Abstract
Zinc (Zn) deficiency during pregnancy may result in a variety of defects in the offspring. We evaluated the influence of marginal Zn deficiency during pregnancy on neonatal bone status. Nine-week-old male Sprague-Dawley rats were divided into two groups and fed AIN-93G-based experimental diets containing 35 mg Zn/kg (Zn adequately supplied, N) or 7 mg Zn/kg (low level of Zn, L) from 14-day preconception to 20 days of gestation, that is, 1 day before normal delivery. Neonates were delivered by cesarean section. Litter size and neonate weight were not different between the two groups. However, in the L-diet-fed dam group, bone matrix formation in isolated neonatal calvaria culture was clearly impaired and was not recovered by the addition of Zn into the culture media. Additionally, serum concentration of osteocalcin, as a bone formation parameter, was lower in neonates from the L-diet-fed dam group. Impaired bone mineralization was observed with a significantly lower content of phosphorus in neonate femurs from L-diet-fed dams compared with those from N-diet-fed dams. Moreover, Zn content in the femur and calvaria of neonates from the L-diet group was lower than that of the N-diet-fed group. In the marginally Zn-deficient dams, femoral Zn content, serum concentrations of Zn, and osteocalcin were reduced when compared with control dams. We conclude that maternal Zn deficiency causes impairment of bone matrix formation and bone mineralization in neonates, implying the importance of Zn intake during pregnancy for proper bone development of offspring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc (Zn) is a constituent of various enzymes and proteins as well as an important regulator of biological functions. Zn deficiency can lead to dermatitis, alopecia, decreased growth, and impaired immune function [1]. Zn also plays important roles in bone formation activity. Animal studies have shown that Zn deficiency results in low bone mass [2, 3], and inadequate intake of Zn via diet results in a decrease in the number of osteoblasts [4] and chondrocytes [5] in animal bone. In humans, low serum Zn concentration and decreased bone turnover have been reported in small-for-gestational age preterm infants [6]. In addition, low Zn intake has been reported to be associated with low bone mass in adult women [7]. Studies in osteoblastic cell cultures have shown that Zn stimulates DNA synthesis in osteoblast-like cells [8] and has an inhibitory effect on bone resorption [9].
Although consequences of severe Zn deficiency during pregnancy have been examined in a number of animal models, such nutritional status is uncommon in human populations. However, marginal Zn deficiency is now recognized as prevalent in both industrialized and developing countries [10]. Despite the likely high prevalence of maternal Zn deficiency, data supporting maternal Zn deficiency as a cause of poor fetal growth are far from conclusive. One human study has been published suggesting that maternal Zn deficiency lowers fetal bone growth [11]. In the rhesus monkey, the Zn status of bone in newborns from dams fed marginally Zn-deficient diets during pregnancy was reported a quarter century ago [12]. Nevertheless, the involvement of Zn status during pregnancy on neonatal bone metabolism has remained unclear until now. The aim of the present study was to investigate the influence of marginal Zn deficiency during pregnancy on bone matrix formation of newborn rats using specific parameters of Zn status and bone metabolism in neonates and dams.
Materials and Methods
Diets
Rats were fed an egg-white-based experimental diet that included other compositions (Table 1) based on the AIN-93G formulation [13]. The diets contained 35 mg Zn/kg (normal control, N) and 7 mg Zn/kg (marginally Zn deficient, L), as Zn carbonate. The Zn content of both diets was confirmed after wet ashing by inductively coupled plasma atomic emission spectroscopy (ICP-AES; ICPS-7510, Shimadzu, Tokyo, Japan).
Experimental Design
The experiments were performed in accordance with the guidelines of the ethics committee on animal use of Meiji Dairies Corporation and Law No. 105 and Notification No. 6 of the Government of Japan. Virgin Sprague-Dawley female rats (purchased from Japan SLC, Hamamatsu, Japan) at 9 weeks of age were housed in a temperature-controlled room with a 12:12-h light–dark cycle and free access to water and a commercial diet for a 7-day acclimation period. The rats (n = 14/diet group) were randomly assigned to N-diet or L-diet groups. After 14 days with the experimental diets, individual female rats were housed together with a single male rat for 1 day to mate; both rats were fed the same diet. After mating, the female rats were maintained with the same experimental diet. Throughout the experiment, food intake and animal weight were recorded every 3 days except for on the day of mating. On 20 days postconception, the pregnant rats were anesthetized with ethyl ether and sacrificed with aortic puncture. Pups (neonates) were aseptically delivered by cesarean section. The litter size, neonate weight, and placenta weight were recorded. The time point of cesarean section was randomized between groups. Neonates were sacrificed by decapitation. All the littermate serum samples from each dam were pooled to one serum sample. Serum samples were maintained at −80°C until analysis of various parameters.
Tissue and Serum Mineral Analyses
A carcass of randomly chosen littermate from each dam was homogenized, then wet-ashed with ultrapure nitric acid. A right femur and whole calvaria of another randomly selected littermate from each dam was wet-ashed with ultrapure nitric acid. Wet-ashed samples were measured for Zn, phosphorus, and calcium concentrations with ICP-AES. Serum mineral concentrations were measured using commercial kits (Zn: Zn Test Wako and calcium: Ca E-Test Wako, Wako Pure Chemical Industries, Osaka, Japan; inorganic phosphorus with Fuji Dry Chem Slide IP-P, Fuji Film Industries, Tokyo, Japan).
Assessment of Bone Matrix Formation Using Calvarial Organ Culture
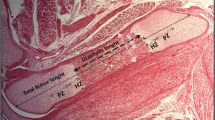
Calvarial organ culture [14] was performed to evaluate bone matrix formation activity in one neonate randomly selected from randomized 12 dams in each diet group. Briefly, aseptically dissected and cleaned neonatal half calvariae were placed concave side down on the sterile plastic grid in 12-well tissue culture plates and cultured for 7 days with BGJb medium (Invitrogen, Carlsbad, CA, USA) supplemented with 1 mg/mL of bovine serum albumin, 100 U/mL each of penicillin/streptomycin, 0.092 mg/mL of glutamine, and 50 μg/mL of ascorbic acid (basal medium). The other set of calvaria halves was divided into two groups and cultured in medium with added basal medium and 20 μmol/L Zn (as Zn sulfate) to investigate the direct and compensatory effect of Zn on bone matrix formation (n = 6/group) or with 100 nmol/L of corticosterone to confirm integrity of the assay system, presuming that negative bone matrix formation would be observed (n = 6/group). The cultured calvariae were fixed in 10% formalin-containing phosphate-buffered saline and decalcified overnight in 14% EDTA containing 10% buffered formalin. The next day, the demineralized calvariae were processed overnight using the following protocol: 70% ethanol for 45 min, twice with 95% ethanol for 45 min, four times with 100% ethanol, twice with xylene, and twice with paraffin for 45 min. The processed calvariae were embedded in paraffin blocks. Three different 5-μm-thick sections, which included the coronal suture area at 400-μm, 800-μm, and 1,200-μm depths, were cut parallel to the midline suture. The sections were mounted onto silane-treated glass microscope slides, deparaffinized, and stained for collagen fiber with Masson’s trichrome staining. With this staining method, the original bone area was stained darker, whereas new bone appeared as a lighter color. With a charge-coupled device camera under microscopy, three images per section were captured, followed by measurement of new bone area with the software program ImageJ (NIH, Bethesda, MD, USA). New bone area was calculated as percentage of total bone area.
Biochemical Parameter Analyses
Serum osteocalcin concentration was analyzed with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Rat-MID Osteocalcin ELISA; Nordic Bioscience Diagnostics a/s, Herlev, Denmark). Serum corticosterone level was measured using an RIA kit (COAT-A-COUNT Rat Corticosterone; Diagnostic Products Corporation, Los Angeles, CA, USA). Serum insulin-like growth factor (IGF)-I level was measured using an ELISA kit (IGF-I HS ELISA; Immunodiagnostic Systems Ltd., Boldon).
Statistics
Data are presented as means ± SE. Statistical analysis was performed with Student’s t test using the software program StatView (SAS Institute Inc., Cary, NC, USA), except for the in vitro bone matrix formation assay. Two-way analysis of variance (ANOVA) was performed for the in vitro bone matrix formation assay using the effects of dam diet and Zn addition; these effects were applied to all groups, except the groups receiving corticosterone addition. In every statistical analysis, a P value of <0.05 was regarded as significantly different.
Results
Growth Parameters of Dams and Neonates
Daily food intake and final body weight of dams were not significantly different between the two dietary groups. Litter size and body weight for the neonates were comparable in both groups. Relative placental weight for neonates was not different between the two groups (Table 2).
Mineral Status of Neonates
Zn content was about 20% lower in carcasses and about 30% lower in the femurs and calvariae of neonates from L-diet-fed dams than those from N-diet-fed dams. Phosphorus content was significantly lower only in femurs of neonates from L-diet-fed dams compared with those from N-diet-fed dams; however, the content was comparable in the carcasses and calvariae between the two diet groups. The calcium content of neonatal carcasses, femurs, and calvariae was not different between the dam groups (Table 3). Serum Zn concentration was lower in neonates from L-diet-fed dams than that from dams fed on N diet (P < 0.0001). However, phosphorus and calcium concentrations for neonatal serum were not significantly different between the two groups (data not shown).
Bone Matrix Formation in Cultured Neonatal Calvaria
Two-way ANOVA for four groups of culture medium alone and culture medium + Zn demonstrated clear impairment of bone matrix formation in the cultures from the L-diet-fed dam group (P = 0.002). The addition of 20 μmol/L Zn to the culture medium, which is a normal serum concentration for adult rats, had no effect on bone matrix formation for the calvariae of neonates from N-diet dams. Addition of 100 nmol/L corticosterone to culture media, the normal serum concentration in pregnant rats [15], resulted in a clear reduction in bone matrix formation in both groups (Fig. 1).
The effects of maternal dietary zinc (Zn) levels and acute Zn exposure on bone matrix formation assay using murine fetal half calvaria. Neonatal half calvaria was cultured for 7 days with basal medium alone (n = 12 per group) or with 20 μmol/L ZnSO4 (n = 6 per group) or with 100 nmol/L corticosterone (CS, n = 6 per group). Bone area was measured in three images per section. New bone area was calculated by subtracting the original bone area from the total bone and shown as the percentage of the total bone. Blank bar, pups from N-diet-fed dams; striped bar, pups from L-diet-fed dams. Data are presented as means ± SEM. Results for two-way ANOVA within the four groups except for two CS groups are as follows: P = 0.0018 as dam effect, P = 0.2712 as Zn addition effect, and P = 0.6178 as interaction effect between dam and Zn addition
Mineral Status of Dams
Notably, low Zn concentration was seen in the serum for the L-diet-fed dam group. The dams that were fed the L diet exhibited significantly lower Zn content in femurs compared with those that were fed the N diet. In contrast, serum concentration and femur content of phosphorus and calcium did not change between the dietary groups (Table 4).
Serum Parameters for Bone Metabolism in Neonates and Dams
A remarkably lower osteocalcin concentration was seen in dam serum in the L-diet group compared with the N-diet group (Table 5). Osteocalcin concentrations of neonatal serum were much lower than those of dam serum and were significantly lower in neonates fed the L diet than those fed the N diet. IGF-I and corticosterone concentrations were not different in the serum for dam groups or in the pooled serum for their littermates.
Discussion
In the present study, we aimed to elucidate the influence of marginal Zn deficiency during prepregnancy and pregnancy periods on bone status of offspring at birth. To our knowledge, the present rodent model study demonstrated three novel findings: (1) the impairment of neonatal bone matrix formation associated with marginal Zn deficiency during pregnancy, (2) the significant influence of Zn and mineral status on neonatal bone, and (3) the significant reduction of serum osteocalcin concentrations in marginal Zn-deficient dams and their offspring.
In the present study, we set a dietary Zn level at 7 mg/kg as marginally Zn deficient during prepregnancy and pregnancy, compared with 35 mg/kg as control level of dietary Zn in the present study. Human populations are more likely to consume diets that are only marginally deficient in Zn. Additionally, low-Zn diets are started at conception in most animal studies, whereas, in practice, women eating low-Zn diets have probably consumed such a diet prior to conception. Severe depletion of Zn can evoke anorexia in experimental animals [16, 17], causing them to develop marked calorie and protein deficiencies. Anorexia associated with low-Zn diets was not observed in the present study because the Zn concentration of 7 mg/kg, although insufficient, was high enough to prevent anorexia. Therefore, the study of the effects of a moderately low-Zn diet is more relevant for understanding marginal Zn deficiency in human than studies using extremely low Zn-level diets.
We cultured neonatal calvaria to examine the effects of neonatal Zn deficiency on bone matrix formation activity. Calvarial collagen fiber formation, which we designated as the parameter of bone matrix formation, was impaired in the neonates from marginally Zn-deficient dams. The impaired bone matrix formation is probably associated with a reduction of osteoblastic activity because the bone matrix formation process in the calvaria mainly consists of intramembranous ossification by osteoblasts [18]. A previous in vitro study using a primary culture of calvaria showed that Zn significantly induced collagen formation [8]. A chick study also demonstrated that Zn deficiency decreased bone collagen turnover [8]. These findings support the results of our study showing a reduction of neonatal calvaria formation with Zn deficiency in dams. On the other hand, the impairment of bone matrix formation could not be recovered even with the addition of Zn to the culture medium to bring it to the normal Zn concentration present in adult rat blood. This suggests that some critical programming effect on bone matrix formation might be evoked during the period of pregnancy with Zn deficiency. According to a recent report, marginal Zn deficiency during fetal life induced an increase in arterial blood pressure; such prenatal influence could not be totally reversed by postnatal Zn repletion [19]. A long-term study would shed light on the hypothetical programming effect of marginal Zn deficiency during fetal life on bone status in later adult life.
We first demonstrated that femoral phosphorus content was significantly decreased in the offspring of dams fed a marginally Zn-deficient diet, and a similar tendency was observed in femoral calcium content. A previous study in the rhesus monkey [12] revealed the impairment of endochondral ossification in the long bone of offspring with marginal Zn deficiency from conception to the postnatal period. We speculate that bone mineralization in the neonatal femur at birth might be impaired by marginal Zn deficiency of dams during pregnancy. Neonatal long-bone matrix formation is mainly the result of endochondral ossification by chondrocytes [18]. On the contrary, phosphorus and calcium content of calvariae in neonates did not differ between the groups in spite of impaired calvarial bone matrix formation in the neonates from marginally Zn-deficient dams.
These results suggest that bone matrix formation and bone mineralization in long bones might be more susceptible to Zn deficiency compared to calvaria.
Serum osteocalcin concentration, as a bone matrix formation parameter, was evaluated in our study to clarify the bone status of neonates from dams with marginal Zn deficiency. We observed significant reductions of serum osteocalcin concentration in dams and their offspring in the marginally Zn-deficient group. Decrease in serum osteocalcin concentration has been reported in deficient models with less than 1-ppm Zn diet [20]. In the skeletal system, osteocalcin is secreted by osteoblasts and chondrocytes [18]. Taken together, we suggest that neonatal bone matrix formation at birth might be impaired through the compromise of fetal osteoblasts and chondrocytes by prolonged marginal Zn deficiency, including the pregnancy period.
We demonstrated that marginal Zn deficiency during pregnancy resulted in the reduction of Zn content of carcasses and bones in the offspring and in bone Zn content of dams at delivery. The flux of Zn from dams to their rapidly growing fetuses is reported to be sustained at the expense of maternal hepatic pools and other slow turnover tissues, such as bone, when dietary Zn is marginal [23]. According to a previous report using a tracer technique, pregnant rats mobilize Zn from metabolically active pools for transfer to the fetus [22]. Nevertheless, in the neonate, the average ratio of Zn content in the deficient group to that of the control group tended to be lower in bones (70.8% for femur and 71.3% for calvaria) than in carcasses (81.2%). We assume that Zn is preferentially transferred from dam to fetus in tissues with high Zn demand other than bones, which may imply that bones are more susceptible to Zn deficiency than other fetal tissues. Although relatively little information is available on Zn toxicity in human [23], high exposure to Zn and a diet rich in Zn may also adversely affect pregnancy outcome. Therefore, the appropriate intake of Zn is necessary during pregnancy in view of Zn transfer from mother to fetus.
In conclusion, marginal Zn deficiency during pregnancy decreases bone matrix formation activity and bone mineral content in neonates. We strongly propose the appropriate intake of Zn during pregnancy for the proper development and growth of offspring.
References
Prasad AS (1991) Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr 53:403–412
Rossi L, Migliaccio S, Corsi A et al (2001) Reduced growth and skeletal changes in zinc-deficient growing rats are due to impaired growth plate activity and inanition. J Nutr 131:1142–1146
McClung JP, Stahl CH, Marchitelli LJ et al (2006) Effects of dietary phytase on body weight gain, body composition and bone strength in growing rats fed a low-zinc diet. J Nutr Biochem 17:190–196
Diamond I, Hurley LS (1970) Histopathology of zinc-deficient fetal rats. J Nutr 100:325–329
Yamaguchi M, Matsui T (1996) Stimulatory effect of zinc-chelating dipeptide on deoxyribonucleic acid synthesis in osteoblastic MC3T3-E1 cells. Peptides 17:1207–1211
Domenech E, Diaz-Gomez NM, Barroso F et al (2001) Zinc and perinatal growth. Early Hum Dev 65 Suppl:S111–S117
Angus RM, Sambrook PN, Pocock NA et al (1998) Dietary intake and bone mineral density. Bone Miner 4:265–277
Yamaguchi M, Oishi H, Suketa Y (1987) Stimulatory effects of zinc on bone matrix formation in tissue culture. Biochem Pharmacol 36:4007–4012
Hall SL, Dimai HP, Farley JR (1996) Effects of zinc human skeletal alkaline phosphatase activity in vitro. Calcif Tissue Int 64:163–172
Christian P, West KP Jr (1998) Interactions between zinc and vitamin A: an update. Am J Clin Nutr 68 Suppl:435S–441S
Merialdi M, Caufield LE, Zavaleta N et al (2004) Randomized controlled trial of prenatal zinc supplementation and fetal bone growth. Am J Clin Nutr 79:826–830
Leek JC, Vogler JB, Gershwin ME et al (1984) Studies on marginal zinc deprivation in rhesus monkeys. V. Fetal and infant skeletal effects. Am J Clin Nutr 40:1203–1212
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Garrett IR (2003) Assessing bone matrix formation using mouse calvarial organ culture. In: Helfrich MP, Ralston SH (eds) Bone research protocols. Humana, Totowa, pp 183–200
Hapon MB, Simoncini M, Via G et al (2003) Effect of hypothyroidism on hormone profiles in virgin, pregnant and lactating rats, and on lactation. Reproduction 126:371–382
McConnell SD, Henkin RI (1974) Altered preference for sodium chloride, anorexia, and changes in plasma and urinary zinc in rats fed a zinc-deficient diet. J Nutr 104:1108–1114
Shay NF, Mangian HF (2000) Neurobiology of zinc-influenced eating behavior. J Nutr 130:1493S–1499S
Favus MJ, Christakos S (eds) (2003) Primer on the metabolic bone diseases and disorders of mineral metabolism, 5th edn. American Society for Bone and Mineral Research, Washington
Stracher BC, Hill CH, Madaras JG (1980) Effect of zinc deficiency on bone collagenase and collagen turnover. J Nutr 110:2095–2102
Tomat AL, Inserra F, Veiras L et al (2008) Moderate zinc restriction during fetal and postnatal growth of rats: effects on adult arterial blood pressure and kidney. Am J Physiol Regul Integr Comp Physiol 295:R543–R549
Hosea HJ, Taylor CG, Wood T et al (2004) Zinc-deficient rats have more limited bone recovery during repletion than diet-restricted rats. Exp Biol Med Maywood 229:303–311
Lowe NM, Woodhouse LR, Wee J et al (1999) Short-term zinc kinetics in pregnant rats fed marginal zinc diets. J Nutr 129:1020–1025
Fairweather-Tait SJ, Wright AJA, Cooke J et al (1984) Studies of zinc metabolism in pregnant and lactating rats. Br J Nutr 54:401–413
Hotz C, Lowe NM, Araya M, Brown KH (2003) Assessment of the trace element status of individuals and populations: the example of zinc and copper. J Nutr 133:1563S–1568S
Acknowledgments
We thank Dr. K. Nakayama (Kyoto Institute of Nutrition and Pathology Inc.) for helping with the histological technique and M. Yamada (Meiji Dairies Corp.) for giving technical assistance. This research was supported by Meiji Dairies Corporation. M. Nagata, M. Kayanoma, T. Takahashi, T. Kaneko, and H. Hara have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagata, M., Kayanoma, M., Takahashi, T. et al. Marginal Zinc Deficiency in Pregnant Rats Impairs Bone Matrix Formation and Bone Mineralization in Their Neonates. Biol Trace Elem Res 142, 190–199 (2011). https://doi.org/10.1007/s12011-010-8760-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-010-8760-8