Abstract
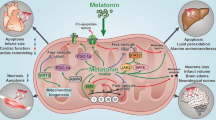
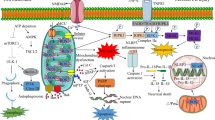
Acute brain reperfusion stress is associated with mitochondrial dysfunction through unknown mechanisms. Accordingly, there is no effective drug to control the development and progression of brain reperfusion stress currently. The aim of our investigation is to verify whether melatonin attenuates acute brain reperfusion stress via affecting mitochondrial function. Our studies demonstrated that melatonin treatment suppressed reperfusion-induced neuron death. At the molecular levels, melatonin treatment modulated mitochondrial homeostasis via activating mitochondrial fusion. At the stage of reperfusion, MFN2 expression was downregulated, contributing to mitochondrial fusion inhibition. Interestingly, MFN2-related mitochondrial fusion was reversed by melatonin. Loss of MFN2-related mitochondrial fusion abrogated the protective actions of melatonin on mitochondrial function. Mechanistically, melatonin sustained MFN2-related mitochondrial fusion via suppressing Mst1-Hippo pathway. Overexpression of Mst1 attenuated the beneficial effects of melatonin on mitochondrial fusion, evoking mitochondrial damage and neuron death in the setting of brain reperfusion stress. Taken together, our results confirmed the protective effects of melatonin on acute brain reperfusion stress. Melatonin treatment activated MFN2-related mitochondrial fusion via suppressing Mst1-Hippo pathway, finally sustaining mitochondrial function and reducing reperfusion-mediated cerebral injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Revascularization is a procedure used to treat stroke via timely opening of the occluded vessels. However, revascularization itself also induces additional damage to ischemic brain, which is termed ischemia-reperfusion (IR) injury (Zhou et al. 2018a). Preventing IR injury could further limit infarct size and improve cerebral function (Rossello et al. 2017). Many studies have focused on the molecular mechanisms underlying neuron death during IR injury and on protective approaches aimed at attenuating IR-induced cerebral damage (Kozlov et al. 2017; Rossello and Yellon 2017; Zhai et al. 2017).
The role of mitochondria in cerebral IR injury has been explored by several in vitro and in vivo studies (Jin et al. 2018; Zhu et al. 2018b). Many brain biological processes are handled by mitochondria, including ATP production, cellular oxidative stress (Liu et al. 2017), intracellular calcium balance (Gadicherla et al. 2017), and apoptosis initiation (Hong et al. 2017). In response to cerebral IR injury, mitochondrial morphology initially converts into mostly small fragments, which occurs by a process identified as mitochondrial fission (Zhou et al. 2017a). Subsequently, excessive mitochondrial fission causes mitochondrial DNA damage. Fragmented mitochondria fail to produce sufficient mitochondrial respiratory complexes, leading to decreased oxidative phosphorylation and increased ROS production (Das et al. 2017). Moreover, damaged mitochondria liberate pro-apoptotic factors such as cyt-c in the nucleus, where cyt-c launches the mitochondria-dependent apoptosis pathway. Based on previous findings, mitochondrial fission is recognized as a potential target to alleviate cerebral IR injury. In contrast to mitochondrial fission, mitochondrial fusion is the repair system that corrects excessive mitochondrial fission by promoting mitochondrial communication (Bikfalvi 2017; Fuhrmann and Brune 2017). With the help of mitofusion-2 (MFN2), a mitochondrial fusion factor, fragmented mitochondria interact with each other, which allows mitochondrial DNA exchange and recovery. Restoration of MFN2 inhibits reperfusion injury in the brain and liver by interfering with mitochondrial fission (Blackburn et al. 2017; Buijs et al. 2017). This notion is further supported by a cardiac reperfusion model, which demonstrated that MFN2 overexpression sustains mitochondrial homeostasis and cardiomyocyte viability (Casadonte et al. 2017; Conradi et al. 2017). However, the upstream regulators of MFN2-related mitochondrial fusion remain unclear.
Although the hormone melatonin is originally used to regulate body rhythms (Tamura et al. 2017), ample evidence supports its therapeutic effects on reperfused brains. Melatonin reduces neuron oxidative stress, attenuates calcium overload, inhibits ER stress, and blocks mitochondrial apoptosis (Cuervo et al. 2017). In addition, the inhibitory action of melatonin on mitochondrial fission has been reported in several careful studies (Zhou et al. 2017b). Interestingly, no studies have investigated the role of melatonin in mitochondrial fusion, especially MFN2-related mitochondrial fusion.
At the molecular level, Mst1, a major downstream effector of the Hippo pathway, has been found to be associated with cerebral protection during reperfusion burden (Gao et al. 2017). Increased Mst1 reduces reperfusion-mediated cardiomyocyte apoptosis by repressing Mst1 expression (Griffiths et al. 2017). The Mst1-Hippo pathway also alleviates cerebral IR injury by inactivating Drp1-related mitochondrial fission (Giatsidis et al. 2018). In rectal cancer and gastric tumors, Mst1 overexpression promotes cancer survival by diminishing mitochondrial fission and enhancing mitochondrial autophagy (Li et al. 2017). Recent evidence has illustrated cross-talk between mitochondrial fission and Mst1-Hippo signaling (Ghiroldi et al. 2017). However, whether Mst1 is also involved in reperfusion-related mitochondrial fusion is poorly understood. The aim of our study was to investigate the beneficial effects of melatonin on MFN2-related mitochondrial fusion with a focus on the Mst1-Hippo pathway in the setting of cerebral IR injury.
Materials and methods
Animal treatment and cerebral IR injury
The surgical protocol used to induce cerebral IR injury was performed according to the methods of a previous study. In brief, 100 mg/kg of pentobarbital was used for anesthesia. Next, the middle cerebral artery was occluded using a 0.22-mm-diameter silicon-covered 6-0 nylon monofilament (Doccol). After 45 min of ischemia, the monofilament was removed to restore the blood flow for approximately 2 h. After IR injury, the brain tissues were isolated and stained with 2,3,5-triphenyltetrazolium chloride (TTC) staining to demonstrate the cerebral infarction zone based on a previous study (Zhao et al. 2018).
Cell culture and hypoxia-reoxygenation injury
A hypoxia-reoxygenation (HR) model was used in mouse N2a neuroblastoma cells (ATCC® CCL-131™) to mimic cerebral IR injury in vitro. N2a cells were cultured in L-DMEM supplemented with 10% FBS. To induce HR injury, the medium was replaced with L-DMEM without FBS in a hypoxia chamber containing 5% CO2 and 95% N2 for 45 min. Subsequently, the medium was replaced with fresh L-DMEM with 10% FBS, and cells were maintained at 37 °C in a 5% CO2 incubator for another 2 h, according to the methods of a previous study (Jin et al. 2018). To prevent Mst1 activation, verteporfin (Sigma, Cat. No. #SML0534) was added to the medium for 2 h before HR injury. Low-dose (10 μM) and high-dose (20 μM) melatonin were added to the medium 24 h before HR injury.
Western blotting
After treatment, infarcted area in brain tissues was collected, and total protein was isolated. Samples (40–80 μg of protein) were separated via 10% SDS-PAGE and transferred to PVDF membranes. After being washed with TBST, the membranes were blocked with 5% nonfat milk at room temperature for 45 min. Then, the membranes were incubated overnight with the primary antibodies at 4 °C. Subsequently, the membranes were washed again with TBST 3 times at room temperature, followed by incubation with secondary antibodies for 45 min at room temperature. After being washed with TBST another three times, the membranes were visualized using an enhanced chemiluminescence (ECL) kit (Beyotime Institute of Biotechnology, China). The following primary antibodies were used for immunoblotting: Drp1 (1:1000, Abcam, #ab56788), MFN2 (1:1000, Abcam, #ab42364), Mfn1 (1:1000, Abcam, #ab57602), Mfn2 (1:1000, Abcam, #ab56889), Mff (1:1000, Cell Signaling Technology, #86668), Bcl2 (1:1000, Cell Signaling Technology, #3498), Bax (1:1000, Cell Signaling Technology, #2772), caspase9 (1:1000, Cell Signaling Technology, #9504), pro-caspase3 (1:1000, Abcam, #ab13847), cleaved caspase3 (1:1000, Abcam, #ab49822), c-IAP (1:1000, Cell Signaling Technology, #4952), survivin (1:1000, Cell Signaling Technology, #2808), Bad (:1000; Abcam; #ab90435), cyt-c (1:1000; Abcam; #ab90529), Mst1(1:1000; Cell Signaling Technology, #14074), complex III subunit core (CIII-core2, 1:1000, Invitrogen, #459220), complex II (CII-30, 1:1000, Abcam, #ab110410), complex IV subunit II (CIV-II, 1:1000, Abcam, #ab110268), GAPDH (1:1000, Cell Signaling Technology, #5174), and β-actin (1:1000, Cell Signaling Technology, #4970).
TUNEL staining and MTT assay
Cell death was measured via a TUNEL assay using an in situ cell death detection kit (Roche, Indianapolis, IN, USA). The TUNEL kit stains nuclei containing fragmented DNA. After HR injury, N2a cells were fixed with 3.7% paraformaldehyde for 30 min at room temperature. Subsequently, an equilibration buffer, nucleotide mix, and rTdT enzyme were incubated with the samples at 37 °C for 60 min. Then, a saline-sodium citrate buffer was used to stop the reaction. After being loaded with DAPI, the samples were visualized via fluorescence microscopy (Olympus BX-61). In addition, an MTT assay was performed to analyze cell viability according to the methods described in a previous study (Brasacchio et al. 2017). Absorbance at 570 nm was determined. The relative cell viability was recorded as the ratio with the control group.
Caspase activity detection and ELISA
Caspase-3 and caspase-9 activity were determined using commercial kits (Beyotime Institute of Biotechnology). The levels of antioxidant factors including GPX, SOD, and GSH were measured with ELISA kits, which were purchased from Beyotime Institute of Biotechnology. ELISA Kits (#PM4000B for TNFɑ, #PM6000B for MCP-1; #PM4030B for IL-8, Cusabio Technology, Wuhan, China) were used to measure the concentrations of TNFα, IL-8, and MCP1 after IR injury.
Transfection
Transfection with siRNA was used to inhibit MFN2 expression in melatonin-treated N2a cell. siRNA (siRNA-MFN2, Yangzhou Ruibo Biotech Co., Ltd. (Yangzhou, China)) were used to infect N2a cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The negative control group was transfected with negative control siRNA. Transfection was performed for approximately 48 h. Then, Western blotting was used to observe the knockdown efficiency after harvesting the transfected cells.
Flow cytometry for mROS
Flow cytometry was used to analyze mitochondrial ROS (mROS) production. After HR injury, N2a cells were washed three times with PBS and then resuspended in PBS using 0.25% trypsin. Subsequently, cells were incubated with the MitoSOX red mitochondrial superoxide indicator (Molecular Probes, USA) for 15 min at 37 °C in the dark. After three washes with PBS, mROS production was analyzed via flow cytometry (Sysmex Partec GmbH, Görlitz, Germany) (Li et al. 2018), and the data were analyzed using Flowmax software (Sysmex Partec, Version 2.3, Germany).
Immunofluorescent staining and mitochondrial potential detection
After HR injury, N2a cells were first fixed with 4% paraformaldehyde for 30 min at room temperature. After incubation with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity, the samples were treated overnight with primary antibodies at 4 °C. Afterwards, the slides were washed with PBS and then incubated with secondary antibody (1:500, Invitrogen, Carlsbad, CA, USA) at room temperature for 45 min. Nuclei were stained using DAPI. Images were acquired via fluorescence microscopy (Olympus BX-61). The following primary antibodies were used in the present study: MFN2 (1:1000, Abcam, #ab42364), Mst1 (1:1000; Cell Signaling Technology, #14074), and cyt-c (1:1000; Abcam; #ab90529). Mitochondrial potential was measured using a JC-1 kit (Beyotime Institute of Biotechnology, China). After HR injury, N2a cells were washed three times with PBS and then incubated with fresh medium supplemented with 10 mg/ml JC-1. Thirty minutes later, the samples were washed three times with PBS to remove the free probe, and then fresh medium was added. The samples were observed via fluorescence microscopy (Olympus BX-61). The red/green fluorescence of JC-1 was analyzed using Image Pro Plus version 4.5 (Media Cybernetics, Inc., Rockville, MD, USA) (Zhang et al. 2016).
Statistical analysis
Statistical analyses were performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). All results in the present study were analyzed by one-way analysis of variance followed by Tukey’s test. P < 0.05 was considered statistically significant.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
Results
Melatonin attenuates reperfusion stress-mediated cerebral damage
In our study, 45 min of ischemia and 4 h of reperfusion were used to establish cerebral IR injury, and low- and high-dose melatonin were administered 24 h before cerebral IR injury. Subsequently, infarction area was measured to evaluate the role of melatonin in brain protection. As shown in Fig. 1a, b. the cerebral infarct area was obviously increased in reperfused brains, and this effect was repressed by melatonin in a concentration-dependent manner (Fig. 1a, b). The formation of the infarct zone entails neuron death in response to reperfusion stress. As shown in Fig. 1c, d, TUNEL staining revealed that the number of TUNEL-positive neuron was markedly elevated after IR injury and was reduced by melatonin treatment in a dose-dependent manner. This finding was supported in vitro using N2a cell in a hypoxia-reoxygenation (HR) model. Caspase-3 activity was significantly increased in HR-treated Na2 cells (Fig. 1e), an effect that was accompanied by a drop in cell viability, as assessed by MTT assay (Fig. 1f). However, melatonin was able to reverse HR-mediated neuron damage in a dose-dependent manner.
Melatonin treatment alleviates cerebral IR injury by reducing neuron death and repressing the inflammatory response. Forty-five min of ischemia and 2 h of reperfusion were used to establish the cerebral IR injury model. Low-dose (10 mg/kg) and high-dose (20 mg/kg) melatonin were administered intraperitoneally 24 h before cerebral IR injury. a, b The infarct area was observed using TTC staining. c, d TUNEL assays were performed to quantify neuron death in response to cerebral IR injury. e In vitro, N2a cell were used and 45 min of hypoxia and 2 h of reoxygenation were used to establish HR injury. Then, cell apoptosis was determined by analyzing caspase-3 activity. f Cell viability was measured using LDH release assays. g–i Blood was collected after cerebral IR injury, and the concentrations of inflammatory factors were analyzed using ELISA. Data represent the mean ± SEM (n = 6 for each group). Asterisk indicates p < 0.05
In addition to cell apoptosis, we further observed inflammatory responses during brain IR injury. Using ELISA, we found that serum MIP1α, IL-8, and MMP9 levels were rapidly upregulated in response to reperfusion stress (Fig. 1g–i), which was reduced by melatonin supplementation. Altogether, our results demonstrated that melatonin was able to reduce cerebral IR injury in a dose-dependent manner by attenuating neuron apoptosis and repressing the inflammatory response.
Melatonin improves MFN2-related mitochondrial fusion
To investigate the beneficial role of melatonin in the reperfused brain, we observed the changes in mitochondrial fusion. In vivo, Western blotting demonstrated that IR injury reduced the expression of mitochondrial fusion-related factors, such as OPA1, MFN1, and MFN2 (Fig. 2a–f). In contrast, mitochondrial fission factors including Mff and Drp1 were significantly upregulated in response to reperfusion stress (Fig. 2a–f). Interestingly, melatonin supplementation restored the levels of mitochondrial fusion-related proteins and repressed the expression of fission-related factors. Interestingly, among the tested proteins, MFN2 expression was increased to the greatest extent by melatonin. This result indicated that melatonin may activate mitochondrial fusion via MFN2.
Melatonin enhances Mfn2-related mitochondrial fusion. a–f In vivo, Western blotting of mitochondrial fusion- and fission-related proteins. g–i Mitochondrial fusion and Mfn2 expression were observed using immunofluorescence. siRNAs against Mfn2 were transfected into melatonin-treated cells. l The average mitochondrial length was determined to quantify mitochondrial fusion. Data represent the mean ± SEM (n = 6 for each group). Asterisk indicates p < 0.05
To further observe mitochondrial fusion, mitochondrial morphology was assessed using immunofluorescence assays. As shown in Fig. 2g–i, HR injury induced mitochondria division into several fragments, indicative of increased mitochondrial fission and decreased fusion. Interestingly, melatonin treatment reversed the mitochondrial interconnective morphology. To verify whether MFN2 was involved in melatonin-mediated mitochondrial integrity, siRNAs against MFN2 were transfected into melatonin-treated cells. Meanwhile, MFN2 expression was determined using coimmunofluorescence. As shown in Fig. 2g–i, compared with the melatonin group (20 μM), MFN2 siRNAs transfection caused the formation of mitochondrial debris. Subsequently, mitochondrial length was measured and used to quantify mitochondrial fusion. The baseline length of mitochondria was ~ 9.3 μm in N2a cells containing abundant MFN2 expression (Fig. 2g–i). However, the average mitochondrial length was rapidly reduced to ~ 4.6 μm upon HR stress, which coincided with a drop in MFN2 expression (Fig. 2g–i). Melatonin treatment restored mitochondrial length to ~ 8.6 μm, and this effect was abrogated by MFN2 siRNAs transfection. Altogether, our results indicated that melatonin activated MFN2-related mitochondrial fusion in the context of cerebral IR injury.
MFN2 knockdown abrogates the protective effects of melatonin on mitochondrial energy metabolism
To explain the protective mechanism of MFN2-related mitochondrial fusion on reperfused brains, mitochondrial function was determined. First, cellular ATP content was reduced in response to HR treatment, and this effect was reversed by melatonin (Fig. 3a). However, MFN2 knockdown abolished the ability of melatonin to promote ATP production. At the molecular level, mitochondria are the energy center of N2a cells, and ATP is primarily generated in mitochondria via conversion of the mitochondrial potential energy into chemical energy. Interestingly, mitochondrial potential, as assessed by JC-1 staining, was significantly dissipated in response to HR stress (Fig. 3b). However, melatonin stabilized the mitochondrial potential depending on MFN2-related mitochondrial fusion. We also found that activity of the mitochondrial respiratory complex was downregulated in HR-treated N2a cell (Fig. 3c–e); this effect was reversed by melatonin supplementation via enhanced MFN2-related mitochondrial fusion.
Mfn2 knockdown abolishes the protective effects of melatonin on mitochondrial energy metabolism. a ATP production was evaluated using ELISA. Two independent siRNAs against Mfn2 were transfected into melatonin-treated cells. b The JC-1 probe was used to measure the mitochondrial membrane potential. Quantification of the mitochondrial membrane potential was performed by detecting the red-to-green fluorescence intensity ratio. c–e After HR injury, N2a cells were isolated, and ELISA was performed to quantify the activity of the mitochondrial respiratory complex. f, g Glucose uptake and lactic acid production were determined using ELISA. Data represent the mean ± SEM (n = 6 for each group). Asterisk indicates p < 0.05
Furthermore, the remaining glucose in the medium was detected to analyze mitochondrial metabolism. Compared with the control group, HR treatment increased the concentration of glucose in the medium (Fig. 3f, g), indicating decreased glucose uptake by N2a cell. This effect was closely associated with a decline in lactic acid production (Fig. 3f, g). However, melatonin supplementation improved glucose uptake and promoted lactic acid generation, and these effects were nullified by MFN2 knockdown. Taken together, our results indicated that melatonin sustained mitochondrial function by modulating MFN2-related mitochondrial fusion.
Loss of MFN2 induces mitochondrial damage
Irreversible mitochondrial damage initiates the mitochondria-dependent endogenous apoptosis pathway, which is defined as mitochondrial oxidative stress, cyt-c liberation, pro-apoptotic protein upregulation, and caspase-9 activation (Zhu et al. 2018a). As shown in Fig. 4a, using flow cytometry, we observed a significant increase in mitochondrial ROS (mROS) production after HR treatment, indicating mitochondrial oxidative stress. This effect was followed by a steep drop in cellular antioxidant factors, such as GSH, GOD, and GPX (Fig. 4b–d). Interestingly, melatonin treatment neutralized excessive mROS and reversed the decline of antioxidant factors. MFN2 knockdown blocked the antioxidative properties of melatonin during cerebral reperfusion stress.
Mfn2 deficiency activates mitochondrial apoptosis. a Mitochondrial ROS (mROS) were analyzed using flow cytometry. b–d The concentrations of cellular antioxidant factors were determined using ELISA. e, f Immunofluorescence assay for cyt-c translocation from the mitochondria to the nucleus. DAPI was used to label the nucleus. g–k After HR injury, N2a cells were isolated, and Western blotting was used to quantify the proteins related to mitochondrial apoptosis. Data represent the mean ± SEM (n = 6 for each group). Asterisk indicates p < 0.05
Excessive mROS production was accompanied by cyt-c translocation into the cytoplasm/nucleus, and this process was verified via immunofluorescence (Fig. 4e–f). Interestingly, melatonin treatment repressed cyt-c liberation, and this effect was achieved via MFN2-related mitochondrial fusion. This finding was also supported by Western blotting. HR increased the level of cytoplasmic cyt-c (cyto cyt-c) and reduced the expression of mitochondrial cyt-c (mito cyt-c) (Fig. 4g–k); these phenotypic alterations were nullified by melatonin in a manner dependent on MFN2-related mitochondrial fusion. As a consequence of cyt-c liberation, the levels of pro-apoptotic proteins, including Bax and caspase-9, were significantly increased by HR treatment (Fig. 4g–k). In contrast, the expression of anti-apoptotic proteins, such as Bcl-2 and survivin, was drastically downregulated (Fig. 4g–k). However, melatonin treatment reversed the changes in anti-apoptotic protein expression and prevented the activation of pro-apoptotic factors, and this effect was blocked by MFN2 siRNAs transfection. Overall, our data illustrated that melatonin inhibited HR-mediated N2a cell mitochondrial apoptosis via MFN2-related mitochondrial fusion.
Melatonin modulates MFN2 via the Mst1-Hippo pathway
The Mst1-Hippo pathway has been associated with cerebral and cardiac IR injury by sustaining mitochondrial homeostasis (Kleinbongard et al. 2017). In the present study, we investigated whether melatonin improves MFN2-related mitochondrial fusion via the Mst1-Hippo pathway. Western blotting revealed that Mst1 expression was upregulated in response to HR injury and was restored to near-normal levels by melatonin supplementation (Fig. 5a–c). To verify whether melatonin modulated MFN2 expression via Mst1-Hippo signaling, the Mst1 adenovirus (Ad-Mst1) was used in melatonin-treated N2a cells. Ad-Mst1 treatment prevented melatonin-mediated Mst1 downregulation, and this effect was accompanied by a drop in MFN2 expression (Fig. 5a–c). These results indicated that the Mst1-Hippo pathway is required for melatonin-mediated MFN2 expression. This finding was further supported by qPCR, as both Mst1 and MFN2 expression were alterated in response to HR injury and reversed to near-normal levels by melatonin treatment (Fig. 5d, e). However, Ad-Mst1 application inhibited melatonin-mediated MFN2 upregulation, recapitulating the essential role played by the Mst1-Hippo pathway in melatonin-induced MFN2 activation.
Melatonin improves Mfn2 expression by activating the Mst1-Hippo pathway. a–c Western blotting was used to analyze the changes in Mfn2 and Mst1. Verteporfin, a Mst1-Hippo pathway antagonist, was added to the medium. d, e qPCR assay for Mfn2 and Mst1. The transcription of Mfn2 and Mst1 was determined in response to melatonin and/or verteporfin treatment. Data represent the mean ± SEM (n = 6 for each group). Asterisk indicates p < 0.05
Mst1-Hippo signaling is also implicated in reperfusion-mediated N2a cell mitochondrial damage
Finally, we explored whether the Mst1-Hippo pathway was involved in reperfusion-mediated mitochondrial stress and N2a cell death. HR-mediated cyt-c liberation (Fig. 6a, b) and ATP depletion (Fig. 6c) were reversed by melatonin in a Mst1-Hippo pathway-dependent manner. In addition, N2a cell death, as evaluated via TUNEL staining (Fig. 6d) and caspase-9 activation (Fig. 6e), was significantly triggered by HR injury and repressed by melatonin treatment. However, activation of Mst1-Hippo signaling via Ad-Mst1 abolished the anti-apoptotic effects of melatonin in HR-treated N2a cell. Altogether, these data indicated that suppression of the Mst1-Hippo pathway by melatonin promoted N2a cell survival and mitochondrial integrity.
Inhibition of the Mst1-Hippo pathway induces neuron death and mitochondrial stress. a, b Cyt-c immunofluorescence. DAPI was used to label the nucleus. The nuclear expression of cyt-c was analyzed. c ATP production was analyzed using ELISA. d Neuron death was analyzed using LDH release assays. e Caspase-9 activity was measured via ELISA. Verteporfin was used to prevent Mst1-Hippo pathway activation. Data represent the mean ± SEM (n = 6 for each group). Asterisk indicates p < 0.05
Discussion
In the present study, we demonstrated that melatonin alleviated cerebral IR injury by activating MFN2-related mitochondrial fusion. Biological analyses illustrated that melatonin reduced N2a cell death, maintained brain function, and corrected cell energy metabolism disorders. At the molecular level, cerebral IR injury was characterized by mitochondrial stress, highlighted by mitochondrial fragmentation due to increased mitochondrial fission and decreased fusion. Interestingly, melatonin improved mitochondrial fusion, and this effect was achieved via upregulation of MFN2 expression. Increased MFN2-related mitochondrial fusion suppressed mitochondrial oxidative stress and disrupted mitochondrial apoptosis, favoring N2a cell survival in the context of cerebral IR injury. However, loss of MFN2 abolished the protective effects of melatonin on mitochondrial homeostasis and N2a cell viability. Although imbalanced mitochondrial dynamics (mitochondrial fission and fusion) have been acknowledged as a pathogenic factor contributing to the progression of cerebral IR injury, little attention has been paid to the role of mitochondrial fusion in reperfusion-related brain damage. Accordingly, this study is the first to explore the molecular features of melatonin-induced mitochondrial fusion in cerebral reperfusion stress. In addition, our study provided evidence to support the regulatory role of melatonin in MFN2-related mitochondrial fusion via the Mst1-Hippo pathway.
Excessive mitochondrial fission has been noted in reperfused brains in several animal and cell studies (Jokinen et al. 2017; Lee and Back 2017). The primary consequence of mitochondrial fission is mitochondrial malfunction and N2a cell apoptosis. In contrast to mitochondrial fission, mitochondrial fusion corrects the aberrant fission by promoting mitochondrial communication (Nuntaphum et al. 2018). The beneficial effects of mitochondrial fusion on mitochondrial genome integrity, mitochondrial oxidative stress, mitochondrial autophagy, mitochondrial calcium homeostasis, and mitochondrial apoptosis have been explored in several disease models, such as amyotrophic lateral sclerosis (Hassanshahi et al. 2017), pulmonary arterial hypertension (Karwi et al. 2017), heart failure (Iggena et al. 2017), and Parkinson’s disease (Hambright et al. 2017). In addition, several cell biological processes are highly modulated by mitochondrial fusion, such as the cell cycle, cell apoptosis, stem cell differentiation, and mitochondrial biogenesis (Han et al. 2017; Hooshdaran et al. 2017; Kelly et al. 2017). Most studies of cerebral IR injury have focused on the influence of mitochondrial fission in reperfusion-induced N2a cell damage, but few studies have explored the contribution of mitochondrial fusion in cerebral IR injury (Zhou et al. 2018c). In the present study, we reported that mitochondrial fusion was largely inhibited by IR injury, as evidenced by the reduced transcription and expression of mitochondrial fusion factors. This finding was similar to those of previous studies in which enhanced mitochondrial fusion alleviated heart (Peng et al. 2018; Zhou et al. 2018b) and liver IR injury (Zhang et al. 2017). These results implicate mitochondrial fusion as an important target to modify mitochondrial homeostasis and N2a cell viability in response to cerebral reperfusion stress.
Our data demonstrated that melatonin activated MFN2-related mitochondrial fusion via the Mst1-Hippo pathway. In cerebral IR injury, Mst1 inhibition attenuates reperfusion-mediated neuronal apoptosis by inhibiting mitochondrial fission (Geng et al. 2018). Similarly, in human rectal cancer, Mst1 affects Drp1-dependent mitochondrial fission, contributing to cancer death (Li et al. 2017). This evidence highlights a strong correlation between Mst1 expression and mitochondrial homeostasis. Notably, no studies have investigated the role of Mst1 in mitochondrial fusion. Our data may provide some answers, showing that Mst1 suppression is required for MFN2 stabilization and that this process is closely regulated by melatonin. However, the molecular mechanisms by which Mst1 controls MFN2 expression remain to be elucidated. In view of the central role played by Mst1 in promoting oncogene expression (Guers et al. 2017), whether Mst1 transcriptionally modulates MFN2 activity is an open question.
Overall, our results show that MFN2-related mitochondrial fusion is repressed by cerebral IR injury due to inactive Mst1-Hippo signaling. Restoring MFN2-related mitochondrial fusion via melatonin treatment attenuates mitochondrial damage and contributes to neuron survival in the context of cerebral reperfusion stress. This work identifies the Mst1-Hippo pathway and MFN2-related mitochondrial fusion as novel targets for the potential development of therapeutic interventions against acute cerebral reperfusion stress.
Change history
19 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12192-021-01206-4
References
Bikfalvi A (2017) History and conceptual developments in vascular biology and angiogenesis research: a personal view. Angiogenesis 20:463–478. https://doi.org/10.1007/s10456-017-9569-2
Blackburn NJR, Vulesevic B, McNeill B, Cimenci CE, Ahmadi A, Gonzalez-Gomez M, Ostojic A, Zhong Z, Brownlee M, Beisswenger PJ, Milne RW, Suuronen EJ (2017) Methylglyoxal-derived advanced glycation end products contribute to negative cardiac remodeling and dysfunction post-myocardial infarction. Basic Res Cardiol 112:57. https://doi.org/10.1007/s00395-017-0646-x
Brasacchio D, Alsop AE, Noori T, Lufti M, Iyer S, Simpson KJ, Bird PI, Kluck RM, Johnstone RW, Trapani JA (2017) Epigenetic control of mitochondrial cell death through PACS1-mediated regulation of BAX/BAK oligomerization. Cell Death Differ 24:961–970. https://doi.org/10.1038/cdd.2016.119
Buijs N, Oosterink JE, Jessup M, Schierbeek H, Stolz DB, Houdijk AP, Geller DA, van Leeuwen PA (2017) A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: dimethylarginine dimethylaminohydrolase 1. Angiogenesis 20:557–565. https://doi.org/10.1007/s10456-017-9567-4
Casadonte L, Verhoeff BJ, Piek JJ, VanBavel E, Spaan JAE, Siebes M (2017) Influence of increased heart rate and aortic pressure on resting indices of functional coronary stenosis severity. Basic Res Cardiol 112:61. https://doi.org/10.1007/s00395-017-0651-0
Conradi LC, Brajic A, Cantelmo AR, Bouché A, Kalucka J, Pircher A, Brüning U, Teuwen LA, Vinckier S, Ghesquière B, Dewerchin M, Carmeliet P (2017) Tumor vessel disintegration by maximum tolerable PFKFB3 blockade. Angiogenesis 20:599–613. https://doi.org/10.1007/s10456-017-9573-6
Cuervo H, Pereira B, Nadeem T, Lin M, Lee F, Kitajewski J, Lin CS (2017) PDGFRbeta-P2A-CreER(T2) mice: a genetic tool to target pericytes in angiogenesis. Angiogenesis 20:655–662. https://doi.org/10.1007/s10456-017-9570-9
Das N, Mandala A, Naaz S, Giri S, Jain M, Bandyopadhyay D, Reiter RJ, Roy SS (2017) Melatonin protects against lipid-induced mitochondrial dysfunction in hepatocytes and inhibits stellate cell activation during hepatic fibrosis in mice. J Pineal Res 62. https://doi.org/10.1111/jpi.12404
Fuhrmann DC, Brune B (2017) Mitochondrial composition and function under the control of hypoxia. Redox Biol 12:208–215. https://doi.org/10.1016/j.redox.2017.02.012
Gadicherla AK, Wang N, Bulic M, Agullo-Pascual E, Lissoni A, de Smet M, Delmar M, Bultynck G, Krysko DV, Camara A, Schlüter KD, Schulz R, Kwok WM, Leybaert L (2017) Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol 112:27. https://doi.org/10.1007/s00395-017-0618-1
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang Z, Li Z, Feng X, Hao J, Zhang K, Xiao B, Chen M, Huang W, Xiong S, Wu X, Deng W (2017) Melatonin synergizes the chemotherapeutic effect of 5-fluorouracil in colon cancer by suppressing PI3K/AKT and NF-kappaB/iNOS signaling pathways. J Pineal Res 62. https://doi.org/10.1111/jpi.12380
Geng C, Wei J, Wu C (2018) Yap-Hippo pathway regulates cerebral hypoxia-reoxygenation injury in neuroblastoma N2a cells via inhibiting ROCK1/F-actin/mitochondrial fission pathways. Acta Neurol Belg. https://doi.org/10.1007/s13760-018-0944-6
Ghiroldi A, Piccoli M, Ciconte G, Pappone C, Anastasia L (2017) Regenerating the human heart: direct reprogramming strategies and their current limitations. Basic Res Cardiol 112:68. https://doi.org/10.1007/s00395-017-0655-9
Giatsidis G, Cheng L, Haddad A, Ji K, Succar J, Lancerotto L, Lujan-Hernandez J, Fiorina P, Matsumine H, Orgill DP (2018) Noninvasive induction of angiogenesis in tissues by external suction: sequential optimization for use in reconstructive surgery. Angiogenesis 21:61–78. https://doi.org/10.1007/s10456-017-9586-1
Griffiths HR, Gao D, Pararasa C (2017) Redox regulation in metabolic programming and inflammation. Redox Biol 12:50–57. https://doi.org/10.1016/j.redox.2017.01.023
Guers JJ, Zhang J, Campbell SC, Oydanich M, Vatner DE, Vatner SF (2017) Disruption of adenylyl cyclase type 5 mimics exercise training. Basic Res Cardiol 112:59. https://doi.org/10.1007/s00395-017-0648-8
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17. https://doi.org/10.1016/j.redox.2017.01.021
Han L, Wang H, Li L, Li X, Ge J, Reiter RJ, Wang Q (2017) Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res 63. https://doi.org/10.1111/jpi.12431
Hassanshahi M, Hassanshahi A, Khabbazi S, Su YW, Xian CJ (2017) Bone marrow sinusoidal endothelium: damage and potential regeneration following cancer radiotherapy or chemotherapy. Angiogenesis 20:427–442. https://doi.org/10.1007/s10456-017-9577-2
Hong H, Tao T, Chen S, Liang C, Qiu Y, Zhou Y, Zhang R (2017) MicroRNA-143 promotes cardiac ischemia-mediated mitochondrial impairment by the inhibition of protein kinase Cepsilon. Basic Res Cardiol 112:60. https://doi.org/10.1007/s00395-017-0649-7
Hooshdaran B, Kolpakov MA, Guo X, Miller SA, Wang T, Tilley DG, Rafiq K, Sabri A (2017) Dual inhibition of cathepsin G and chymase reduces myocyte death and improves cardiac remodeling after myocardial ischemia reperfusion injury. Basic Res Cardiol 112:62. https://doi.org/10.1007/s00395-017-0652-z
Iggena D, Winter Y, Steiner B (2017) Melatonin restores hippocampal neural precursor cell proliferation and prevents cognitive deficits induced by jet lag simulation in adult mice. J Pineal Res 62:e12397. https://doi.org/10.1111/jpi.12397
Jin Q, Li R, Hu N, Xin T, Zhu P, Hu S, Ma S, Zhu H, Ren J, Zhou H (2018) DUSP1 alleviates cardiac ischemia/reperfusion injury by suppressing the Mff-required mitochondrial fission and Bnip3-related mitophagy via the JNK pathways. Redox Biol 14:576–587. https://doi.org/10.1016/j.redox.2017.11.004
Jokinen R, Pirnes-Karhu S, Pietilainen KH, Pirinen E (2017) Adipose tissue NAD(+)-homeostasis, sirtuins and poly(ADP-ribose) polymerases -important players in mitochondrial metabolism and metabolic health. Redox Biol 12:246–263. https://doi.org/10.1016/j.redox.2017.02.011
Karwi QG, Bice JS, Baxter GF (2017) Pre- and postconditioning the heart with hydrogen sulfide (H2S) against ischemia/reperfusion injury in vivo: a systematic review and meta-analysis. Basic Res Cardiol 113:6. https://doi.org/10.1007/s00395-017-0664-8
Kelly P, Denver P, Satchell SC, Ackermann M, Konerding MA, Mitchell CA (2017) Microvascular ultrastructural changes precede cognitive impairment in the murine APPswe/PS1dE9 model of Alzheimer's disease. Angiogenesis 20:567–580. https://doi.org/10.1007/s10456-017-9568-3
Kleinbongard P, Skyschally A, Gent S, Pesch M, Heusch G (2017) STAT3 as a common signal of ischemic conditioning: a lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res Cardiol 113:3. https://doi.org/10.1007/s00395-017-0660-z
Kozlov AV, Lancaster JR Jr, Meszaros AT, Weidinger A (2017) Mitochondria-meditated pathways of organ failure upon inflammation. Redox Biol 13:170–181. https://doi.org/10.1016/j.redox.2017.05.017
Lee K, Back K (2017) Overexpression of rice serotonin N-acetyltransferase 1 in transgenic rice plants confers resistance to cadmium and senescence and increases grain yield. J Pineal Res 62:e12392. https://doi.org/10.1111/jpi.12392
Li H, He F, Zhao X, Zhang Y, Chu X, Hua C, Qu Y, Duan Y, Ming L (2017) YAP inhibits the apoptosis and migration of human rectal cancer cells via suppression of JNK-Drp1-mitochondrial fission-HtrA2/Omi pathways. Cell Physiol Biochem 44:2073–2089. https://doi.org/10.1159/000485946
Li R, Xin T, Li D, Wang C, Zhu H, Zhou H (2018) Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol 18:229–243. https://doi.org/10.1016/j.redox.2018.07.011
Liu D, Zeng X, Li X, Mehta JL, Wang X (2017) Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res Cardiol 113:5. https://doi.org/10.1007/s00395-017-0663-9
Nuntaphum W, Pongkan W, Wongjaikam S, Thummasorn S, Tanajak P, Khamseekaew J, Intachai K, Chattipakorn SC, Chattipakorn N, Shinlapawittayatorn K (2018) Vagus nerve stimulation exerts cardioprotection against myocardial ischemia/reperfusion injury predominantly through its efferent vagal fibers. Basic Res Cardiol 113:22. https://doi.org/10.1007/s00395-018-0683-0
Peng C, Rao W, Zhang L, Gao F, Hui H, Wang K, Dai S, Yang Y, Luo P, Ma Y, Ma W, Yu X, Fei Z (2018) Mitofusin 2 exerts a protective role in ischemia reperfusion injury through increasing autophagy. Cell Physiol Biochem 46:2311–2324. https://doi.org/10.1159/000489621
Rossello X, Yellon DM (2017) The RISK pathway and beyond. Basic Res Cardiol 113:2. https://doi.org/10.1007/s00395-017-0662-x
Rossello X, Riquelme JA, He Z, Taferner S, Vanhaesebroeck B, Davidson SM, Yellon DM (2017) The role of PI3Kalpha isoform in cardioprotection. Basic Res Cardiol 112:66. https://doi.org/10.1007/s00395-017-0657-7
Tamura H, Kawamoto M, Sato S, Tamura I, Maekawa R, Taketani T, Aasada H, Takaki E, Nakai A, Reiter RJ, Sugino N (2017) Long-term melatonin treatment delays ovarian aging. J Pineal Res 62:e12381. https://doi.org/10.1111/jpi.12381
Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, Yu L, Liu Z, Yu B, Ren K, Gao E, Yang Y, Liang H, Jin Z, Yu S (2017) Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res 63:e12419. https://doi.org/10.1111/jpi.12419
Zhang Y, Zhou H, Wu W, Shi C, Hu S, Yin T, Ma Q, Han T, Zhang Y, Tian F, Chen Y (2016) Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radic Biol Med 95:278–292. https://doi.org/10.1016/j.freeradbiomed.2016.03.035
Zhang C, Huang J, An W (2017) Hepatic stimulator substance resists hepatic ischemia/reperfusion injury by regulating Drp1 translocation and activation. Hepatology 66:1989–2001. https://doi.org/10.1002/hep.29326
Zhao H, Luo Y, Chen L, Zhang Z, Shen C, Li Y, Xu R (2018) Sirt3 inhibits cerebral ischemia-reperfusion injury through normalizing Wnt/beta-catenin pathway and blocking mitochondrial fission. Cell Stress Chaperones 23:1079–1092. https://doi.org/10.1007/s12192-018-0917-y
Zhou H, Hu S, Jin Q, Shi C, Zhang Y, Zhu P, Ma Q, Tian F, Chen Y (2017a) Mff-dependent mitochondrial fission contributes to the pathogenesis of cardiac microvasculature ischemia/reperfusion injury via induction of mROS-mediated cardiolipin oxidation and HK2/VDAC1 disassociation-involved mPTP opening. J Am Heart Assoc 6:e005328. https://doi.org/10.1161/JAHA.116.005328
Zhou H, Zhang Y, Hu S, Shi C, Zhu P, Ma Q, Jin Q, Cao F, Tian F, Chen Y (2017b) Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J Pineal Res 63:e12413. https://doi.org/10.1111/jpi.12413
Zhou H, Ma Q, Zhu P, Ren J, Reiter RJ, Chen Y (2018a) Protective role of melatonin in cardiac ischemia-reperfusion injury: from pathogenesis to targeted therapy. J Pineal Res 64. https://doi.org/10.1111/jpi.12471
Zhou H, Wang J, Hu S, Zhu H, Toanc S, Ren J (2018b) BI1 alleviates cardiac microvascular ischemia-reperfusion injury via modifying mitochondrial fission and inhibiting XO/ROS/F-actin pathways. J Cell Physiol doi:https://doi.org/10.1002/jcp.27308
Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y (2018c) Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ 25:1080–1093. https://doi.org/10.1038/s41418-018-0086-7
Zhu H, Jin Q, Li Y, Ma Q, Wang J, Li D, Zhou H, Chen Y (2018a) Melatonin protected cardiac microvascular endothelial cells against oxidative stress injury via suppression of IP3R-[Ca(2+)]c/VDAC-[Ca(2+)]m axis by activation of MAPK/ERK signaling pathway. Cell Stress Chaperones 23:101–113. https://doi.org/10.1007/s12192-017-0827-4
Zhu P, Hu S, Jin Q, Li D, Tian F, Toan S, Li Y, Zhou H, Chen Y (2018b) Ripk3 promotes ER stress-induced necroptosis in cardiac IR injury: a mechanism involving calcium overload/XO/ROS/mPTP pathway. Redox Biol 16:157–168. https://doi.org/10.1016/j.redox.2018.02.019
Author information
Authors and Affiliations
Contributions
SL, XYL, CLB, and JFL were involved in the conception and design, performance of experiments, data analysis and interpretation, and manuscript writing. CLB was involved in data analysis and interpretation.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://dx.doi.org/10.1007/s12192-021-01206-4
About this article
Cite this article
Lan, S., Liu, J., Luo, X. et al. RETRACTED ARTICLE: Effects of melatonin on acute brain reperfusion stress: role of Hippo signaling pathway and MFN2-related mitochondrial protection. Cell Stress and Chaperones 24, 235–245 (2019). https://doi.org/10.1007/s12192-018-00960-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-018-00960-2