Abstract
Purpose
The aim of this study was to evaluate the diagnostic capability of simultaneous 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/MRI compared to 18F-FDG PET/CT as well as their single components in head and neck cancer patients.
Methods
In a prospective study 17 patients underwent 18F-FDG PET/CT for staging or follow-up and an additional 18F-FDG PET/MRI scan with whole-body imaging and dedicated examination of the neck. MRI, CT and PET images as well as PET/MRI and PET/CT examinations were evaluated independently and in a blinded fashion by two reader groups. Results were compared with the reference standard (final diagnosis determined in consensus using all available data including histology and follow-up). Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated.
Results
A total of 23 malignant tumours were found with the reference standard. PET/CT showed a sensitivity of 82.7 %, a specificity of 87.3 %, a PPV of 73.2 % and a NPV of 92.4 %. Corresponding values for PET/MRI were 80.5, 88.2, 75.6 and 92.5 %. No statistically significant difference in diagnostic capability could be found between PET/CT and PET/MRI. Evaluation of the PET part from PET/CT revealed highest sensitivity of 95.7 %, and MRI showed best specificity of 96.4 %. There was a high inter-rater agreement in all modalities (Cohen’s kappa 0.61–0.82).
Conclusion
PET/MRI of patients with head and neck cancer yielded good diagnostic capability, similar to PET/CT. Further studies on larger cohorts to prove these first results seem justified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Computed tomography (CT), magnetic resonance imaging (MRI) and 18F-fluorodeoxyglucose (FDG) positron emission tomography with combined CT (PET/CT) are well-established diagnostic imaging tools for investigation of initial staging and post-treatment settings in head and neck cancer [1–6].
The new hybrid imaging modality PET/MRI is expected to be of special use in body regions with difficult anatomy and in organs where the inherent soft tissue contrast of MRI is superior to that of CT [7, 8]. One of these regions is the head and neck area, where MRI has the additional advantage of lower susceptibility to dental filling artefacts compared to CT [9]. A general advantage of PET/MRI over PET/CT is the lower radiation exposure due to the omission of CT, especially if contrast-enhanced CT is performed. Although this might be considered to be of lower importance in tumour patients as their life expectancy is generally reduced, this effect should not be underrated, especially in younger patients who need follow-up studies.
Especially due to the high mobility and the anatomical complexity of the head and neck region, performing simultaneous PET/MRI may result in a better alignment of both imaging data in comparison to retrospectively fused PET and MR imaging data. This is because patient movements that can occur during patient repositioning are minimized when using two separate or sequential examination approaches [10].
Two feasibility studies have already shown the possibility of PET/MRI in head and neck cancer imaging. One of them was performed with a prototype PET insert into a 3 T MRI [11], and the other one was done on a sequential PET/MRI system, showing that PET and MR image quality were not impaired [12].
This report presents the first results of a prospective clinical study, in which PET/CT and integrated PET/MRI were used to evaluate patients with suspected or known cancer of the head and neck region. Diagnostic capability of the two combined modalities as well as of the three independent components MRI, CT and PET was compared, when the same subjects were examined by PET/CT and PET/MRI on the same day.
Materials and methods
Patients
This prospective study was approved by the local Ethics Committee and all patients gave their written informed consent. A total of 22 consecutive head and neck cancer patients were investigated. Inclusion and exclusion criteria for the analysis are presented in Table 1.
Of the 22 patients, 17 fulfilled the inclusion criteria, among them 7 patients for primary staging and 10 patients for restaging from 6 months to 4 years after therapy (3 after radiochemotherapy, 2 after surgery and radiochemotherapy, 2 after surgery, 1 after surgery and radiotherapy, 1 after surgery and chemotherapy and 1 after chemotherapy). Of these 17 patients, 4 were female, and their mean age was 60 years (range 42–78 years). All patients underwent an 18F-FDG PET/CT and subsequently a 3 T simultaneous PET/MRI on the same day without further radiopharmaceutical administration.
PET/CT acquisition
All patients underwent an 18F-FDG PET/CT protocol using a Siemens Biograph 16 PET/CT scanner (Siemens Medical Solutions, Erlangen, Germany). Before the investigation, patients fasted for at least 6 h. After administration of 275–445 MBq of 18F-FDG depending on body weight (5 MBq/kg) and a median uptake time of 90 min, the whole-body PET data were acquired with 3 min per bed position. Contrast-enhanced CT scan was performed after intravenous injection of 120 ml contrast agent (Imeron 300, Bracco Imaging, Constance, Germany) with a collimation of 16 × 0.75 mm, a tube voltage of 120 kVp and the use of angular and longitudinal dose modulation (CARE Dose4D®, Siemens Medical Solutions, Erlangen, Germany). Patients were placed in the supine position.
Depending on the clinical situation and their inclusion in radiochemotherapy studies patients underwent different (PET)/CT protocols:
-
Eight patients with clinically proven tumour recurrence or suspicion of metastasized tumour were placed with their arms up and received a contrast-enhanced scan from skull base to the groins during free breathing (150 mAs per protocol), followed by a low-dose chest CT during inspiration (50 mAs per protocol).
-
Nine patients with clinically suspected local tumour only or high-risk patients (former head and neck cancer International Union Against Cancer stage III or IV) underwent a dedicated CT of the neck (150 mAs per protocol, patient positioning with arms down). As three of these nine patients already had a recent CT of the chest, only six underwent an additional full-dose chest CT during inspiration (100 mAs per protocol) after a change in arm positioning. All nine received a low-dose CT scan from skull base to the groins for attenuation correction of the PET data.
PET/MRI acquisition
Subsequently, PET/MR imaging was performed on the integrated PET/MR scanner (Siemens Biograph mMR, Siemens Healthcare, Erlangen, Germany). The examination protocol developed at our institution combined a whole-body scan with a dedicated examination of the head and neck area which resulted in an examination time of 70 min and was used for primary staging, follow-up examinations and tumour search in patients with cancer of unknown primary (CUP). Patients were placed in the supine position with arms down. Specially designed coils were placed on the patient: one head and neck coil and four body phased array coils which together covered the region from skull to thigh.
Then, whole-body PET/MR imaging without contrast medium was performed in six bed positions: head, neck, thorax, abdomen, pelvis and proximal thighs (for details see Table 2). This investigation already included a coronal Dixon-volumetric interpolated breath-hold examination (VIBE) sequence for attenuation correction. Chosen b values for diffusion-weighted imaging (DWI) were 0 s/mm2 to allow fast scanning without the need for an additional gradient and 800 s/mm2, in concordance with other whole-body DWI studies [13–15]. The diffusion-sensitizing gradients were applied in all three orthogonal directions. These MRI measurements took 5 min per bed position, during which PET data were acquired. Compared to PET/CT this longer acquisition time leads to a higher count rate and slightly better image quality of PET/MRI, although this effect was not separately evaluated in this study. Respiratory triggering was used in the bed positions of thorax and abdomen only, other bed positions were scanned during free breathing. Subsequently, a dedicated MRI of the neck was performed (for details see Table 3), which included a coronal Dixon-VIBE sequence for attenuation correction. For contrast-enhanced MR imaging, an axial dynamic contrast-enhanced T1-weighted fast low-angle shot (FLASH) sequence with 40 measurements over 4 min was performed immediately during intravenous administration of a single dose of 0.1 mmol/kg gadobutrol (Gadovist®, Bayer HealthCare, Leverkusen, Germany) at a rate of 3 ml/s and flushing with 10 ml of normal saline using a power injector (Spectris Solaris, Medrad/Bayer HealthCare, Leverkusen Germany). A dedicated evaluation of tumour perfusion was not performed in this study. Exemplary images of the neck protocol are shown in Fig. 1.
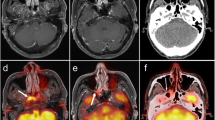
A 52-year-old female patient with glottic carcinoma and a nodal metastasis at level V on the right side. Metastasis seen in all modalities. a T2-weighted HASTE image from whole-body MRI showing necrotic nodal metastasis (arrow). b Fat-saturated T2-weighted TSE image from dedicated neck MRI with the same metastasis better visualized (arrow). c Fat-saturated T1-weighted TSE post contrast with circular enhancement of the metastasis (arrow). d Maximum intensity projection (MIP) image of the 18F-FDG PET with focally increased glucose metabolism in the vocal chord (open arrow) and in the outline contour of the mostly necrotic nodal metastasis (arrow). e Axial PET image in the region of the nodal metastasis (arrow). f Fused image of simultaneously acquired PET and T1-weighted TSE MR images
Image analysis
The PET/CT and PET/MRI data sets were evaluated on dedicated workstations (PET/CT and PET/MRI: syngo.via, Siemens Healthcare, Erlangen, Germany; CT only and MRI only: MagicView 1000, Siemens Medical Solutions, Erlangen, Germany; PET only: Hermes Hybrid Viewer, Hermes Medical Solutions, Stockholm, Sweden).
The reference standard was defined as the final collective diagnosis determined in consensus between an experienced board-certified radiologist, specialized in head and neck radiology, an experienced board-certified nuclear medicine physician and a head and neck surgeon. They had access to all relevant data including medical history, prior examinations, results of panendoscopy or surgery as well as all imaging data (PET/CT and PET/MRI). Histopathological correlates following surgery were available for six patients. Histological diagnosis after biopsy could be used in five patients. In the remaining six patients follow-up examinations showed tumour progression in three (two with PET/CT and one with CT), tumour regression after chemotherapy in one and confirmed the absence of tumour in two patients (CT). Follow-up time ranged between 2 and 14 months.
For image interpretation, the investigation of the CT only and MRI only was done independently and in a blinded fashion by two radiologists (radiologists A and B) with a 4-week gap between the readings of MRI and CT. The readers only took into account all images from MRI and CT without the knowledge of any findings from the corresponding PET component. Evaluation of MRI included all morphological sequences and DWI.
A lesion was considered malignant if there was a visible soft tissue mass, atypical contrast enhancement or central necrosis in a mass on CT. Lymph nodes were considered malignant if they were enlarged in their short axis above 1.5 cm, had a round shape or an unusually strong contrast enhancement. With regard to MRI, decreased diffusion in a circumscribed region on the apparent diffusion coefficient (ADC) map was also considered a sign of malignancy as well as hyperintensity on T2-weighted images.
The evaluation of the PET part from PET/CT and PET/MRI was also performed independently and in a blinded fashion by two nuclear medicine physicians (nuclear physicians 1 and 2), without considering any diagnostic MRI or CT images. This was done with a 4-week gap between the analysis of the two PET parts, using the whole-body exam PET data. The PET data of the head and neck scan were only used for the three PET/MRI examinations without whole-body imaging. The semi-quantitative standardized uptake value (SUVmax) was used as a tool to supplement visual interpretation. Visual assessment of the PET images focused on the pattern and asymmetry of FDG distribution as well as contrast to background ratio.
Subsequently, PET/MRI and PET/CT were assessed independently, in a blinded fashion and in a randomized order by two reader groups, each composed of one radiologist and one nuclear physician (reader groups I and II). In these evaluations, all of the above-mentioned characteristics for malignant lesions of the combined modalities were used. Especially for lymph nodes, an intense and asymmetrical FDG uptake was regarded as a sign of malignancy, even in normal sized nodes. For one and the same reader there was always a 4-week gap between evaluation of the single and combined modalities.
Statistical analysis
For statistical analysis in comparison to the reference standard, evaluation was done on a lesion-by-lesion basis. Only the correct detection and classification as a malignant lesion was evaluated as positive. Failure in lesion detection, correct classification of a lesion as non-malignant or benign classification of a malignant lesion were each evaluated as negative. Thus statistical analysis was exclusively based on lesions that could be detected in at least one of the three imaging modalities.
Statistical assessment was based on sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) analysis of each multimodal imaging modality (PET/CT, PET/MRI) and each single part of the combined diagnostic tools (CT, MRI, PET). The McNemar test with Bonferroni correction for multiple testing was used to compare sensitivities, specificities, PPVs and NPVs between the different diagnostic modalities of a single investigator or within the same reader group (statistically significant difference in single test when p < 0.0083). Cohen’s kappa coefficient was used to assess the inter-rater agreement between different observer groups within the corresponding single or combined diagnostic modality.
Results
All patients tolerated the PET/CT well. With regard to the PET/MRI study protocol, in three patients only dedicated sequences for the neck region were obtained and no whole-body protocol due to their constrained compliance. Another patient received his whole-body PET/MRI examination only from the liver region upwards to the head due to unknown technical problems in the two lower bed positions (MRI scans did not start, so there were no anatomical images and no possibilities to reconstruct attenuation-corrected PET images in this region). But in any case there were no relevant findings in the uncovered body regions. All other 14 patients tolerated the whole PET/MRI protocol well.
In the study population the definitive diagnoses from the reference standard revealed 23 malignant findings. Of these, eight lesions were primary, residual or recurrent tumours. There were three carcinomas of the base of the tongue, two laryngeal carcinomas, one tonsillar carcinoma, one carcinoma of the oropharynx and one hypopharyngeal carcinoma. Furthermore, 15 foci of metastatic spread were diagnosed. In particular there were ten lesions with metastatic cervical or mediastinal lymph nodes, four pulmonary metastases and one metastasis in the chest wall. The size of the lesions ranged between 1.1 and 8.5 cm (mean 2.5 cm).
Besides these 23 positive findings, 55 benign, inflammatory or non-specific changes were established and classified as negative. These findings were rated as non-specific/inflammatory lymph nodes (n = 23), inflammatory/post-operative changes in pharynx, paravascular cervical space, chest and abdominal wall (n = 16), renal and hepatic cysts (n = 5), uterine fibroadenoma (n = 2), thyroid nodes (n = 2) and one case each of thrombosed carotid artery, pleomorphic adenoma, follicular nodular hyperplasia, lateral cleft branch cyst, discitis, silicoanthracosis and old focal cerebellar infarcts.
The findings of the different investigators are summarized in Table 4. In this study, PET/CT as well as PET/MRI reached comparable good specificity of 85.5–89.1 % for PET/CT and 81.8–94.5 % for PET/MRI and very good NPV of 90.7–94 % for PET/CT and 91.2–93.8 % for PET/MRI, respectively (see Fig. 2). Both combined diagnostic modalities showed no statistically significant differences in diagnostic capability for both reader groups (see Table 5).
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the single MRI and CT part of radiologists A and B, of the single PET part from PET (CT) and PET (MRI) of nuclear medicine physicians 1 and 2 as well as of the combined PET/CT and PET/MRI evaluation of the two reader groups I and II, respectively
The two radiological readers A and B detected slightly fewer malignant lesions on MRI as compared to the other single or combined modalities, which resulted in a sensitivity of 73.9 % and a NPV from 88.7 to 89.8 %. For instance, the laryngeal tumour of one patient was overlooked by both investigators, because only a very slight asymmetry in the vocal cord was present (see Fig. 3).
A 52-year-old female patient with right glottic carcinoma and a nodal metastasis at level V on the right side (same patient as Fig. 1). TM seen on PET and CT, but missed on MRI by both examiners. a CT shows thickened vocal chord with slightly pronounced enhancement (arrows) and necrotic nodal metastasis (open arrow). b Fat-saturated T2-weighted TSE with only slight asymmetry of the vocal chord (arrow). c Inverted PET (CT) image with asymmetrically increased glucose metabolism at the level of the vocal chord (arrow). d Fusion of simultaneously acquired PET and T2-weighted TSE MR image
In contrast, both nuclear medicine physicians found more false-positive lesions (12–15) with both single PET parts from PET/CT and PET/MRI than all the other reader groups, resulting in a lower specificity from 72.7 to 78.2 %, associated with a PPV of 59.4–60.6 %. For example, the elevated 18F-FDG uptake (SUV values between 5.3 and 10.8) in multiple mediastinal lymph nodes of a patient with histologically confirmed tuberculosis were falsely considered to be caused by tumoural growth by all single PET evaluations and the PET/CT as well as PET/MRI observer group I. In contrast, all single CT and MRI evaluations plus PET/CT and PET/MRI reader group II correctly categorized these lymph nodes as benign or inflammatory changes (see Fig. 4).
A 70-year-old male patient with CUP (squamous cell carcinoma within the resected submandibular gland). PET/CT and consecutive PET/MRI for search of primary tumour and further metastases. Several lymph nodes were initially suspicious for metastasis due to increased glucose metabolism on 18F-FDG PET; however, pathohistology of the lymph node at level Vll (see b and c) showed tuberculosis. a MIP reconstruction of PET data with several suspicious lymph nodes in mediastinum and abdomen. b CT with only slightly enlarged lymph node in upper mediastinum/level VII (arrow). c MRI (whole-body T2-weighted HASTE) of the same lymph node (arrow). d) PET image with increased glucose metabolism in the same node (arrow) and in a lymph node further right (open arrow). e) Fused PET/CT image
Similarly, there were also false-positive and false-negative diagnoses in difficult cases, which could be observed by almost every single investigator or reader group. For instance, in one patient a histopathologically confirmed nodular fibrosis with anthracosis in the left lung was falsely declared as intrapulmonary tumour or metastasis by all investigators and modalities except the single MRI evaluation. In the combined evaluation these false-positive diagnoses were based on elevated radiotracer accumulation within the lesion (SUV = 9).
The highest sensitivity of 95.7 % was reached by the single PET evaluation of nuclear physician 1. The best result for specificity was 96.4 % for the sole MRI evaluation of radiologist A.
The McNemar test revealed a statistically significant difference of the diagnostic capability between the single CT and MRI evaluation by radiologist B (p value = 0.007). No statistically significant differences were shown between all the other single or combined modalities (Table 5).
Finally, in this study very good inter-rater agreement was found between both nuclear physicians concerning the single PET evaluation of PET/MRI with a Cohen’s kappa coefficient of 0.82. For the remaining observer groups, good agreement with Cohen’s kappa coefficients between 0.61 and 0.78 could be determined (Table 6).
Discussion
Prior studies in head and neck cancer patients so far concentrated on the feasibility of simultaneous [11] or sequential [12] PET/MRI acquisition and the resulting image quality. This study provides results of the first 17 patients with suspected cancer of the head and neck region undergoing simultaneous 18F-FDG PET/MRI after routine PET/CT imaging at our institution. No statistically significant differences in sensitivity, specificity, PPV and NPV could be found between the two hybrid imaging modalities in these patients. This proves the diagnostic capabilities of the newly developed, integrated PET/MRI scanner, although the high expectations concerning superior performance of this method could not yet be verified [7, 8, 10]. This might be due to the high sensitivity of the PET examination, which helps to overcome limitations of tumour detection on CT and thus levels out the advantages of MRI in soft tissue tumour detection.
Evaluations concerning the image fusion from MRI and PET recently indicated that synchronous assessment of morphological (MRI) and functional (PET) data sets can be expected to reach a higher diagnostic accuracy in the evaluation of head and neck malignancies, compared to both imaging modalities applied solely. In the study conducted by Nakamoto et al. image fusion showed a slightly higher sensitivity (100 %) in diagnosing the primary tumour in comparison to MRI alone (98 %). Notably, in cases of recurrent tumour growth or occurrence of second malignancies, fused images were highly superior to MRI with an overall sensitivity of 92 instead of 67 %, respectively. In one patient with cancer of unknown origin the primary tumour and in other cases eight further lesions were correctly identified by image fusion only [16]. This assumption is confirmed by the results of our study with an overall sensitivity of 74 % for the MRI part, not distinguishing between primary and recurrent tumours.
Another comparative study aimed to investigate suspected masticator space invasion of advanced buccal squamous cell carcinoma by using different imaging modalities, namely 3 T MRI, CT, PET/CT and retrospectively fused 18F-FDG PET/MRI [17]. Seventeen patients were included in this prospective study. Eventually, fused PET/MRI was superior to the other imaging tools with its sensitivity and specificity of 90.0% and 90.9 %. Moreover, concerning the maximal tumour size, there was better agreement between fused PET/MR images and pathological findings compared to the other three modalities, which all showed overestimation in delineation of lesion diameter. The results of our study did not show such a high sensitivity for the simultaneous PET/MRI in detection of head and neck cancers; in fact they were comparable to PET/CT. However, PET/MRI is an upcoming diagnostic modality and so far there are no standards concerning imaging protocols or interpretation of the resulting images, whereas at our institution there already is a 6-year experience with PET/CT imaging, which might bias the results somewhat towards this modality. Furthermore, these patients represent the first sample of an ongoing, prospective study, so in a larger number of patients the PET/MRI might well prove to have superior diagnostic capability due to its better soft tissue contrast, especially in difficult cases of suspected tumour recurrence.
The MRI investigation on its own showed lower sensitivity in comparison to the other single or combined modalities. This finding emphasizes that the criteria of size, morphology and contrast enhancement are not always sufficient for correct classification of findings. In this study, several times the elevated radiotracer uptake was the only suggestive finding to indicate tumour growth or metastatic spread. This can be seen by the highest sensitivity achieved in the PET evaluation of the PET/CT by reader 1 (95.7 %). On the other hand, this evaluation resulted in the lowest specificity of all modalities in this study (72.7 %). Sensitivity and specificity of the PET evaluation of PET/MRI and PET/CT were comparable for both readers, although PET/MRI was constantly performed as the second examination. One can conclude that the order of the examinations does not influence detection of focal lesions on PET, although its influence on SUV is well known. SUV cut-off levels to distinguish benign from malignant lesions were not used since there is only a trend to higher SUV in malignant lesions. Furthermore, as Boellaard et al. described, SUV depends strongly on several parameters (e.g. effects of noise, image resolution and region of interest definition) and it can only be used for diagnostic purposes when data acquisition and processing are performed in a standardized way [18].
One reason for the lower specificity of PET can be the blinded diagnostic evaluation, as clinical information plays an influential role in difficult cases. In the clinical setting of restaging and the corresponding question regarding presence or absence of vital tumour tissue, it is more than helpful to be aware of all information about the clinical history of a patient. This information eases differentiation between elevated tracer uptake as an effect of radiochemotherapy or as a sign of vital tumour tissue and will diminish false-positive and false-negative diagnoses. Additionally, nuclear medicine physicians at our institution routinely evaluate PET data in combination with CT instead of the PET component only. For this study, the restriction to PET images only could have had a negative effect on specificity. However, a study by Guenzel et al. compared MR with PET imaging in 120 patients suffering from malignant head and neck tumours and found no difference in sensitivity and specificity with regard to diagnosis of primary tumours, tumour recurrence or CUP [19]. Still they conclude that “a combination of both techniques is likely to be the future of diagnostic imaging”.
Limitations of the study presented here comprise the small size of the study group, the inhomogeneity of the study group and the incomplete histopathological confirmation of suspected tumours. The strict order of PET/CT first followed by PET/MRI might also bias the results of glucose-avid findings.
Although the sample size is quite small, this is the first report about the performance of simultaneous PET/MRI in patients with head and neck cancer. As great expectations have been imposed on this method, especially in this patient group, the results help in forming a realistic view of the capabilities of PET/MRI. It will be interesting to investigate whether PET/MRI proves to be advantageous in more specific questions, e.g. detection of tumour recurrence, T staging of primary head and neck cancer or lymph node assessment.
Use of different CT protocols can be explained by different requirements of different chemotherapy studies, for which some of our patients were evaluated. Three patients received only dedicated PET/MRI sequences for the neck region and no whole-body protocol, as they did not agree to a procedure of more than 1 h duration. However, the differences in examined regions did not have any influence on the results of our study, because none of these patients showed a finding in the region not covered by one of the two modalities.
The strict order of the examinations can lead to bias; however, this study was planned keeping the standard PET/CT workflow for two reasons. First, it should give comparable results to earlier examinations. Second, it was not clear what the results of the PET/MRI would be like. As comparable sensitivity and specificity were found in this work, future studies can confidently use alternating strategies or place PET/MRI prior to PET/CT.
After all, these are the first results of patients with head and neck tumours examined in an integrated whole-body PET/MRI scanner and they prove both the technical possibility of a whole-body PET/MRI scan plus an additional, dedicated PET/MRI of the head and neck region as well as the diagnostic capability of the PET/MRI, which is comparable to PET/CT. Whether the new hybrid imaging technology is actually superior to PET/CT will have to be shown by comparisons of PET/CT versus PET/MRI in a larger cohort of patients and also in subgroup analyses (primary cancer staging—tumour recurrence—CUP).
References
Wippold FJ. Head and neck imaging: the role of CT and MRI. J Magn Reson Imaging 2007;25:453–65.
de Bondt RBJ, Nelemans PJ, Bakers F, Casselman JW, Peutz-Kootstra C, Kremer B, et al. Morphological MRI criteria improve the detection of lymph node metastases in head and neck squamous cell carcinoma: multivariate logistic regression analysis of MRI features of cervical lymph nodes. Eur Radiol 2009;19:626–33.
Castelijns JA, van den Brekel MWM. Imaging of lymphadenopathy in the neck. Eur Radiol 2002;12:727–38.
Nakamura T, Sumi M. Nodal imaging in the neck: recent advances in US, CT and MR imaging of metastatic nodes. Eur Radiol 2007;17:1235–41.
Yoon DY, Hwang HS, Chang SK, Rho YS, Ahn HY, Kim JH, et al. CT, MR, US,18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol 2009;19:634–42.
Ghanooni R, Delpierre I, Magremanne M, Vervaet C, Dumarey N, Remmelink M, et al. 18F-FDG PET/CT and MRI in the follow-up of head and neck squamous cell carcinoma. Contrast Media Mol Imaging 2011;6:260–6.
Beyer T, Freudenberg LS, Czernin J, Townsend DW. The future of hybrid imaging-part 3: PET/MR, small-animal imaging and beyond. Insights Imaging 2011;2:235–46.
Antoch G, Bockisch A. Combined PET/MRI: a new dimension in whole-body oncology imaging? Eur J Nucl Med Mol Imaging 2009;36 Suppl 1:S113–20.
Klinke T, Daboul A, Maron J, Gredes T, Puls R, Jaghsi A, et al. Artifacts in magnetic resonance imaging and computed tomography caused by dental materials. PLoS One 2012;7:e31766.
Castelijns JA. PET-MRI in the head and neck area: challenges and new directions. Eur Radiol 2011;21:2425–6.
Boss A, Stegger L, Bisdas S, Kolb A, Schwenzer N, Pfister M, et al. Feasibility of simultaneous PET/MR imaging in the head and upper neck area. Eur Radiol 2011;21:1439–46.
Platzek I, Beuthien-Baumann B, Schneider M, Gudziol V, Langner J, Schramm G, et al. PET/MRI in head and neck cancer: initial experience. Eur J Nucl Med Mol Imaging 2013;40:6–11.
Pearce T, Philip S, Brown J, Koh DM, Burn PR. Bone metastases from prostate, breast and multiple myeloma: differences in lesion conspicuity at short-tau inversion recovery and diffusion-weighted MRI. Br J Radiol 2012;85:1102–6.
Sommer G, Klarhöfer M, Lenz C, Scheffler K, Bongartz G, Winter L. Signal characteristics of focal bone marrow lesions in patients with multiple myeloma using whole body T1w-TSE, T2w-STIR and diffusion-weighted imaging with background suppression. Eur Radiol 2011;21:857–62.
Heusner T, Kuemmel S, Koeninger A, Hamami ME, Hahn S, Quinsten A, et al. Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging 2010;37:1077–86.
Nakamoto Y, Tamai K, Saga T, Higashi T, Hara T, Suga T, et al. Clinical value of image fusion from MR and PET in patients with head and neck cancer. Mol Imaging Biol 2009;11:46–53.
Huang S, Chien C, Lin W, Fang FM, Wang PW, Lui CC, et al. A comparative study of fused FDG PET/MRI, PET/CT, MRI, and CT imaging for assessing surrounding tissue invasion of advanced buccal squamous cell carcinoma. Clin Nucl Med 2011;36:518–25.
Boellaard R, Krak NC, Hoekstra OS, Lammertsma AA. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 2004;45:1519–27.
Guenzel T, Franzen A, Wiegand S, Kraetschmer S, Jahn JL, Mironczuk R, et al. The value of PET compared to MRI in malignant head and neck tumors. Anticancer Res 2013;33:1141–6.
Acknowledgments
The simultaneous PET/MRI device (Siemens Biograph mMR, Siemens Healthcare, Erlangen, Germany) was promoted and funded by the Deutsche Forschungsgemeinschaft (German Research Foundation), Bonn, Germany.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
K. Kubiessa and S. Purz contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kubiessa, K., Purz, S., Gawlitza, M. et al. Initial clinical results of simultaneous 18F-FDG PET/MRI in comparison to 18F-FDG PET/CT in patients with head and neck cancer. Eur J Nucl Med Mol Imaging 41, 639–648 (2014). https://doi.org/10.1007/s00259-013-2633-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-013-2633-2