Abstract
It has been shown that the trace elements and lipids play role in the growth, development and maintenance of bones. We aimed to investigate serum selenium (Se), zinc (Zn), copper (Cu) and lipid (total cholesterol, triglyceride (TG), high density lipoprotein-cholesterol, low-density lipoprotein–cholesterol) levels in postmenopausal women with osteoporosis, osteopenia and in healthy controls, and to determine the relationship between Se, Zn, Cu and lipid parameters and bone mineral density (BMD). The study included 107 postmenopausal women; 35 healthy (group 1), 37 osteopenic (group 2) and 35 osteoporotic (group 3). The women in all three groups were carefully matched for body mass index (BMI). Serum concentrations of Se, Zn and Cu were measured by atomic absorption spectrophotometry. Plasma Se, Cu, Zn and lipid levels were similar in all groups (p > 0.05). When we combined the women in each of the three groups, and considered them as one group (n = 107) we found a positive correlation between BMI and lumbar vertebra BMD, femur neck BMD, femur total BMD; a positive correlation between TG and femur neck BMD, femur total BMD; a positive correlation between Zn and lumbar vertebra BMD (total T score) (p < 0.05). There was no correlation between Se, Cu, Zn, P and lipid parameters (p > 0.05). Although BMI has a positive effect on BMD, trace elements and lipids, except Zn and TG, did not directly and correlatively influence BMD. Further studies are needed to clarify the role and relationship of trace elements and lipid parameters in postmenopausal osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is defined as a disease characterised by low bone mass and microarchitectural deterioration of bone tissue resulting in increased bone fragility and therefore, a higher fracture risk [1]. Osteoporosis in women following menopause is a major health problem that affects up to 50% of postmenopausal women [2]. Although there are many risk factors that contribute to the development of osteoporosis such as genetics, ethnicity, sex, age, menopausal state, smoking, alcohol intake, exercise, body weight, sunlight and thyroid, nutritional factors, such as low calcium intake, deficiency of trace elements and vitamins, precise etiological factors and pathogenesis of osteoporosis are still unclear [3–6].
Previous studies [7, 8] have shown that many trace elements are required for the growth, development and maintenance of healthy bones, and recently, some studies [9, 10] have investigated the relationship between postmenopausal osteoporosis and these trace elements. Since it has been reported that supplements of trace minerals, with or without calcium (Ca), can have beneficial effects on bone density in postmenopausal women [7], abnormal metabolism of trace elements may play an important role in the pathogenesis of osteoporosis [8, 10, 11]. Individual studies performed for each of the trace elements of selenium (Se), zinc (Zn) and copper (Cu), demonstrated that deficiency in any of these trace elements can cause an increase in the risk of bone resorption by inhibiting bone growth and thus, may play a role in the onset and progression of osteoporosis [8, 12–14].
Although osteoporosis and atherosclerosis are often associated and common genes may be involved in their pathogenesis, the mechanism governing the relationship between lipids and bone mineral density (BMD) is unclear [15]. While some studies have found an association between BMD and serum lipids in postmenopausal women [16–18], others have not [19, 20]. Similarly, there is an inconsistency in the literature among studies that investigated the relationship between trace elements and lipids, with some studies identifying a correlation between these trace elements and lipids [21, 22], while others did not [23, 24].
As seen in these studies, there is inadequate and contradictory data present in the literature concerning the relationship between plasma Se, Zn, Cu and lipid levels and osteoporosis in postmenopausal women. There were, however, no existing studies that evaluated both trace element and lipid levels in the same study population. Therefore, for the first time, we aimed to determine serum Se, Zn, Cu and lipid levels in the same study population with postmenopausal normal, osteopenic and osteoporotic women. In addition, we considered the relationships between Se, Zn, Cu and BMD and lipid levels in these subjects.
Material and Methods
This case–control study included 107 postmenopausal women divided into three groups according to BMD; 35 healthy (group 1) (T scores <−1.0), 37 osteopenic (group 2) (T score between −1.0 and −2.5) and 35 osteoporotic (group 3) (T score >−2.5) postmenopausal women. The women in all three groups were carefully matched for body mass index (BMI). They were recruited from among patients who applied to outpatient clinics in the Department of Gynecology and Obstetrics of the Medical Faculty of Kahramanmaras Sutcu Imam (Kahramanmaras, Turkey). Research ethics approval was obtained from the Ethics Committee of Kahramanmaras Sutcu Imam University before the commencement of the study and signed-informed consent was obtained from all patients and volunteers.
All of the participants were in natural menopause for more than 6 months. The exclusion criteria from the study were surgical menopause and secondary osteoporosis or other medical conditions that might affect bone metabolism or trace element status such as kidney disease, diabetes mellitus or drug use (e.g. diuretics). Patients who were treated with bisphosphonates, calcitonin, anabolic steroids, hormone replacement therapy, calcium or vitamin D up to 6 months before the investigation were also excluded.
BMD Measurements
The diagnosis of osteoporosis was based on BMD measurements. BMD was measured at the lumbar spine (L1–L4), femoral neck, trochanter, Ward's triangle and total hip by dual energy X-ray absorptiometry (Hologic QDR-4500, USA).
The diagnosis of osteoporosis was based on the WHO criteria [25], in which a loss of bone mass ≤1 standard deviation (SD) was considered as normal, 1 SD <loss of bone mass, ≤2.5 SD was diagnosed as osteopenia and loss of bone mass >2.5 SD was diagnosed as osteoporosis. A subject was diagnosed as osteoporotic if the T score of any measured eight parts of the subject was less than −2.5 SD, normal if T scores were greater than or equal to −1.0 SD for all parts and osteopenic for all other results.
Serum Parameters
A blood sample was taken from each participant before administration of any medication and before any medical or surgical intervention. The blood samples, which were obtained from the antecubital area, were collected between 0800 and 0900 hours following 10–12 h of fasting. Fasting venous blood specimens were drawn from the antecubital vein and collected in no additive vacutainer (Becton-Dickinson, Franklin Lakes, NJ, USA) blood-collecting tubes according to standard hospital guidelines for venipuncture and sample collection. The serum separator tube specimens were allowed to clot and then centrifuged for 10 min at 3,000×g to separate the serum. Plasma were separated and stored at −70°C until the analysis.
Serum total cholesterol (TC) (milligrammes per decilitre), high-density lipoprotein cholesterol (HDL-C) (milligrammes per decilitre), low-density lipoprotein cholesterol (LDL-C) (milligrammes per decilitre), triglyceride (TG) (milligrammes per decilitre), Ca (milligrammes per decilitre) and phosphorus (P) (milligrammes per decilitre) levels were measured the same day using a Dade Behring RXL calibrated autoanalyzer (USA).
Measurement of Serum Se Level
Selenium measurement was done in graphite furnace atomic absorption spectrophotometer (PerkinElmer Analyst 800) using Zeeman background correction. Matrix modifiers were palladium (4 mg in 20-mL sample) and magnesium sulphate (3 mg in 20-mL sample). Samples and calibration standards were diluted in 1:3 with 0.05% Triton X-100 to improve the sample viscosity and reproducibility of the results. Se levels in all groups were evaluated according to a standard curve as microgrammes per litre. Selenium calibration standards were prepared from the commercial Se Standard (1,000 mg/L) by serial dilutions [26].
Measurement of Serum Cu Levels
Serum Cu levels were analysed in flame photometerof atomic absorption spectrophotometer (PerkinElmer Analyst 800). Samples and calibration standards for Cu measurement were 1:2 dilutions with 10% glycerol. Commercial Cu calibrators were used as standards (1,000 mg/L) by serial dilutions, and samples were evaluated according to a standard curve [27].
Measurement of Serum Zn Levels
Serum Zn levels were analysed inflame photometerof atomic absorption spectrophotometer (PerkinElmer Analyst 800). Samples and calibration standards for Zn measurement were prepared in 1:4 dilutions with 5% glycerol. Commercial Zn standards (1,000 mg/L) were used by serial dilutions, and samples were evaluated according to standard curve [28]. Cu and Zn results were given as microgrammes per decilitre.
Statistical Analyses
All data were analysed using the Statistical Package for the Social Sciences for Windows version 15.0 (SPSS, Chicago, IL, USA). One-way ANOVA test was used for statistical significance of differences in variables among groups and the Bonferroni test was used as a post-hoc test for multiple comparisons when a significant result was obtained. Correlations between variables were evaluated using Pearson's correlation test. The data were presented as mean ± SD. Statistical significance was defined as p < 0.05.
Results
The demographic and clinical characteristics of the groups are shown in Table 1. When compared with group 1, a significant increase in the mean age and duration of menopause was found in groups 2 and 3 (p < 0.05).
The plasma Se, Cu, Zn levels and other laboratory findings of the groups are shown in Table 2. Plasma levels of all parameters were similar across each group (p > 0.05). Although plasma Zn levels were higher in the healthy group when compared to the osteopenic and osteoporotic groups, the difference was not statistically significant (p > 0.05).
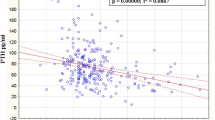
When we combined the women in each of the three groups, and considered them as one group (n = 107) we found a positive correlation between BMI and lumbar vertebra BMD, femur neck BMD, femur total BMD; a positive correlation between TG and femur neck BMD, femur total BMD; a positive correlation between Zn and lumbar vertebra BMD (total T score); and a positive correlation between Ca and femur neck BMD (Table 3) (p < 0.05). There was no correlation between Se, Cu, Zn, P and TC, TG, HDL-C, LDL-C (Table 4) (p > 0.05). The only correlation between serum elements and lipids was the positive correlation between Ca and TC (Table 4) (p < 0.05).
Discussion
Studies that investigate the correlation between trace elements and osteoporosis have mostly been focused on Cu and Zn. Similar to our study, Reginster et al. [29] reported no significant difference in Cu and Zn plasma levels between postmenopausal women with osteoporosis and the non-osteoporotic control subjects. In contrast, several studies found that serum Zn and Cu levels were lower among patients with postmenopausal osteoporosis than with the controls [30, 31].
Previously, it has been reported that there is an association between Zn deficiency and many kinds of skeletal abnormalities in fetal and postnatal development [32]. Zinc is an essential mineral, forming a component part in more than 200 enzymes and, most importantly, Zn is a cofactor of alkaline phosphatase that is involved in the synthesis of various bone matrix constituents and plays a particularly important role for normal collagen synthesis and bone mineralization [33]. Yamaguchi et al. [34] demonstrated that Zn enhanced the ability of 1,25-dihydroxycholecalciferol to increase alkaline phosphatase activity and DNA content in rat calvaria. Therefore, osteoblastic activity, collagen and proteoglycan synthesis, alkaline phosphatase activity and bone deposition of Ca and magnesium could be reduced due to Zn deficiency, thus, causing bone fragility [35]. Although we found similar Zn levels in all groups, there was a positive correlation between Zn and lumbar vertebrae BMD (total T score). This result supports the hypothesis that Zn deficiency results in osteoporosis.
Similarly, it has been known for a long time that Cu deficiency inhibits bone growth and promotes pathological changes characteristic of osteoporosis [36]. In a study performed by Conlan et al. [37], elderly patients with fractures of the femoral neck were found to have significantly lower serum Cu levels than age and sex-matched controls. Although the Cu content of bone was negatively correlated with bone Ca, bone density and collagen content in ageing mice [38], bone Cu levels in human subjects with osteoporosis were found to be the same or slightly higher than in normal individuals of the same age [9, 29]. Cu is also a cofactor of lysyl oxidase, an enzyme involved in the initiation and regulation of collagen and elastin [39]. In Cu deficiency, the activity of this enzyme in bone areas is greatly reduced [40], and it is presumed that this causes a reduction in collagen crosslinking that may influence the synthesis and stability of bone collagen and induce skeletal development disorders, leading to osteoporosis [41]. Inconsistent with this, we found similar Cu levels in osteoporotic and non-osteoporotic postmenopausal women.
Selenium plays a fundamental role in human health by providing protection against oxidative damage [42], and Se deficiency has been shown to affect bone tissue, potentially leading to impaired bone metabolism [14, 43]. In regions of Tibet, there is an endemic osteoarthropathy called Kashin–Beck disease that occurs due to Se deficiency caused by the low Se content of the soil [44]. There are only two studies present in the literature assessing Se status in patients with osteoporosis and, as with our study, both of them reported similar Se levels in postmenopausal osteoporotic and healthy women [9, 10]. Our results of similar Se, Cu and Zn levels in all groups support the hypothesis of Liu et al. [10] in which they indicated that the contents of these trace elements in serum did not directly and mutually influence BMD and may have little effect on the pathogenesis of osteoporosis.
Since the accumulation of lipids in the osteons of human osteoporotic bone had previously been identified [45] in 2003, Parhami hypothesized that accumulation of lipoproteins and lipids in bone may affect the osteoblastic cells and inhibit their proper bone-forming activity [46]. The mechanism by which lipids cause osteoporosis is a reduction in antioxidant defence systems that comes with age, resulting in an increase in the circulating levels of lipids and lipoproteins which can in turn lead to an increase in lipid and lipoprotein accumulation and oxidation in bone tissue [47]. These oxidized lipids may inhibit osteoblastic differentiation and bone formation and increased adipogenesis of marrow stromal cells may induce osteoclastic differentiation, ultimately resulting in net bone loss [48]. However, there are contradictory studies related to BMD and lipid parameters in postmenopausal osteoporotic women in which it is assumed that obesity has a protective effect against the development of osteoporosis [49, 50]. Our results showed that TC, TG, HDL-C, LDL-C levels did not differ significantly between groups and are consistent with results of Demir et al. [49]. In the present study, only TG levels show a significant positive correlation with the BMD of the femur neck and femur total. In a study performed to investigate the relationship between BMI and BMD, Langsetmo et al. [50] found a strong positive correlation between BMI and femoral neck BMD, which is consistent with our data. However, published data on this topic are still scarce, and the reports that exist are inconsistent: some studies have found a positive association between BMD and serum lipids in postmenopausal women [16, 51] and some have found a negative association [17, 52, 53], whereas Samelson et al. [19] have found no association. Bagger et al. [53] reported that these discrepancies could be explained by the fact that the association of lipids to bone is not detectable before the severity of vascular intraluminal lesions reaches a critical level as blood supply to the anatomical region is considerably disrupted.
Previous studies investigating the relationship between trace elements and lipids mostly take into account the cardiovascular risk factors for humans [24, 54–57]. For the first time, this study presents data on Se, Cu, Zn and lipids in the serum of Turkish postmenopausal osteoporotic women. Our results showed that serum Se, Zn, Cu and lipid levels did not differ significantly between the groups. In addition, no significant correlation was found between these parameters. Previous studies confirm that Cu deficiency causes an increase in plasma cholesterol [58, 59]. One possible mechanism of this condition may be due to an increase in the removal of cholesterol from the hepatic pool into the plasma pool [60]. It has been shown in rats that Cu deficiency causes an increase in hepatic reduced glutathione concentrations, which increases the activity of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, the rate-limiting enzyme in cholesterol synthesis and, as a result, causes hypercholesterolemia [61]. Most other reported human studies found no changes in cholesterol with short-term experimental Cu deprivation [62, 63]. In modelling with rats, it has been shown that Zn deficiency causes higher liver concentrations of total lipids (TL), cholesterol, TG and LDL-C, while HDL-C declined significantly in a dose-dependent manner [64]. In contrast to this study, Tiber et al. [24] found no association between plasma Zn or Cu and the serum levels of lipids such as TC, TG, HDL-C, LDL-C in adult male patients with confirmed coronary artery disease. Selenium absorption and metabolism may be reduced after menopause, and this may promote cholesterol metabolism and/or decreased LDL-C receptors [54, 55]. In some investigational studies, it has been shown that oral Se administration in rats fed a high-cholesterol diet decreased blood TC and LDL-C levels and liver TC, and it has been suggested that hypercholesterolemia may consume Se after menopause to protect endothelial cells against LDL-C oxidation [65, 66]. In addition, it has been shown that increased Se intake induces increased glutathione peroxidase activity and decreased concentrations of lipid parameters (TC, LDL-C, TG) in animal blood [56, 66]. Karita et al. [21] in their study reported that lower levels of Se in erythrocytes were associated with higher levels of serum TC and LDL-C and that this relationship was marked only in the postmenopausal group. However, in parallel with our results, they could not find any relation between serum Se level and lipids. Recently, Laclaustra et al. [57] reported that high-serum Se concentrations were associated with increased serum concentrations of TC and LDL-C in men and women aged ≥40 years old. Similar to our results, Viegas et al. [67] found similar serum levels of Se, Cu and Zn in normal and hyperlipidemic individuals of both sexes. In addition, they reported no significant correlation between the level of each trace element and lipid parameters such as TG, TC, HDL-C, LDL-C [67].
Although we could not find any correlation between plasma Se, Zn, Cu and lipid levels, as can be seen in the studies of deficiencies in these trace elements, there is a tendency towards an increase in lipid levels [58, 59, 61, 64–66]. Karita et al. [21] explained the reason for similar serum Se levels as being that serum Se reflects short-term Se status and fluctuates widely according to daily food intake. They emphasised that serum Se levels could not reflect nutritional Se status in well-nourished humans because serum Se can easily reach a saturated level. In addition, they concluded that serum Se levels could only be useful as a nutritional status indicator in populations where Se intake is insufficient [21]. Because this mechanism may also be valid for Zn and Cu, future studies should evaluate both serum and erythrocyte levels of these trace elements to reveal the relationship of these trace elements to lipids and osteoporosis.
As a conclusion, the main findings of the present study were as follows: (1) Se, Zn, Cu and lipid levels in plasma were similar in postmenopausal women with osteoporosis in contrast to healthy and osteopenic postmenopausal women, (2) BMI and TG significantly correlated with lumbar and hip BMD, (3) no correlation was present between trace elements and lipid parameters. In contrast to studies that report that a lack of these elements in diet may affect the bone structure, our results reveal that these trace elements and lipids, with the exception of Zn and TG, did not directly and correlatively influence BMD. This aspect should be investigated in future studies with larger populations that include pre-, peri- and post-menopausal women with osteoporosis to find the relationship between trace elements and lipids and their role in etiopathogenesis of osteoporosis.
References
Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018
Ross PD (1998) Risk factors for osteporotic fracture. Endocr Metab Clin N Am 27:289–296
Kimble RB (1997) Alcohol, cytokines, and estrogen in the control of bone remodeling. Alcohol Clin Exp Res 21:385–391
Palacios C (2006) The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr 46:621–628
Williams FM, Spector TD (2007) The genetics of osteoporosis. Acta Reumatol Port 32:231–240
Saltman P, Strause L (1991) Trace elements in bone metabolism. J Inorg Biochem 3:284
Saltman PD, Strause LG (1993) The role of trace minerals in osteoporosis. J Am Coll Nutr 12:384–389
Odabasi E, Turan M, Aydin A, Akay C, Kutlu M (2008) Magnesium, zinc, copper, manganese, and selenium levels in postmenopausal women with osteoporosis. Can magnesium play a key role in osteoporosis? Ann Acad Med Singapore 37:564–567
Liu SZ, Yan H, Xu P, Li JP, Zhuang GH, Zhu BF, Lu SM (2009) Correlation analysis between bone mineral density and serum element contents of postmenopausal women in Xi'an urban area. Biol Trace Elem Res 131:205–214
Nieves JW (2005) Osteoporosis: the role of micronutrients. Am J Clin Nutr 81:1232–1239
Strain JJ (1988) A reassessment of diet and osteoporosis-possible role for copper. Med Hypotheses 27:333–338
Relea P, Revilla M, Ripoll E, Arribas I, Villa LF, Rico H (1995) Zinc, biochemical markers of nutrition, and type I osteoporosis. Age Ageing 24:303–307
Moreno-Reyes R, Egrise D, Nève J, Pasteels JL, Schoutens A (2001) Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J Bone Miner Res 16:1556–1563
Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ, AtheroGene Investigators (2003) Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med 349:1605–1613
Adami S, Braga V, Zamboni M, Gatti D, Rossini M, Bakri J, Battaglia E (2004) Relationship between lipids and bone mass in two cohorts of healthy women and men. Calcif Tissue Int 74:136–142
Yamaguchi T, Sugimoto T, Yano S, Yamauchi M, Sowa H, Chen Q, Chihara K (2002) Plasma lipids and osteoporosis in postmenopausal women. Endocr J 49:211–217
Brownbill RA, Ilich JZ (2006) Lipid profile and bone paradox: higher serum lipids are associated with higher bone mineral density in postmenopausal women. J Women's Health (Larchmt) 15:261–270
Samelson EJ, Cupples LA, Hannan MT, Wilson PW, Williams SA, Vaccarino V, Zhang Y, Kiel DP (2004) Long-term effects of serum cholesterol on bone mineral density in women and men: the Framingham Osteoporosis Study. Bone 34:557–561
Solomon DH, Avorn J, Canning CF, Wang PS (2005) Lipid levels and bone mineral density. Am J Med 118:1414
Karita K, Yamanouchi Y, Takano T, Oku J, Kisaki T, Yano E (2008) Associations of blood selenium and serum lipid levels in Japanese premenopausal and postmenopausal women. Menopause 15:119–124
Hiller R, Seigel D, Sperduto RD, Blair N, Burton TC, Farber MD, Gragoudas ES, Gunter EW, Haller J, Seddon JM et al (1995) Serum zinc and serum lipid profiles in 778 adults. Ann Epidemiol 5:490–496
Bukkens SG, de Vos N, Kok FJ, Schouten EG, de Bruijn AM, Hofman A (1990) Selenium status and cardiovascular risk factors in healthy Dutch subjects. J Am Coll Nutr 9:128–135
Tiber AM, Sakhaii M, Joffe CD, Ratnaparkhi MV (1986) Relative value of plasma copper, zinc, lipids and lipoproteins as markers for coronary artery disease. Atherosclerosis 62:105–110
Miller PD (2006) Guidelines for the diagnosis of osteoporosis: T-scores vs fractures. Rev Endocr Metab Disord 7:75–89
Correia PRM, Oliveira E, Oliveira PV (2002) Simultaneous determination of manganese and selenium in serum by electrothermal atomic absorption spectrometry. Talanta 57:527–535
Evenson MA (1988) Measurement of copper in biological samples by flame or electrothermal atomic absorption spectrometry. Methods Enzymol 158:351–357
Smith JC, Butrimovitz GP, Purdy WC (1979) Direct measurement of zinc in plasma by atomic absorption spectroscopy. Clin Chem 25:1487–1491
Reginster JY, Strause L, Saltman P, Frachimont P (1998) Trace elements and osteoporosis: a preliminary study of decreased serum manganese. Med Sci Res 16:337–338
Gür A, Colpan L, Nas K, Cevik R, Saraç J, Erdoğan F, Düz MZ (2002) The role of trace minerals in the pathogenesis of postmenopausal osteoporosis and new effect of calcitonin. J Bone Miner Metab 20:39–43
Steidl L, Ditmar R (1990) Blood zinc findings in osteoporosis. Acta Univ Palacki Olomuc Fac Med 126:129–138
Hurley LS (1981) Teratogenic aspects of manganese, zinc, and copper nutrition. Physiol Rev 61:249–295
Hyun TH, Barret-Connor J, Milne DB (2004) Zinc intakes and plasma concentrations in men with osteoporosis the Rancho Bernardo Study. Am J Clin Nutr 80:715–721
Yamaguchi M, Oishi H (1989) Effect of 1,25-dihydroxyvitamin D3 on bone metabolism in tissue culture. Enhancement of the steroid effect by zinc. Biochem Pharmacol 38:3453–3459
Salgueiro MJ, Torti H, Meseri E, Weill R, Orlandini J, Urriza R, Zubillaga M, Janjetic M, Barrado A, Boccio J (2006) Dietary zinc effects on zinc, calcium, and magnesium content in bones of growing rats. Biol Trace Elem Res 110:73–78
Dollwet HH, Sorenson JR (1988) Roles of copper in bone maintenance and healing. Biol Trace Elem Res 18:39–48
Conlan D, Korula R, Tallentire D (1990) Serum copper levels in elderly patients with femoral-neck fractures. Age Ageing 19:212–214
Massie HR, Aiello VR, Shumway ME, Armstrong T (1990) Calcium, iron, copper, boron, collagen, and density changes in bone with aging in C57BL/6J male mice. Exp Gerontol 25:469–481
Rucker RB, Murray J (1978) Cross-linking amino acids in collagen and elastin. Am J Clin Nutr 31:1221–1236
Siegel RC, Page RC, Martin GR (1970) The relative activity of connective tissue lysyl oxidase and plasma amine oxidase on collagen and elastin substrates. Biochim Biophys Acta 222:552–555
Lowe NM, Lowe NM, Fraser WD, Jackson MJ (2002) Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc Nutr Soc 61:181–185
Chariot P, Bignani O (2003) Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve 27:662–668
Turan B, Can B, Delilbasi E (2003) Selenium combined with vitamin E and vitamin C restores structural alterations of bones in heparin-induced osteoporosis. Clin Rheumatol 22:432–436
Moreno-Reyes R, Mathieu F, Boelaert M, Begaux F, Suetens C, Rivera MT, Nève J, Perlmutter N, Vanderpas J (2003) Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am J Clin Nutr 78:137–144
Ramseier E (1962) Untersuchungen uber arteriosklerotische Veranderungen der Konchenarterien. Virchows Arch Pathol Anat 336:77–86
Parhami F (2003) Possible role of oxidized lipids in osteoporosis: could hyperlipidemia be a risk factor? Prostaglandins Leukot Essent Fatty Acids 68:373–378
Parhami F, Demer LL (1997) Arterial calcification in face of osteoporosis in ageing: can we blame oxidized lipids? Curr Opin Lipidol 8:312–314
Arjmandi BH, Juma S, Beharka A, Bapna MS, Akhter M, Meydani SN (2002) Vitamin E improves bone quality in the aged but not in young adult male mice. J Nutr Biochem 13:543–549
Demir B, Haberal A, Geyik P, Baskan B, Ozturkoglu E, Karacay O, Deveci S (2008) Identification of the risk factors for osteoporosis among postmenopausal women. Maturitas 60:253–256
Langsetmo L, Hanley DA, Prior JC, Barr SI, Anastassiades T, Towheed T, Goltzman D, Morin S, Poliquin S, Kreiger N, CaMos Research Group (2011) Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged ≥50 y: a population-based cohort study. Am J Clin Nutr 93:192–199
Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A (2007) Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet 370(9588):657–666
Orozco P (2004) Atherogenic lipid profile and elevated lipoprotein (a) are associated with lower bone mineral density in early postmenopausal overweight women. Eur J Epidemiol 19:1105–1112
Bagger YZ, Rasmussen HB, Alexandersen P, Werge T, Christiansen C, Tankó LB, PERF study group (2007) Links between cardiovascular disease and osteoporosis in postmenopausal women: serum lipids or atherosclerosis per se? Osteoporos Int 18:505–512
Kwiterovich PO Jr (1997) The effect of dietary fat, antioxidants, and prooxidants on blood lipids, lipoproteins, and atherosclerosis. J Am Diet Assoc 97:31–41
Aviram M (1996) Interaction of oxidized low density lipoprotein with macrophages in atherosclerosis, and the antiatherogenicity of antioxidants. Eur J Clin Chem Clin Biochem 34:599–608
Wójcicki J, Rózewicka L, Barcew-Wiszniewska B, Samochowiec L, Juźwiak S, Kadłubowska D, Tustanowski S, Juzyszyn Z (1991) Effect of selenium and vitamin E on the development of experimental atherosclerosis in rabbits. Atherosclerosis 87:9–16
Laclaustra M, Stranges S, Navas-Acien A, Ordovas JM, Guallar E (2010) Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 210:643–648
Petering HG, Murthy L, O'Flaherty E (1977) Influence of dietary copper and zinc on rat lipid metabolism. J Agric Food Chem 25:1105–1109
Murthy L, Petering HG (1976) Effect of dietary zinc and copper on blood parameters of the rat. J Agric Food Chem 24:808–811
Lin IM, Lei KY (1981) Cholesterol kinetic analyses in copper-deficient rats. J Nutr 111:450–457
Kim S, Chao PY, Allen KG (1992) Inhibition of elevated hepatic glutathione abolishes copper deficiency cholesterolemia. FASEB J 6:2467–2471
Milne DB, Klevay LM, Hunt JR (1988) Effects of ascorbic acid supplements and a diet marginal in copper on indices of copper nutriture in women. Nutr Res 8:865–873
Turnlund JR, Keen CL, Smith RG (1990) Copper status and urinary and salivary copper in young men at three levels of dietary copper. Am J Clin Nutr 51:658–664
Yousef MI, El-Hendy HA, El-Demerdash FM, Elagamy EI (2002) Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175:223–234
Dhingra S, Bansal MP (2005) Hypercholesterolemia and apolipoprotein B expression: regulation by selenium status. Lipids Health Dis 4:28–41
Iizuka Y, Sakurai E, Tanaka Y (2001) Effect of selenium on serum, hepatic and lipoprotein lipids concentration in rat fed on a high-cholesterol diet. Yakugaku Zasshi 12:93–96
Viegas-Crespo AM, Pavão ML, Paulo O, Santos V, Santos MC, Nève J (2000) Trace element status (Se, Cu, Zn) and serum lipid profile in Portuguese subjects of San Miguel Island from Azores'archipelago. J Trace Elem Med Biol 14:1–5
Conflict of Interest
None of the authors had any financial or other potential conflicts of interests concerning this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arikan, D.C., Coskun, A., Ozer, A. et al. Plasma Selenium, Zinc, Copper and Lipid Levels in Postmenopausal Turkish Women and Their Relation with Osteoporosis. Biol Trace Elem Res 144, 407–417 (2011). https://doi.org/10.1007/s12011-011-9109-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-011-9109-7