Abstract
Zinc (Zn), copper (Cu), and iron (Fe) are essential trace elements for the growth, development, and maintenance of healthy bones. However, there are conflicting reports as to the relationship between serum level of Zn, Cu, or Fe and osteoporosis (OP). The purpose of the present study is to clarify the relationship between serum Zn, Cu, or Fe and OP using a meta-analysis approach. We searched all articles indexed in PubMed published up to May 2014 concerning the association between serum level of Zn, Cu, or Fe and OP. Eight eligible articles involving 2,188 subjects were identified. Overall, pooled analysis indicated that patients with OP had a lower serum level of Zn, Cu, or Fe than the healthy controls (Zn standardized mean difference (SMD) = −1.396, 95 % confidence interval (CI) = [−2.129, −0.663]; Cu SMD = −0.386, 95 % CI = [−0.538, −0.234]; Fe SMD = −0.22, 95 % CI = [−0.30, −0.13]). Further subgroup analysis found that geographical location and gender had an influence on the serum level of Zn in OP and healthy controls, but not on the serum level of Cu or Fe. No evidence of publication bias was observed. In conclusion, this meta-analysis suggests that low serum levels of Zn, Cu, and Fe seem to be important risk factors for OP and well-designed studies with adequate control for confounding factors are required in future investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trace elements are essential for normal growth and development of the skeleton in human and animals. Although they are minor building components in bones, they play important functional roles in bone metabolism and bone turnover [1]. Our previous study finds that low serum level of magnesium is a risk factor for osteoporosis (OP), which drives us to explore more on the association between OP and other trace elements [2].
Trace element disturbances, such as zinc (Zn), copper (Cu), or iron (Fe) deficiency, have become an increasing important risk factor for OP [3–5]. Zn is essential for the growth of human and animals and is required for the growth, development, and maintenance of healthy bones [5–7]. Zn has been demonstrated to stimulate osteoblastic bone formation and to inhibit osteoclastic bone resorption, thereby increasing bone mass [8–10]. Cu plays a pivotal role in crosslinking of collagen and elastin, and it is a necessary mineral for bone development and maintenance [11, 12]. Fe is an integral part of many enzymes and an essential element for life cycle of cells and plays a role in the synthesis of collagen and other proteins that form the structure of bones [13, 14]. Animal studies indicate that bone growth retardation is associated with Zn, Cu, or Fe deficiency and that deficiencies lead to OP [15–21]. Human studies show that there is a significant relationship between low serum level of Zn, Cu, or Fe and low bone mineral density (BMD), and supplementation of these trace elements is found to restore both skeletal growth and maturation [22–28]. However, other human studies find no association between serum level of Zn, Cu, or Fe and OP [29–31]. Although serum Zn, Cu, or Fe deficiency is plausibly linked to an increase risk of OP, the inconsistency among the findings of previous studies precludes definitive recommendations at present.
Meta-analysis is an important tool for revealing trends that might not be apparent. Therefore, we performed a comprehensive and critical meta-analysis of the studies, in order to draw a more clear and evidence-based conclusion on the association between serum level of Zn, Cu, or Fe and OP.
Methods
Search Strategy
We searched all English-written articles indexed in PubMed published up to May 2014. Literature searches were performed using medical subject heading (MeSH) or free text words. The searching keywords were as follows: “serum zinc” or zinc or “serum copper” or copper or “serum iron” or iron and osteoporosis. Reference lists of all eligible studies were screened to identify potentially eligible studies. Emails were sent to the authors of identified studies for additional information if necessary.
Selection Criteria
Three authors (Jianmao Zheng, Jingjing Quan, Xueli Mao) conducted the search independently. Titles and abstracts were screened for subject relevance. Studies that could not be definitely excluded based on abstract information were also selected for full-text screening. Two authors (Jianmao Zheng, Jingjing Quan) independently selected eligible studies for inclusion possibility. Where there was a disagreement for study inclusion, a discussion was held (with Xueli Mao) to reach a consensus.
Eligible studies should meet the following criteria: (1) human study; (2) case-control study or cohort study or randomized clinical trial; (3) studies focusing on the association between serum level of Zn, Cu, or Fe and OP; (4) studies providing data of serum level of Zn, Cu, or Fe for both osteoporotic and non-osteoporotic subjects; and (5) subjects with no diseases and no drugs intake which might influence the serum level of Zn, Cu, or Fe.
Exclusion criteria included the following: (1) animal study; (2) in vitro or laboratory study; (3) review or case report; (4) studies not providing serum level of Zn, Cu, or Fe for both osteoporotic and non-osteoporotic subjects; (5) subjects with diseases and drugs intake which might influence the serum level of Zn, Cu, or Fe; and (6) sample size less than 20.
Data Extraction and Quality Assessment
Two authors (Xueli Mao, Jianmao Zheng) independently extracted data using a standard form. The following information was extracted from each included study: first author’s family name; year of publication; country; demography of subjects (number of subjects and gender); and data on serum Zn, Cu, or Fe.
The qualities of all included studies were assessed using the Newcastle-Ottawa scale (NOS). The assessment tool focused on three aspects, including participant selection, comparability, and exposure. The studies would be assigned stars of nine if all items were satisfied. Two authors (Xueli Mao, Jianmao Zheng) assessed the quality independently.
Statistical Analysis
The extracted data were used to perform meta-analysis to obtain the standardized mean difference (SMD) and 95 % confidence interval (CI). The SMDs were calculated using either fixed-effects models or, in the presence of heterogeneity, random-effects models. Heterogeneity between studies was tested through the Chi-square and I-square tests. If the I 2 value was less than 50 % and the p value was greater than 0.05, the meta-analysis was not considered as homogeneous.
Subgroup analysis was used to identify associations between the serum level of Zn, Cu, or Fe and other relevant study characteristics (such as geographical location and gender) as possible sources of heterogeneity. Publication bias was measured using Begg’s tests and visualization of funnel plots. The stability of the study was also detected by sensitivity analysis, through re-meta-analysis with one involved study excluded each time. All statistical analyses were performed with Stata version 11.0 (StataCorp, College Station, TX).
Results
Literature Search
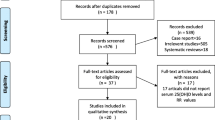
The literature search yielded a total of 637 primary articles. These articles were included for full-text assessment, of which 629 were excluded for one of the following reasons: (1) irrelevant to our topic (n = 303), (2) non-original studies (reviews etc.) (n = 158), (3) non-human studies (n = 153), and (4) articles not providing serum level of Zn or Cu or Fe for both osteoporotic and non-osteoporotic subjects (n = 15). Overall, 8 eligible articles with 2,188 subjects met the inclusion criteria for meta-analysis, in which 7 articles with 13 case-control studies for Zn [23–25, 29–32], 5 articles with 10 case-control studies for Cu [23, 24, 30–32], and 3 articles with 9 case-control studies for Fe [27, 30, 32]. Of note, five articles involved more than one risk factor and were included in more than one group [23, 24, 30–32]. A flow diagram of the study selection process is presented in Fig. 1.
Study Characteristics and Quality Assessment
The detailed characteristics of the included studies and the results of the quality assessment were summarized in Tables 1, 2, and 3. The number of subjects in each study ranged from 60 [29] to 576 [32]. The earliest study was published in 1995 [29], and the latest in 2013 [32]. By geographic location, five case-control studies were conducted in western countries [25, 27, 29], and 10 case-control studies in oriental countries [23, 24, 30–32]. Thirteen case-control studies were conducted with the post-menopausal women [23, 24, 27, 29–32], and 2 case-control studies with men [25]. The overall study quality averaged 6.9 stars on a scale of 0 to 9.
Serum Zn and OP
The random-effects meta-analysis results indicated that patients with OP had a lower serum level of Zn than the healthy controls (SMD = −1.396, 95 % CI = [−2.129, −0.663]) (Fig. 2). The 13 sets of results showed a statistically significant amount of heterogeneity (I 2 = 98.3 %, p < 0.001) (Fig. 2). The subgroup analysis showed that geographical location and gender had an influence on the serum level of Zn in OP and healthy controls. The difference of serum level of Zn between OP and healthy controls in western countries (or male) was higher than that in oriental countries (or female) (Table 4).
Serum Cu and OP
The random-effects meta-analysis results indicated that patients with OP had a lower serum level of Cu than healthy controls (SMD = −0.386, 95 % CI = [−0.538, −0.234]) (Fig. 3). The 10 sets of results showed a statistically significant amount of heterogeneity (I 2 = 57.5 %, p = 0.012) (Fig. 3). The subgroup analysis showed that geographical location was the main sources of heterogeneity. Further analysis found that the subgroup which excluded the only one Chinese study (Liu in 2009) showed no significant amount of heterogeneity (I 2 = 39.7 %, p = 0.103) (Table 4).
Serum Fe and OP
The fixed-effects meta-analysis results indicated that patients with OP had a lower serum level of Fe than healthy controls (SMD = −0.22, 95 % CI = [−0.30, −0.13]) (Fig. 4). The nine sets of results showed no significant amount of heterogeneity (I 2 = 0, p = 0.934) (Fig. 4).
Publication Bias and Sensitivity Analysis
Publication bias was measured using Begg’s tests and visualization of funnel plots. There was no evidence of publication bias for Zn (Begg’s test: p = 0.300), Cu (Begg’s test: p = 0.999), and Fe (Begg’s test: p = 0.251) (Fig. 5). Sensitivity analysis showed that excluding any one involved study from the pooled analysis did not vary the results substantially, except for the study performed by Liu in 2009, which can eliminate the heterogeneity of studies for serum Cu (Table 5).
Discussion
The result of random-effects meta-analysis indicated that the low serum level of Zn was associated with OP. This result is in keeping with some data showing that Zn deficiency might have harmful effects on osseous metabolism, leading to OP. First, Zn has been shown to be concentrated in the layer of osteoid prior to calcification; the deficiency of Zn causes the deterioration of bone formation, which can be completely prevented by the supplementation of Zn [16, 33–35]. Second, Zn stimulates osteoblastic bone formation and the deficiency of Zn decreases osteoblastogenesis. The proliferation of osteoblastic cells was stimulated after culture with Zn [36]. Zn has been shown to stimulate cell differentiation of osteoblastic cells and can remarkably increase alkaline phosphatase activity and the expression of Runx2 [8, 37]. Deficiency of Zn decreases osteoblastogenesis associated with the reduced expression of Runx2 through the inhibition of Wnt/beta-catenin signaling [9]. Third, Zn has been shown to have suppressive effects on osteoclastogenesis and osteoclastic cell death [38]. Zn has an inhibitory effect on RANKL-induced osteoclast-like cell formation and TNFа-induced osteoclastogenesis [39, 40]. Finally, Zn modulates anabolic effect of 1,25-dihydroxyvitamin D3 or estrogen on bone metabolism in vitro and in vivo [41–43]. Thus, Zn deficiency may directly result in a decrease in bone formation.
The outcome of this meta-analysis also suggested that low serum level of Cu was an important risk factor for OP. This finding is consistent with previous studies finding that deficiency or low intake of Cu leads to OP. Michelle et al. found that serum Cu deficiency can cause bone lesions in infants, and therapeutic supplementation with Cu corrected their deficits and clinical and radiologic findings [28]. Cu plays a pivotal role in the crosslinking of collagen and elastin, which participates in the production of bony matrix and, when deficient, would result in OP [12, 44, 45]. Cu has a positive effect on the proliferation and function of osteoblast, which is responsible for bone formation deriving from mesenchymal stem cells (MSCs) present in bone marrow stroma. Previous studies find that Cu not only improves the viability of osteoblast but also stimulates MSCs differentiation towards the osteogenic lineage [46, 47]. Thus, Cu deficiency may decrease the bone formation.
The present study still found that low serum level of Fe was associated with OP. Previous studies find that the patients with OP are Fe deficient, and this reduced Fe bioavailability could influence bone metabolism, since Fe acts as a cofactor in enzymes involved in collagen bone matrix synthesis as well as in 25 OH vitamin D hydroxylase, an enzyme involved in activating vitamin D and hence in calcium absorption [27, 48]. Animal studies show that Fe deficiency decreased serum osteocalcin concentration, bone mineral content, bone mineral density, and mechanical strength of the femur [14]. Depletion of Fe in cultured osteoblast cells impaired mineralization, similar to that occurring among some human populations, and reduced bone microarchitecture [19]. Impaired mineralization with Fe deficiency appears to be a possible mechanism for OP.
To the best of our knowledge, this is the first comprehensive meta-analysis to estimate the association between serum level of Zn, Cu, or Fe and OP. However, the possible limitations of our study must be considered. First, only 2,188 subjects included in the meta-analysis might weaken the quality of the results. In addition, because of the heterogeneity which might have been introduced by geographical location and gender, the results are polemic and the conclusion should be more conservative. Despite these limitations, our findings point out new direction for future research, like what is the compound effect of multiple risk factors on OP? For instance, what is the risk of OP with Zn and Cu deficiency or Fe and Cu deficiency? To answer this question, several well-designed studies with adequate control for confounding factors should be considered.
Conclusion
In conclusion, this meta-analysis suggests that low levels of Zn, Cu, and Fe seem to be important risk factors for OP, and well-designed studies with adequate control for confounding factors are required in future investigations.
References
Zofkova I, Nemcikova P, Matucha P (2013) Trace elements and bone health. Clin Chem Lab Med 51:1555–1561
Zheng J, Mao X, Ling J, He Q, Quan J et al (2014) Association between serum level of magnesium and postmenopausal osteoporosis: a meta-analysis. Biol Trace Elem Res. doi:10.1007/s12011-014-9961-3
Wynchank S, Saltman PD (1997) Trace elements and osteoporosis. S Afr Med J 87:473–474
Davey DA (1997) Trace elements and osteoporosis. S Afr Med J 87:902
Hsieh HS, Navia JM (1980) Zinc deficiency and bone formation in guinea pig alveolar implants. J Nutr 110:1581–1588
Burch RE, Hahn HK, Sullivan JF (1975) Newer aspects of the roles of zinc, manganese, and copper in human nutrition. Clin Chem 21:501–520
Nagata M, Kayanoma M, Takahashi T, Kaneko T, Hara H (2011) Marginal zinc deficiency in pregnant rats impairs bone matrix formation and bone mineralization in their neonates. Biol Trace Elem Res 142:190–199
Yamaguchi M, Goto M, Uchiyama S, Nakagawa T (2008) Effect of zinc on gene expression in osteoblastic MC3T3-E1 cells: enhancement of Runx2, OPG, and regucalcin mRNA expressions. Mol Cell Biochem 312:157–166
Hie M, Iitsuka N, Otsuka T, Nakanishi A, Tsukamoto I (2011) Zinc deficiency decreases osteoblasts and osteoclasts associated with the reduced expression of Runx2 and RANK. Bone 49:1152–1159
Yamaguchi M, Segawa Y, Shimokawa N, Tsuzuike N, Tagashira E (1992) Inhibitory effect of beta-alanyl-L-histidinato zinc on bone resorption in tissue culture. Pharmacology 45:292–300
Shaw JC (1988) Copper deficiency and non-accidental injury. Arch Dis Child 63:448–455
Rucker RB, Murray J, Riggins RS (1977) Nutritional copper deficiency and penicillamine administration: some effects on bone collagen and arterial elastin crosslinking. Adv Exp Med Biol 86B:619–648
Tranquilli AL, Lucino E, Garzetti GG, Romanini C (1994) Calcium, phosphorus and magnesium intakes correlate with bone mineral content in postmenopausal women. Gynecol Endocrinol 8:55–58
Katsumata S, Tsuboi R, Uehara M, Suzuki K (2006) Dietary iron deficiency decreases serum osteocalcin concentration and bone mineral density in rats. Biosci Biotechnol Biochem 70:2547–2550
Leek JC, Vogler JB, Gershwin ME, Golub MS, Hurley LS et al (1984) Studies of marginal zinc deprivation in rhesus monkeys. V. Fetal and infant skeletal effects. Am J Clin Nutr 40:1203–1212
Da CFR, Marquiegui IM, Elizaga IV (1989) Teratogenicity of zinc deficiency in the rat: study of the fetal skeleton. Teratology 39:181–194
Oner G, Bhaumick B, Bala RM (1984) Effect of zinc deficiency on serum somatomedin levels and skeletal growth in young rats. Endocrinology 114:1860–1863
Medeiros DM, Plattner A, Jennings D, Stoecker B (2002) Bone morphology, strength and density are compromised in iron-deficient rats and exacerbated by calcium restriction. J Nutr 132:3135–3141
Parelman M, Stoecker B, Baker A, Medeiros D (2006) Iron restriction negatively affects bone in female rats and mineralization of hFOB osteoblast cells. Exp Biol Med (Maywood) 231:378–386
Rico H, Roca-Botran C, Hernandez ER, Seco C, Paez E et al (2000) The effect of supplemental copper on osteopenia induced by ovariectomy in rats. Menopause 7:413–416
Rico H, Gomez-Raso N, Revilla M, Hernandez ER, Seco C et al (2000) Effects on bone loss of manganese alone or with copper supplement in ovariectomized rats. A morphometric and densitomeric study. Eur J Obstet Gynecol Reprod Biol 90:97–101
Ronaghy HA, Reinhold JG, Mahloudji M, Ghavami P, Fox MR et al (1974) Zinc supplementation of malnourished schoolboys in Iran: increased growth and other effects. Am J Clin Nutr 27:112–121
Gur A, Colpan L, Nas K, Cevik R, Sarac J et al (2002) The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and a new effect of calcitonin. J Bone Miner Metab 20:39–43
Mutlu M, Argun M, Kilic E, Saraymen R, Yazar S (2007) Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res 35:692–695
Hyun TH, Barrett-Connor E, Milne DB (2004) Zinc intakes and plasma concentrations in men with osteoporosis: the Rancho Bernardo Study. Am J Clin Nutr 80:715–721
Strause L, Saltman P, Smith KT, Bracker M, Andon MB (1994) Spinal bone loss in postmenopausal women supplemented with calcium and trace minerals. J Nutr 124:1060–1064
D’Amelio P, Cristofaro MA, Tamone C, Morra E, Di Bella S et al (2008) Role of iron metabolism and oxidative damage in postmenopausal bone loss. Bone 43:1010–1015
Marquardt ML, Done SL, Sandrock M, Berdon WE, Feldman KW (2012) Copper deficiency presenting as metabolic bone disease in extremely low birth weight, short-gut infants. Pediatrics 130:e695–e698
Relea P, Revilla M, Ripoll E, Arribas I, Villa LF et al (1995) Zinc, biochemical markers of nutrition, and type I osteoporosis. Age Ageing 24:303–307
Liu SZ, Yan H, Xu P, Li JP, Zhuang GH et al (2009) Correlation analysis between bone mineral density and serum element contents of postmenopausal women in Xi’an urban area. Biol Trace Elem Res 131:205–214
Arikan DC, Coskun A, Ozer A, Kilinc M, Atalay F et al (2011) Plasma selenium, zinc, copper and lipid levels in postmenopausal Turkish women and their relation with osteoporosis. Biol Trace Elem Res 144:407–417
Okyay E, Ertugrul C, Acar B, Sisman AR, Onvural B et al (2013) Comparative evaluation of serum levels of main minerals and postmenopausal osteoporosis. Maturitas 76:320–325
Yamaguchi M, Ozaki K (1990) Aging affects cellular zinc and protein synthesis in the femoral diaphysis of rats. Res Exp Med (Berl) 190:295–300
Segawa Y, Tsuzuike N, Tagashira E, Yamaguchi M (1993) Preventive effect of beta-alanyl-L-histidinato zinc on the deterioration of bone metabolism in ovariectomized rats. Biol Pharm Bull 16:486–489
Yamaguchi M, Uchiyama S (2003) Preventive effect of zinc acexamate administration in streptozotocin-diabetic rats: restoration of bone loss. Int J Mol Med 12:755–761
Hashizume M, Yamaguchi M (1993) Stimulatory effect of beta-alanyl-L-histidinato zinc on cell proliferation is dependent on protein synthesis in osteoblastic MC3T3-E1 cells. Mol Cell Biochem 122:59–64
Hashizume M, Yamaguchi M (1994) Effect of beta-alanyl-L-histidinato zinc on differentiation of osteoblastic MC3T3-E1 cells: increases in alkaline phosphatase activity and protein concentration. Mol Cell Biochem 131:19–24
Yamaguchi M, Kishi S (1996) Zinc compounds inhibit osteoclast-like cell formation at the earlier stage of rat marrow culture but not osteoclast function. Mol Cell Biochem 158:171–177
Yamaguchi M, Uchiyama S (2004) Receptor activator of NF-kappaB ligand-stimulated osteoclastogenesis in mouse marrow culture is suppressed by zinc in vitro. Int J Mol Med 14:81–85
Zou W, Hakim I, Tschoep K, Endres S, Bar-Shavit Z (2001) Tumor necrosis factor-alpha mediates RANK ligand stimulation of osteoclast differentiation by an autocrine mechanism. J Cell Biochem 83:70–83
Yamaguchi M, Inamoto K (1986) Differential effects of calcium-regulating hormones on bone metabolism in weanling rats orally administered zinc sulfate. Metabolism 35:1044–1047
Yamaguchi M, Yamaguchi R (1986) Action of zinc on bone metabolism in rats. Increases in alkaline phosphatase activity and DNA content. Biochem Pharmacol 35:773–777
Yamaguchi M, Kitajima T (1991) Effect of estrogen on bone metabolism in tissue culture: enhancement of the steroid effect by zinc. Res Exp Med (Berl) 191:145–154
Dahl SL, Rucker RB, Niklason LE (2005) Effects of copper and cross-linking on the extracellular matrix of tissue-engineered arteries. Cell Transplant 14:367–374
Rucker RB, Kosonen T, Clegg MS, Mitchell AE, Rucker BR et al (1998) Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am J Clin Nutr 67:996S–1002S
Milkovic L, Hoppe A, Detsch R, Boccaccini AR, Zarkovic N (2013) Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J Biomed Mater Res A. doi:10.1002/jbm.a.35032
Ding H, Gao YS, Wang Y, Hu C, Sun Y, et al. (2014) Dimethyloxaloylglycine increases the bone healing capacity of adipose-derived stem cells by promoting osteogenic differentiation and angiogenic potential. DOI: 10.1089/scd.2013.0486.
Sato K, Nohtomi K, Demura H, Takeuchi A, Kobayashi T et al (1997) Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1,25-dihydroxy-vitamin D production in renal tubules. Bone 21:57–64
Acknowledgments
The project was supported by the Guangzhou Municipal Science and Technology Project (11C32060748) and the National Natural Science Foundation of China (81100728).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, J., Mao, X., Ling, J. et al. Low Serum Levels of Zinc, Copper, and Iron as Risk Factors for Osteoporosis: a Meta-analysis. Biol Trace Elem Res 160, 15–23 (2014). https://doi.org/10.1007/s12011-014-0031-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0031-7