Abstract
Intrahepatic cholangiocarcinoma (ICC) is a rare life-threatening disease, whose only treatment with potential for cure is surgical resection. However, only 27% of patients at most are suitable for surgery when first diagnosed. For patients with unresectable disease, therapeutic options are chemotherapy or chemoradiation. We evaluated the feasibilty and safety of oxaliplatin-eluting microspheres transarterial chemoembolization (OEM-TACE) associated with chemotherapy (ChT) in patients affected by unresectable ICC. Between December 2005 and May 2008 we treated nine patients (six female and three male) with unresectable ICC. All patients had undergone OEM-TACE associated with chemotherapy with oxaliplatin and gemcitabine. A retrospective comparison was carried out with a historical group of 11 patients treated with ChT only, estimating the prevalence of adverse effects and the median survival of the two groups. A total of 30 TACEs were performed during the observational time (ranging from one to seven procedures per patient). OEM-TACEs were followed by few adverse effects (AEs), without G4 AEs, according to CTACAE 3.0. According to RECIST criteria, 44% (4/9) of patients achieved partial responses and 56% (5/9) stabililization of disease. Overall survival analysis in the two groups showed a significantly increased survival in patients treated with ChT and OEM-TACE, with respect to those treated with ChT (30 vs. 12.7 months; p = 0.004). In conclusion, in our experience OEM-TACE associated with ChT in the treatment of advanced unresectable ICC is a safe and feasible treatment causing no major adverse events. Although RECIST criteria can underestimate the rate of responses in patients treated with locoregional therapies, we achieved very encouraging results. A randomized multicentric trial is warranted to assess the actual superiority of OEM-TACE associated with ChT compared to conventional chemotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic biliary malignancies (intrahepatic cholangiocarcinoma; ICC) account for 13% of annual cancer-related deaths worldwide and for 3% in Western countries. Cholangiocarcinoma (CCA) arises from the ductal epithelium of the intra- or extrahepatic biliary tree. Currently, CCAs are anatomically classified as intrahepatic (including tumors arising from bifurcation of the hepatic duct or Klatskin’s tumors) and extrahepatic [1]. The prevalence of CCA shows a wide geographic variability, with the highest rates in Asia, due to endemic infections with Opisthorchis Viverrini and Clonorchis sinensis [2–4]. The only curative treatment for CCA is complete surgical resection with histologically negative resection margins, but only between 13 and 27% of patients are suitable for surgery [5–7]. Patients with unresectable disease can benefit from palliative therapies such as systemic chemotherapy (ChT). In the past, several cytotoxic drugs were used in palliative treatment of CCA, including mitomycin-C, 5 FU/leucovorin, cisplatin, and doxorubicin, affording little or no improvement in survival. Currently, ChT based on gemcitabine, alone or in combination with platinum derivatives such as oxaliplatin and cisplatin, has shown a statistically significant but moderate improvement in overall survival compared with palliative care [8]. New treatment options have been proposed in ICC, including the locoregional approach based on transarterial chemoembolization (TACE), which has been widely used in hepatocellular carcinoma, where it has shown better results than conventional palliative care [9–11]. TACE is performed by combining a chemotherapeutic agent, the most widely used being doxorubicin, with an ethiodized oil (lipiodol; Savage Laboratories, Melville, NY, USA) whose role is to emulsify the drugs and carry them into the lesions. Embolization particles or Gelfoam (Pharmacia and Upjohn, Kalamazoo, MI, USA) are then used to reduce arterial inflow and decrease washout of the chemotherapeutic agents into the systemic circulation in order to prolong the contact time between cancer cells and drugs. New drug-eluting microspheres, now available, seem to optimize TACE procedures, loading the drugs and slowly delivering them directly into the tumor, thus achieving high intratumoral concentrations and low plasma concentrations [12]. The capability of these microspheres to be loaded with other types of chemotherapeutic agents gives us a new tool to deliver high doses of effective drugs in tumors with different histotypes, such as ICC, while decreasing plasma levels of drug and limiting systemic side effects.
In this work, the safety and feasibility of oxaliplatin-eluting microsphere TACE (OEM-TACE) were evaluated in patients affected by unresectable ICC.
Patients and Methods
Between December 2005 and May 2008 we treated nine patients (six female and three male) affected by unresectable ICC (mean age, 66.5 years; SD, 8.01 years). Patient characteristics are reported in Table 1. Study inclusion criteria were histologically proven disease by percutaneous core needle biopsy and an ECOG status ≤1. Exclusion criteria included Child-Pugh class C disease, evidence of extrahepatic disease, renal failure, hepatic failure (bilirubin >2 mg/dl, serum albumin <35 g/L), impaired clotting test (platelet count <50,000/mm3 or prothrombin activity <50%), ascites, or evidence of portal thrombosis. Liver function was assessed using Child-Pugh score in all patients, regardless of history of cirrhosis. However cirrhotic patients were equally distributed in the two groups of the study (Table 1). Written informed consent was obtained from all patients prior to treatment.
All patients underwent OEM-TACE followed by ChT containing oxaliplatin and gemcitabine (oxaliplatin, 85 mg/mq, and gemcitabine, 1000 mg/mq d1 q21). A retrospective comparison was carried out with a historical group of 11 patients (4 female and 7 male) affected by ICC. These patients were treated at our institution between September 2002 and April 2005 only with systemic chemotherapy based on oxaliplatin, 85 mg/mq, and gemcitabine, 1000 mg/mq d1 q21.
We retrospectively estimated the incidence of adverse effects (AEs), the progression free-survival (PFS) and the overall survival (OS) of the two groups (OEM-TACE vs. ChT).
Treatment Schedule
All patients underwent OEM-TACE followed by ChT containing oxaliplatin and gemcitabine (oxaliplatin, 85 mg/mq, and gemcitabine, 1000 mg/mq d1q21). The TACE session was considered completed when complete devascularization of all target lesions was obtained at the end of the last procedure. The first cycle of chemotherapy was administered 2–4 weeks after TACE was completed. Before TACE every patient received proton pump inhibitors (PPIs) and antiemetic prophylactic drugs. OEM-TACE was perfomed with Hepaspheres, 50 mg, with a diameter ranging from 50 to 100 μm, preloaded by mixing them for at least 10–15 min with 50 mg oxaliplatin (Eloxatin; Sanofi-Aventis) and diluting the mixture with 5 ml of the nonionic contrast medium (iodixanol; Visipaque 270; Amersham Health, Milan, Italy). The particles were then further diluted with the same contrast medium to obtain a 30-ml solution and continuously mixed with a stopcock between two 20-ml syringes before being injected through a 1-ml Luer-Lock syringe. Antibiotic prophylaxis (amoxicillin-clavulonate, 2 g/day i.v.) was given before and after the procedure. Femoral approach was obtained through a 25-cm-long, 5- or 6-Fr introducer in order to straighten the sharp bending of elongated iliac arteries. Baseline selective angiography of celiac trunk and common hepatic and mesenteric arteries was performed with a properly shaped tip catheter, prolonging the time of fluorography to obtain diagnostic imaging of the portal system. The particles were injected manually under continuous fluoroscopy assessment with a Luer-Lock syringe through the microcatheter positioned as distally as possible into the right or left hepatic artery, avoiding any dangerous reflux inside the arteries feeding the gallbladder or the gastric wall. The angiographic end point was achieved when stagnant flow in the pathologic area was appreciated. Serum liver transaminases, total bilirubin, alkaline phosphatase, cell blood and platelet counts, serum amylase, and lipase were monitored for each patient just before and 24 h after each OEM-TACE procedure.
Evaluation of Response and Safety
Carcinoembrionary antigen (CEA) and carbohydrate antigen 19.9 (CA 19.9) were measured at baseline in both populations of patients. The baseline value of CA 19.9 exceeded the normal upper limit in 3 of 9 patients in the OEM-TACE group (range, 142–4677 IU/ml) and in 4 of 11 patients in the ChT group (range, 165–5000 IU/ml), while CEA exceeded the normal upper limit in only 1 patient in the OEM-TACE, group so they were not considered useful in monitoring the response to both treatments. All patients were evaluated with contrast-enhanced abdominal CT scan every 3 months. Response were evaluated by RECIST criteria [13]. Adverse events were evaluated by CTCAE 3.0 [14].
Statistical Analysis
Analysis of the survival parameters was performed on 31 October 2008. OS was defined as the interval from the first therapy (start of follow-up period) to the time of death; PFS was defined as the interval from the first therapy to the time of disease progression, according to RECIST criteria. Patients undergoing surgical intervention or withdrawal (lost to follow-up) were censored. Survival rates were estimated with the use of the Kaplan–Meier method with R software for Linux (R Software, Vienna, Austria) [15, 16]. A retrospective comparison was carried out with a historical group of 11 patients (4 female and 7 male) treated only with ChT containing oxaliplatin and gemcitabine, estimating the incidence of AEs, PFS, and OS in the two groups (OEM-TACE vs. ChT). Comparisons between baseline characteristics of the two groups were carried out by means of chi-square test or unpaired t-test for discrete or continuous variables, respectively. Statistical analysis for significance was performed using log-rank (Mantel-Cox) test; statistical significance was assigned for a p-value of <0.05. Differences in adverse events were tested with chi-square test or Fischer exact test where appropriate.
Results
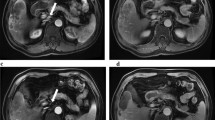
The median observation time was 20 months for the historical ChT group and 35 months for the OEM-TACE group, respectively. A total of 64 cycles of ChT were performed during the observational time in the historical group of patients (ranging from 3 to 11 cycles for each patient). Several AEs were recorded in the ChT group, including nausea, vomiting, peripheral neuropathy, asthenia, and leucopoenia, ranging from grade 1 to grade 3, according to CTCAE 3.0 criteria. In the nine patients in the OEM-TACE group, a total of 30 OEM-TACEs and 37 cycles of ChT were performed, ranging from 1 to 7 TACE procedures and from 3 to 7 cycles of ChT for each patient. In the OEM-TACE group a low number of AEs were recorded, ranging from grade 1 to grade 3; no G4 AEs occurred. AEs were calculated on a total of 67 procedures administered; abdominal pain was observed in 24% of procedures (28/67), cholangitis G3, and hypertensive crisis G3 in 3% (2/67). Table 2 presents major AEs according to the study groups. Compared with ChT only, the combination of OEM-TACE and ChT was associated with less grade 3 asthenia, gastrointestinal AEs, and peripheral neuropathy, but significantly more grade 3 pain and liver toxicity. Response evaluation was obtained with CT scan according to RECIST criteria. All patients in the historical group were reassessed between three and six cycles of ChT, and 73% (8/11) were found to be in PD. Patients in group OEM-TACE were re-evaluated by CT scan 3 months after the first procedure, 44% (4/9) of patients achieving a partial response (PR), while 56% (5/9) of patients reached stable disease (SD). Among the four patients who achieved PR, three met the inclusion criteria for surgical resection: to date, after debulking surgery, two of the three patients are still alive and disease-free (Fig. 1). The only patient with CT evidence of partial response who did not meet the surgical criteria for resection underwent 18FDG PET, which did not reveal any areas of residual metabolic hyperactivity, thus confirming complete necrosis of the voluminous lesion located in the right liver lobe (Fig. 2). Among the patients in the historical group, the median PFS was 2.9 months, and OS was 12.7 months, ranging from 4 to 24 months, in agreement with the literature [17–20]. Four of nine patients treated with OEM-TACE are still alive, with a median PFS of 8.4 months and a median OS of 30 months (Fig. 3). Analysis of survival data points out a statistically significant difference in OS: patients treated with OEM-TACE benefit from an advantage in survival compared to patients treated only with ChT (p = 0.004).
Microscopic pathology in a patient who underwent hepatic resection of the residual tumor following three cycles of OEM-TACE. A cluster of microspheres is shown within tumoral arteries surrounded by necrotic (thick arrow) and fibrosclerotic tissue (thin arrow), without evidence of viable tumoral tissue (Hematoxylin and eosin; original magnification, ×100)
Discussion
In this work we have reported our experience in treating patients affected by unresectable ICC with OEM-TACE followed by ChT. The PFS, OS, and toxicity profiles found for these patients were compared with those for a historical group of patients affected by unresectable ICC treated only with ChT. The addition of OEM-TACE to ChT led to an increased incidence of abdominal pain and a transient elevation of hepatic enzyme but did not increase the incidence of hematological side effects. As we have reported elsewhere [21] the administration of oxaliplatin with TACE allows higher concentrations of drug within the lesion compared to the systemic route, while minimizing systemic side effects. Moreover, OEM-TACE patients received fewer systemic ChT cycles, which may partially account for the differences observed in ChT-related gastrointestinal, hematologic, and/or neurologic side effects.
A significantly increased median survival was observed in the OEM-TACE group compared to the ChT group (30 vs. 12.7 months; p = 0.004), without greater toxicity.
However several limits are inherent in our study. First, the comparison between the two groups was retrospective, so the significance of the results we obtained is different from that of a prospective randomized trial, although prognostic factors were nearly equally distributed in the two groups. However, the differences found could not be attributed to improved diagnostic and therapeutic procedures, since at our institution these were not significantly changed during the time encompassing the treatment of the two groups. Another relevant consideration is the absence of extrahepatic disease in the OEM-TACE group compared to the historical ChT group. Although this difference in disease stage was slight (2 of 11 patients) it could have negatively influenced the OS of the ChT group.
Finally, new methods of response evaluation are warranted, because of the inadequacy of RECIST criteria in determining tumor response to locoregional therapy. As shown by our results, PFS was not significantly different between the two groups (p = 0.10), in contrast with the evidence of a significant increase in OS (p = 0.004). In fact, following OEM-TACE a decrease in tumor size fulfilling the partial response criteria is rarely achieved, although OEM-TACE may produce the complete necrosis of the tumor.
Locoregional therapy seems to play a promising role in the treatment of unresectable patients with ICC. Burger et al. [22] first used TACE as locoregional treatment for 17 patients with unresectable ICC, obtaining a median survival of 23 months. Aliberti et al. [23] used doxorubicin-eluting beads TACE in 11 unresectable and ChT-naive patients with ICC, achieving a median survival of 13 months. Tanaka et al. [24] combined intraarterial delivery of ChT followed by transarterial embolization (TAE) with lipiodol in 11 patients, achieving a median survival of 26 months. Similar experiences are reported by Vogl et al. [25] using hepatic intraarterial chemotherapy with gemcitabine and embolization with starch microspheres.
No studies of direct comparison between locoregional treatment and chemotherapy in ICC are found in the literature. Only Aliberti et al. reported a comparison between the two treatments, but in their experience the two groups were unbalanced, because patients in the control arm underwent an inhomogeneous treatment (chemotherapy or other unspecified palliative care). Despite the limitations previously outlined, in our experience both groups were treated with chemotherapy, but one of them also received OEM-TACE. Therefore the differences in OS observed in the two patient populations can be attributed, with stronger evidence, to the effect of the additional locoregional treatment. However, due to the very small number of patients, this has to be considered an exploratory study, in anticipation of a future prospective study designed to correctly quantify the advantage that OEM-TACE offers to these patients.
Conclusion
In our experience OEM-TACE associated with ChT in advanced unresectable ICC is a safe and feasible treatment causing no major adverse events. Although RECIST criteria can underestimate the rate of responses in patients treated with locoregional therapies, we achieved very encouraging results. A randomized multicentric trial is warranted to assess the actual superiority of OEM-TACE associated with ChT compared to conventional chemotherapy.
References
Shaib Y, El-Serag HB (2004) The epidemiology of cholangiocarcinoma. Semin Liver Dis 24:115–125
Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL (1996) Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 224:463–473
Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD (2004) Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol 99:523–526
Berthiaume EP, Wands J (2004) The molecular pathogenesis of cholangiocarcinoma. Semin Liver Dis 24:127–137
Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH (2008) Surgical management of intrahepatic cholangiocarcinoma—a population-based study. Ann Surg Oncol 15:600–608
Shaib YH, Davila JA, Henderson L, McGlynn KA, El-Serag HB (2007) Endoscopic and surgical therapy for intrahepatic cholangiocarcinoma in the United States: a population-based study. J Clin Gastroenterol 41:911–917
Jarnagin WR, Fong Y et al (2001) Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 234(4):507–519
Alberts SR, Gores GJ, Kim GP, Roberts LR, Kendrick ML, Rosen CB, Chari ST, Martenson JA (2007) Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin Proc 82:628–637
Llovet JM, Real MI, Montana X et al (2002) Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359:1734–1739
Lo CM, Ngan H, Tso WK et al (2001) Randomized controlled trial of transarterial lipiodol chemoembolization for hepatocellular carcinoma: Is there room for a new studies? Clin Gastroenterol 32:383–389
Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M (2002) Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 224:47–54
Lewis AL, Taylor RR, Brenda H et al (2006) Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol 17:1335–1343
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Kaplan EL, Meier P (1958) Non parametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
R Development Core Team (2005) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org
Endo I, Gonen M, Yopp AC et al (2008) Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 248:84–96
Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, Boku N, Tanaka K, Nagase M, Saisho H, Sato T (2007) A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol 37:843–851
Charoentum C, Thongprasert S, Chewaskulyong B, Munprakan S (2007) Experience with gemcitabine and cisplatin in the therapy of inoperable and metastatic cholangiocarcinoma. World J Gastroenterol 13:2852–2854
Paule B, Herelle MO, Rage E, Ducreux M, Adam R, Guettier C, Bralet MP (2007) Cetuximab plus gemcitabine-oxaliplatin (GEMOX) in patients with refractory advanced intrahepatic cholangiocarcinomas. Oncology 72:105–110
Poggi G, Quaretti P, Minoia C et al (2008) Transhepatic arterial chemoembolization with oxaliplatin-eluting microsphere (OEM-TACE) for unresectable hepatic tumors. Anticancer Res 28:3835–3842
Burger I, Hong K, Schulick R, Georgiades C, Thuluvath P, Choti M, Kamel I, Geschwind JF (2005) Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol 16:353–361
Aliberti C, Benea G, Tilli M, Fiorentini G (2008) Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. CardioVasc Interv Radiol 5:883–888
Tanaka N, Yamakado K, Nakatsuka A, Fujii A, Matsumura K, Takeda K (2002) Arterial chemoinfusion therapy through an implanted port system for patients with unresectable intrahepatic cholangiocarcinoma—initial experience. Eur J Radiol 41:42–48
Vogl TJ, Schwarz W, Eichler K, Hochmuth K, Hammerstingl R, Jacob U, Scheller A, Zangos S, Heller M (2006) Hepatic intraarterial chemotherapy with gemcitabine in patients with unresectable cholangiocarcinomas and liver metastases of pancreatic cancer: a clinical study on maximum tolerable dose and treatment efficacy. J Cancer Res Clin Oncol 132:745–755
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poggi, G., Amatu, A., Montagna, B. et al. OEM-TACE: A New Therapeutic Approach in Unresectable Intrahepatic Cholangiocarcinoma. Cardiovasc Intervent Radiol 32, 1187–1192 (2009). https://doi.org/10.1007/s00270-009-9694-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-009-9694-4