Abstract
Objective
The aim of this study was to systematically review the literature to evaluate the clinical performance of integrated 18F-FDG PET/MR as compared with 18F-FDG PET/CT in oncologic imaging.
Methods
The literature was searched using MEDLINE and EMBASE via OVID. Studies comparing the diagnostic accuracy of integrated 18F-FDG PET/MR and 18F-FDG PET/CT in the diagnosis, staging/restaging, assessment of treatment response, or evaluation of metastasis in patients with suspected or diagnosed cancers were deemed eligible for inclusion. Risk of bias and applicability concerns were assessed using the QUADAS-2 tool.
Results
Twenty studies met the inclusion criteria. The overall quality of the studies was rated favorably with bias or applicability concerns in a few studies. Our review suggests that 18F-FDG PET/MR performs comparably to 18F-FDG PET/CT in the detection of local lymph node and distant metastases and superiorly in determining the local extent of tumor. SUV obtained from 18F-FDG PET/MR correlated highly with those obtained from 18F-FDG PET/CT.
Conclusions
Based on early evidence, 18F-FDG PET/MR is comparable to 18F-FDG PET/CT in the clinical scenarios examined in this review. The potential for interchangeability of 18F-FDG PET/MR with 18F-FDG PET/CT will vary by indication and the body site that is being imaged, with PET scanners integrated with MRI predicted to provide greater detail in the evaluation of local tumor extent, where 18F-FDG PET/CT can be limited.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography (PET) performed with 18F-fluorodeoxyglucose (18F-FDG) provides unique information regarding tumour metabolism in cancer patients, which cannot be determined by conventional imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI). PET has changed the way cancer patients are managed by providing critical information regarding tumour staging and prognosis. The integration of PET with low-dose CT (PET/CT) has resulted in its widespread use in cancer imaging by allowing rapid collection of accurate attenuation correction data, which enable quantification of metabolic activity, and by providing anatomic detail allowing improved interpretation of studies [1, 2].
The use of hybrid PET/CT imaging is not without its shortcomings. First, CT adds to the amount of ionizing radiation (6.40–19.70 mSv) delivered to the patient during the examination [3]. Radiation exposure in general should be minimized, particularly in the pediatric population and in women of child-bearing age [4]. Second, CT provides relatively poor soft tissue contrast, notably in the head and neck, and in gynecologic malignancies. With the evolving transition of PET/MR from the research to clinical arena, there is a growing interest in determining its clinical capabilities, particularly for indications where MRI has been shown to be superior to CT.
Several strategies have emerged for combining PET and MRI data. Initial solutions comprised retrospective fusion of independently or sequentially acquired PET and MRI data using dedicated software registration algorithms. However, these approaches are time consuming and can limit the accuracy of the evaluation due to differences in patient position during each imaging step. More recently, fully integrated PET/MR scanners have become available to enable simultaneous or sequential acquisition of PET and MR data in order to obtain more accurate image registration with reduced examination times.
To date, the use of integrated PET/MR in clinical settings is restricted due to its limited availability, cost, and the technical challenges associated with implementing the system. An important challenge has been to generate tissue attenuation maps to allow accurate quantification of metabolic activity [5]. Nonetheless, early data regarding the feasibility and potential oncologic applications of integrated PET/MR have been promising. As a result, the purpose of this study was to systematically review the literature to evaluate the clinical performance of integrated PET/MR as compared with PET/CT in oncologic imaging and the possibility of using existing PET/MR systems interchangeably with PET/CT for common clinical indications.
Materials and methods
Search strategy
The literature was searched using MEDLINE and EMBASE via OVID up to June 9, 2016. See Supplemental Table 1 for the search strategy. The reference lists from relevant articles were searched for additional studies, as were the reference lists from relevant review articles. In addition, the National Guidelines Clearinghouse, the Canadian Medical Association Infobase, the National Institute for Health and Care Excellence, the Scottish Intercollegiate Guidelines Network, and the National Health and Medical Research Council were searched up to June 2016 for existing evidence-based guidelines. Identified systematic reviews were evaluated based on their clinical content and relevance.
Study selection criteria and process
After duplicates of the retrieved articles were removed, the following criteria were used to screen for eligibility: (1) published as a full article in a peer-reviewed journal; (2) evaluated the use of PET/CT and PET/MR with 18F-FDG; (3) studies that used an integrated simultaneous or sequential PET/MR system; (4) histopathologic results, clinical or radiologic follow-up were used as the reference standard; and (5) studies that reported numeric data on diagnostic performance (e.g., sensitivity, specificity, positive predictive value, negative predictive value, accuracy) with a p value less than 0.05 to indicate statistical significance. The exclusion criteria were: (1) conference abstracts, literature or narrative reviews, letters, editorials, historical articles, or commentaries; (2) single case reports or case series with fewer than 12 patients; and (3) reports published in a language other than English because translation was not available. A review of the titles and abstracts that resulted from the search was conducted independently by one author, as were the items that warranted full-text review.
Data extraction and assessment of study quality and potential for bias
One author extracted all study data, such as study characteristics, imaging sequence protocol, reference standard, and diagnostic performance. All extracted data and information were audited by an independent auditor. Furthermore, an assessment of study quality was performed for each eligible study by one author. Due to variable population characteristics and outcome measurements among the eligible studies, a meta-analysis was not conducted. Instead, a narrative synthesis of the results according to disease site was presented.
Results
Literature search results
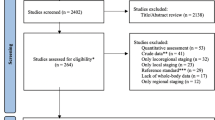
A total of 8678 unique citations were identified from the electronic searches, of which 8598 were excluded after a review of titles and abstracts. Eighty citations were considered as candidates, but upon full-text review, 60 did not meet the inclusion criteria. Finally, the remaining 20 studies were included in this systematic review. See Fig. 1 for the literature flow diagram. PET/MR images were obtained with an integrated, simultaneous PET/MR device in 18 studies [6–23], while the other two studies used a sequential-acquisition PET/MR system [24, 25]. No existing guidelines, systematic reviews, or randomized controlled trials were found that specifically evaluated the comparability of diagnostic performance between PET/CT and PET/MR imaging.
Study design and quality
Twelve studies enrolled patients prospectively, whereas the rest were evaluated retrospectively. The study quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool (Table 1). For the domains relating to bias, two studies were judged to have high risk of bias in patient selection. One study included only male patients [24]. The other study enrolled only patients who had confirmed diagnoses, which may lead to an overestimation of the accuracy [6]. Moreover, readings for PET/MR and PET/CT were either not blinded to the results of the reference standard [7, 8] or were unclear as to whether the results were interpreted without knowledge of the reference standard [9, 10, 17, 22, 24]. In the same way, most of the studies did not provide sufficient information to determine whether the reference standard results were interpreted without knowledge of the index test results [7, 9–25]. In terms of applicability concerns, one study was noted to have an underrepresentation of patients with higher tumour stages in the cohort [22], while another had an atypical distribution of lymphoma subtypes which frequently show low or no FDG uptake [19]. Despite these limitations, the overall quality of the studies was rated favourably, with bias or applicability concerns in only a few studies.
Diagnostic performance
The clinical characteristics and diagnostic results reported in each eligible study are shown in Table 2.
Breast cancer
A recent study indicated comparable results of PET/MR and PET/CT in the characterization of primary tumours and the detection of axillary lymph node metastases; however, PET/MR enabled a correct identification of the T-stage in significantly more cases (n = 50, 82 versus 68%; p < 0.05) [6]. Furthermore, results from another study showed that PET/MR detected a higher number of bony metastases than PET/CT (141 versus 90; p < 0.001). The estimated sensitivity of PET/MR and PET/CT were 96.3 and 85.2%, respectively. Overall, PET/MR identified additional sites of bony metastases in 12% of cases that were not demonstrated on PET/CT. These findings led to management changes that included the immediate start of radiation therapy, modification to hormone therapy, and initiation of chemotherapy [11]. PET/MR was also demonstrated to have great diagnostic potential in staging recurrent breast cancer compared to PET/CT with a sensitivity and specificity of 100% and 88.9% versus 95.7% and 88.9%, respectively [21].
Colorectal cancer
To date, two studies have shown promising results for PET/MR in colorectal cancer. PET/MR with diffusion weighted imaging (DWI) proved to be more sensitive (71 versus 30%; p < 0.05) and more accurate (74 versus 56%, p = 0.006) than PET/CT in the evaluation of liver metastases. No significant differences were seen in diagnosing intestinal lesions, peritoneal lesions, or lymph node and pulmonary metastases [18]. Likewise, PET/MR showed at least comparable accuracy to PET/CT (91.7 versus 83.3%, respectively) in N and M staging/restaging of colorectal and rectal cancer patients [25].
Esophageal cancer
One study demonstrated no significant differences in accuracy (n = 12, 83.3 versus 66.7%; p > 0.99) or area under the curve (0.80 versus 0.63; p = 0.163) between PET/MR and PET/CT for diagnosing nodal metastasis in patients with resectable esophageal cancer [24].
Gynecologic cancer
For gynecologic cancer applications, one prospective study showed equal sensitivity (n = 58, 100%) for detecting malignant lesions in recurrent ovarian and cervical cancer [12]. Another study also reported a comparably high diagnostic performance between PET/MR and PET/CT in the restaging of patients with a suspected tumor recurrence of a pelvic malignancy [20].
Head and neck cancer
The evidence comparing PET/MR with PET/CT in head and neck cancer was illustrated in two studies. No significant difference in diagnostic capability was seen between the two multimodality imaging techniques for local tumour staging and cancer recurrence diagnosis in patients with suspected or known cancer of the head and neck [13, 22].
Lymphoma
PET/MR with or without DWI was of similar efficacy as PET/CT in assessing nodal and extranodal involvement in patients with Hodgkin and non-Hodgkin lymphoma with an accuracy of 100% with DWI, 87.5% without DWI for PET/MR, and 87.5% for PET/CT [19].
Non-small cell lung cancer
There were two prospective studies that compared PET/MR with PET/CT in the non-small cell lung cancer population. PET/MR did not provide significant advantages over PET/CT in terms of detecting lymph node metastases (p = 0.48) [14] or determining resectability [15].
Thyroid cancer
In patients with differentiated thyroid cancer suspected or known to have become dedifferentiated, PET/MR was inferior to PET/CT in characterizing pulmonary metastases (accuracy: n = 81, 79.0 versus 97.5%; <0.001), but no significant differences were found in detecting local relapse, or lymph node and bone metastases [23].
Various sites
A number of studies have compared PET/MR with PET/CT in patients with different primary cancers. In this heterogeneous population, one retrospective study reported that PET/MR impacted the care of patients more often than PET/CT (p < 0.001). PET/MR revealed additional findings not seen on PET/CT in 41.0% of patients (n = 134) and affected clinical management in 17.9% (e.g., avoidance of biopsy, close follow-up instead of chemotherapy, surgery, initiation of chemoradiation, radiofrequency ablation, or radiation). Conversely, PET/CT revealed additional findings not seen on PET/MR in 4.5% of patients (n = 134) and affected clinical management in 1.5% (e.g., chest CT follow-up) [7]. In primary tumour staging (p = 0.74), regional lymph node staging (p > 0.05), and distant metastasis staging (p > 0.05), the diagnostic performance did not differ significantly [8]. Other studies have also reported no differences in sensitivity between PET/MR and PET/CT for the detection of malignant liver (n = 26, 100% for both) or bone (n = 48, 100 versus 93.8%) lesions as well as a wide spectrum of tumours or non-tumour lesions (n = 278, 98.9 versus 93.5%) [9, 10, 16]. In a more recent study, PET/MR was demonstrated to have a significantly higher sensitivity (92.2 versus 67.8%; p < 0.01), negative predictive value (NPV) (95.1 versus 82.0%; p < 0.05) and accuracy (96.1 versus 82.4%; p < 0.001) than PET/CT for the detection of liver metastases [17].
Discussion
To the best of our knowledge, this is the first systematic review performed for the purpose of comparing the performance of PET/CT and integrated PET/MR for oncologic indications. This review has been limited to 18F-FDG as most of the current clinically approved indications and accepted evidence based practices involve this tracer. Certainly, a comprehensive comparison of PET/CT and PET/MR would include other radiotracers, but this is beyond the scope of this work. Literature of high methodological quality on this topic is limited due to the nascent nature and availability of this modality. This will likely change in the coming years but at this time it was necessary that this summary be performed in a survey format of multiple tumor types and clinical scenarios. Most of the included studies address solid tumor types. Notably, there has been limited work so far in the evaluation of lymphoma, which typically represents a large component of clinical PET imaging.
Only a subset of the available literature was composed of prospective cohort studies, many with low sample sizes. Many of the studies are considered pilot or preliminary studies. It is not possible from the available literature to consistently compare PET obtained from PET/CT to PET obtained with MR-based attenuation correction. Furthermore, in multiple studies showing superiority of PET/MR, it is not possible to determine whether that is solely due to the contribution of MR, or whether PET/MR fusion was explicitly superior to composite data from scans obtained separately. The impact of the additional site-specific or indication-specific MR sequences on diagnostic performance is unknown and would likely skew the comparison of performance characteristics in favor of PET/MR.
There are several challenges when designing a study comparing PET/CT to PET/MRI. In an effort to minimize radiation dose and maximize patient convenience and validity of comparisons, the studies must be done in one imaging session in sequence. This precludes direct comparison of standardized uptake values (SUVs) derived from MR based versus CT based attenuation maps, as SUV is known to increase with time. Only a correlation factor can be calculated. The most direct comparison between the two modalities would be to use a Dixon sequence for MR imaging but that reduces the real world utility of the MRI component as this modality offers a number of sequences that provide advantages based on the tumor type and location. This however makes inter-study comparison difficult. The reference standard is most often a composite of biopsy and imaging follow-up, which can be prone to bias and confounding. This limitation is difficult to eliminate due to patient preference and study ethics. There is also a selection bias resulting from the selection of candidates who can actually tolerate the time to complete both studies in sequence.
As mentioned above, a major criticism of PET/MR is the inability to accurately calculate SUV with MR based attenuation correction. Several studies addressed this concern showing good correlation (Spearman’s or Pearson’s coefficient 0.72–0.91) between SUV derived from PET/CT and PET/MR devices [10, 14, 16, 17, 19, 21, 23, 25]. Absolute SUV correlation remains unknown as the studies are performed in sequence and are affected by differences in time from tracer injection to scanning. For example, in Paspulati et al. [25], patients who underwent PET/MRI first yielded lower SUV than PET/CT. The converse was true when PET/CT was performed first. In the remaining studies, PET/MR was performed second and consistently yielded higher SUV values. Historically problematic areas for MR based SUV calculation including bone and lungs were not specifically addressed in most studies. For clinical applications, current differences in calculated SUV values may not be a limiting factor for the use of PET/MRI in oncology, but this question remains unresolved.
Differences in the imaging protocol among these studies related to indication-based dedicated MR sequences precluded intra-modality comparison and generation of summary statistics for diagnostic performance and also objective comparison with PET/CT (Supplemental Table 2). Studies performed with retrospective fusion of PET and MR images were not analyzed due to the heterogeneity of MR techniques, inability to accurately assess MR-based SUV, and potential bias created by selecting studies that were retrospectively technically adequate. Although it would be expected that additional MR protocols would be performed on an integrated scanner tailored to disease site and indication, current non-standardization of PET/MR protocols along with absence of randomized controlled trials or expert guidelines on this topic limit the generalizability of these findings.
The studies summarized in this review are limited both in design and number due to the novelty of PET/MR as an imaging modality. However, this work provides some important insights into the advantages and limitations of PET/MR imaging. For example, one study suggests that PET/MR performs superiorly to PET/CT in determining the local extent of tumour in breast cancer [6] as well as assessment for disease recurrence. This finding is expected, and in keeping with current understanding of the superiority of MRI over PET/CT in local tumour extent evaluation. In another study evaluating thyroid cancer, PET/MR was less effective in detecting lung metastases [23]. For the question of interchangeability of these modalities, PET/CT generally is not used for local staging. However, the source literature does suggest the possibility of a “one-stop-shop” approach for complete TNM staging [8]. PET/CT in general has been shown to be of greatest benefit in the detection of local lymph node and distant metastases. PET/MR and PET/CT are comparable for this purpose for all the malignancy sites examined. Overall, the anticipated advantages of PET/CT versus PET/MR arising from the CT or MR components of the integrated scanners hold true. Predictably, PET/CT is superior in the evaluation of lung parenchyma in comparison to PET/MRI. PET/MRI excels at evaluating local tumor extent, particularly for malignancies of the head and neck, female pelvis and breast. PET/MRI would also predictably yield greater accuracy for the diagnosis of brain metastases given its superior contrast resolution when compared to the CT component of PET/CT and limited utility of 18F-FDG in the brain due to normal physiologic cerebral uptake.
Although the general trend appears to show equivalency between PET/CT and PET/MR, additional work is required. Further work and confirmatory studies to ensure accurate quantification and reproducibility of SUV from integrated scanners is needed as this can affect patient management decisions. To begin evaluating the interchangeability or benefit of PET/MR over PET/CT, there needs to be a consensus of required MRI sequences for specific disease sites and across research centres to allow sharing and aggregation of evidence to support the transition to this modality.
Well-designed prospective cohort or randomized controlled trials evaluating impact on predefined clinical outcomes such as management changes and survival are necessary. In an increasing fiscally challenging health care environment, cost-effectiveness analyses are vital to justify the increased cost of PET/MR examinations, specifically comparing integrated PET/MR with the current standard practice of obtaining PET/CT and site specific MRI on separate visits.
Conclusions
Based on the early evidence to date, PET/MR appears to be comparable to PET/CT and in some specific use scenarios, superior. PET/MR excels at local tumor characterization and is comparable when assessing nodal and distant metastatic disease. However, given the scarcity of data, as well as heterogeneity of the imaging protocols and study methodologies, the role of PET/MR in clinical practice remains unknown. Therefore, specific recommendations where PET/MR may be superior to PET/CT in routine clinical work cannot be made at this time. Based on the accelerating pace of work in this field, this will soon change. Further work will be needed to standardize imaging protocols, determine reliability of PET/MR-derived SUV, and identify clinical indications where PET/MR may improve clinical outcomes.
References
Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44:1200–9.
Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–79.
Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. 2009;251:166–74.
Czernin J, Ta L, Herrmann K. Does PET/MR imaging improve cancer assessments? Literature evidence from more than 900 patients. J Nucl Med. 2014;55:59S–62S.
Wagenknecht G, Kaiser HJ, Mottaghy FM, Herzog H. MRI for attenuation correction in PET: methods and challenges. MAGMA. 2013;26:99–113.
Grueneisen J, Nagarajah J, Buchbender C, Hoffmann O, Schaarschmidt BM, Poeppel T, et al. Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast cancer: a comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Invest Radiol. 2015;50:505–13.
Catalano OA, Rosen BR, Sahani DV, Hahn PF, Guimaraes AR, Vangel MG, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients—a hypothesis-generating exploratory study. Radiology. 2013;269:857–69.
Heusch P, Nensa F, Schaarschmidt B, Sivanesapillai R, Beiderwellen K, Gomez B, et al. Diagnostic accuracy of whole-body PET/MRI and whole-body PET/CT for TNM staging in oncology. Eur J Nucl Med Mol Imaging. 2015;42:42–8.
Beiderwellen K, Gomez B, Buchbender C, Hartung V, Poeppel TD, Nensa F, et al. Depiction and characterization of liver lesions in whole body (18F)-FDG PET/MRI. Eur J Radiol. 2013;82:e669–e675.
Beiderwellen K, Huebner M, Heusch P, Grueneisen J, Ruhlmann V, Nensa F, et al. Whole-body (18F)FDG PET/MRI vs. PET/CT in the assessment of bone lesions in oncological patients: initial results. Eur Radiol. 2014;24:2023–30.
Catalano OA, Nicolai E, Rosen BR, Luongo A, Catalano M, Iannace C, et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br J Cancer. 2015;112:1452–60.
Beiderwellen K, Grueneisen J, Ruhlmann V, Buderath P, Aktas B, Heusch P, et al. ((18)F)FDG PET/MRI vs. PET/CT for whole-body staging in patients with recurrent malignancies of the female pelvis: initial results. Eur J Nucl Med Mol Imaging. 2015;42:56–65.
Kubiessa K, Purz S, Gawlitza M, Kuhn A, Fuchs J, Steinhoff KG, et al. Initial clinical results of simultaneous 18F-FDG PET/MRI in comparison to 18F-FDG PET/CT in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2014;41:639–48.
Heusch P, Buchbender C, Kohler J, Nensa F, Gauler T, Gomez B, et al. Thoracic staging in lung cancer: prospective comparison of 18F-FDG PET/MR imaging and 18F-FDG PET/CT. J Nucl Med. 2014;55:373–8.
Fraioli F, Screaton NJ, Janes SM, Win T, Menezes L, Kayani I, et al. Non-small-cell lung cancer resectability: diagnostic value of PET/MR. Eur J Nucl Med Mol Imaging. 2015;42:49–55.
Tian J, Fu L, Yin D, Zhang J, Chen Y, An N, et al. Does the novel integrated PET/MRI offer the same diagnostic performance as PET/CT for oncological indications? PLoS One. 2014;9:e90844.
Beiderwellen K, Geraldo L, Ruhlmann V, Heusch P, Gomez B, Nensa F, et al. Accuracy of [18F]FDG PET/MRI for the detection of liver metastases. PLoS One. 2015;10:e0137285.
Brendle C, Schwenzer NF, Rempp H, Schmidt H, Pfannenberg C, la Fougere C, et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J Nucl Med Mol Imaging. 2016;43:123–32.
Giraudo C, Raderer M, Karanikas G, Weber M, Kiesewetter B, Dolak W, et al. 18F-fluorodeoxyglucose positron emission tomography/magnetic resonance in lymphoma: comparison with 18F-fluorodeoxyglucose positron emission tomography/computed tomography and with the addition of magnetic resonance diffusion-weighted imaging. Invest Radiol. 2016;51:163–9.
Grueneisen J, Schaarschmidt BM, Heubner M, Suntharalingam S, Milk I, Kinner S, et al. Implementation of FAST-PET/MRI for whole-body staging of female patients with recurrent pelvic malignancies: a comparison to PET/CT. Eur J Radiol. 2015;84:2097–102.
Sawicki LM, Grueneisen J, Schaarschmidt BM, Buchbender C, Nagarajah J, Umutlu L, et al. Evaluation of 18F-FDG PET/MRI, 18F-FDG PET/CT, MRI, and CT in whole-body staging of recurrent breast cancer. Eur J Radiol. 2016;85:459–65.
Schaarschmidt BM, Heusch P, Buchbender C, Ruhlmann M, Bergmann C, Ruhlmann V, et al. Locoregional tumour evaluation of squamous cell carcinoma in the head and neck area: a comparison between MRI, PET/CT and integrated PET/MRI. Eur J Nucl Med Mol Imaging. 2016;43:92–102.
Lee G, Hoseok I, Kim SJ, Jeong YJ, Kim IJ, Pak K, et al. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med. 2014;55:1242–7.
Vrachimis A, Burg MC, Wenning C, Allkemper T, Weckesser M, Schafers M, et al. [(18)F]FDG PET/CT outperforms [(18)F]FDG PET/MRI in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2016;43:212–20.
Paspulati RM, Partovi S, Herrmann KA, Krishnamurthi S, Delaney CP, Nguyen NC. Comparison of hybrid FDG PET/MRI compared with PET/CT in colorectal cancer staging and restaging: a pilot study. Abdom Imaging. 2015;40:1415–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singnurkar, A., Poon, R. & Metser, U. Comparison of 18F-FDG-PET/CT and 18F-FDG-PET/MR imaging in oncology: a systematic review. Ann Nucl Med 31, 366–378 (2017). https://doi.org/10.1007/s12149-017-1164-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-017-1164-5