansen MS, Ringgaard S, Refsgaard J, Kim WY, Petersen AK, Botker HE, Schmidt MR (2017) Effect of long-term remote ischemic conditioning in patients with chronic ischemic heart failure. Basic Res Cardiol 112:67. https://doi.org/10.1007/s00395-017-0658-6
Randriamboavonjy V, Kyselova A, Elgheznawy A, Zukunft S, Wittig I, Fleming I (2017) Calpain 1 cleaves and inactivates prostacyclin synthase in mesenteric arteries from diabetic mice. Basic Res Cardiol 112:10. https://doi.org/10.1007/s00395-016-0596-8
Ronchi C, Torre E, Rizzetto R, Bernardi J, Rocchetti M, Zaza A (2017) Late sodium current and intracellular ionic homeostasis in acute ischemia. Basic Res Cardiol 112:12. https://doi.org/10.1007/s00395-017-0602-9
Rossello X, Riquelme JA, He Z, Taferner S, Vanhaesebroeck B, Davidson SM, Yellon DM (2017) The role of PI3 Kalpha isoform in cardioprotection. Basic Res Cardiol 112:66. https://doi.org/10.1007/s00395-017-0657-7
Rovira-Llopis S, Banuls C, Diaz-Morales N, Hernandez-Mijares A, Rocha M, Victor VM (2017) Mitochondrial dynamics in type 2 diabetes: pathophysiological implications. Redox Biol 11:637–645. https://doi.org/10.1016/j.redox.2017.01.013
Salminen A, Kauppinen A, Kaarniranta K (2016) AMPK/Snf1 signaling regulates histone acetylation: impact on gene expression and epigenetic functions. Cell Signal 28:887–895. https://doi.org/10.1016/j.cellsig.2016.03.009
Shi C, Cai Y, Li Y, Li Y, Hu N, Ma S, Hu S, Zhu P, Wang W, Zhou H (2018) Yap promotes hepatocellular carcinoma metastasis and mobilization via governing cofilin/F-actin/lamellipodium axis by regulation of JNK/Bnip3/SERCA/CaMKII pathways. Redox Biol 14:59–71. https://doi.org/10.1016/j.redox.2017.08.013
Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ (2016) Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 351:275–281. https://doi.org/10.1126/science.aab4138
Van Nostrand JL, Bowen ME, Vogel H, Barna M, Attardi LD (2017) The p53 family members have distinct roles during mammalian embryonic development. Cell Death Differ 24:575–579. https://doi.org/10.1038/cdd.2016.128
van Tiel CM, Kurakula K, Koenis DS, van der Wal E, de Vries CJ (2012) Dual function of Pin1 in NR4A nuclear receptor activation: enhanced activity of NR4As and increased Nur77 protein stability. Biochim Biophys Acta 1823:1894–1904. https://doi.org/10.1016/j.bbamcr.2012.06.030
Vargas LA, Velasquez FC, Alvarez BV (2017) Compensatory role of the NBCn1 sodium/bicarbonate cotransporter on Ca(2+)-induced mitochondrial swelling in hypertrophic hearts. Basic Res Cardiol 112:14. https://doi.org/10.1007/s00395-017-0604-7
Wang WN, Zhang WL, Zhou GY, Ma FZ, Sun T, Su SS, Xu ZG (2016) Prediction of the molecular mechanisms and potential therapeutic targets for diabetic nephropathy by bioinformatics methods. Int J Mol Med 37:1181–1188. https://doi.org/10.3892/ijmm.2016.2527
Xu A, Liu J, Liu P, Jia M, Wang H, Tao L (2014) Mitochondrial translocation of Nur77 induced by ROS contributed to cardiomyocyte apoptosis in metabolic syndrome. Biochem Biophys Res Commun 446:1184–1189. https://doi.org/10.1016/j.bbrc.2014.03.089
Yu E, Mercer J, Bennett M (2012) Mitochondria in vascular disease. Cardiovasc Res 95:173–182. https://doi.org/10.1093/cvr/cvs111
Zhang W, Ren H, Xu C, Zhu C, Wu H, Liu D, Wang J, Liu L, Li W, Ma Q, Du L, Zheng M, Zhang C, Liu J, Chen Q (2016) Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife. https://doi.org/10.7554/eLife.21407
Zheng J, Wei CC, Hase N, Shi K, Killingsworth CR, Litovsky SH, Powell PC, Kobayashi T, Ferrario CM, Rab A, Aban I, Collawn JF, Dell’Italia LJ (2014) Chymase mediates injury and mitochondrial damage in cardiomyocytes during acute ischemia/reperfusion in the dog. PLoS One 9:e94732. https://doi.org/10.1371/journal.pone.0094732
Zhou H, Du W, Li Y, Shi C, Hu N, Ma S, Wang W, Ren J (2018) Effects of melatonin on fatty liver disease: the role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J Pineal Res. https://doi.org/10.1111/jpi.12450
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y (2017) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc. https://doi.org/10.1161/JAHA.116.005328
Zhou H, Li D, Shi C, Xin T, Yang J, Zhou Y, Hu S, Tian F, Wang J, Chen Y (2015) Effects of Exendin-4 on bone marrow mesenchymal stem cell proliferation, migration and apoptosis in vitro. Sci Rep 5:12898. https://doi.org/10.1038/srep12898
Zhou H, Li D, Zhu P, Hu S, Hu N, Ma S, Zhang Y, Han T, Ren J, Cao F, Chen Y (2017) Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res. https://doi.org/10.1111/jpi.12438
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018) Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res. https://doi.org/10.1111/jpi.12471
Zhou H, Shi C, Hu S, Zhu H, Ren J, Chen Y (2018) BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk-Nox2-Drp1-mitochondrial fission pathways. Angiogenesis. https://doi.org/10.1007/s10456-018-9611-z
Zhou H, Wang S, Zhu P, Hu S, Chen Y, Ren J (2017) Empagliflozin rescues diabetic myocardial microvascular injury via AMPK-mediated inhibition of mitochondrial fission. Redox Biol 15:335–346. https://doi.org/10.1016/j.redox.2017.12.019
Zhou H, Yang J, Xin T, Li D, Guo J, Hu S, Zhou S, Zhang T, Zhang Y, Han T, Chen Y (2014) Exendin-4 protects adipose-derived mesenchymal stem cells from apoptosis induced by hydrogen peroxide through the PI3K/Akt-Sfrp2 pathways. Free Radical Biol Med 77:363–375. https://doi.org/10.1016/j.freeradbiomed.2014.09.033
Zhou H, Yang J, Xin T, Zhang T, Hu S, Zhou S, Chen G, Chen Y (2015) Exendin-4 enhances the migration of adipose-derived stem cells to neonatal rat ventricular cardiomyocyte-derived conditioned medium via the phosphoinositide 3-kinase/Akt-stromal cell-derived factor-1alpha/CXC chemokine receptor 4 pathway. Mol Med Rep 11:4063–4072. https://doi.org/10.3892/mmr.2015.3243
Zhou H, Yue Y, Wang J, Ma Q, Chen Y (2018) Melatonin therapy for diabetic cardiomyopathy: a mechanism involving Syk-mitochondrial complex I-SERCA pathway. Cell Signal. https://doi.org/10.1016/j.cellsig.2018.03.012
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y (2017) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res. https://doi.org/10.1111/jpi.12413
Zhou H, Zhu P, Guo J, Hu N, Wang S, Li D, Hu S, Ren J, Cao F, Chen Y (2017) Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol 13:498–507. https://doi.org/10.1016/j.redox.2017.07.007
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. https://doi.org/10.1038/s41418-018-0086-7
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Zhou H, Chen Y (2018) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23:101–113. https://doi.org/10.1007/s12192-017-0827-4
Zong C, Qin D, Yu C, Gao P, Chen J, Lu S, Zhang Y, Liu Y, Yang Y, Pu Z, Li X, Fu Y, Guan Q, Wang X (2017) The stress-response molecule NR4A1 resists ROS-induced pancreatic beta-cells apoptosis via WT1. Cell Signal 35:129–139. https://doi.org/10.1016/j.cellsig.2017.03.012
Funding
This study was supported by Grants from the National Natural Science Foundation of China (no. 81770237). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
HZ is involved in conception and design, performance of experiments, data analysis and interpretation, and manuscript writing. HZ, JW, and PJZ are involved in the development of methodology. HZ and JR are involved in the data acquisition. SYH and PJZ are involved in data analysis and interpretation. HZ and YDC are involved in study supervision and final approval of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhou, H., Wang, J., Zhu, P. et al. RETRACTED ARTICLE: NR4A1 aggravates the cardiac microvascular ischemia reperfusion injury through suppressing FUNDC1-mediated mitophagy and promoting Mff-required mitochondrial fission by CK2α. Basic Res Cardiol 113, 23 (2018). https://doi.org/10.1007/s00395-018-0682-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-018-0682-1