Abstract
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary hepatic malignancy after hepatocellular carcinoma. Complete surgical resection remains the only potentially curative option for patients with ICC. However, until now, early diagnosis with potential surgical intervention has been the exception rather than the rule with only 30% of patients qualifying for attempted surgical cure. Many patients are unresectable because of disease stage, anatomic conditions, medical comorbidities, and small future remnant liver. Interventional radiology procedures are available for these types of patients with intra-arterial therapies and/or ablative treatments both for curative and for palliative treatment. The goals of interventional therapy are to control local tumor growth, to relieve symptoms, and to improve and preserve quality of life. The choice of treatment depends largely on tumor extent and patient performance. No randomized studies exist to compare treatments. The present review describes the current evidence of the interventional treatments in the management of the ICC. Moreover, interventional procedures available to increase the future liver reserve before surgery were analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholangiocarcinoma (CC) is the second most frequent type of primary liver cancer, and it represents 3% of all gastrointestinal neoplasms [1].
Cholangiocarcinoma (CC) can arise from every point of the biliary system: intrahepatic (ICC) or extrahepatic (ECC); the extrahepatic CC is classified as perihilar (pCC) or distal CCA (dCC).
Approximately up to 10% of all cholangiocarcinomas are intrahepatic, whereas 50–60% are pCC and the remaining involve the distal bile duct (dCC) [2].
The majority of CCs occur in the absence of an evident chronic liver disease or other risk factors. However, the most relevant risk factors for CC are: primary sclerosing cholangitis (PSC), liver flukes, HCV-related and HBV-related liver diseases. ICC occurs in patients with chronic liver disease and/or cirrhosis more often than pCC and dCC [3, 4].
On the basis of the macroscopic appearance, the ICC can present in three different patterns of growth: mass-forming (MF-ICC), periductal infiltrating (PI-ICC), and intraductal growing (IG-ICC). The mass-forming type represents the most frequent macroscopic presentation of ICC (>90%) [5, 6].
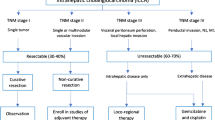
Treatment for cholangiocarcinoma depends on the clinical condition of the patient and the stage of disease. Surgery with complete resection represents the only treatment for CC with potential curative intent [7]. Survival after resection mainly depends on the presence of tumor-negative margins, absence of vascular invasion and lymph node metastasis, and adequate functional liver remnant [8]. Overall, 5-year survival after resection has ranges 22–44% for ICC; 1–41% for pCC, and 27–37% for dCC [9]. Unfortunately, the majority of patients develop recurrence following surgical resection. Liver transplantation has been associated with rapid tumor recurrence and low survival rates (10–25%) and is not recommended as a treatment for CC [10].
Many patients that are technically resectable cannot undergo surgery as a result of medical comorbidities, disease stage, or small future liver remnant (FLR) [6].
The prognosis of patients with advanced ICC is poor, with an estimated median survival of 3–8 months [11, 12]. Intra-arterial therapies (IAT) and/or ablative treatments (AT) are available with the potential for cure or for palliation, to relieve symptoms, and to improve and/or preserve quality of life.
IAT for primary liver tumors are efficient, safe and can improve survival [13–15]. IAT include bland embolization (TAE), transarterial chemoembolization (TACE), drug-eluting bead TACE (DEB-TACE) or radioembolization (yttrium-90). No statistically significant difference has been found in small RCT evaluating these therapies in the management of HCC. To date, there is no RCT comparing these different IAT for ICC [15].
Thermal ablation has been used as a curative option for HCC and is currently recommended instead of surgery for small HCC that can be treated with margins. However, the efficacy of thermal ablation for ICC lacks sufficient evidence [16].
Percutaneous image-guided thermal ablation using MWA or RFA is safe and effective in the treatment of ICC. It may be an alternative treatment approach in some cases of ICC, particularly for smaller tumors that can be treated with margins in patients that are not candidates for surgery. It has satisfactory local therapeutic efficacy, low complication rate, and acceptable survival [17].
The purpose of the present review is to describe the current evidence of the interventional treatments in the management of the intrahepatic cholangiocarcinoma. Moreover, we analyzed the interventional procedures used to increase the future liver reserve before surgery.
Materials and methods
Study selection
A systematic literature search was performed in PubMed, with the syntax interventional radiology and intrahepatic cholangiocarcinoma; intrahepatic cholangiocarcinoma and portal vein embolization; intrahepatic cholangiocarcinoma and intra-arterial therapies or IAT or transarterial chemoembolization or TACE, drug-eluting bead TACE or DEB-TACE or radioembolization or yttrium-90; intrahepatic cholangiocarcinoma and ablative treatments or radiofrequency ablation or RFA or microwave ablation or MWA, including only studies published in English from January 2005 to June 2016. All titles and abstracts of identified studies in the initial search were screened to select those reporting interventional radiology treatments in patients with unresectable intrahepatic cholangiocarcinoma.
We also included studies in which tumors were treated simultaneously with percutaneous ablation and transarterial embolization. We identified additional studies through manual search of the primary studies references, review articles, and key journals. We excluded papers that included data reported previously.
The primary endpoint was to investigate safety and complications of these techniques. Secondary endpoint was to present the current status of interventional radiology in the treatment of unresectable intrahepatic cholangiocarcinoma.
The following variables were extracted, where available, from the included articles: number of patients; number of lesions and their diameter; number of sessions of treatment; complications; mean follow-up; technical and clinical success. Median survival was calculated.
Clinical evidence
Portal vein embolization and radioembolization to bridge for surgery
Only a minority of patients with ICC are candidates for surgical resection [2]. In general, a FLR of 20–25% is recommended after surgery, although higher volumes are needed for patients with compromised liver function [18]. Percutaneous portal vein embolization can induce contralateral lobe hypertrophy and can therefore reduce the risks of postoperative liver insufficiency and increase the number of patients who may be eligible for surgical resection [19]. One of the major disadvantages of portal vein embolization is the time required to induce hypertrophy, which can be more than 1 month. This delay before surgery may allow for interval tumor growth, which could theoretically worsen prognosis or exclude patients from surgical resection [20].
In most cases, a right portal vein embolization is needed (Fig. 1a–d). Left portal vein embolization is rarely performed because a left trisegmentectomy typically results in a FLR of greater than 30%. After access into the portal vein is obtained, a variety of embolic agents have been used, including particles, coils, and liquid adhesives (i.e., n-butyl cyanoacrylate or Onyx) [21].
Interestingly, postembolization syndrome is uncommon compared with the incidence observed in transarterial embolization. Complications are rare and include issues at the access site such as hematoma, bleeding and rarely pneumothorax. Portal vein thrombosis can also occur; therefore, the ipsilateral (to the resection site) approach is favored so as not to compromise FLR [22].
Hypertrophy starts immediately and is often seen on imaging 1 month after the procedure. The degree of hypertrophy varies significantly based on different published studies [18].
Presently, unilobar radioembolization or selective internal radiation therapy (SIRT) with Yttrium-90 has been described as technique to afford a significant hypertrophy of the contralateral liver lobe [23]. This finding is relatively recent and has the potential of increasing resectability rates.
Apart from few case reports, two institutions have reported their experiences with radioembolization in downstaging [24, 25].
Rayar et al. [25] reported successful downstaging to resection with radioembolization and chemotherapy in eight patients with initially unresectable ICC. R0 resections were achieved in all patients, with a median of six resected segments. Mouli et al. [24] reported successful downstaging to resection in 5/46 patients with unresectable ICC and successful liver transplantation in one patient.
Recently, some studies have compared the hypertrophy seen with portal vein embolization to that seen with yttrium-90 (90Y) radioembolization. Seemingly, portal vein embolization tends to induce more significant contralateral hypertrophy at an early time point compared with radioembolization [26, 27]. Adverse events following radioembolization were fatigue, abdominal pain, nausea, vomiting, fever/chills, weight loss, hyperbilirubinemia, diarrhea and pseudoaneurysm at the puncture site [27].
Intra-arterial therapies
The role of intra-arterial therapy (IAT) for hepatocellular carcinoma is well established, with two randomized trials demonstrating a survival benefit over best supportive care [13, 14].
IAT can involve bland embolization (TAE), transarterial chemoembolization (TACE), drug-eluding bead TACE (DEB-TACE) or radioembolization (yttrium-90) and hepatic arterial infusion of chemotherapy (HAIC).
Because hepatocellular carcinoma is typically a very vascular tumor, IAT can take advantage of the fact that the majority of the tumor’s blood supply comes from the hepatic artery compared with normal hepatocytes, which are primarily supplied by the portal vein [15]. In contrast, ICC is often more hypovascular in nature, potentially making IAT more technically challenging as well as possibly less effective [11].
All the reports known are small single-institution series [15]. In addition, the type of IAT utilized varied considerably from center to center, making interpretation of the data difficult and generalization of the findings limited.
Hyder et al. [15] stratified overall survival on the basis of type of tumor response considered (mRECIST or EASL criteria): complete or partial response and stable disease were 25–35 and 50–60%, respectively. Moreover, ECOG performance status was associated with outcome, as patients with worse ECOG status before IAT had worse results. Adverse effects associated with IAT were relatively uncommon. Only 16 (8.1%) patients had a severe complication, including renal failure, hepatic failure, and liver abscess. Lastly, no statistical differences in overall survival were observed between the types of IAT utilized.
Transarterial chemoembolization (TACE)
Inclusion and exclusion criteria for chemoembolization in patients with ICC are similar to those for patients with hepatocellular carcinoma.
Patients undergoing chemoembolization should have adequate hepatic function (Child-Pugh Class A or B) and acceptable functional performance status [Eastern Cooperative Oncology Group (ECOG) 0–2]. In patients with elevated bilirubin levels, the possibility of biliary obstruction by the tumor should be considered. These cases represent a high-risk situation regarding postprocedural biliary infection. Techniques such as bowel preparation and prolonged antibiotic use have been employed in small case series [18].
In Table 1 [11, 28–35], we reported all published series of patients with inoperable intrahepatic cholangiocarcinoma treated with transarterial chemoembolization. These studies were very heterogeneous in regard to several variables (number and dimensions of the lesions), chemotherapeutic agent administered, number of treatment sessions. Most of the studies utilized modified RECIST criteria [36] to evaluate tumor response. Except for the series of Vogl et al. [34], all authors [11, 29–35] reported median survival from diagnosis.
Statistically significant survival benefit was registered for patients treated with TACE compared with patients receiving best supportive care (BSC), even in patients with extrahepatic metastases [31, 33, 34, 37].
Ray et al. [38] recently reported the results of a meta-analysis regarding chemoembolization for ICC. Over 16 studies, the overall median survival was 15.7 months from the date of diagnosis. Complete or partial response on follow-up imaging based on RECIST criteria was observed in 23% of patients. The overall severe complication rate was 19%, but there was a trend for even higher rates of severe complications (i.e., Common Terminology Criteria for Adverse Events grade 3 or higher) [39] in patients undergoing chemoembolization with irinotecan.
Drug-eluting beads-transarterial chemoembolization (DEB-TACE)
Chemoembolization with drug-eluting beads (DEB) combines the release of drugs from beads with a reduction of blood flow by embolization. Thus, beads with the ability to control drug release and drug dose offer the possibility to continuously apply the chemotherapeutic agent(s) into the tumor area [40].
Table 2 [40–44] reports all series published with patients with inoperable intrahepatic cholangiocarcinoma treated with DEB-TACE. The same considerations about the heterogeneity of the data available performed for patients treated with conventional TACE may be performed. Studies resulted were very heterogeneous for several variables (number and dimensions of the lesions), chemotherapeutic agent administered, sessions of treatments.
Transarterial embolization (TAE)
Even in cases of “hypovascular tumours” on imaging, such as colorectal carcinoma and cholangiocarcinoma, the process of neovascularization from the arterial system is clearly demonstrated by injection of contrast medium via hepatic arterial catheterization while performing computed tomography (CT) angiography [45, 46] (Fig. 2a–d).
This technique is used preferentially over TACE in selected centers. In literature series of patients with HCC and neuroendocrine, liver metastases are more commonly reported [47, 48].
No randomized series on ICC are reported. A small number of patients (n = 13) treated with TAE were included in a multi-institutional analysis of patients with advanced ICC treated with IAT [15]. Median overall survival was 14 months, similar to that observed in the groups of patients treated with TACE, DEB-TACE, and 90Y Radioembolization (p = 0.46).
90Y radioembolization
90Y radioembolization consists of the delivery of highly radioactive microspheres via the hepatic artery. Both resin (SIR- Sphere, Sirtex, Australia) and glass (TheraSphere, BTG, Canada) 90Y microspheres have shown efficacy in patients with ICC.
Several studies regarding the use of radioembolization in ICC have been published in Table 3 [15, 24, 49–54]. Resin microspheres were the most used. In most cases, tumor was multifocal. Heterogeneity of results may be observed, and in particular RECIST criteria were the most used to evaluate treatment response; EASL and WHO criteria [55] were alternatively applied.
When patients were stratified by response rate, the tumor response rate (using RECIST criteria) was found to be correlated with overall survival [51].
Response rates using modified RECIST criteria with specific assessment of delayed-phase imaging at 3 months were found to be an even stronger predictor of overall survival in another series [54]. Overall survival is higher than historical survival rates and shows similar survival to those patients treated with systemic chemotherapy and/or transarterial chemoembolization therapy. Complication profile is similar to that of other IAT [56].
Therefore, the use of Y-90 microspheres should be considered in the list of available treatment options for ICC.
Hepatic arterial infusion chemotherapy (HAIC)
Hepatic arterial infusion chemotherapy, via surgically inserted devices and, more recently, percutaneously implanted port-catheter systems by interventional specialists, proved to be an effective treatment option for selected patients with unresectable colorectal liver metastases [57]. In unresectable ICC, a few earlier studies suggested that HAIC is relatively safe and can improve survival [58–62]. These studies were underpowered due to the small number of patients and included a combination of ICC, HCC, and gallbladder cancer, making the interpretation of results difficult. In 2016, Konstantinidis et al. [63] performed a retrospective analysis of 236 unresectable patients with ICC treated with systemic chemotherapy and/or HAIC. They found that the group of patients with liver-confined disease (n = 104) who were treated with HAIC in addition to systemic chemotherapy (n = 78) experienced improved survival (30.8 months) in comparison with those who were treated with systemic chemotherapy only (n = 26; 18.4 months; p < 0.001). Currently, several phase 1 and 2 prospective trials investigating the effects of HAIC combined with systemic chemotherapy [64, 65] in patients with unresectable ICC are ongoing.
Ablation
Image-guided thermal ablation by means of radiofrequency ablation (RFA) or microwave ablation (MWA) has been proven to be a reasonable therapeutic option for the treatment of relatively small liver tumors, in selected patients (Fig. 3a–c). Advantages of image-guided local ablation include minimal invasiveness, easy performance, repeatability with relative low morbidity and diminished hospital stay that provides for a favorable cost-effectiveness or risk–benefit profile [66].
In suitable cases, thermal ablation can be used as a curative option for HCC [16]; however, the efficacy of thermal ablation for ICC lacks sufficient evidence.
ICC is nonencapsulated and always has a tendency to infiltrate adjacent tissues. It is the invasion of local and hepatic ducts by ICC that leads to the low rate of curative resection and the high recurrence rate after treatment. In general, it is necessary to completely destroy the tumor and an additional 0.5–1 cm of adjacent liver in an attempt to ensure a tumor-free margin [56].
On the basis of the data available in the literature in Table 4 [17, 67–75], RFA was the most used.
A recent meta-analysis established that RFA may be considered a locoregional treatment option that can prolong survival in patients with ICC who are ineligible for surgery [76].
Recently, Yang et al. [75] reported the first experience of 26 inoperable patients treated with percutaneous microwave ablation combined with simultaneous transarterial chemoembolization. Their results are encouraging with a high complete ablation rate without any major complication.
Conclusions
Currently multiple treatment options are available for inoperable ICC. The choice of treatment depends largely on tumor extent and patient performance status. No randomized studies exist to compare treatments; populations treated are very heterogeneous; the selection of therapy often varies from a center to another. Similarly to the approach used for hepatocellular carcinoma, thermal ablation is preferred when lesions are no more than 3 and size is suitable (ideally under 3 and arguably up to 5 cm). In conclusion, the current study provides important data demonstrating the feasibility, safety, and potential efficacy of interventional procedures for ICC, both in inoperable patients and in borderline ones to make them suitable for surgery.
The specific role of the interventional oncology in the management of ICC is growing. Larger studies with the help of international registries and ideally large, randomized, prospective trials are much needed to better demonstrate the benefits of local therapies in the management of ICC.
References
Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol. 2015;1:505527.
Esnaola NF, Meyer JE, Karachristos A, Maranki JL, Camp ER, Denlinger CS. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma cancer. 2016;122(9):1349–69.
Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76.
Patel T. Cholangiocarcinoma—controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8(4):189–200.
Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(9):512–22.
Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10(4):288–91.
Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11(11):1488–96.
Zaydfudim VM, Rosen CB, Nagorney DM. Hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23(2):247–63.
Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–69.
Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23(7):692–7.
Burger I, Hong K, Schulick R, Thuluvath P, Choti M, Kamel I, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16(3):353–61.
Cunningham SC, Choti MA, Bellavance EC, Pawlik TM. Palliation of hepatic tumors. Surg Oncol. 2007;16(4):277–91.
Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–9.
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–71.
Hyder O, Marsh JW, Salem R, Petre EN, Kalva S, Liapi E, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20(12):3779–86.
Bruix J, Sherman M. Practice guidelines committee, American Association for the Study of Liver, management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36.
Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 1016;2012(85):1078–84.
Padia SA. Intrahepatic cholangiocarcinoma. Tech Vasc Interv Rad. 2015;18(5):227–35.
Li YY, Li H, Lv P, Liu G, Li XR, Tian BN, et al. Prognostic value of cirrhosis for intrahepatic cholangiocarcinoma after surgical treatment. J Gastrointest Surg. 2011;15(4):608–13.
Ebata T, Yokoyama Y, Igami T, Sugawara G, Takahashi Y, Nagino M. Portal vein embolization before extended hepatectomy for biliary cancer: current technique and review of 494 consecutive embolizations. Dig Surg. 2012;29(1):23–9.
de Baere T, Teriitehau C, Deschamps F, Catherine L, Rao P, Hakime A, et al. Predictive factors for hypertrophy of the future remnant liver after selective portal vein embolization. Ann Surg Oncol. 2010;17(8):2081–9.
Kodama Y, Shimizu T, Endo H, Miyamoto N, Miyasaka K. Complications of percutaneous transhepatic portal vein embolization. J Vasc Interv Radiol. 2002;13(12):1233–7.
Teo JY, Allen JC Jr, Ng DC, Choo SP, Tai DW, Chang JP, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford). 2016;18(1):7–12.
Mouli S, Memon K, Baker T, Benson AB 3rd, Mulcahy MF, Gupta R, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24(8):1227–34.
Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22(9):3102–8.
Garlipp B, de Baere T, Damm R, Irmscher R, van Buskirk M, Stübs P, et al. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014;59(5):1864–73.
Vouche M, Lewandowski R, Atassi R, Memon K, Gates VL, Ryu RK, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59(5):1029–36.
Herber S, Otto G, Schneider J, Manzl N, Kummer I, Kanzler S, et al. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2007;30(6):1156–65.
Gusani NJ, Balaa FK, Steel JL, Geller DA, Marsh JW, Zajko AB, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg. 2008;12(1):129–37.
Kim JH, Yoon HK, Sung KB, Ko GY, Gwon DI, Shin JH, et al. Transcatheter arterial chemoembolization or chemoinfusion for unresectable intrahepatic cholangiocarcinoma: clinical efficacy and factors influencing outcomes. Cancer. 2008;113(7):1614–22.
Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;17(7):1498–505.
Andrašina T, Válek V, Pánek J, Kala Z, Kiss I, Tuček S, et al. Multimodal oncological therapy comprising stents, brachytherapy, and regional chemotherapy for cholangiocarcinoma. Gut Liver. 2010;1(Suppl 1):S82–8.
Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66(4):322–8.
Vogl TJ, Naguib NN, Nour-Eldin NE, Hochmuth K, Hammerstingl R, Jacob U, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer. 2012;131(3):733–40.
Scheuermann U, Kaths JM, Heise M, Pitton MB, Weinmann A, Hoppe-Lotichius M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma—a single-center experience. Eur J Surg Oncol. 2013;39(6):593–600.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Kuhlmann JB, Blum HE. Locoregional therapy for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29(3):324–8.
Ray CE Jr, Edwards A, Smith MT, Leong S, Kondo K, Gipson M, et al. Metaanalysis of survival, complications, and imaging response following chemotherapy-based transarterial therapy in patients with unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2013;24(8):1218–26.
Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 (2010).
Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol. 2008;31(5):883–8.
Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy Eur J Gastroenterol Hepatol. 2012;24(4):437–43.
Harder J, Euringer W, Langer M, Blum HE, Spangenberg HC. Transarterial chemoembolization of intrahepatic cholangiocarinoma with irinotecan-eluting beads: preliminary results. Z Gastroenterol. 2009;47:101.
Poggi G, Amatu A, Montagna B, Quaretti P, Minoia C, Sottani C, et al. OEM-TACE: a new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Interv Radiol. 2009;32(6):1187–92.
Schiffman SC, Metzger T, Dubel G, Andrasina T, Kralj I, Tatum C, et al. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol. 2011;18(2):431–8. doi:10.1245/s10434-010-1333-4.
Amini A, Gamblin TC. Palliation: treating patients with inoperable biliary tract and primary liver tumors. Surg Oncol Clin N Am. 2014;23(2):383–97.
Sato KT, Omary RA, Takehana C, Ibrahim S, Lewandowski RJ, Ryu RK, et al. The role of tumor vascularity in predicting survival after yttrium-90 radioembolization for liver metastases. J Vasc Interv Radiol. 2009;20(12):1564–9.
Maluccio MA, Covey AM, Porat LB, Schubert J, Brody LA, Sofocleous CT, et al. Transcatheter arterial embolization with only particles for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2008;19(6):862–9.
Gupta S, Johnson MM, Murthy R, Ahrar K, Wallace MJ, Madoff DC, et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: variables affecting response rates and survival. Cancer. 2005;104(8):1590–602.
Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113(8):2119–28.
Saxena A, Bester L, Chua T, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17(2):484–91.
Hoffmann RT, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr EM, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intra- hepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35(1):105–16.
Shridhar R, Sweeney J, Biebel B, Hoffe S.E., Choi J., Arslan B. Short-term outcomes of intrahepatic cholangiocarcinoma treated with glass based yttrium 90 microspheres. J Vasc Interv Radiol 2012; 23 S84–5. Conference: 37th Annual Scientific Meeting of the Society of Interventional Radiology 2012 San Francisco, CA United States.
Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36(2):440–8.
Camacho JC, Kokabi N, Xing M, Prajapati HJ, El-Rayes B, Kim HS. Modified response evaluation criteria in solid tumors and European Association for point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol. 2014;25(2):256–65.
Gonzalez-Guindalini FD, Botelho MP, Harmath CB, Sandrasegaran K, Miller FH, Salem R, et al. Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics. 2013;33(6):1781–800.
Al-Adra DP, Gill RS, Axford SJ, Shi X, Kneteman N, Liau SS. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol. 2015;41(1):120–7.
Kemeny NE, Chou JF, Boucher TM, Capanu M, DeMatteo RP, Jarnagin WR, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol. 2016;113(5):477–84.
Tanaka N, Yamakado K, Nakatsuka A, Fujii A, Matsumura K, Takeda K. Arterial chemoinfusion therapy through an implanted port system for patients with unresectable intrahepatic cholangiocarcinoma—initial experience. Eur J Radiol. 2002;41(1):42–8.
Jarnagin WR, Schwartz LH, Gultekin DH, Gönen M, Haviland D, Shia J, D’Angelica M, et al. Regional chemotherapy for unresectable primary liver cancer: results of a phase II clinical trial and assessment of DCE-MRI as a biomarker of survival. Ann Oncol. 2009;20(9):1589–95.
Inaba Y, Arai Y, Yamaura H, Sato Y, Najima M, Aramaki T, et al. Phase I/II study of hepatic arterial infusion chemotherapy with gemcitabine in patients with unresectable intrahepatic cholangiocarcinoma (JIVROSG-0301). Am J Clin Oncol. 2011;34(1):58–62.
Kemeny NE, Schwartz L, Gönen M, Yopp A, Gultekin D, D’Angelica MI, et al. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results? Oncology. 2011;80(3–4):153–9.
Subbiah IM, Subbiah V, Tsimberidou AM, Naing A, Kaseb AO, Javle M, et al. Targeted therapy of advanced gallbladder cancer and cholangiocarcinoma with aggressive biology: eliciting early response signals from phase 1 trials. Oncotarget. 2013;4(1):156–65.
Konstantinidis IT, Groot Koerkamp B, Do RK, Gönen M, Fong Y, Allen PJ, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758–65.
Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009;20(7 Suppl):S377–90.
Chiou YY, Hwang JI, Chou YH, Wang HK, Chiang JH, Chang CY. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci. 2005;21(7):304–9.
Carrafiello G, Laganà D, Cotta E, Mangini M, Fontana F, Bandiera F, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Interv Radiol. 2010;33(4):835–9.
Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196(2):W205–9.
Giorgio A, Calisti G, De Stefano G, Farella N, Di Sarno A, Amendola F, et al. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single centre experience. Anticancer Res. 2011;31(12):4575–80.
Yu MA, Liang P, Yu XL, Cheng ZG, Han ZY, Liu FY, et al. Liver abscess as a complication of microwave ablation for liver metastatic cholangiocarcinoma after bilioenteric anastomosis. Int J Hyperth. 2011;27(5):503–9.
Haidu M, Dobrozemsky G, Schullian P, Widmann G, Klaus A, Weiss H, et al. Stereotactic radiofrequency ablation of unresectable intrahepatic cholangiocarcinomas: a retrospective study. Cardiovasc Interv Radiol. 2012;35(5):1074–82.
Fu Y, Yang W, Wu W, Yan K, Xing BC, Chen MH. Radiofrequency ablation in the management of unresectable intrahepatic cholangiocarcinoma. J Vasc Interv Radiol. 2012;23(5):642–9.
Butros SR, Shenoy-Bhangle A, Mueller PR, Arellano RS. Radiofrequency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcome. Clin Imaging. 2014;38(4):490–4.
Yang GW, Zhao Q, Qian S, Zhu L, Qu XD, Zhang W, et al. Percutaneous microwave ablation combined with simultaneous transarterial chemoembolization for the treatment of advanced intrahepatic cholangiocarcinoma. Onco Targets Ther. 2015;8:1245–50.
Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. Vasc Interv Radiol. 2015;26(7):943–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Ierardi, A.M., Angileri, S.A., Patella, F. et al. The role of interventional radiology in the treatment of intrahepatic cholangiocarcinoma. Med Oncol 34, 11 (2017). https://doi.org/10.1007/s12032-016-0866-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0866-1