Abstract
Purpose
The purpose was to investigate the diagnostic performance of different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT for the evaluation of metastatic colorectal cancer lesions.
Methods
Image data of 15 colorectal cancer patients (FDG-PET/CT and subsequent FDG-PET/MRI) were retrospectively evaluated by two readers in five reading sessions: MRI (morphology) alone, MRI/diffusion-weighted MRI (DWI), MRI/PET, MRI/DWI/PET; and PET/CT. Diagnostic performance of lesion detection with each combination was assessed in general and organ-based. The reference standard was given by histology and/or follow-up imaging. Separate analysis of mucinous tumours was performed.
Results
One hundred and eighty lesions (110 malignant) were evaluated (intestine n = 6, liver n = 37, lymph nodes n = 55, lung n = 4, and peritoneal n = 74). The overall lesion-based diagnostic accuracy was 0.46 for MRI, 0.47 for MRI/DWI, 0.57 for MRI/PET, 0.69 for MRI/DWI/PET and 0.66 for PET/CT. In the organ-based assessment, MRI/DWI/PET showed the highest accuracy for liver metastases (0.74), a comparable accuracy to PET/CT in peritoneal lesions (0.55), and in lymph node metastases (0.84). The accuracy in mucinous tumour lesions was limited in all modalities (MRI/DWI/PET = 0.52).
Conclusions
PET/MRI including DWI is comparable to PET/CT in the evaluation of colorectal cancer metastases, with a markedly higher accuracy when using combined imaging data than the modalities separately. Further improvement is needed in the imaging of peritoneal carcinomatosis and mucinous tumours.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with colorectal cancer suffer from primary metastatic disease in 19 % of cases, while recurrence occurs in 30–50 % of cases during the course of the disease [1]. Current data of various imaging techniques in staging and restaging of colorectal cancer patients is complex. While magnetic resonance imaging (MRI) is recommended for local staging and restaging of the primary tumour, lymph node and peritoneal staging is challenging [2]. Although additional diffusion-weighted imaging (DWI) may improve diagnostic accuracy, more accurate assessment of lymph node involvement has been reported for hybrid positron emission tomography/computed tomography (PET/CT) [3–7]. The major strength of PET/CT is the detection of distant colorectal cancer metastases with an accuracy of up to 97 % [8–10]. While whole-body MRI does not seem to be advantageous in the staging of distant metastases, a first study using whole-body DWI reported high sensitivity but limited specificity [4, 5].
Concerning metastases in particular organs, MRI is the first choice for imaging of liver metastases, especially if small in size; however, PET/CT and DWI may also be used [11–14]. In the diagnosis of peritoneal carcinomatosis, PET/CT is superior to MRI alone [15–17]. In a recent study performed by Soussan et al. DWI has been shown to provide results comparable to PET/CT [18]. Moreover, the quantitative assessment of FDG uptake and diffusivity has been reported to correlate significantly [18, 19].
The accuracy of PET/CT imaging with FDG is however dependent on histology. Mucinous tumours, which occur in 17 % of all colorectal cancers, were shown to cause severe difficulties for PET/CT imaging due to the lack of FDG uptake [20]. Literature about imaging these tumours is sparse, and the role of MRI and DWI has not been evaluated so far.
In the last few years, hybrid PET/MRI scanners have been implemented as a new modality in clinical routine and show promising results in oncological imaging [21–23]. In view of the growing number of different morphological and functional imaging techniques and modalities available, the most accurate combination of imaging techniques for different disease entities and treatment regimens remains to be identified. Therefore, studies are needed to investigate the influence of multiparametric image information on diagnostic performance and confidence. Direct comparison studies between PET/CT and PET/MRI can provide useful data for this task. Redundant image techniques or MR sequences might be identified, which can be skipped because of the lack of additional diagnostic benefit. This could reduce the number and duration of hybrid imaging studies, concomitant with a reduction of patient’s discomfort and expenditure of scan time in clinical routine.
Therefore, the aim of our study was to assess the diagnostic performance of different combinations of morphological and functional imaging techniques for lesion evaluation in patients with metastatic colorectal cancer: MRI alone, MRI with DWI, MRI with PET, MRI with DWI and PET, and PET/CT.
Materials and methods
Patients
The study was approved by the institutional review board. All patients gave their informed consent for the examinations and for the scientific use of their data.
Fifteen patients with metastatic colorectal cancer who underwent a clinically indicated 18F-FDG-PET/CT and subsequent 18F-FDG-PET/MRI were consecutively enrolled in this retrospective study (nine males, six females, mean age 45 years, range 10–62 years). The patients suffered from colon cancer (n = 9), sigmoid cancer (n = 4), and rectal cancer (n = 2). All patients had adenocarcinoma; five patients had a mucinous subtype in histology. The indications for PET/CT were recurrence suspected due to elevated tumour marker (n = 2) or suspicious imaging findings (liver, n = 4; peritoneal, n = 2), therapy evaluation after chemotherapy of metastatic primary tumours (n = 2) or recurrent tumours (local recurrence, n = 2; peritoneal lesions, n = 1; multifocal recurrence, n = 1) and primary staging (n = 1). Prior therapies included surgery (n = 1), chemotherapy (n = 3), surgery and chemotherapy (n = 9), surgery, radiation and chemotherapy (n = 1), and no therapy (n = 1). The time interval between the last chemotherapy and the examination was on average 4 months (range 11 days to 10 months). A reference standard was given by histology in 10 patients in case of surgical therapy (50 lesions) and by imaging follow-up in all patients (130 lesions; MRI in 6 patients, CT in 10 patients, PET/CT in 8 patients). Mean follow-up period was 15 months (range 4–30 months), with the first follow-up examination averaging after 4 months (range 0–9 months) and surgery after 1 month (range 0–4 months).
PET/CT
The PET/CT examinations were performed on a Biograph mCT (Siemens Healthcare, Erlangen, Germany). The CT examination (peak voltage 120 kVp, tube current 250 mAs) was performed as whole-body scan from the base of the skull to the upper thigh. Contrast-enhanced CT was acquired in portal venous phase (n = 13). In case of contraindication against the use of intravenous contrast agent an unenhanced CT scan was performed (n = 2). The CT images were used for attenuation correction and for diagnostic purposes (axial slice thickness 3 mm, increment 2.5 mm, table feed 30.7 mm). There was 1000 ml mannitol (2.5 %) administered to each patient as a negative oral contrast agent 30 min before the examination. PET was acquired over 6–8 bed positions (acquisition time 2 min/bed) with an uptake time of 62 ± 5 min after the injection of 337 ± 59 MBq of 18F-Fluoro-Deoxyglucose (18F-FDG), and reconstructed using an 3D ordered-subset expectation maximization (OSEM) algorithm (2 iterations, 21 subsets, Gaussian filter 2 mm).
PET/MRI
The PET/MRI examinations were performed subsequently to the PET/CT on a Biograph mMR (Siemens Healthcare, Erlangen, Germany) consisting of a 3T MRI (maximum gradient strength 45 mT/m, slew rate 200 mT/m/s) with an integrated PET system, as examinations of the abdomen and the pelvis. Due to the study set-up consisting of a PET/MRI directly following the PET/CT examination, a shortened MRI protocol was performed to reduce overall examination duration. The following MRI sequences were available for analysis: a T1-weighted spoiled gradient echo sequence with Dixon-based fat-water separation for attenuation correction (n = 15), a coronal T2-weighted short-tau inversion recovery sequence (STIR, inversion time 220 ms, echo time (TE) 53 ms, repetition time (TR) 5940 ms, slice thickness 5.0 mm), a navigator triggered axial fat-saturated T2-weighted turbo-spin echo (TSE) sequence of the abdomen (TE 101 ms, TR 2130 ms, slice thickness 5.0 mm), an axial fat-saturated T2-weighted TSE sequence of the pelvis (TE 86 ms, TR 3810 ms, slice thickness 5.0 mm), and an echo-planar imaging sequence for diffusion-weighted imaging (TE 57 ms, TR 6100 ms, b = 50, 800 s/mm2, slice thickness 6.0 mm) with calculation of an apparent diffusion coefficient map (ADC). PET was acquired over 2–4 bed positions (acquisition time 8 min/bed), with a mean uptake time of 120 ± 9 min after the injection of 18F-FDG. PET was reconstructed using a 3D OSEM algorithm (3 iterations, 21 subsets, Gaussian filter 3 mm). For PET attenuation correction of PET/MRI, a segmentation-based attenuation-correction map was calculated by the vendor-provided software using the Dixon-sequence in the coronal plane.
Image interpretation
Five different reading sessions were carried out, each with a time interval of 4 weeks apart: reading 1) morphological MRI alone (using the T2 and STIR images), reading 2) MRI and DWI = MRI/DWI, reading 3) MRI and PET = MRI/PET, reading 4) MRI and DWI and PET = MRI/DWI/PET, and reading 5) PET/CT. For PET/CT, just the abdominal and pelvic regions and the basal lungs were used for evaluation, equivalent to the scan field of PET/MRI. Two readers independently analyzed the image data (C.S., 10 years of experience, and H.R., 7 years of experience in oncological imaging) on dedicated fusion software for PET/CT and PET/MRI (TrueD, Siemens). The readers were aware of the clinical history but were blinded to any other imaging results. The datasets were searched in a visual analysis for the presence of lesions. Lesions were rated as a) malignant, b) benign, or, if they could not be assigned to the preceding, as c) equivocal, according to their appearance. Characterization of malignant lesions was performed according to standard clinical practice on a visual basis, quantitative parameters were not assessed. Concerning morphology, criteria of malignancy were enlarged size and irregular shape. Diffusion restriction as criteria for malignancy was assigned in lesions with a high signal in the diffusion-weighted images with the highest b value, corresponding to a decreased ADC in the ADC map when visually compared to the surrounding tissue. Increased glucose metabolism in PET as criteria of malignancy was attributed to lesions with focally increased FDG uptake compared to the surrounding tissue and above the level of liver uptake. The ratings were based on MRI appearance (reading 1), primarily on DWI or PET appearance with referring to MRI for lesion localisation (reading 2 and 3) and on synopsis of the modalities (reading 4 and 5).
There was no lower size limit for the lesions in the reference standard, each macroscopically visible tumour lesion, e.g. in the peritoneum, and tumour infiltration in follow-up surgery was included in the evaluation.
Statistical analysis
Sensitivity, specificity, and accuracy obtained by the different combinations of imaging techniques for the detection or exclusion of metastatic lesions were calculated for each reading and compared using McNemar’s test. Bonferroni correction for multiple testing was applied. The interobserver variability was calculated using the kappa coefficient. A kappa coefficient of 0–0.2 indicated poor agreement, 0.2–0.4 fair agreement, 0.4–0.6 moderate agreement, 0.6–0.8 strong agreement, and 0.8–1.0 almost perfect agreement [24]. Equivocal lesions were classified as false negative (in case of a malignant lesion in the reference standard) or false positive (in case of a benign lesion in the reference standard) in the final overall and organ-based evaluation. In addition to this conservative rating of equivocal lesions, an additional statistical analysis was performed, with all equivocal lesions definitely classified as malignant or benign, respectively. All statistical analyses were performed using statistical software (JMP 10.0.2., SAS Institute, Cary, NC).
Results
Lesion characteristics
In total, 180 lesions were identified, with 110 being malignant and 70 being benign according to the reference standard. The number of malignant lesions per patient ranged from 0 to 28 lesions (mean 7 malignant lesions/patient), one patient had no metastases. Six malignant lesions were located in the intestine, one benign lesion in the bone, 37 lesions in the liver (15 benign, 22 malignant), 55 in lymph nodes (39 benign, 16 malignant), four malignant lesions in the basal lungs as far as was displayed in PET/MRI, three benign lesions in the adrenal glands and 74 lesions had peritoneal localisation (12 benign, 62 malignant). Forty-two of the malignant lesions had mucinous histology, while 68 lesions were non-mucinous. The lesion size was not measurable in 16 lesions due to small size or diffuse tumour infiltration. The mean size of the 164 measurable lesions was 1.3 cm (range 0.3–6.2 cm).
Diagnostic accuracy
The overall diagnostic performance of the different combinations of imaging techniques is summarized in Table 1, including different rating possibilities of the equivocal lesions either assigned as malignant, benign or incorrectly identified with regard to the reference standard. The mean accuracy was slightly higher in MRI/DWI/PET than in PET/CT, with no significant difference (0.69 and 0.66; p = 0.2752). Sensitivity and specificity of MRI/DWI/PET and PET/CT were within the same range, with an overall high specificity of both modalities (sensitivity: 0.56/0.48; p = 0.0134, specificity: 0.87/0.94; p = 0.0719). The mean accuracy of MRI alone, MRI/DWI and MRI/PET was significantly lower than in MRI/DWI/PET (0.46; p < 0.0001, 0.47; p < 0.0001, and 0.57; p < 0.0001, respectively). The number of equivocal lesions was substantially lower in MRI/DWI/PET and PET/CT readings (n = 5 and n = 7, respectively) as compared to MRI alone (n = 19), MRI/DWI (n = 14) and MRI/PET (n = 22). The overall lesion-based interobserver agreement was strong, with a kappa value of 0.60. The highest kappa values were obtained for MRI/DWI/PET (0.72) and for PET/CT (0.73). The separate reading results of the two readers and the interobserver variability for all modalities are shown in Table 2.
The detailed results of the organ-based evaluation are summarized in Table 3. The accuracy of MRI/DWI/PET and PET/CT did not differ significantly regarding evaluation of peritoneal lesions (0.55 versus 0.58; p = 0.6121), while MRI/DWI/PET was markedly superior for the diagnostic evaluation of liver lesions (accuracy 0.74 versus 0.56; p = 0.0060). As to the diagnosis of lymph node and pulmonary metastases, PET/CT and MRI/DWI/PET showed comparable accuracy (0.84 and 0.50 for both modalities). In intestinal lesions, PET/CT was slightly superior compared to MRI/DWI/PET, though without a statistically significant difference (accuracy 0.67 versus 0.50; p = 0.1573). The single benign bone lesion was correctly identified as bone island in all readings. The specificity for the three benign lesions in the adrenal glands was 0.33 in MRI alone and MRI/DWI, 0.67 in MRI/PET, 0.84 in MRI/DWI/PET and 0.67 in PET/CT.
Concerning the overall sensitivity, specificity, and accuracy for the evaluation of metastatic lesions (N- and M-state), there was no significant difference between any modality combination.
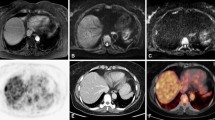
In mucinous tumour lesions, the sensitivity was rather low for all modalities and PET did not provide an additional benefit when combined with MRI and DWI (Table 4). The accuracy of MRI/DWI/PET and PET/CT for mucinous and non-mucinous tumour lesions was comparable with markedly lower values in mucinous tumour lesions as compared to non-mucinous tumour lesions. In the organ-based evaluation of mucinous tumour lesions, the diagnostic accuracy of MRI/DWI/PET was slightly superior to PET/CT for the evaluation of lymph nodes, but without significant difference (0.89 and 0.73; p = 0.1025). Comparable diagnostic accuracy was obtained in the evaluation of peritoneal lesions (0.42 and 0.43: p = 0.8185) and liver lesions (0.56 and 0.50; p = 0.5637). Exemplarily, image data with different combinations of imaging findings in the evaluated modalities are shown with discordant imaging findings in a liver metastasis in Fig. 1, with discordant findings in mucinous peritoneal lesions in Fig. 2 and with discordant findings in lymph node metastases in Fig. 3.
Fifty-nine year-old male patient with hepatic metastasis of an adenocarcinoma of the transverse colon. Status post resection of primary tumour and chemotherapy. Discordant imaging finding consisting of a liver metastasis with high metabolic activity and only slight diffusion restriction. Liver metastasis (white arrow) presenting as hypodense liver lesion in CT a, with increased FDG uptake in PET (b, fused PET/CT, and f, PET of PET/MRI). In MRI, the lesion is T2 hyperintense (c, T2-weighted fat-saturated TSE) with moderately high signal in the diffusion-weighted image with high b value (e, b = 800 s/mm2), with relatively high ADC as compared to liver parenchyma (d, ADC map)
Forty-three year-old male patient with mucinous adenocarcinoma of the rectosigmoid. Status post peritoneal resection, hyperthermic intraperitoneal chemotherapy (HIPEC) and systemic chemotherapy. Peritoneal carcinomatosis with omental cake (white arrows), shrinking of the mesentery and associated ascites (a, portal venous CT, and c, T2-weigthed fat-saturated TSE). In PET, the peritoneal cancer lesion shows only slight FDG uptake (b, fused PET/CT, and f, PET of PET/MRI). In DWI, the omental tumour masses show relatively slight diffusion restriction (d, ADC map, and e, DWI with b = 800 s/mm2)
Forty-seven year-old male patient with adenocarcinoma of the colon. Status post surgery and chemotherapy; discordant imaging findings in two retroperitoneal lymph node metastases (white arrow and white arrow head): both with enlargement (a, portal venous CT, and c, T2-weighted fat-saturated TSE) and diffusion restriction (d, ADC map, and e, DWI with b = 800 s/mm2), but increased FDG uptake only in the medial lymph node metastasis (white arrowhead in b, fused PET/CT, and f, PET from PET/MRI) with no increased FDG uptake in the lateral lymph node metastasis (white arrow in b, fused PET/CT, and f, PET from PET/MRI)
Discussion
In this pilot study, the diagnostic performance of different combinations of anatomical and functional imaging techniques in hybrid PET/MRI was evaluated in comparison to PET/CT in the diagnostic evaluation of metastatic colorectal cancer. As the local staging was not within the scope of this study, PET/MRI scans did not include a dedicated pelvic protocol for local staging of colorectal cancer.
Based on our study results, hybrid imaging, namely the combination of MRI with DWI with PET (MRI/DWI/PET) was clearly superior to the imaging techniques alone. As compared to PET/CT, MRI/DWI/PET showed slightly higher accuracy for colorectal cancer staging, however, with no significant difference (0.69 vs. 0.66). Moreover, the interobserver agreement rose markedly in MRI/DWI/PET and PET/CT, indicating a higher clarity of imaging features in lesions and, thus, resulting in a higher diagnostic confidence.
Previous studies investigating whole-body MRI in colorectal cancer imaging reported a relatively high overall diagnostic accuracy of MRI (0.83) with a sensitivity of 0.72 and a specificity of 0.93 [6, 25]. Contrarily, several studies investigating PET/CT in recurrent colorectal cancers revealed a mean accuracy of 0.90, a mean sensitivity of 0.90 and a specificity of 0.83 [10, 26, 27]. Compared to these data reported in the literature, the diagnostic performance of the imaging techniques evaluated in our study was inferior. This may be explained by the following reasons: First, we did not define a minimum lesion size in the reference standard and the limited spatial resolution of diagnostic imaging naturally causes lower detection rates, especially of small lesions. Second, our data contained a relatively high percentage of mucinous tumours, which are known to be challenging for both DWI and PET evaluation. Third, we performed a shortened MRI protocol without application of intravenous contrast agent due to the ethical committee recommendations. This might be the cause for the observed limited accuracy and also for the higher interobserver variability. Finally, due to the retrospective study design, patients’ imaging data could have been more heterogeneous due to prior therapies, which might have complicated the diagnostic interpretation. However, this reflects the clinical routine where patients are administered to imaging not only for initial diagnostic work-up but also for post-treatment evaluation and for the assessment of recurrent disease.
It is interesting that - based on the present study - the additional functional information provided by PET seems to be more important than the functional information obtained by DWI for the overall diagnostic performance in colorectal cancer evaluation. However, this may be based on a relatively high percentage (38 %) of mucinous tumour lesions. Moreover, the use of DWI in oncological imaging is currently developing, and the technique might benefit from technical improvements regarding robustness and spatial resolution [4]. It is worth noting that the highest diagnostic accuracy was obtained when using the combination of MRI, DWI and PET in synopsis. This combination of modalities was also associated with a markedly reduced number of equivocal findings. The existence of equivocal lesions is a general problem in clinical oncological reading and their implication on patient management is not yet clear. In clinical routine, equivocal lesions are often ignored or undergo a limited follow-up imaging. However, follow-up imaging of equivocal lesions may delay treatment and may also cause additional costs for the healthcare system. In light of these considerations, it is interesting that the number of equivocal lesions was decreased to one third using hybrid imaging.
Concerning the organ-based diagnostic accuracy of the different combinations of imaging techniques, MRI/DWI/PET was markedly superior to PET/CT in liver lesions. Morphological MRI alone already showed higher accuracy and sensitivity compared to PET/CT in the detection of liver metastases, which is in accordance with the literature [28, 11]. On the other hand, the addition of PET to MRI increased the specificity, which emphasizes the complementary role of the two modalities [13]. Lesion detection in peritoneal carcinomatosis is known to be a challenge. In our study, both PET/CT and MRI/DWI/PET had a limited accuracy in peritoneal lesions and similarly in intestinal lesions, especially a rather low sensitivity (0.50 and 0.52). Satoh et al. reported sensitivities of 0.89 for PET/CT and 0.84 for MRI/DWI in peritoneal lesions [17]. A reason for the relatively low diagnostic values in our study may be, again, the high percentage of mucinous tumours. Moreover, our study design was not focussed on peritoneal carcinomatosis only. Furthermore, peritoneal lesions are often small and intestine motility may handicap the anatomical allocation, which is a problem when dealing with longer acquisition times as encountered in MRI. In the diagnosis of colorectal lymph node metastases, both MRI/DWI/PET and PET/CT showed acceptable performance in the present study (both with an accuracy of 0.84), though with a weakness in sensitivity (both 0.60). In our study, adding DWI to morphological MRI had considerable impact on sensitivity, while the addition of PET significantly improved specificity. In the literature, a variable range of diagnostic accuracy (0.66–0.87) is reported for PET/CT with high specificity, but limited sensitivity, and contrarily, a high sensitivity is reported for MRI when combined with DWI [29–31].
In the present study, a considerable percentage (42 of 110) of the lesions had a mucinous histopathology. Mucinous tumours are characterized by low FDG uptake and only slight diffusion restriction compared to solid tumours [32, 33]. This complicates diagnostic imaging, especially as DWI and FDG uptake both depend on the tumour histology [19]. In a study by Berger et al. mucinous carcinoma could be detected in only 59 % of patients using PET [34].
In our study, both PET/CT and MRI/DWI/PET showed a limited accuracy (0.50 and 0.52) for the overall evaluation of mucinous tumour lesions. This was mainly ascribable to a considerably low sensitivity (0.38 and 0.36). In the organ-based evaluation, MRI/DWI/PET diagnosed mucinous lymph node metastases very well (accuracy 0.89) and was also superior in liver metastases when compared to PET/CT (accuracy 0.74 vs. 0.56), while the diagnosis of peritoneal lesions was difficult in both modalities. A recent PET study with mucinous and partially mucinous tumours reports a detection rate of 0.95 in lymph node metastases and 0.58 in distant metastases [35].
Our study has several limitations. First, the study population was heterogeneous regarding tumour histology and preceding therapies, with possible influence on lesion characteristics in imaging. The patients of this retrospective study were enrolled consecutively according to their clinical indication for PET/CT. So far, there exist no generally accepted guidelines for PET/CT examinations in colorectal cancer patients. Therefore, the study population represents the clinical reality including patients with different histological subtypes and also various prior therapies. Prospective studies in the future might overcome this shortcoming of our study. Second, the number of patients was relatively small. Although the time period for patient inclusion lasted over 21 months, there were only fifteen patients who met the inclusion criteria of the study. However, all patients had subsequent PET/CT and PET/MRI at the same time point, and we performed a lesion-based analysis of 180 lesions. Furthermore, histological confirmation as a reference standard was not available for all lesions. In lesions without histological confirmation, classification was based on generally established image findings (signal characteristics, margin, infiltration of neighbouring structures, diffusion properties, FDG avidity) as well as changes over time, e.g., newly occurring lesions, enlargement of pre-existing lesions. Changes under therapy were also included in the final categorization. In-depth analysis of all available image data was performed and lesions were followed over time.
Finally, a shortened MRI protocol without intravenous contrast was performed in accordance with the institutional review board because of subsequent PET/CT and PET/MRI. Dedicated MRI protocols e.g. with application of intravenous contrast agents might improve lesion evaluation in MRI. However, the comparison of unenhanced with enhanced MR was not considered a crucial aspect of this comparison study focusing on a more general evaluation of combinations of functional and anatomical techniques.
In conclusion, PET/MRI including DWI shows a slightly higher overall diagnostic accuracy as compared to PET/CT for the diagnosis of colorectal cancer metastases. The diagnostic performance of hybrid modalities is markedly better than that of single modalities. In hybrid examinations, interobserver agreement rose and the number of equivocal lesions decreased, indicating a higher clarity of imaging features in lesions and, thus, a higher reliability in the diagnosis. The addition of PET information seems to be more important for the performance of MRI than the addition of DWI. In all modalities, further improvement is needed in the imaging of peritoneal carcinomatosis and mucinous tumours.
References
Chen LB, Tong JL, Song HZ, Zhu H, Wang YC. (18)F-DG PET/CT in detection of recurrence and metastasis of colorectal cancer. World J Gastroenterol : WJG. 2007;13(37):5025–9.
Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23(9):2522–31. doi:10.1007/s00330-013-2864-4.
van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S. Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis. Radiology. 2013;269(1):101–12. doi:10.1148/radiol.13122833.
Lambregts DM, Maas M, Cappendijk VC, Prompers LM, Mottaghy FM, Beets GL, et al. Whole-body diffusion-weighted magnetic resonance imaging: current evidence in oncology and potential role in colorectal cancer staging. Eur J Cancer. 2011;47(14):2107–16. doi:10.1016/j.ejca.2011.05.013.
Schmidt G. Importance of whole body MRI for staging of colorectal cancer. Radiologe. 2012;52(6):537–44. doi:10.1007/s00117-011-2285-9.
Squillaci E, Manenti G, Mancino S, Ciccio C, Calabria F, Danieli R, et al. Staging of colon cancer: whole-body MRI vs. whole-body PET-CT--initial clinical experience. Abdom Imaging. 2008;33(6):676–88. doi:10.1007/s00261-007-9347-5.
Kim DJ, Kim JH, Ryu YH, Jeon TJ, Yu JS, Chung JJ. Nodal staging of rectal cancer: high-resolution pelvic MRI versus (1)(8)F-FDGPET/CT. J Comput Assist Tomogr. 2011;35(5):531–4. doi:10.1097/RCT.0b013e318225720f.
Cho YB, Chun HK, Kim MJ, Choi JY, Park CM, Kim BT, et al. Accuracy of MRI and 18F-FDG PET/CT for restaging after preoperative concurrent chemoradiotherapy for rectal cancer. World J Surg. 2009;33(12):2688–94. doi:10.1007/s00268-009-0248-3.
Lambregts DM, Maas M, Riedl RG, Bakers FC, Verwoerd JL, Kessels AG, et al. Value of ADC measurements for nodal staging after chemoradiation in locally advanced rectal cancer-a per lesion validation study. Eur Radiol. 2011;21(2):265–73. doi:10.1007/s00330-010-1937-x.
Maas M, Rutten IJ, Nelemans PJ, Lambregts DM, Cappendijk VC, Beets GL, et al. What is the most accurate whole-body imaging modality for assessment of local and distant recurrent disease in colorectal cancer? A meta-analysis : imaging for recurrent colorectal cancer. Eur J Nucl Med Mol Imaging. 2011;38(8):1560–71. doi:10.1007/s00259-011-1785-1.
Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257(3):674–84. doi:10.1148/radiol.10100729.
Bonanni L, De’liguori Carino N, Deshpande R, Ammori BJ, Sherlock DJ, Valle JW, et al. A comparison of diagnostic imaging modalities for colorectal liver metastases. Eur J Surg Oncol : J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2014;40(5):545–50. doi:10.1016/j.ejso.2013.12.023.
Floriani I, Torri V, Rulli E, Garavaglia D, Compagnoni A, Salvolini L, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging : JMRI. 2010;31(1):19–31. doi:10.1002/jmri.22010.
Eiber M, Fingerle AA, Brugel M, Gaa J, Rummeny EJ, Holzapfel K. Detection and classification of focal liver lesions in patients with colorectal cancer: retrospective comparison of diffusion-weighted MR imaging and multi-slice CT. Eur J Radiol. 2012;81(4):683–91. doi:10.1016/j.ejrad.2011.01.072.
Chang MC, Chen JH, Liang JA, Huang WS, Cheng KY, Kao CH. PET or PET/CT for detection of peritoneal carcinomatosis: a meta-analysis. Clin Nucl Med. 2013;38(8):623–9. doi:10.1097/RLU.0b013e318299609f.
Klumpp BD, Schwenzer N, Aschoff P, Miller S, Kramer U, Claussen CD, et al. Preoperative assessment of peritoneal carcinomatosis: intraindividual comparison of 18F-FDG PET/CT and MRI. Abdom Imaging. 2013;38(1):64–71. doi:10.1007/s00261-012-9881-7.
Satoh Y, Ichikawa T, Motosugi U, Kimura K, Sou H, Sano K, et al. Diagnosis of peritoneal dissemination: comparison of 18F-FDG PET/CT, diffusion-weighted MRI, and contrast-enhanced MDCT. AJR Am J Roentgenol. 2011;196(2):447–53. doi:10.2214/AJR.10.4687.
Soussan M, Des Guetz G, Barrau V, Aflalo-Hazan V, Pop G, Mehanna Z, et al. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy. Eur Radiol. 2012;22(7):1479–87. doi:10.1007/s00330-012-2397-2.
Schwenzer NF, Schmidt H, Gatidis S, Brendle C, Muller M, Konigsrainer I, et al. Measurement of apparent diffusion coefficient with simultaneous MR/positron emission tomography in patients with peritoneal carcinomatosis: comparison with 18F-FDG-PET. J Magn Reson Imaging : JMRI. 2014;40(5):1121–8. doi:10.1002/jmri.24497.
Grassetto G, Capirci C, Marzola MC, Rampin L, Chondrogiannis S, Musto A, et al. Colorectal cancer: prognostic role of 18F-FDG-PET/CT. Abdom Imaging. 2012;37(4):575–9. doi:10.1007/s00261-011-9789-7.
Heusch P, Nensa F, Schaarschmidt B, Sivanesapillai R, Beiderwellen K, Gomez B, et al. Diagnostic accuracy of whole-body PET/MRI and whole-body PET/CT for TNM staging in oncology. Eur J Nucl Med Mol Imaging. 2014. doi:10.1007/s00259-014-2885-5.
Bailey DL, Barthel H, Beuthin-Baumann B, Beyer T, Bisdas S, Boellaard R, et al. Combined PET/MR: where are we now? Summary report of the second international workshop on PET/MR imaging April 8–12, 2013, Tubingen, Germany. Mol Imaging Biol : MIB : Off Publ Acad Mol Imaging. 2014;16(3):295–310. doi:10.1007/s11307-014-0725-4.
Schwenzer NF, Schraml C, Muller M, Brendle C, Sauter A, Spengler W, et al. Pulmonary lesion assessment: comparison of whole-body hybrid MR/PET and PET/CT imaging--pilot study. Radiology. 2012;264(2):551–8. doi:10.1148/radiol.12111942.
Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res. 1998;7(3):301–17.
Schmidt GP, Baur-Melnyk A, Haug A, Utzschneider S, Becker CR, Tiling R, et al. Whole-body MRI at 1.5 T and 3 T compared with FDG-PET-CT for the detection of tumour recurrence in patients with colorectal cancer. Eur Radiol. 2009;19(6):1366–78. doi:10.1007/s00330-008-1289-y.
Kitajima K, Murakami K, Yamasaki E, Domeki Y, Tsubaki M, Sunagawa M, et al. Performance of integrated FDG PET/contrast-enhanced CT in the diagnosis of recurrent colorectal cancer: Comparison with integrated FDG PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging. 2009;36(9):1388–96. doi:10.1007/s00259-009-1081-5.
Deleau C, Buecher B, Rousseau C, Kraeber-Bodere F, Flamant M, des Varannes SB, et al. Clinical impact of fluorodeoxyglucose-positron emission tomography scan/computed tomography in comparison with computed tomography on the detection of colorectal cancer recurrence. Eur J Gastroenterol Hepatol. 2011;23(3):275–81. doi:10.1097/MEG.0b013e328343eaa0.
Rojas Llimpe FL, Di Fabio F, Ercolani G, Giampalma E, Cappelli A, Serra C, et al. Imaging in resectable colorectal liver metastasis patients with or without preoperative chemotherapy: results of the PROMETEO-01 study. Br J Cancer. 2014;111(4):667–73. doi:10.1038/bjc.2014.351.
Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS, Kim JC. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J Surg. 2012;36(8):1898–905. doi:10.1007/s00268-012-1575-3.
Lu YY, Chen JH, Ding HJ, Chien CR, Lin WY, Kao CH. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18F-FDG PET or PET/CT. Nucl Med Commun. 2012;33(11):1127–33. doi:10.1097/MNM.0b013e328357b2d9.
Mizukami Y, Ueda S, Mizumoto A, Sasada T, Okumura R, Kohno S, et al. Diffusion-weighted magnetic resonance imaging for detecting lymph node metastasis of rectal cancer. World J Surg. 2011;35(4):895–9. doi:10.1007/s00268-011-0986-x.
Au-Yeung AW, Luk WH, Lo AX. Imaging features of colorectal liver metastasis in FDG PET-CT: a retrospective correlative analysis between CT attenuation and FDG uptake. Nucl Med Commun. 2012;33(4):403–7. doi:10.1097/MNM.0b013e32834f4d54.
Nasu K, Kuroki Y, Minami M. Diffusion-weighted imaging findings of mucinous carcinoma arising in the ano-rectal region: comparison of apparent diffusion coefficient with that of tubular adenocarcinoma. Jpn J Radiol. 2012;30(2):120–7. doi:10.1007/s11604-011-0023-x.
Berger KL, Nicholson SA, Dehdashti F, Siegel BA. FDG PET evaluation of mucinous neoplasms: correlation of FDG uptake with histopathologic features. AJR Am J Roentgenol. 2000;174(4):1005–8. doi:10.2214/ajr.174.4.1741005.
Yang ZY, Hu SL, Shi W, Zhu BL, Xu JY, Zhang YJ. The clinical value of fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography in postoperative patients with gastrointestinal mucinous adenocarcinoma. Nucl Med Commun. 2011;32(11):1018–25. doi:10.1097/MNM.0b013e32834bbd22.
Compliance with ethical standards
Conflict of interest
The Department of Diagnostic and Interventional Radiology has a collaboration contract with Siemens concerning the technical development of PET/MRI Biograph mMR. This work has not received any funding.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study (retrospective study) formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brendle, C., Schwenzer, N.F., Rempp, H. et al. Assessment of metastatic colorectal cancer with hybrid imaging: comparison of reading performance using different combinations of anatomical and functional imaging techniques in PET/MRI and PET/CT in a short case series. Eur J Nucl Med Mol Imaging 43, 123–132 (2016). https://doi.org/10.1007/s00259-015-3137-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3137-z