Abstract
Purpose
In various tumours PET/CT with [18F]FDG is widely accepted as the diagnostic standard of care. The purpose of this study was to compare a dedicated [18F]FDG PET/MRI protocol with [18F]FDG PET/CT for TNM staging in a cohort of oncological patients.
Methods
A dedicated [18F]FDG PET/MRI protocol was performed in 73 consecutive patients (mean age of 59 years, range 21 – 85 years) with different histologically confirmed solid primary malignant tumours after a routine clinical FDG PET/CT scan (60 min after injection of 295 ± 45 MBq [18F]FDG). TNM staging according to the 7th edition of the AJCC Cancer Staging Manual was performed by two readers in separate sessions for PET/CT and PET/MRI images. Assessment of the primary tumour and nodal and distant metastases with FDG PET/CT and FDG PET/MRI was based on qualitative and quantitative analyses. Histopathology, and radiological and clinical follow-up served as the standards of reference. A McNemar test was performed to evaluate the differences in diagnostic performance between the imaging procedures.
Results
From FDG PET/CT and FDG PET/MRI T stage was correctly determined in 22 (82 %) and 20 (74 %) of 27 patients, N stage in 55 (82 %) and 56 (84 %) of 67 patients, and M stage in 32 (76 %) and 35 (83 %) of 42 patients, respectively. Sensitivity, specificity, positive predictive value, negative predictive value and diagnostic accuracy for lymph node metastases were 65 %, 94 %, 79 %, 89 % and 87 % for PET/CT, and 63 %, 94 %, 80 %, 87 % and 85 % for PET/MRI. The respective values for the detection of distant metastases were 50 %, 82 %, 40 %, 88 % and 76 % for PET/CT, and 50 %, 91 %, 57 %, 89 % and 83 % for PET/MRI. Differences between the two imaging modalities were not statistically significant (P > 0.05).
Conclusion
According to our results, FDG PET/CT and FDG PET/MRI are of equal diagnostic accuracy for TNM staging in patients with solid tumours.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In malignant diseases, patient survival and the therapeutic regimen depend on individual tumour stage. Therefore, adequate staging of the tumour, and nodal and distant metastases (TNM staging) encompassing the entire body is required to provide the best available care and best prognosis for patients. Integrated [18F]FDG PET/CT enables simultaneous acquisition of morphological and functional datasets, providing a precise assessment of malignant tumours based on their increased glucose metabolism [1, 2]. In various studies the diagnostic abilities of whole-body (WB) MRI, PET and PET/CT for cancer staging have been evaluated and compared [3–8]. FDG PET/CT has been considered indispensable in oncological imaging since it provides a higher accuracy in TNM staging than either PET or CT alone [3]. MRI with its excellent soft-tissue contrast, which enables a more detailed evaluation of different soft tissues, offers a higher sensitivity than CT in lesion detection in many organs. However, for WB tumour staging Antoch et al. have demonstrated that in overall TNM staging FDG PET/CT provides a superior performance to WB MRI [3].
With the recently introduced integrated hybrid PET/MRI, PET and MRI have been combined into one imaging modality enabling the simultaneous acquisition and highly accurate spatial coregistration of PET and MRI datasets [9–12]. Besides the advantage over CT of the lack of ionizing radiation, MRI in combination with PET is expected to provide a new quality in functional cancer imaging, mainly due to the combination of high soft-tissue contrast and functional MR with PET [9, 12]. Recently published clinical studies using WB PET/MRI in oncological patients have confirmed its feasibility for WB cancer staging and have shown image quality comparable to the image quality with PET/CT for lesion detection [13–15]. For thoracic staging in patients with non-small-cell lung cancer (NSCLC), FDG PET/MRI using a dedicated pulmonary MRI protocol and FDG PET/CT are in good agreement in thoracic T and N staging [16]. Eiber et al. have recently demonstrated the superiority of PET/MR over PET/CT for the anatomical delineation and identification of bone lesions [17]. Furthermore, PET/MRI provides higher lesion conspicuity and diagnostic confidence than PET/CT in the depiction of liver lesions [18].
The aim of this study was to determine the staging accuracies of WB PET/CT and WB PET/MRI for different malignant diseases and to compare these two imaging tools. Histopathology, radiological and clinical follow-up served as the standards of reference.
Material and methods
Patients
A total of 73 patients with a mean age of 59 years (range 21 – 85 years) and different histologically confirmed malignant solid primary tumours were retrospectively included in this study. In all 73 patients a dedicated [18F]FDG PET/MRI protocol was performed after a routine clinical FDG PET/CT scan (60 min after injection of 295 ± 45 MBq [18F]FDG). The malignant diseases included NSCLC (25 patients), breast carcinoma (4), cancer of unknown primary site (1), head and neck tumour (2), melanoma of the uvea (2), genitourinary tumour (2), tumour of the gastrointestinal tract (2), malignant melanoma (33), pleural mesothelioma (1), and liver tumour (1). Histopathological work-up was performed according to institutional standards, current diagnostic criteria of the WHO/IARC were applied, and staging was performed according to the TNM Classification of Malignant Tumors (7th edition). This study was performed in accordance with the regulations of the local institutional ethics committee, and written informed consent was obtained from all participants.
PET/CT imaging
WB FDG PET/CT scans were obtained on a mCT™ PET/CT scanner (Siemens Molecular Imaging, Hoffman Estates, IL). Before imaging, patients fasted for at least 6 h. All patients had blood glucose levels below 150 mg/dL at the time of [18F]FDG injection. [18F]FDG (295 ± 45 MBq) was intravenously injected 60 min before the scan. The contrast-enhanced CT scan was acquired with the following parameters: caudocranial scan direction, field of view (FoV) skull base to upper thighs, 120 kV, automatic mA/s adjustment (CARE Dose4D™, preset 210 mAs), 5 mm slice thickness, 5 mm increment, pitch 1, iodinated contrast agent injection (100 mL, Ultravist™ 300; Bayer Healthcare, Berlin, Germany) at a flow rate of 2 mL/s, followed by a flush of 2.5 mL/s saline, start delay to CT scan 70 s. The PET scan was acquired with the following parameters: 3-D mode, 2 min emission time per bed position (45 % overlap), reconstruction according to the ordered-subsets expectation maximization (OSEM) algorithm with four iterations and eight subsets, 3-D Gaussian filter, 4.0 mm, full-width at half-maximum (FWHM), scatter correction. Attenuation correction was based on the portal venous phase of the WB CT scan.
PET/MR imaging
WB FDG PET/MRI was performed on a Magnetom Biograph mMR™ (Siemens Healthcare, Erlangen, Germany). FDG PET/MRI was performed following the FDG PET/CT scan with a mean delay of 81 ± 51 min to the start of the PET/CT scan. Patients were positioned supine, and imaged from the head to the upper thighs in the caudocranial direction. A dedicated multichannel mMR head and neck coil and, depending on the patient’s height, up to four multichannel mMR™ body flex surface coils were used for MR imaging. MR imaging was performed simultaneously with PET using the following protocol for each bed position: coronal 3-D VIBE sequence (TR 3.6 ms, TE1 1.23 ms, TE2 2.46 ms, 3.12 mm slice thickness, FoV 500 mm) for Dixon-based attenuation correction; transverse T1-weighted turbo-fast angle low shot (FLASH) sequence (TR 1,800 ms, TE 2.4 ms, matrix size 256, 5-mm slices, FoV 450 mm); transverse echo planar diffusion-weighted imaging (DWI; TR 10,500 ms, TE 78 ms, b-values 0, 500 and 1,000 s/mm2, matrix size 160, 5-mm slices, FoV 450 mm, generalized autocalibrating partially parallel acquisition, acceleration factor 2, two averages).
All patients received an injection of a body weight-adapted dose of a gadolinium-based contrast medium (Gadovist™; Bayer Healthcare Pharmaceuticals, Berlin, Germany). Therefore, an additional transverse T1-weighted turbo-FLASH sequence with fat saturation (TR 1,700 ms, TE 3.33 ms, matrix size 256, 5-mm slices, FoV 450 mm) was acquired. The PET acquisition time was 8 min per bed position using list mode. PET images were reconstructed using the iterative OSEM algorithm, three iterations and 21 subsets, Gaussian filter, FWHM 5.0 mm, scatter correction. Default attenuation correction maps using a four-compartment body-segmentation algorithm based on fat and water separation (Dixon method) were used for PET attenuation correction. PET/MRI image fusion was performed for the postcontrast T1-weighted turbo-FLASH images. For patients with diagnosed NSCLC an additional dedicated pulmonary PET/MRI protocol was performed.
Image analysis
TNM staging was performed by two readers with 4 and 5 years experience in MRI and 3 and 4 years experience in hybrid FDG PET/CT imaging, respectively. Both readers were supplied with the same clinical information about each patient. FDG PET/CT and FDG PET/MRI images were analysed separately, in separate sessions and in random order using a picture archiving and communication system (Centricity; General Electric Medical Systems, Milwaukee, WI) and dedicated viewing software for hybrid imaging (syngo.via; Siemens, Healthcare Sector, Erlangen, Germany). There was an interval of at least 6 weeks between reading the FDG PET/CT and the FDG PET/MRI datasets. Discrepancies between the two readers were resolved in a consensus reading by both readers in a separate session.
Assessment of the primary tumour and nodal and distant metastases on FDG PET/CT and FDG PET/MRI scans was based on qualitative and quantitative analyses. FDG PET/CT and FDG PET/MRI data were evaluated qualitatively for regions of focally increased tracer uptake by visual comparison of a lesion’s signal with the FDG uptake of the liver parenchyma on PET. Furthermore, for FDG PET/CT, lymph nodes were graded as malignant or benign based on these functional criteria and based on their size. Determination of lymph node size was based on measurement of the short-axis diameter. Region-specific size criteria were applied when assessing lymph nodes for malignancy [19]. Central necrosis was considered a sign of malignant tumour spread independent of lymph node size. For FDG PET/MRI, readers were instructed to identify all malignant lymph nodes according to the following criteria: increased short-axis diameter, pathological signal intensity (heterogeneous vs. homogeneous), central necrosis, shape (smooth vs. irregular), contrast enhancement, high signal on b-1,000 DWI images with low signal intensity on corresponding ADC maps, and focal FDG uptake. PET images were assessed with and without attenuation correction using the PET data to avoid false-positive findings due to attenuation correction artefacts.
Standard of reference
Both clinical/radiological follow-up and histopathology served as standards of reference. Malignant disease was confirmed by histopathological verification in all patients. Evaluation of the performance in T, N and M staging was limited to those patients in whom an adequate reference standard data were available. Tumour resection with pathological T stage verification was performed in 27 patients; comparison of PET/CT and PET/MRI for accurate assessment of T stage was limited to these patients. For N staging 67 patients were eligible: Pathological N stage verification by lymph node sampling was available in 57 patients, while radiological follow-up served as the standard of reference in 10 patients. For M staging 42 patients were evaluated. Radiological follow-up served as the standard of reference in 41 patients while M stage was pathologically verified in only 1 patient. The mean follow-up time in all patients was 273 days (range 75 – 515 days). The radiological follow-up examinations included CT, MRI and/or PET/CT. The reference standard data were collected by a physician unaware of the results of the two imaging procedures.
Statistics
Statistical analysis was performed using SPSS 22™ (SPSS Inc., Chicago, IL). Data are presented as means ± standard deviation (SD). The McNemar test was used to evaluate the differences in diagnostic performance between the different imaging procedures. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy in the detection of nodal and distant metastases were calculated for FDG PET/MRI and FDG PET/CT. A P value <0.05 was considered to indicate statistical significance.
Results
FDG PET/CT and FDG PET/MRI acquisitions were completed successfully in all 73 patients.
Primary tumour staging
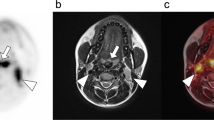
There was agreement between FDG PET/CT and FDG PET/MRI in T staging in 25 of 27 patients (93 %). Compared to resection specimens as the reference standard, the primary tumour was correctly staged using FDG PET/CT in 22 of 27 patients (82 %) and using FDG PET/MRI in 20 of 27 patients (74 %). T stage was overstaged in one patient and understaged in four patients using FDG PET/CT compared to histopathology. Using FDG PET/MRI, T stage was overstaged in two patients (Fig. 1) and understaged in five patients compared to histopathology. Differences between the two imaging modalities were not statistically significant (P = 0.74). For both PET/CT and PET/MRI there was high interobserver agreement in T staging (κ = 0.92).
Primary tumour staging in a 53-year-old male patient with an [18F]FDG-avid histologically proven squamous cell carcinoma in the right anterior floor of the mouth. a–c PET/CT imaging: the tumour mass is seen on the CT image (a), on the PET image with CT-based attenuation correction (b) and on the FDG PET/CT image (c). d–f PET/MRI imaging: the tumour mass is also seen on the contrast-enhanced T1-W MR image (d), on the PET image with MR attenuation correction (e) and on the fused FDG PET/MR image (f). The primary tumour (pT2) was correctly staged as a T2 tumour by FDG PET/CT, but was overstaged by FDG PET/MRI due to suspected mandibular bone infiltration
Regional lymph node staging
Regional lymph node stage was correctly characterized in 55 (82 %) and 56 (84 %) of 67 patients with FDG PET/CT and FDG PET/MRI, respectively (Table 1). The N stage was underestimated in eight and nine patients by PET/MRI and PET/CT (Fig. 2), respectively, and was overestimated in three patients by both imaging modalities. To additionally determine sensitivity, specificity, PPV, NPV and diagnostic accuracy, regional lymph node involvement was characterized as N-positive (N1–N3) or N-negative (N0) with FDG PET/CT and FDG PET/MRI, respectively. Sensitivity, specificity, PPV, NPV and diagnostic accuracy were 63 %, 94 %, 80 %, 87 % and 85 % with PET/MRI. The respective values with FDG PET/CT were 65 %, 94 %, 79 %, 89 % and 87 %. Thus, there was no statistically significant difference between FDG PET/MRI and FDG PET/CT in the detection of lymph node metastases (P > 0.05). For both PET/CT and PET/MRI there was high interobserver agreement in N staging (κ = 0.92).
N staging in a 72-year-old male patient with a histologically proven NSCLC of the left upper lung (pT3). a–c PET/CT imaging: a mediastinal lymph node without pathological enlargement is seen on the CT image (a); the lymph node shows no pathological FDG uptake on the PET image with CT-based attenuation correction (b) or on the fused FDG PET/CT image (c). d–f PET/MRI imaging: the mediastinal lymph node is also seen on the T2-W MR image (d), on the PET image with MR attenuation correction (e) and on the fused FDG PET/MR image (f). The lymph node was falsely staged as benign by FDG PET/CT (N0) and correctly staged as malignant by FDG PET/MRI (N2)
Distant metastasis staging
FDG PET/MRI accurately differentiated between M0 and M1 disease in 35 (83 %) of 42 patients. FDG PET/MRI overstaged the M stage in three patients and understaged it in four patients in relation to the standard of reference. With FDG PET/CT the M stage was determined correctly in 32 (76 %) of 42 patients. FDG PET/CT overestimated the M stage in 6 patients and underestimated the M stage in 4 patients (Table 1). The sensitivity, specificity, PPV, NPV and diagnostic accuracy in the discrimination of M-positive and M-negative disease were 50 %, 91 %, 57 %, 89 % and 83 % by FDG PET/MRI, and 50 %, 82 %, 40 %, 88 % and 76 % by FDG PET/CT, respectively. Consequently, there was no statistically significant difference between FDG PET/MRI and FDG PET/CT (P > 0.05) (Fig. 3).
Distant metastasis staging in a 73-year-old male patient with NSCLC in the right lower lobe. a–c PET/CT imaging: an osteolytic lesion in the left scapula is seen on the CT image (a); this bone lesion shows pathological FDG uptake on the PET image with CT-based attenuation correction (b) and on the FDG PET/CT image (c). d–f PET/MRI imaging: the osteolytic lesion is also seen on the T2-W MR image (d), on the PET image with MR attenuation correction (e) and on the fused FDG PET/MR image (f)
Discussion
Precise knowledge of the local tumour stage as well as detection of potential spread of the tumour to regional lymph nodes and distant organs is required for decision-making in cancer therapy. In this study the diagnostic accuracy of integrated FDG PET/MRI for WB TNM staging in patients with solid tumours was compared with that of FDG PET/CT.
We did not detect any statistically significant differences between FDG PET/CT and FDG PET/MRI for the correct definition of T, N and M stages. With regard to T staging, our results contradict the a priori assumed higher accuracy of FDG PET/MRI over FDG PET/CT for local tumour invasion due to the higher soft-tissue contrast of MRI. Al-Nabhani et al. also found a 10 % improvement in local staging using FDG PET/MRI over FDG PET/CT [20]. In that study follow-up imaging was used as the standard of reference, while in our study only patients with surgical tumour resection and pathological work-up of the primary tumour were included for evaluation of the T staging performance of FDG PET/MRI and FDG PET/CT. The stricter reference standard used in our study might account for the differences in the findings between the two studies. A recent study by Catalano et al. showed a significantly higher number of additional findings on FDG PET/MRI than on FDG PET/CT with an impact on patient management [21]. Looking more closely at the results of that study, with regard to T staging a change in therapeutic concept was related to the detection of local tumour infiltration in only two patients, who received neoadjuvant chemotherapy before resection [22].
Summarizing the available evidence, the differences between FDG PET/MRI and FDG PET/CT for primary tumour staging seem to be marginal. One explanation might be that the higher soft-tissue contrast of MRI is an advantage over CT in only a few specific tumour entities (e.g. soft-tissue sarcoma, rectal carcinoma and cervical carcinoma). In other frequent tumours, such as NSCLC, MRI would not frequently be expected to provide a higher accuracy for the evaluation of the primary tumour, as has recently been demonstrated [16]. Still, for primary tumour staging the detection of invasion of the adjacent anatomical structures is a potential advantage of FDG PET/MR over FDG PET/CT. In our recent study, we did not have any patients in whom the invasion of adjacent anatomical structures could not be excluded by FDG PET/CT [16]. The heterogeneity and numbers of the studied oncological cohorts with different tumour entities may not have been powered to demonstrate potential advantages of FDG PET/MRI. We understand this as a limitation of our own study and propose that the T staging accuracy of FDG PET/MRI might still be an advantage over FDG PET/CT in some entities and certainly has to be further investigated.
The equal diagnostic accuracy of FDG PET/MRI and FDG PET/CT for N staging matches the results of recent studies evaluating the practical clinical significance of FDG PET/MRI in comparison to FDG PET/CT which showed a very high agreement with regard to lesion detection and localization [20]. In the same study the authors found no additional clinical value for the evaluation of lymph node metastases compared to FDG PET/CT. Since the detection of lymph node metastases is predominantly based on the high sensitivity of PET, differences between FDG PET/MRI and FDG PET/CT were not expected. In a few studies, additional functional MRI sequences, such as DWI, facilitated the detection of metastases in lymph nodes showing no enlargement [23]. However, in our study, which included the information derived from DWI, we were not able to demonstrate any advantage of FDG PET/MRI over FDG PET/CT in the detection of lymph node metastases and the determination of N stage, a finding supporting those of previous studies that showed no additional value of DWI for the detection of metastases using FDG PET/MRI [24].
For M staging, we found a low sensitivity compared to recent FDG PET/CT studies [3] and we did not observe any significant differences between FDG PET/MRI and FDG PET/CT in the accuracy of determining M stage. These discrepancies in M staging performance might have been due to our strict reference standard. Furthermore, our study cohort included only nine patients with metastatic disease, and therefore the results concerning the accuracy in determining M stage should be considered as preliminary. Due to technical differences between FDG PET/CT and FDG PET/MRI, M staging performance might also depend on primary tumour type, the target organ of potential metastases and the applied imaging protocols. While FDG PET/MRI due to its high soft-tissue contrast may be superior for hepatic staging [18] or detection of subcutaneous metastases, for example in melanoma patients, FDG PET/CT can be expected to be advantageous in the detection of small pulmonary metastases. We stress that we did not use an organ-specific imaging protocol or contrast material, for example for the detection of hepatic metastases, and we did not use any dynamic contrast-enhanced MRI sequences in this study that was focused on WB staging. Therefore, we cannot exclude significant differences in accuracy between FDG PET/CT and FDG PET/MRI in determining M stage using any primary tumour-specific MRI protocols.
The sequence of the FDG PET/CT and subsequent FDG PET/MRI acquisitions may have biased the comparison of these modalities, since tracer accumulation in malignant tissue potentially increases the sensitivity of the delayed FDG PET acquired during FDG PET/MRI. However, since it has been repeatedly demonstrated that SUV values derived from FDG PET/CT and subsequent FDG PET/MRI show a strong correlation [14, 25], and moreover since we did not find higher diagnostic accuracies of FDG PET/MRI for metastasis detection, we believe that the this bias had no relevant impact on the interpretation of our results.
In conclusion, we did not find any significant differences between FDG PET/CT and FDG PET/MRI for TNM staging in patients with solid tumours using a WB MRI staging protocol. Still, we cannot exclude significant differences in the diagnostic accuracy between FDG PET/CT and FDG PET/MRI using a dedicated MRI protocol depending on the specific primary tumour entity. Therefore, further studies are needed to evaluate the diagnostic staging performance of hybrid FDG PET/MRI using a tumour entity-specific protocol to provide all the expected advantages of MRI.
References
Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med. 2003;44(8):1200–9.
Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41(8):1369–79.
Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, et al. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290(24):3199–206.
Kuehl H, Antoch G, Stergar H, Veit-Haibach P, Rosenbaum-Krumme S, Vogt F, et al. Comparison of FDG-PET, PET/CT and MRI for follow-up of colorectal liver metastases treated with radiofrequency ablation: initial results. Eur J Radiol. 2008;67(2):362–71.
Schmidt GP, Baur-Melnyk A, Haug A, Utzschneider S, Becker CR, Tiling R, et al. Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. Eur Radiol. 2009;19(6):1366–78.
Schmidt GP, Baur-Melnyk A, Herzog P, Schmid R, Tiling R, Schmidt M, et al. High-resolution whole-body magnetic resonance image tumor staging with the use of parallel imaging versus dual-modality positron emission tomography-computed tomography: experience on a 32-channel system. Invest Radiol. 2005;40(12):743–53.
Fischer MA, Nanz D, Hany T, Reiner CS, Stolzmann P, Donati OF, et al. Diagnostic accuracy of whole-body MRI/DWI image fusion for detection of malignant tumours: a comparison with PET/CT. Eur Radiol. 2011;21(2):246–55.
Ohno Y, Koyama H, Onishi Y, Takenaka D, Nogami M, Yoshikawa T, et al. Non-small cell lung cancer: whole-body MR examination for M-stage assessment – utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology. 2008;248(2):643–54.
Antoch G, Bockisch A. Combined PET/MRI: a new dimension in whole-body oncology imaging? Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S113–20.
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med. 2012;53(6):928–38
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G. Oncologic PET/MRI, part 2: bone tumors, soft-tissue tumors, melanoma, and lymphoma. J Nucl Med. 2012;53(8):1244–52
Herzog H, Van Den Hoff J. Combined PET/MR systems: an overview and comparison of currently available options. Q J Nucl Med Mol Imaging. 2012;56(3):247–67.
Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Fürst S, Martinez-Möller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53(6):845–55.
Schwenzer NF, Schraml C, Muller M, Brendle C, Sauter A, Spengler W, et al. Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging – pilot study. Radiology. 2012;264(2):551–8.
Kubiessa K, Purz S, Gawlitza M, Kühn A, Fuchs J, Steinhoff KG, et al. Initial clinical results of simultaneous 18F-FDG PET/MRI in comparison to 18F-FDG PET/CT in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2014;41(4):639–48.
Heusch P, Buchbender C, Kohler J, Nensa F, Gauler T, Gomez B, et al. Thoracic staging in lung cancer: prospective comparison of 18F-FDG PET/MR imaging and 18F-FDG PET/CT. J Nucl Med. 2014;55(3):373–8.
Eiber M, Takei T, Souvatzoglou M, Mayerhoefer ME, Fürst S, Gaertner FC, et al. Performance of whole-body integrated 18F-FDG PET/MR in comparison to PET/CT for evaluation of malignant bone lesions. J Nucl Med. 2014;55(2):191–7.
Beiderwellen K, Gomez B, Buchbender C, Hartung V, Poeppel TD, Nensa F, et al. Depiction and characterization of liver lesions in whole body [18F]-FDG PET/MRI. Eur J Radiol. 2013;82(11):e669–75.
Glazer GM, Gross BH, Quint LE, Francis IR, Bookstein FL, Orringer MB. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol. 1985;144(2):261–5.
Al-Nabhani KZ, Syed R, Michopoulou S, Alkalbani J, Afaq A, Panagiotidis E, et al. Qualitative and quantitative comparison of PET/CT and PET/MR imaging in clinical practice. J Nucl Med. 2014;55(1):88–94.
Catalano OA, Rosen BR, Sahani DV, Hahn PF, Guimaraes AR, Vangel MG, et al. Clinical impact of PET/MR imaging in patients with cancer undergoing same-day PET/CT: initial experience in 134 patients – a hypothesis-generating exploratory study. Radiology. 2013;269(3):857–69.
Di Martino M, Marin D, Guerrisi A, Baski M, Galati F, Rossi M, et al. Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology. 2010;256(3):806–16.
Vandecaveye V, De Keyzer F, Vander Poorten V, Dirix P, Verbeken E, Nuyts S, et al. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology. 2009;251(1):134–46.
Buchbender C, Hartung-Knemeyer V, Beiderwellen K, Heusch P, Kühl H, Lauenstein TC, et al. Diffusion-weighted imaging as part of hybrid PET/MRI protocols for whole-body cancer staging: does it benefit lesion detection? Eur J Radiol. 2013;82(5):877–82.
Heusch P, Buchbender C, Beiderwellen K, Nensa F, Hartung-Knemeyer V, Lauenstein TC, et al. Standardized uptake values for [18F]FDG in normal organ tissues: comparison of whole-body PET/CT and PET/MRI. Eur J Radiol. 2013;82(5):870–6.
Conflicts of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heusch, P., Nensa, F., Schaarschmidt, B. et al. Diagnostic accuracy of whole-body PET/MRI and whole-body PET/CT for TNM staging in oncology. Eur J Nucl Med Mol Imaging 42, 42–48 (2015). https://doi.org/10.1007/s00259-014-2885-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2885-5