Abstract
Purpose
To assess the overall survival, efficacy, and safety of radioembolization with yttrium-90 (Y90) for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma (ICC).
Methods
Patients with unresectable standard-chemorefractory ICC treated with Y90 were studied. Survival was calculated from the date of first Y90 procedure. Tumor response was assessed with the Response Evaluation Criteria in Solid Tumors criteria on follow-up computed tomography or magnetic resonance imaging scans. National Cancer Institute Common Terminology Criteria (NCI CTCAE), version 3, were used for complications. Statistical analysis was performed by the Kaplan–Meier estimator by the log rank test.
Results
Nineteen patients underwent a total of 24 resin-based Y90 treatments. Median survival from the time of diagnosis and first Y90 procedure was 752 ± 193 [95 % confidence interval (CI) 374–1130] and 345 ± 128 (95 % CI 95–595) days, respectively. Median survival with Eastern Cooperative Oncology Group (ECOG) performance status 1 (n = 15) and ECOG performance status 2 (n = 4) was 450 ± 190 (95 % CI 78–822) and 345 ± 227 (95 % CI 0–790) days, respectively (p = .214). Patients with extrahepatic metastasis (n = 11) had a median survival of 404 ± 309 (95 % CI 0–1010) days versus 345 ± 117 (95 % CI 115–575) days for patients without metastasis (n = 8) (p = .491). No mortality was reported within 30 days from first Y90 radioembolization. One patient developed grade 3 thrombocytopenia as assessed by NCI CTCAE. Fatigue and transient abdominal pain were observed in 4 (21 %) and 6 (32 %) patients, respectively.
Conclusion
Y90 radioembolization is effective for unresectable standard-chemorefractory ICC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a rapidly progressing malignancy of the intrahepatic bile duct epithelium. It is the second most common primary liver cancer, after hepatocellular carcinoma, accounting for 10 to 20 % of primary liver tumors [1]. The age-adjusted incidence rate has rapidly increased from 0.32 per 100,000 in 1975–1979 to 0.85 per 100,000 in 1995–1999 [2, 3]. Early intervention by complete surgical resection or liver transplantation represents the only curative therapy; however, the majority of ICC patients present with advanced disease or the tumor recurs after surgery [4].
Currently there is no recognized standard of care for palliative treatment of advanced ICC. Numerous traditional systemic chemotherapies have demonstrated little benefit for advanced biliary tract carcinomas [5]. The first randomized phase III clinical trial compared gemcitabine versus gemcitabine plus cisplatin for locally advanced or metastatic biliary tract cancer. The study revealed median overall survival of 11.7 months in the gemcitabine–cisplatin group and 8.1 months in the gemcitabine group [6]. Locoregional therapies, such as transarterial chemoembolization [7, 8] and radiofrequency ablation [9, 10], have led to varying degrees of survival benefit. Relative radiosensitivity of normal liver parenchyma has historically resulted in limited use of external-beam radiation for liver malignancies [11, 12]. Supportive treatment (e.g., pain control, biliary drainage) is usually the last option, with a median survival of 3.3 months from the time of diagnosis [13]. The optimal palliative approach to patients with unresectable ICC remains unclear.
Yttrium-90 (Y90) as an effective palliative therapy for primary hepatocellular carcinoma and metastatic colorectal lesions is well reported [14]. However, to date, only three small series have examined this modality for patients with unresectable ICC. The median survivals reported in these series for a subgroup of patients with prior systemic chemotherapy were 4.4, 9.9, and 11 months, respectively [15–17]. The objective of our study was to present data on survival outcomes, clinical and biochemical toxicities, and tumor response with Y90 radioembolization in patients with inoperable ICC refractory to standard systemic chemotherapy.
Materials and Methods
Patient Selection
This prospective cohort study was approved by the local institutional review board and was Health Insurance Portability and Accountability Act compliant. Consecutive patients with unresectable ICC refractory to standard chemotherapy who received care between December 2002 and October 2010 at a single institution were identified. The patients were referred for Y90 radioembolization therapy by a medical oncologist, and/or their case was discussed at multidisciplinary hepatobiliary tumor conferences. Patients were eligible for inclusion if they met the following criteria: histologically proven diagnosis of ICC that was deemed unsuitable for resection or transplantation; progressive disease (PD) while receiving standard systemic chemotherapy; Eastern Cooperation Oncology Group (ECOG) performance status of 0, 1, or 2; adequate hematology (granulocyte count ≥1.5 × 109/L, platelets >50 × 109/L), renal function (creatinine level ≤2.0 mg/dL), and hepatic function (bilirubin level ≤2.0 mg/dL); and pulmonary shunt fraction of less than 20 % [18, 19]. Main portal vein tumor thrombus was a contraindication for therapy, but treatment of branch portal vein disease was permitted. Patients who had received prior treatment for ICC were not excluded. The algorithm for patient selection is shown in Fig. 1.
Procedure Details
All patients underwent superior mesenteric and celiac angiography to define the arterial supply of the liver, tumor, and stomach. Potential hepaticoenteric arterial communications were investigated (gastroduodenal, supraduodenal, retroduodenal, right or accessory gastric, falciform, and accessory or inferior phrenic arteries) and embolized with coils to prevent unintended radiation-induced injuries [20]. A pulmonary shunt fraction of less than 20 % was considered acceptable for treatment [21].
Therapy was administered as previously described [22]. Briefly, radioembolization was performed via a 3F microcatheter via a lobar approach with Y90 resin-based microspheres (SIR-Spheres; Sirtex Medical, Sydney, Australia). The Y90 dose was based on the extent of tumor involvement in the liver, which was calculated by computed tomography (CT) or magnetic resonance volumetric imaging, adjusted by lung shunt fraction. The actual dose was calculated by the body surface area method, decreased by the degree of lung shunting, as recommended by a consensus report, to avoid radiation-induced liver disease [23]. Microsphere administration was performed in an angiography suite with a standard angiographic technique similar to that of chemoembolization. The end point of the injection was either delivering the entire calculated and supplied dose or reaching the vascular flow stasis. Once vessel stasis was detected, no further injection of remaining Y90 dose was performed.
Data Collection: Survival, Safety, Efficacy
All patients had an initial clinic visit, where a complete history was taken and a physical examination performed, and written informed consent was obtained. Patients were followed up at 4 weeks, then every 12 weeks after treatment. All clinical, biochemical, and imaging data were obtained prospectively. Hospital and outpatient records for each patient were inspected to confirm disease status, treatment response, toxicity of therapy, and survival. Patients were contacted with letters and follow-up telephone calls until death, according to a scripted protocol approved by institutional review board. The study was not funded by any organization or institution.
Overall survival was the primary outcome of this study. The Kaplan–Meier method was used to calculate median survival, starting from the time of first treatment with Y90 until the date of last follow-up or death. The number of days until day 0—the date of first treatment—was less than 28. Mortality data was collected from hospital medical records, from the Social Security Death Index interactive search engine, and through telephone contacts. Secondary outcomes were radiological tumor response and posttreatment clinical and serological toxicities. Liver function tests, complete blood count, coagulation profiles, and albumin and total bilirubin levels were obtained on the first day of Y90 treatment for all patients. To standardize reporting, the National Cancer Institute Common Terminology Criteria (NCI CTCAE), version 3, was used to grade treatment complications. Grades 1, 2, 3, and 4 represented mild, moderate, severe, and life-threatening toxicity, respectively. Grade 5 represented toxicity resulting in death. Treatment-related toxicity within 30 days of the first Y90 radioembolization was recorded.
Patients were evaluated for treatment response with helical three-phase, thin-cut CT and magnetic resonance imaging 4 weeks after treatment and then every 12 weeks if stable. Patients receiving incomplete treatment at the first setting were retreated at 4 weeks and then assessed 12 weeks later. Tumor response based on comparative evaluation of pre- and post-treatment axial imaging was graded by the Response Evaluation Criteria in Solid Tumors (RECIST). Briefly, complete response was defined as disappearance of all target lesions; partial response (PR) was defined as a minimum of 30 % decrease in the sum of the longest diameter of the target lesions, taking as reference the baseline sum of longest diameter; stable disease (SD) was defined as neither of sufficient shrinkage to qualify for PR nor of sufficient increase to qualify for PD, taking as reference the smallest sum of longest diameter since the treatment started; PD was defined as at least a 20 % increase in the sum of the longest diameter of the target lesions, taking as reference the smallest sum of the longest diameter recorded since the treatment started or the appearance of new lesions. For patients with bilobar disease who received unilobar therapy, response was assessed according to the first side treated.
Statistical Analysis
Data from all patients who underwent Y90 treatment were analyzed and outcomes were evaluated. Means of continuous variables were tested by independent t-test; the chi-square test was used to compare categorical variables. The Kaplan–Meier method was used to calculate median survivals, pertaining 95 % confidence intervals, and survival curves with last date of follow-up or death used for censoring. Median survivals of different categories of variables were compared by the log rank test. A p-value of less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS 16.0 software (SPSS, Chicago, IL).
Results
Patient Characteristics
Nineteen patients with a mean ± standard deviation age of 63 ± 15.1 years and histologically confirmed unresectable standard-chemorefractory cholangiocarcinoma underwent Y90 radioembolization therapy during the specified time period. The diagnosis was confirmed by ultrasound or CT-guided fine-needle biopsy and by clinical and radiological findings.
Baseline clinicopathologic characteristics of patients are summarized in Table 1.
The ECOG performance status was 0 in 1 patient (5 %), 1 in 14 patients (74 %) and 2 in 4 patients (21 %). All 19 patients had received prior systemic chemotherapy; 4 patients (21 %) received transcatheter arterial chemoembolization (TACE) with drug-eluting beads. No additional therapy such as partial hepatectomy, liver transplantation, or other liver-directed treatments (radiofrequency ablation or cryoablation) had been previously administered. The mean ± standard deviation duration from diagnosis of ICC to the first Y90 radioembolization was 9.9 ± 6.5 months.
Dosimetry
All Y90 radioembolization procedures were performed on an outpatient basis. Treatment was not abandoned in any patient, and no reduction was made in the calculated dose as a result of extensive shunting of resin microspheres to the lung. A total of 24 Y90 sessions were performed; 15 patients (79 %) received one treatment, 3 patients (16 %) received two treatments, and 1 patient (5 %) received three treatment sessions. The reason for multiple sessions was incomplete tumor coverage from the first treatment session. The mean activity of Y90 per treatment was 1.195 GBq.
Survival
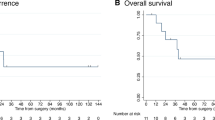
The median follow-up for all patients after Y90 radioembolization was 15 months. At the time of last follow-up, 7 patients (37 %) remained alive and 12 patients (63 %) had died. No patient was lost to follow-up. The median survival from diagnosis and after first Y90 radioembolization was 25.1 and 11.5 months, respectively. The 1-year survival rate after first Y90 therapy was 56 % (Fig. 2). Figure 3 displays the Kaplan–Meier survival estimate for the entire cohort of 19 patients. Table 2 summarizes the median survivals of the dichotomized baseline characteristics.
Radiologic Tumor Response
Tumor response was assessed according to RECIST criteria. Follow-up contrast-enhanced CT and magnetic resonance imaging scans were available for all patients. On average, three postradioembolization scans were obtained for each patient and analyzed by experienced radiologists. Results of therapy are summarized in Table 3.
Overall, when the RECIST criteria at 3-month follow-up was used, PR to treatment was observed in 2 patients (11 %), SD in 13 patients (68 %), and PD in 4 patients (21 %).
Clinical and Biochemical Toxicity
The categorized toxicity profile of patients is listed in Table 4. There was no mortality within 30 days for patients treated with Y90 radioembolization. NCI CTCAE were used to document complications. Clinical toxicities included grade 1 abdominal pain in 6 patients (32 %) and grade 1 fatigue in 4 patients (21 %). All patients were observed for 2–6 h and were discharged on the day of treatment. No serious gastrointestinal complications, such as gastritis or ulceration of gastric mucosa, due to the presence of microspheres were observed.
One patient (5 %) developed grade 3 and 2 patients (11 %) developed grade 2 bilirubin toxicities. One patient (5 %) developed grade 3 thrombocytopenia. Grade 2 aspartate aminotransferase (AST) toxicity was observed in 3 patients (16 %). The mean ± standard deviation worsening from baseline levels of bilirubin, alanine aminotransferase, and AST were, respectively, 0.71 ± 0.22 mg/dL, 6.56 ± 1.77 U/L, and 14.25 ± 4.72 U/L. In 16 patients (84 %), there was no change in posttreatment bilirubin from baseline levels. Posttreatment AST levels remain unchanged from baseline in 14 patients (74 %).
Discussion
ICC is a tumor arising from the second-order branches of intrahepatic bile ducts or the more peripheral bile duct branches [24]. Median survival for those presenting with advanced cholangiocarcinoma is approximately 3–6 months, and overall 5-year survival is less than 5 % [25]. It is a rare yet invariably lethal tumor causing significant morbidity and mortality as a result of its late presentation. The only cure for ICC is surgical resection. Despite the surgical improvements achieved over the last decade, the 5-year survival rates still range from 13 to 44 % [26, 27], and many patients die of local tumor recurrence [28, 29]. However, most patients manifest unresectable disease because the tumor is clinically latent in early stages, and surgery is not an option.
In our study, encouraging overall median survival (11.5 months) was observed compared to similar subgroups in recent studies [15–17]. Contrary to other studies, significantly improved survivals were not observed for subgroups with extrahepatic metastasis or large and multifocal tumors. Future studies should be done with control groups and a large sample size to validate the results. We think that the safety profile was acceptable, with no patient mortality within 30 days of treatment.
Several palliative therapies have been attempted for advanced unresectable ICC. Several chemotherapeutic regimens that used gemcitabine alone or in combination with other agents for biliary tract cancers have resulted in limited responses and survival benefits for unresectable ICC [5]. Valle et al. recently published the first randomized phase III clinical trial that compared gemcitabine plus cisplatin versus gemcitabine alone in patients with locally advanced or metastatic hepatobiliary cancers and found median survivals of 11.7 and 8.1 months, respectively [6]. Limited data are available on the use of external-beam radiotherapy for advanced ICC and liver exposure to high-dose radiation results in significant morbidity [30]. Reported median survival ranges between 10 and 12 months, with no survival benefit in patients with postoperative radiotherapy [11, 12].
Radiofrequency ablation represents another cytoreductive therapy for small-size tumors [10]. The limited literature published on this subject failed to find any significant outcome benefit of radiofrequency ablation for ICC [9]. Within the past few years, minimally invasive liver-directed therapies such as TACE have been investigated in patients with ICC [7, 8, 13]. Although these studies have demonstrated that TACE may be effective in some patients with inoperable ICC, the use of different chemoembolization agents, highly selected patients, and different methods to report survival outcomes limit the ability to interpret results and to make direct comparison between series.
Radioembolization is not a new concept; experimentation with regional infusion of radioisotopes for unresectable liver cancer dates back to the 1970s [31]. A better understanding of the principles of shunt fraction and administration have made this form of therapy more accessible. Encouraging data exist for the use of Y90 in the treatment of ICC, but results are varied and limited. In a prospective study by Ibrahim et al., 29 % of patients were referred for radioembolization after failure of first- and second-line systemic chemotherapies [15]. The median survival in this subgroup of patients was reported to be 4.4 months. Subsequently, larger series supporting this early study were published. Saxena et al. reported a median survival of 9.9 months in their subgroup of patients with previous systemic chemotherapy, compared with 4.6 months in patients without prior chemotherapy [16]. Recently, Hoffmann et al. reported their experience with Y90 in a retrospective analysis of 33 patients with unresectable ICC [17]. They documented a longer survival in chemotherapy-naive group (14.2 months) than in the group of patients with previous systemic chemotherapy (11 months). In our study, the better survival outcome of 11.5 months in patients with previous chemotherapy is encouraging, but because of the lack of a control group, it does not indicate that Y90 treatment has truly affected the natural history of the disease. The prolonged overall survivals reported by Hoffmann et al. could potentially be due to lead-time bias. The mean period from the date of diagnosis until first radioembolization in their study was 21.2 months, compared to 9.6 months in our study. Patients who are diagnosed sooner seem to live longer. Lead-time bias can be corrected by measuring back-end survival.
All patients in our cohort had undergone previous systemic chemotherapy with gemcitabine and were referred for radioembolization because they experienced tumor progression while receiving chemotherapy. From the standpoint of their baseline characteristics, 63 % patients were more than 60 years old, only 5 % patients had an ECOG performance status of 0, and 84 % of patients had a tumor size of >5 cm. Hence, patients were treated at later stages of their advanced disease, and Y90 was offered as a palliative or salvage therapy.
The current study identified one factor that may be associated with significantly improved survival. Patients who received previous treatment with TACE had a better prognosis than the patients who did not receive this therapy (p = 0.047). All the patients who received TACE had an ECOG performance status of 1; it is likely that the results simply reflect a better survival in patients with less severe cancer-related symptoms. Patients with an ECOG performance status of 0 and 1 had a relatively better prognosis than patients with an impaired performance status of ECOG 2, but it was not statistically significant. Only 1 patient (5 %) had a pretreatment performance status of ECOG 0, while the rest of the patients presented with advanced disease (95 %). According to RECIST response criteria, patients with a PR and SD (79 %) to Y90 therapy had a substantially longer median survival of 11.5 months compared to 2.8 months with PD (21 %), but results did not correlate significantly. Our study did not reveal poor prognosis, as would be expected, in patients with extrahepatic metastasis (13.5 months) compared with patients without metastasis (11.5 months) (p = 0.491). Similarly, worse survivals were not observed in patients with multifocal tumor burden versus focal tumors (13.5 months vs. 11.5 months); tumor size of >5 cm versus tumor of ≤5 cm (13.5 months vs. 4.9 months); and bilobar (15 months) tumor distribution compared with unilobar (11.5 months) tumor distribution.
The above observations may not necessarily corelate with similar studies. One explanation is the small sample size due to the relative rarity of ICC patients who undergo Y90 after their disease becomes refractory to chemotherapy. Moreover, complete illustration of survival benefits from any liver-directed therapy also depends on parameters such as failure of systemic chemotherapy, and all patients in our cohort had chemorefractory disease. Nonetheless, the findings need to be further evaluated in large studies; these observations might be proven otherwise. In observing toxicity profiles of Y90 according to the NCI CTCAE, we noted two grade 3 biochemical toxicities (bilirubin toxicity, thrombocytopenia) on posttreatment serology. No patient died within 30 days of treatment. The most common clinical symptoms were grade 1 abdominal pain (32 %) and fatigue (21 %). No other treatment-related severe adverse events were observed.
Y90 radioembolization, unlike radiofrequency ablation, is effective for large-size tumors (>5 cm), as indicated by the results of our study. Moreover, compared to TACE, radioembolization is well tolerated with less overall toxicity, resulting in a shorter hospital stay [21]. We acknowledge the limitations of our small study size and the nonrandomized design, which did not allow us to identify all the prognostic factors. Our study was of a group of patients with a poor prognosis, which may have had some effect on the results. Newly reported data of the randomized study on gemcitabine–cisplatin chemotherapy [6] will be a guide for future studies on Y90 for unresectable ICC.
Our study evaluated the safety and efficacy of Y90 for the palliative treatment of unresectable ICC. The encouraging survival outcomes should be evaluated in future studies to demonstrate that the natural history of the disease is truly affected by the treatment.
References
Shaib Y, El-Serag HB (2004) The epidemiology of cholangiocarcinoma. Semin Liver Dis 24:115–125
Patel T (2001) Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 33:1353–1357
Shaib YH, Davila JA, McGlynn K, El-Serag HB (2004) Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 40:472–477
Farley DR, Weaver AL, Nagorney DM (1995) “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc 70:425–429
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902
Valle J, Wasan H, Palmer DH, for the ABC-02 Trial Investigators et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Burger I, Hong K, Schulick R, Georgiades C et al (2005) Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 16:353–361
Herber S, Otto G, Schneider J et al (2007) Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Interv Radiol 30:1156–1165
Chiou YY, Hwang JI, Chou YH et al (2005) Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung J Med Sci 21:304–309
Kim JH, Won HJ, Shin YM, Kim KA, Kim PN (2011) Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 196:W205–W209
Veeze-Kuijpers B, Meerwaldt JH, Lameris JS et al (1990) The role of radiotherapy in the treatment of bile duct carcinoma. Int J Radiat Oncol Biol Phys 18:63–67
Pitt HA, Nakeeb A, Abrams RA et al (1995) Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg 221:788–797
Park SY, Kim JH, Yoon HJ et al (2011) Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol 66:322–328
Vente MA, Wondergem M, van der Tweel I et al (2009) Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 19:951–959
Ibrahim SM, Mulcahy MF, Lewandowski RJ et al (2008) Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer 113:2119–2128
Saxena A, Bester L, Chua TC et al (2009) Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol 17:484–491
Hoffmann RT, Paprottka PM, Schön A et al (2012) Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Interv Radiol 35:105–116
Salem R, Parikh P, Atassi B et al (2008) Incidence of radiation pneumonitis after hepatic intra-arterial radiotherapy with yttrium-90 microspheres assuming uniform lung distribution. Am J Clin Oncol 31:431–438
Bester L, Salem R (2007) Reduction of arteriohepatovenous shunting by temporary balloon occlusion in patients undergoing radioembolization. J Vasc Interv Radiol 18:1310–1314
Lewandowski RJ, Sato KT, Atassi B et al (2007) Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Interv Radiol 30:571–592
Kooby DA, Egnatashvili V, Srinivasan S et al (2009) Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 21:224–230
Salem R, Thurston KG (2006) Radioembolization with 90yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol 17:1251–1278
Kennedy A, Nag S, Salem R et al (2007) Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the Radioembolization Brachytherapy Oncology Consortium. Int J Radiat Oncol Biol Phys 68:13–23
Chen MF (1999) Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol 14:1144–1149
American Cancer Society (2010) Cancer facts and figures. http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-and-figures-2010
Nathan H, Pawlik TM, Wolfgang CL et al (2007) Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 11:1488–1496
Lieser MJ, Barry MK, Rowland C et al (1998) Surgical management of intrahepatic cholangiocarcinoma: a 31-year experience. J Hepatobiliary Pancreat Surg 5:41–47
Miwa S, Miyagawa S, Kobayashi A et al (2006) Predictive factors for intrahepatic cholangiocarcinoma recurrence in the liver following surgery. J Gastroenterol 41:893–900
Paik KY, Jung JC, Heo JS et al (2008) What prognostic factors are important for resected intrahepatic cholangiocarcinoma? J Gastroenterol Hepatol 23:766–770
Czito BG, Anscher MS, Willett CG (2006) Radiation therapy in the treatment of cholangiocarcinoma. Oncology (Williston Park) 20:873–884
Grady ED (1979) Internal radiation therapy of hepatic cancer. Dis Colon Rectum 22:371–375
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rafi, S., Piduru, S.M., El-Rayes, B. et al. Yttrium-90 Radioembolization for Unresectable Standard-chemorefractory Intrahepatic Cholangiocarcinoma: Survival, Efficacy, and Safety Study. Cardiovasc Intervent Radiol 36, 440–448 (2013). https://doi.org/10.1007/s00270-012-0463-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-012-0463-4