Abstract
Bone-related diseases are very common problems, especially in the elderly population. Zinc takes part in the growth and maintenance of healthy bones. This meta-analysis aims to evaluate the effects of zinc supplementation or dietary zinc intake on serum zinc levels and bone turnover markers. A systematical research was performed with 2899 articles in PubMed, WoS, and Scopus for relevant articles in English which have mean/standard deviation values of serum zinc levels, dietary zinc intake/zinc supplementation (mg/day), and bone turnover markers up to February 2020. In the overall analysis, serum zinc level was significantly lower in patients with osteoporosis compared with controls (p 0.0002). Dietary zinc intake decreased in the fracture group compared with controls according to subgroup analysis patients with fracture (p 0.02). Zinc supplementation was effective on the femoral neck (p < 0.0001) and lumbar spine (p 0.05) bone mineral density (BMD). In the correlation analysis of the data obtained from all of the included studies, serum osteocalcin (p 0.0106, r − 0.9148) correlated with serum zinc level. In conclusion, serum zinc level and dietary zinc intake could have an essential role in preventing osteoporosis. Zinc supplementation might improve bone turnover markers for bone formation such as serum osteocalcin and serum alkaline phosphatase and also, BMD at the site of the femoral neck.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone remodeling is a lifelong process in which bone resorption followed by bone formation. However, an imbalance in the homeostasis between resorption and formation processes leads to a change in bone mass in the case of aging, menopause, fracture, and other bone metabolism problems such as osteoporosis [1].
Osteoporosis, a major public health problem, is characterized by low bone mass and micro-architectural deterioration of bone tissue, resulting in an increased risk of bone fragility [2] and fractures of the hip, spine, and other skeletal sites [3]. The World Health Organization has defined osteoporosis criteria based on bone mineral density (BMD) [4] or bone mineral content (BMC), i.e., normal (within 1 SD of the young adult reference mean for the population) osteopenia (between − 1 and − 2.5 SD of the young adult reference mean), osteoporosis (more than − 2.5 SD below the young adult reference mean), and established osteoporosis as the same mass definition but associated with a fragility fracture [2]. Osteoporotic fractures are a serious health concern in populations aged 50 years or older. Malnutrition and low intake of nutrients have been found more prevalent and severe among hip fracture patients as compared with the general elderly populations. In elderly people, nutrient deficiency may accelerate bone loss, micro-architectural deterioration, and increase the risk for subsequent fractures [5].
Nutrition has an important influence on the maintenance of bone mass. Besides macronutrients, minerals such as calcium (Ca), magnesium (Mg), fluoride (Fl), zinc (Zn), copper (Cu), iron (Fe), selenium (Se), and vitamins D, A, C, K, B2, B6, folate, and B12 are required for normal bone metabolism [6]. Imbalances of nutritional intake, especially mineral deficiencies as a result of reduced intake and absorption of these nutrients, could be an important factor in the pathophysiological mechanisms of osteopenia or osteoporosis [7].
Especially, zinc is well known as an essential trace element for the growth, development, and maintenance of bone health [8]. The adult human body contains closely 2–3 g of zinc. Approximately 60% of the total body, Zn content was found in the skeletal muscle, 0% in the bone, 5% in the liver and skin, and the remaining 2–3% in other tissues [9]. Zn affects bone metabolism via its role in RANKL/RANK/OPG and Wnt signaling pathways [10] and its action on gene expression of the Runx2/Cbfa1 transcription factor, type I collagen, alkaline phosphatase, and osteocalcin within the cells [11]. RANKL is secreted from osteoblasts, and it is a member of the tumor necrosis factor (TNF) superfamily. RANKL/RANK pathway is essential for osteoclast differentiation. RANKL expression is induced in osteoblastic cells and bone marrow stromal cells in response to osteotropic factors such as PTH, 1,25-dihydroxyvitaminD3, and PGE2. The effect of RANKL was completely abolished by adding a natural antagonist of RANKL, osteoprotegerin (OPG), which is produced in osteoblastic cells. Zinc has a suppressive effect on the receptor activator of nuclear factor (NF)-κB ligand (RANKL) –induced osteoclastogenesis, indicating that the metal inhibits RANKL signaling in pre-osteoclasts [12].
Zinc is a cofactor in bone-related enzymes such as alkaline phosphatase, collagenase, and it affects protein synthesis through activation of DNA polymerase, RNA polymerase, and tRNA polymerase synthetase [13]. In vitro and in vivo studies demonstrated that zinc stimulates bone growth and mineralization, osteoblasts proliferation, differentiation, and increases IgG-1 activity [14].
It was found that zinc intake is lower in osteoporotic or fracture patients, and this might be important for etiopathogenesis and be related to disease prognosis [15, 16]. However, it was also established that zinc supplementation with or without calcium had no significant effect on the bone health of postmenopausal women [3]. It can be seen from different studies that zinc effects on bone turnover and related complications are still unclear.
As osteoporosis, a major public health problem is becoming increasingly prevalent with the aging of the world population [3], the preventive and therapeutic factors are gaining importance. Besides medication, nutritional intervention would be beneficial to maintain bone health throughout life. The reasons mentioned above have taken the attention to dietary zinc intake, zinc supplementation, or serum zinc status in bone metabolism. However, the effects of zinc on bone homeostasis in related diseases remain unclear. The aim of this meta-analysis is to investigate for the first time the effects of zinc supplementation and dietary zinc intake on bone turnover markers and serum zinc status in bone-related comorbidities with data given in 40 studies after systematical search.
Methods
Eligibility Criteria
To show the relationship between zinc and bone metabolism in the case of complications such as osteoporosis, fracture, and fragility, all human studies including serum zinc status/dietary zinc intake/zinc intervention were searched and reviewed. The mean and standard deviation data were collected and assessed to meta-analysis. There were no restrictions imposed on age, gender, or on any other population characteristic such as race or body mass index (BMI).
The inclusion criteria were determined as studies determining the serum zinc levels/zinc intake/zinc supplementation, including the mean and the standard deviation values, reporting the zinc values having a suitable parameter that can be converted with each other between studies, giving the sample size of the groups, making the diagnosis of the disease according to the criteria accepted in the literature, and indicating that appropriate conditions were met for the collection of samples.
Sources and Search
PRISMA procedures were followed for searching and evaluating the data. PubMed, Web of Science, and Scopus databases were searched without any date restrictions, and relevant articles were detected. Searching was being performed until March 2020. Searching keywords were “zinc” OR ”zinc intake” OR ”zinc supplementation” AND “osteoporosis” OR “bone (clinical trial)” OR “fragility (clinical trial)” OR “fracture (clinical trial)” for all databases.
Statistical Analysis
Pooled data were calculated to assess the relationship of serum zinc level/zinc intake/zinc supplementation with bone metabolism in osteoporosis. The I2 was used for measuring of heterogeneity as described before (I2% values of 0–25, 25–50, 50–75, and 75–100 represent no, low, moderate, and high heterogeneity) [17]. The fixed and effect models were used according to heterogeneity chi-square value to combine the results [18]. Meta-analysis was performed with RevMan 5.3. (Cochrane Collaboration, Copenhagen, 2014). GraphPad Prism 6 was used for correlation analyses and figures.
Risk of Bias Assessment of Studies
The risk of bias for each study was assessed either as low, unclear, or high risk for each of the following criteria: selection bias, performance bias, detection bias, attrition bias, and reporting bias, and other as described in the Cochrane Handbook [19].
Results
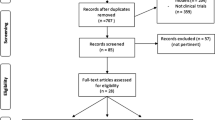
As a result of the systematical search on PubMed, Scopus, and Web of Science, a total of 2899 publications were screened. Seven hundred forty-four publications were review, book chapters, conference papers, etc. Among 2155 articles, 2115 articles were not related/did not include the inclusion criteria according to the information obtained from the titles, abstracts, or full texts, and a totally 40 articles [6, 7, 13,14,15,16, 20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53] met with the inclusion criteria which mentioned above (Fig. 1). Publishing dates of articles were included in the meta-analysis ranged from 1994 to 2020. Characteristics of the included studies were given in Table 1.
Cumulative Meta-analysis
In all bone health complications related to osteoporosis, serum zinc level and dietary zinc intake were evaluated with the random effect model. The case groups include osteoporosis, osteopenia, postmenopausal, and fracture patients’ data. The heterogeneity was found to be a high level in serum zinc meta-analysis (99%) and dietary zinc intake meta-analysis (96%). The random effect model performed on sixteen studies for serum zinc level and twelve studies for dietary zinc intake. Serum zinc level did not show significant difference between cases and controls (p 0.10, mean difference − 3.24 [− 7.05, 0.57]) (Fig. 2a). Similarly, dietary zinc intake was not significantly different in cases compared with control groups (p 0.14, mean difference − 0.33 [− 0.77, 0.11]) (Fig. 2b).
Subgroup Analysis
The random effect model performed on eight studies for osteoporosis, four studies for osteopenia, and ten studies for postmenopausal women subgroups to analyze serum zinc level. In order to analyze dietary zinc intake status, the random effect model was performed on four studies for osteoporosis, two studies for osteopenia, five studies for postmenopausal women, and three studies for fracture subgroups.
Osteoporosis and Osteopenia Subgroups
Following the cumulative analysis of overall cases of serum zinc and dietary zinc intake status, each complication was analyzed. Serum zinc level was significantly lower in osteoporosis subgroup compared with controls (REM p 0.0002, mean difference − 12.68 [− 19.31, − 6.05] (Fig. 3a). However, there was no any difference between osteoporosis and healthy controls in dietary zinc intake (REM p 0.99, mean difference − 0.01, [− 1.60, 1.57]). It should be mentioned that heterogeneity among included studies was low (42%) in serum zinc evaluation, but moderate heterogeneity was seen in dietary zinc intake analysis (70%) (Fig. 4a).
In osteopenia subgroup, both serum zinc level (REM p 0.14, mean difference − 8.32 [− 19.34, 2.70]) and dietary zinc intake status (REM p 0.88, mean difference − 0.25 [− 2.88, 3.37]) did not show significant difference with control groups (Figs. 3b and 4b).
Postmenopausal Subgroup
The effect of menopause on bone health and its relation with serum zinc levels in postmenopausal cases were evaluated in cumulative analysis and subgroup analysis. There was no significant difference in serum zinc level between postmenopausal women and healthy controls (REM p 0.22, mean difference − 6.77 [− 17.56, 4.01]) with high heterogeneity (97%) (Fig. 3c). Similarly, dietary zinc intake did not show the difference between groups (REM p 0.49, mean difference − 0.40 [− 1.55, 0.70]), and there was high heterogeneity among studies (87%) (Fig. 4c).
Fracture Subgroup
In fracture studies, we could not obtain enough serum zinc level data for subgroup analysis; however, dietary zinc intake status was evaluated. There was no/low heterogeneity between included studies (25%). Dietary zinc intake decreased in fracture group compared with controls according to subgroup analysis result (REM p 0.02, mean difference − 0.50 [− 0.90, 0.09]) (Fig. 4d). In addition to zinc intake, the dietary protein decreased in the fracture group compared with controls according to subgroup analysis result (REM p 0.02, mean difference − 0.41 [− 7.47, − 0.56]).
Zinc Supplementation and Bone Markers
Several common bone markers detected that studies include such as BMD of the femoral neck and lumbar spine BMD, and also serum levels of alanine phosphatase (ALP), bone alkaline phosphatase (BAP), osteocalcin and parathyroid hormone (PTH). Respectively, heterogeneity i2 levels were high (85%), low (40%), no (0%), high (77%), high (94%), and high (100%) of each bone marker analysis. According to heterogeneity chi-square value, fixed and random effect models were used. The number of studies included for each bone marker analyses was mentioned at the end of the analysis results. Zinc supplementation was effective on the femoral neck (FEM p < 0.0001, mean difference 0.02 [0.01, 0.02])(n = 3) and lumbar BMD (FEM p 0.05, mean difference − 0.01 [− 0.01, 0.00]) (n = 4). However, it showed a different effect on these different areas that while femoral neck BMD was higher in zinc supplementation groups, lumbar BMD was affected negatively. These results might be depended on differences in study protocols, supplementation duration, and characteristics among studies.
While serum ALP levels were found to be higher with zinc supplementation groups compared with control groups (FEM p < 0.0001, mean difference 33.70 [22.79, 44.61]) (n = 12), serum BAP levels did not show the difference between groups (REM p 0.73, mean difference 0.84 [− 3.96, 5.64]) (n = 2), notably serum osteocalcin levels were lower in supplementation groups compared with controls in the random effect model (p 0.003, mean difference − 4.14 [− 6.92, − 1.36])(n = 4); at last, there was no any significant difference in serum PTH levels between zinc supplementation (+) and (−) groups (REM p 0.76, mean difference 3.55 [− 18.88, 25.98]) (n = 5) (Fig. 5).
Zinc supplementation effects on several bone markers. a The fixed effect model of femoral neck BMD data. b The fixed effect model of lumbar BMD data. c The fixed effect model of serum ALP status data. d The random effect model of serum BAP status data. e The random effect model of serum osteocalcin data. f The random effect model of serum PTH status data obtained from different studies
Correlation Analysis
Correlation analysis was done to consolidate the relationship between serum, dietary, or supplementary zinc and bone health. The number of studies included for each correlation analysis was mentioned at the end of the result. As an important result, it was showed that both dietary zinc intake (p 0.8678) (n = 11) and zinc supplementation (p 0.35) (n = 7) did not affect the serum zinc levels (Fig. 6a, b). There was a correlation between dietary energy intake and serum zinc status (p 0.0215, r 0.6063) (n = 27) (Fig. 6c). When bone markers and serum zinc level status relation was investigated, serum osteocalcin (p 0.0106, r − 0.9148) (n = 6) were correlated with serum zinc level, however serum ALP level was correlated (p 0,1453, r 0.4468) (n = 12) (Fig. 6d, e). Neither lumbar (p 0.4102, r 0.2167) (n = 16) nor femoral BMD (p 0.1537, r 0.4608) (n = 11) was correlated with serum zinc status (Fig. 6f, g).
Correlations between serum zinc level (μg/dL) and (a) dietary zinc intake (mg/day), (b) zinc supplementation (mg/day), (c) dietary energy intake (kcal/day), (d) serum ALP level (U/L), (e) serum osteocalcin level (μg/L), (f) lumbar bone mineral density (BMD) (g/cm2), (g) femoral bone mineral density (BMD) (g/cm2)
In addition, to understand zinc sources of individuals, dietary macronutrient intakes in relation with dietary zinc intake were examined. There were correlations with dietary protein (p 0.0129, r 0.4995) (n = 24) and dietary fat (p 0.0010, r 0.8216) (n = 12) intakes, however dietary carbohydrate intake did not show significant correlation with dietary zinc status but it was tend to have positive correlation too (p 0.0543, r 0.5446) (n = 13). Also, other dietary micronutrients which might have an effect on zinc absorption were evaluated, and it was seen that dietary zinc intake was correlated with dietary phosphorus (p 0.0118, r 0.6505) (n = 14), calcium (p 0.0117, r 0.4215) (n = 35), magnesium (p 0.0003, 0.7896) (n = 16), iron (Fe) (p 0.002, r 0.6937) (n = 17), potassium (p 0.007, r 0.891) (n = 6), and folate (p 0.03, r 0.681) (n = 8) intakes, however, there was no any correlation with dietary copper (p 0.479, r − 0.266) (n = 8) and sodium (p 0.659, r − 0.186) (n = 7) status (Fig. 7).
Risk of Bias Assessments
The funnel plot shown in Fig. 8a and b do not suggest evidence of publication bias in the studies included in this meta-analysis. The assessment of the bias status of each study is shown in Fig. 8c and d. There was a low risk of bias in studies included in this meta-analysis. The high risk of bias was detected among the studies that had not given clear study group selection, exclusion, and inclusion criteria, and also had not given clear statistical analysis methodology in the study paper.
Discussion
This meta-analysis established that serum zinc level did not show a significant difference in overall bone turnover–related complications, such as osteoporosis, osteopenia, fracture, or postmenopause from control groups (Fig. 2a), however the subgroup analysis of osteoporosis patients’ data showed that serum zinc level was lower in osteoporosis (Fig. 3a). The dietary zinc intake status has not differed between groups, but it was found to be lower in fracture subgroup analysis (Fig. 4). The correlation analysis also showed that both dietary zinc intake and zinc supplementation did not affect the serum zinc levels. Also, serum zinc levels and BMD were not found to be correlated (Fig. 6). To better interpret these results, zinc homeostasis, changing bone metabolism, and dietary factors are examined.
In a meta-analysis of Zheng et al. which examine serum zinc, iron, and copper status in osteoporotic patients, the random effect meta-analysis results show that patients with osteoporosis had a lower serum level of Zn than the healthy controls (SMD = − 1.396, 95% CI = [− 2.129, − 0.663]). The 13 sets of results showed a statistically significant amount of heterogeneity (I2 = 98.3%, p < 0.001) [54]. In our meta-analysis, there was no significant difference in the overall analysis of bone turnover–related complications. However, osteoporotic patients’ data analysis result was similar to the meta-analysis of Zheng et al. The different results may arise due to the data obtained from different patients’ groups between these meta-analyses. Zheng et al. examined serum zinc levels in osteoporotic postmenopausal women; however, our data was obtained from both men and women with osteoporosis, osteopenia, fracture, and also postmenopausal women.
The subgroup analysis of osteoporotic patients showed that there was a significant difference in serum zinc level and dietary zinc intake than their non-osteoporotic controls (Figs. 3a and 4). In the study of Hyun et al., which is also included in our meta-analysis, when the zinc intakes and plasma concentrations were examined in men with osteoporosis, plasma zinc was found to be correlated with total zinc intake, including intake from supplements, but not with dietary zinc intake alone. Also, zinc intake and measured plasma zinc levels were significantly lower in men with osteoporosis than in men without osteoporosis [27].
In another subgroup analysis, postmenopausal women were investigated. Neither serum zinc level nor dietary zinc intake showed a significant difference between postmenopausal and control groups (Figs. 3c and 4c). These results might indicate that despite postmenopausal women having a high risk for osteoporosis, all postmenopausal women should not be thought of as osteoporotic patients, and their dietary pattern and serum status should be well examined before any supplement intervention.
As it is known, total body zinc has two metabolic pools that named as the rapid and slow pool. The rapid pool includes zinc in plasma, extracellular fluid, and in the liver, pancreatic, kidney, and intestinal tissue; in contrast, a slow pool consists of the skeletal muscle and bone, which has almost 90% of the whole-body zinc. Severe dietary zinc restriction (< 1 mg/day for 4 to 5 weeks) causes a decrease (approximately 35%) of zinc in the rapid pool but has little or no measurable effect on the slow pool [55]. The homeostatic mechanisms were insufficient to maintain body zinc in case of extreme intake and that can lead to loss or accumulation of zinc in the body [56]. The similar Zn intakes in the two groups could explain why we did not observe significant differences in serum Zn levels in this meta-analysis. Also, in a previous study in the literature, it was shown that bone zinc status was significantly lower in patients with fractures compared with healthy controls [57]. Thus, it is thought that in the case of bone turnover and fracture, the zinc needs of the body might be increased.
In this meta-analysis, we examine the effects of zinc supplementation on bone mineral density at the site of the femoral neck and lumbar spine, and some biochemical markers related to bone metabolism such as serum PTH, ALP, BAP, and osteocalcin levels (Fig. 5). However, it was established that the femoral neck BMD of the zinc-supplemented group were significantly higher than controls. Interestingly, lumbar spine BMD tended to be decreased in the zinc-supplemented group compared with lean controls. Differences between study groups or measurements might cause these results. It should be well examined in further studies the underlying mechanism of zinc supplementation on BMD. Also, it should be considered that supplementation interventions did not include only zinc; in some studies, multi-supplements were used, such as vitamins and other trace elements. In the study of Braam et al., which is meta-analyzed in our study, the effects of complex mineral supplementation (contains 10 mg Zn) on BMD were examined in postmenopausal women during 3-year trail. They demonstrated that the femoral neck BMD had declined significantly in the treatment group, although the rate of bone loss was lower in the supplemented group than the placebo. In the study of Nielsen et al. that is meta-analyzed in our study, the supplement contains 600 mg Ca, 2 mg Cu, and 12 mg Zn, or placebo plus 600 mg Ca supplement were daily given to healthy women aged 51–80 years during 2-year trail. It was found that neither Ca + placebo nor Ca + Cu + Zn supplementation has a preventive effect on whole-body bone mineral content, density, or T score from decreasing from baseline during the supplementation period [43]. In the study of Rodondi et al. that is meta-analyzed in our study, the influence of additional 30-mg zinc on IGF-I and bone turnover responses to 4 weeks of essential amino acids-whey (EAA-W) protein supplements in frail elderly was demonstrated. The results showed that in the elderly, zinc supplementation accelerated the serum IGF-I response to EAA-W protein by 1 week and decreased a biochemical marker of bone resorption [46].
The limitation of zinc supplementation analysis is that in clinical studies, other trace elements or vitamins accompany zinc application, which limits us to see zinc effect on bone metabolism directly. To eliminate other supplement effects, animal studies might be examined. In an animal study, diabetes depended on osteoporotic bone loss in rats was investigated in the case of zinc supplementation with 0.25 mg/kg/day of zinc sulfate administration. It was observed that zinc application increased the BMD, decreased serum ALP, and RANKL increased serum OPG and RUNX 2 levels, as well as OPG/RANKL ratio [58]. Also, there are several studies that zinc supplementation have protective effects on bone structure in ovariectomized rats [59,60,61] and on potential promotions on bone formation [62, 63].
Our meta-analysis results showed that serum ALP and BAP levels of zinc-supplemented groups were higher than controls, however significant difference was found only in serum ALP levels between groups (Fig. 5c and d) This result may be related to limited data on serum BAP levels. Besides, serum zinc level and serum ALP levels were not found to be correlated (Fig. 6). ALP is a well-known biochemical marker used in the diagnosis and follow-up of the liver and metabolic bone disease. BAP is one of the several different isoenzymes of ALP [64]. BAP is synthesized by the osteoblasts and is presumed to be involved in the calcification of bone matrix. It is considered to be a highly specific marker of the bone-forming activity of osteoblasts [65]. BAP catalyzes the hydrolysis of pyrophosphate and provides the extracellular phosphate pool, which determines the rate of hydroxyapatite crystal formation in the bone. In vitro, Zn has been shown to stimulate osteoblastic bone formation by activation of ALP, while Zn deficiency reduced bone mineralization by decreasing the synthesis of ALP [13]. The results of the study of Peretz et al. showed that zinc supplementation results with a significant increase in serum total ALP as well as in bone-specific ALP. [14]. In the study of Cho et al., thirty rats were grouped as Zn-adequate (ZA, 35 mg/kg), pair-fed (PF, 35 mg/kg), Zn-deficient (ZD, 1 mg/kg) diet, and fed for 10 weeks. It was showed that ALP activity was decreased in plasma (p < 0.05) in Zn-deficient rats compared with ZA or PF controls [66].
Another important outcome of our meta-analysis is that serum osteocalcin levels of zinc-supplemented groups were significantly lower than controls (Fig. 5e). Also, we found a significant correlation between serum osteocalcin and serum zinc status, and that was a negative relationship (Fig. 6e). Osteocalcin is synthesized during the bone formation, and it exhibits a compact, calcium-dependent, alpha-helical confirmation, in which the gamma-carboxyglutamic acid (GLA) residues bind and promote absorption to hydroxyapatite in the bone matrix. In this way, bone mineralization takes place [67]. It has been hypothesized that lower female sex hormone levels cause changes in the osteocalcin homeostasis, leading to a decrease of osteocalcin and, in consequence, an increase in uncarboxylated osteocalcin levels in blood serum. Fluctuations in osteocalcin levels in the course of postmenopausal osteoporosis were presented in the study carried out by Gurban et al. Levels of osteocalcin among women who had not been menstruating for at least 15 years reached values of 20.12 ± 0.87 ng/mL, whereas, in the group where this period was less than 15 years, concentrations of osteocalcin were significantly lower (15.12 ± 1.55 ng/mL). These results allow for putting forward a thesis that sustained a decrease in the function of osteoblasts after the last menstrual period is reflected by increased levels of uncarboxylated osteocalcin in the blood serum [68, 69].
In the study of Singh et al., the results showed that serum osteocalcin levels were significantly higher in postmenopausal osteopenic (p < 0.005) and osteoporotic women (p < 0.001) compared with healthy ones. BMD at the femoral neck and lumbar spine was significantly lower (p < 0.001) than in women with normal BMD (p < 0.001) [70]. In the study with cells derived from a bone mesenchymal stem cell (BMSC) and ovariectomized rats (OVX), it was shown that zinc supplementation resulted in a modest increase in BMD and a significant increase in serum osteocalcin and ALP activity in BMSC. Serum levels of RANKL and TRAP were lower in OVX + Zn (vs OVX) rats. Osteocalcin level was significantly upregulated ex vivo in cultured OVX − Zn (vs OVX) cells [71]. In a different animal study, zinc effects on diabetic osteoclast bone resorption were examined, and it was found that zinc might prevent the diabetes-induced increase in osteoclastogenesis and decrease in osteoblastogenesis by inhibiting the RANK expression and stimulating IGF-1/IGF-1R/Akt/GSK3β/β-catenin signaling [72]. Nagata et al. showed the cellular zinc trafficking effect on osteoblastic cell lines in their study that resulted in a correlation between osteocalcin mRNA levels and zinc exposure. They mentioned that zinc might have an important role in osteoblast mineralization through zinc storage proteins and zinc transporters [73].
In the subgroup analysis of fracture, groups showed that patients with fractures had significantly lower dietary Zn than their controls (Fig. 4d). In addition, we examined dietary protein intake between patients with fracture and control groups. Patients with fractures had significantly lower protein intake compared with control groups. It was shown with the correlation analysis that dietary zinc intake was correlated with dietary protein, fat, and total energy intakes (Fig. 7a). In the study of Kim et al. that is meta-analyzed in our study, the bone mineral density of vegetarian and non-vegetarian postmenopausal women was investigated. The results showed that the vegetarian group had significantly lower dietary zinc intake and serum zinc levels than non-vegetarian controls [32]. These results indicated that a healthy and balanced diet might be beneficial to prevent fracture and osteoporosis. Especially, adequate dietary protein intake may provide sufficient micronutrients such as zinc, iron, and copper. However, it should be considered that zinc sources and bioavailability studies are still unclear and should be examined with further studies. There were also correlations between zinc intake and dietary protein, dietary phosphorus (P), Ca, Mg, Fe, potassium (K), and folate intake. Similarly, our results also demonstrated that dietary zinc intake is correlated with protein, fat, magnesium, iron, phosphorus, potassium, folate, and calcium. There was also a significant correlation between serum zinc status and dietary energy intake (Fig. 7). The study of New et al. that is meta-analyzed in our study, suggest that high current intakes of the nutrients, potassium, magnesium, vitamin C, fiber, and zinc, were associated with a higher bone mass, and that a high past consumption of fruit had a positive effect on adult bone mass. These findings appear to indicate that high long-term intakes of nutrients found in abundance in fruit and vegetables may be important to bone health, possibly because of their beneficial effect on acid-base balance [41].
It should be mentioned that we accept the limits of our meta-analysis study based on the quality of the studies and data in the literature. Some of the limitations of our current meta-analysis are due to differences in cases or patients, supplementation procedures, dietary factors, and heterogeneity. The statistical heterogeneity of the data was high in some of our analyses. However, clinical heterogeneity can be observed, and is a natural result for meta-analysis and should be considered when interpreting the results of this study. It was performed by evaluating the PRISMA checklist. On the other hand, to the best of our knowledge, this study is the first meta-analysis in the literature evaluating the relationship between dietary zinc intake, zinc supplementation, serum zinc levels, and bone turnover–related diseases or outcomes.
Conclusion
In conclusion, as a result of the meta-analysis, it was found that serum zinc level and dietary zinc intake could have an essential role in preventing osteoporosis. Also, it was seen that zinc supplementation might improve bone turnover markers for bone formation such as serum osteocalcin and serum alkaline phosphatase, and also BMD, especially on the femoral neck. This paper is the first meta-analysis that investigated the effects of zinc supplementation and dietary zinc status on serum zinc levels and bone turnover markers on osteoporosis, osteopenia, postmenopause, and fracture. For further understanding of the underlying mechanism, different clinical studies are needed to enlighten the role of zinc supplementation or dietary zinc intake on bone turnover.
References
Bayliss L, Mahoney DJ, Monk P (2012) Normal bone physiology, remodelling and its hormonal regulation. Surgery 30(2):47–53
Cashman KD (2007) Diet, nutrition, and bone health. J Nutr 137(11):2507S–2512S
Lane NE (2006) Epidemiology, etiology, and diagnosis of osteoporosis. Am J Obstet Gynecol 194(2):S3–S11
Mutlu M, Argun M, Kilic E, Saraymen R, Yazar S (2007) Magnesium, zinc and copper status in osteoporotic, osteopenic and normal post-menopausal women. J Int Med Res 35(5):692–695
Dai Z, Wang R, Ang L, Yuan J-M, Koh W-P (2013) Dietary B vitamin intake and risk of hip fracture: the Singapore Chinese Health Study. Osteoporos Int 24(7):2049–2059
Ilich J, Brownbill R, Tamborini L (2003) Bone and nutrition in elderly women: protein, energy, and calcium as main determinants of bone mineral density. Eur J Clin Nutr 57(4):554–565
Mahdavi-Roshan M, Ebrahimi M, Ebrahimi A (2015) Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin Cases Miner Bone Metab 12(1):18–21
Yamaguchi M (2007) Role of zinc in bone metabolism and preventive effect on bone disorder. Biomed Res Trace Elem 18(4):346–366
Kambe T, Tsuji T, Hashimoto A, Itsumura NJ (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiological reviews 95(3):749–784
Kenkre J, Bassett J (2018) The bone remodelling cycle. Ann Clin Biochem 55(3):308–327
Yamaguchi M (2010) Role of nutritional zinc in the prevention of osteoporosis. Mol Cell Biochem 338(1-2):241–254
Yamaguchi M, Uchiyama S (2004) Receptor activator of NF-κB ligand-stimulated osteoclastogenesis in mouse marrow culture is suppressed by zinc in vitro. Int J Mol Med 14(1):81–85
Shiota J, Tagawa H, Izumi N, Higashikawa S, Kasahara H (2015) Effect of zinc supplementation on bone formation in hemodialysis patients with normal or low turnover bone. Ren Fail 37(1):57–60
Peretz A, Papadopoulos T, Willems D, Hotimsky A, Michiels N, Siderova V, Bergmann P, Neve J (2001) Zinc supplementation increases bone alkaline phosphatase in healthy men. J Trace Elem Med Biol 15(2–3):175–178
de Luis RD, Aller R, Castrillon JP, De Luis J, Sagrado MG, Izaola O, Romero E, Escudero JM, Herreros V (2004) Effects of dietary intake and life style on bone density in patients with diabetes mellitus type 2. Ann Nutr Metab 48(3):141–145
Elmståhl S, Gullberg B, Janzon L, Johnell O, Elmståhl B (1998) Increased incidence of fractures in middle-aged and elderly men with low intakes of phosphorus and zinc. Osteoporos Int 8(4):333–340
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Bmj 327(7414):557–560
Borenstein M, Hedges LV, Higgins JP, Rothstein HR (2009) Fixed-effect versus random-effects models. Introduction to Meta-analysis, 77–85
Higgins JP (2011) Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. https://www.cochrane-handbook.org
Arikan DC, Coskun A, Ozer A, Kilinc M, Atalay F, Arikan T (2011) Plasma selenium, zinc, copper and lipid levels in postmenopausal Turkish women and their relation with osteoporosis. Biol Trace Elem Res 144(1–3):407–417
Braam L, Knapen M, Geusens P, Brouns F, Hamulyak K, Gerichhausen M, Vermeer C (2003) Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int 73(1):21–26
Candan F, Gültekin F, Candan F (2002) Effect of vitamin C and zinc on osmotic fragility and lipid peroxidation in zinc-deficient haemodialysis patients. Cell Biochem Funct 20(2):95–98
Canhao H, Fonseca JE, Caetano-Lopes J, Saldanha C, Queiroz MV (2008) Assessment of laboratory measurements and− 308 TNFα gene promoter polymorphisms in normal bone mineral density. Clin Rheumatol 27(3):301–307
Gunn C, Weber J, Kruger M (2014) Diet, weight, cytokines and bone health in postmenopausal women. J Nutr Health Aging 18(5):479–486
Gür A, Çolpan L, Nas K, Çevik R, Saraç J, Erdoğan F, Düz MZ (2002) The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and a new effect of calcitonin. J Bone Miner Metab 20(1):39–43
Farrell VA, Harris M, Lohman TG, Going SB, Thomson CA, Weber JL, Houtkooper LB (2009) Comparison between dietary assessment methods for determining associations between nutrient intakes and bone mineral density in postmenopausal women. J Am Diet Assoc 109(5):899–904
Hyun TH, Barrett-Connor E, Milne DB (2004) Zinc intakes and plasma concentrations in men with osteoporosis: the Rancho Bernardo Study. Am J Clin Nutr 80(3):715–721
Ilich JZ, Cvijetic S, Baric IC, Cecic I, Saric M, Crncevic-Orlic Z, Blanusa M, Korsic M (2009) Nutrition and lifestyle in relation to bone health and body weight in Croatian postmenopausal women. Int J Food Sci Nutr 60(4):319–332
Jensen C, Holloway L, Block G, Spiller G, Gildengorin G, Gunderson E, Butterfield G, Marcus R (2002) Long-term effects of nutrient intervention on markers of bone remodeling and calciotropic hormones in late-postmenopausal women. Am J Clin Nutr 75(6):1114–1120
Kadam N, Chiplonkar S, Khadilkar A, Divate U, Khadilkar V (2010) Low bone mass in urban Indian women above 40 years of age: prevalence and risk factors. Gynecol Endocrinol 26(12):909–917
Kamp F, Donangelo CM (2008) Supplementing young women with both zinc and iron protects zinc-related antioxidant indicators previously impaired by iron supplementation. J Nutr 138(11):2186–2189
Kim M-H, Choi M-K, Sung C-J (2007) Bone mineral density of Korean postmenopausal women is similar between vegetarians and nonvegetarians. Nutr Res 27(10):612–617
Geinoz G, Rapin C-H, Rizzoli R, Kraemer R, Buchs B, Slosman D, Michel J, Bonjour J (1993) Relationship between bone mineral density and dietary intakes in the elderly. Osteoporos Int 3(5):242–248
Krebs J, Schneider V, LeBlanc A (1988) Zinc, copper, and nitrogen balances during bed rest and fluoride supplementation in healthy adult males. Am J Clin Nutr 47(3):509–514
Kruger MC, Schollum LM, Kuhn-Sherlock B, Hestiantoro A, Wijanto P, Li-Yu J, Agdeppa I, Todd JM, Eastell R (2010) The effect of a fortified milk drink on vitamin D status and bone turnover in post-menopausal women from South East Asia. Bone 46(3):759–767
Kruger MC, Chan YM, Kuhn-Sherlock B, Lau LT, Lau C, Chin Y, Todd JM, Schollum LM (2016) Differential effects of calcium- and vitamin D-fortified milk with FOS-inulin compared to regular milk, on bone biomarkers in Chinese pre-and postmenopausal women. Eur J Nutr 55(5):1911–1921
Li SK, Wan MM, Siu FP, Chung S, Pang MY (2017) Relationship between nutritional factors and hip bone density in individuals with chronic stroke. Calcif Tissue Int 101(3):259–270
Lim H, Kim HJ, Hong SJ, Kim S (2014) Nutrient intake and bone mineral density by nutritional status in patients with inflammatory bowel disease. J Bone Metab 21(3):195–203
Liu S-Z, Yan H, Xu P, Li J-P, Zhuang G-H, Zhu B-F, Lu S-M (2009) Correlation analysis between bone mineral density and serum element contents of postmenopausal women in Xi’an urban area. Biol Trace Elem Res 131(3):205–214
Mahdaviroshan M, Golzarand M, Taramsari MR, Mahdaviroshan M (2013) Effect of zinc supplementation on serum zinc and calcium levels in postmenopausal osteoporotic women in Tabriz, Islamic Republic of Iran. East Mediterr Health J 19(3):271–275
New SA, Bolton-Smith C, Grubb DA, Reid DM (1997) Nutritional influences on bone mineral density: a cross-sectional study in premenopausal women. Am J Clin Nutr 65(6):1831–1839
Nielsen F, Milne D (2004) A moderately high intake compared to a low intake of zinc depresses magnesium balance and alters indices of bone turnover in postmenopausal women. Eur J Clin Nutr 58(5):703–710
Nielsen FH, Lukaski HC, Johnson LK, Roughead ZF (2011) Reported zinc, but not copper, intakes influence whole-body bone density, mineral content and T score responses to zinc and copper supplementation in healthy postmenopausal women. Br J Nutr 106(12):1872–1879
Okyay E, Ertugrul C, Acar B, Sisman AR, Onvural B, Ozaksoy D (2013) Comparative evaluation of serum levels of main minerals and postmenopausal osteoporosis. Maturitas 76(4):320–325
Relea P, Revilla M, Ripoll E, Arribas I, Villa L, Rico H (1995) Zinc, biochemical markers of nutrition, and type I osteoporosis. Age Ageing 24(4):303–307
Rodondi A, Ammann P, Ghilardi-Beuret S, Rizzoli R (2009) Zinc increases the effects of essential amino acids-whey protein supplements in frail elderly. J Nutr Health Aging 13(6):491–497
Sadighi A, Roshan MM, Moradi A, Ostadrahimi A (2008) The effects of zinc supplementation on serum zinc, alkaline phosphatase activity and fracture healing of bones. Saudi Med J 29(9):1276–1279
Samieri C, Coupez GV, Lorrain S, Letenneur L, Allès B, Féart C, Paineau D, Barberger-Gateau P (2012) Nutrient patterns and risk of fracture in older subjects: results from the Three-City Study. Osteoporos Int 24:1295–1305
Andriollo-Sanchez M, Hininger-Favier I, Meunier N, Toti E, Zaccaria M, Brandolini-Bunlon M, Polito A, O'Connor JM, Ferry M, Coudray C, Roussel A M (2005) Zinc intake and status in middle-aged and older European subjects: the ZENITH study. European journal of clinical nutrition 59(2): S37–S41
Strause L, Saltman P, Smith KT, Bracker M, Andon MB (1994) Spinal bone loss in postmenopausal women supplemented with calcium and trace minerals. J Nutr 124(7):1060–1064
Sugiyama TTH, Kawai S (2000) Improvement of periarticular osteoporosis in postmenopausal women with rheumatoid artritis by beta-alanyl-l-histidinato zinc: a pilot study. J Bone Miner Metab 18(6):335–338
Sun LLLB, Xie HL, Fan F, Yu WZ, Wu BH, Xue WQ, Chen YM (2014) Associations between the dietary intake of antioxidant nutrients and the risk of hip fracture in elderly Chinese: a case–control study. Br J Nutr 112(10):1706–1714
Zhou YAD, Dixon PM, Messina M, Reddy MB (2011) The effect of soy food intake on mineral status in premenopausal women. J Women's Health (Larchmt) 20(5):771–780
Zheng J, Mao X, Ling J, He Q, Quan J (2014) Low serum levels of zinc, copper, and iron as risk factors for osteoporosis: a meta-analysis. Biol Trace Elem Res 160(1):15–23
Hess SY, Peerson JM, King JC, Brown KH (2007) Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull 28(3_suppl3):S403–S429
King JC, Shames DM, Woodhouse LR (2000) Zinc homeostasis in humans. J Nutr 130(5):1360S–1366S
Karaaslan FMM, Mermerkaya MU, Karaoğlu S, Saçmaci Ş, Kartal Ş (2014) Comparison of bone tissue trace-element concentrations and mineral density in osteoporotic femoral neck fractures and osteoarthritis. Clin Interv Aging 18(9):1375–1382
Qi S, He J, Zheng H, Chen C, Jiang H, & Lan S (2020) Zinc Supplementation Increased Bone Mineral Density, Improves Bone Histomorphology, and Prevents Bone Loss in Diabetic Rat. Biological Trace Element Research 194(2):493–501
Li BLH, Jia S (2014) Zinc enhances bone metabolism in ovariectomized rats and exerts anabolic osteoblastic/adipocytic marroweffects ex vivo. Biol Trace Elem Res 163:202–207
Bhardwaj PRD, Garg ML (2013) Zinc as a nutritional approach to bone loss prevention in an ovariectomized rat model. Menopause 20:1184–1193
Shanshan Q (2018) Synergistic effects of genistein and zinc on bone metabolism and the femoral metaphyseal histomorphology in the ovariectomized rats. Biol Trace Elem Res 183:288–295
Li XSY, Ito A (2009) The optimum zinc content in set calcium phosphate cement for promoting bone formation in vivo. Mater Sci Eng 29:969–975
Suzuki TKS, Matsuzaki H, Suzuki K (2016) A shortterm zinc-deficient diet decreases bone formation through downregulated BMP 2 in rat bone. Biosci Biotechnol Biochem 24:1–3
Woitge H, Seibel M, Ziegler R (1996) Comparison of total and bone-specific alkaline phosphatase in patients with nonskeletal disorder or metabolic bone diseases. Clin Chem 42(11):1796–1804
Roudsari JM, Mahjoub S (2012) Quantification and comparison of bone-specific alkaline phosphatase with two methods in normal and Paget’s specimens. Caspian J Intern Med 3(3):478
Cho Y-E, Lomeda R-AR, Ryu S-H, Sohn H-Y, Shin H-I, Beattie JH, Kwun I-S (2007) Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr Res Pract 1(2):113–119
Kalaiselvi V, Prabhu K, Mani Ramesh VV (2013) The association of serum osteocalcin with the bone mineral density in post menopausal women. J Clin Diagn Res 7(5):814
Gurban C, Tanasie G, Gotia S, Cornianu M, Glaja R, Faur A, Anghel S, Ceacli C (2007) Osteocalcin and estradiol markers of bone cells in postmenopausal osteoporosis. Physiology 17(4):273–282
Rahnama M, Jastrzębska-Jamrogiewicz I, Jamrogiewicz R, Trybek G (2015) Analysis of the influence of hormone replacement therapy on osteocalcin gene expression in postmenopausal women. Biomed Res Int 2015:1–8
Singh S, Kumar D, Lal AK (2015) Serum osteocalcin as a diagnostic biomarker for primary osteoporosis in women. J Clin Diagn Res 9(8):RC04–RC07
Li BLH, Jia S (2015) Zinc enhances bone metabolism in ovariectomized rats and exerts anabolic osteoblastic/adipocytic marrow effects ex vivo. Biol Trace Elem Res 63(1–2):202–207
Iitsuka NHM, Tsukamoto I (2013) Zinc supplementation inhibits the increase in osteoclastogenesis and decrease in osteoblastogenesis in streptozotocin-induced diabetic rats. Eur J Pharmacol 4(1–3):41–47
Nagata MLB (2011) Role of zinc in cellular zinc trafficking and mineralization in a murine osteoblast-like cell line. J Nutr Biochem 22(2):172–178
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ceylan, M.N., Akdas, S. & Yazihan, N. Is Zinc an Important Trace Element on Bone-Related Diseases and Complications? A Meta-analysis and Systematic Review from Serum Level, Dietary Intake, and Supplementation Aspects. Biol Trace Elem Res 199, 535–549 (2021). https://doi.org/10.1007/s12011-020-02193-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02193-w