Abstract
The effect of zinc sulfate on the mRNA expressions in Runx2, osteocalcin, α1(I) collagen, insulin-like growth factor-I (IGF-I), transforming growth factor-β1 (TGF-β1), osteoprotegerin (OPG), regucalcin, zinc transporter 1 (ZIP1), or glyceroaldehyde-3-phosphate dehydrogenase (G3PDH) in osteoblastic MC3T3-E1 cells in vitro was investigated. Cells with subconfluency were cultured for 48 h in a medium containing either vehicle or zinc sulfate (10−6–10−4 M) without fetal bovine serum. Culture with zinc sulfate (10−5 M) caused a significant increase in Runx2, OPG, or regucalcin mRNA expressions in the cells, while it did not have a significant effect on osteocalcin, α1(I) collagen, IGF-I, TGF-β1, ZIP1, or G3PDH mRNA expressions. The effect of zinc sulfate (10−4 M) in increasing Runx2 mRNA expression was seen at 24–72 h after culture. A significant increase in OPG mRNA expression was observed at 24 or 48 h after culture. Regucalcin mRNA expression was significantly increased at 48 or 72 h after culture with zinc sulfate (10−4 M). The stimulatory effects of zinc sulfate on Runx2, OPG, or regucalcin mRNAs were significantly prevented in the presence of cycloheximide (10−7 M), an inhibitor of protein synthesis, or 5,6-dichloro-1-β-d-ribofuranosyl-benzimidazole (10−6 M), an inhibitor of transcription activity. Culture with β-alanyl-l-histidinato zinc (10−5 M) caused a significant increase in Runx2 or regucalcin mRNA expressions, while zinc acexamate (10−5 M) did not have a significant effect on Runx2, OPG, ZIP1, or regucalcin mRNA expressions. This study demonstrates that zinc sulfate has a role in the enhancement of Runx2, OPG, or regucalcin mRNA expression in osteoblastic cells in vitro, suggesting its role in the regulation of gene expression in the cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is known to be an essential trace element for the growth of human and animals [1, 2]. Zinc deficiency results in a retardation of bone growth [3, 4], indicating that the element is required for the growth, development, and maintenance of healthy bone. The pathophysiologic role of zinc in osteopenia and osteoporosis has also been shown. Bone zinc content is reduced with increasing age [5] and skeletal unloading in rats [6]. Osteoporosis patients have been shown to have lower levels of skeletal zinc than normal individuals [7]. Women with osteoporosis excrete a great amount of zinc in urine [8]. Zinc supplementation has been shown to have a preventive and therapeutic effect on bone loss [9, 10]. Zinc has been shown to have a role as a nutritional and pharmacologic tool in the prevention of osteoporosis with increasing age [11, 12].
Zinc can stimulate osteoblastic bone formation [13–15] and inhibit osteoclastic bone resorption [16–18]. Zinc has been shown to have stimulatory effects on alkaline phosphatase activity [19], protein tyrosine phosphatase activity [20], protein [21], and deoxyribonucleic acid (DNA) syntheses [22] in osteoblastic MC3T3-E1 cells, indicating that the metal has anabolic effects on cellular function in vitro. Zinc has also been shown to inhibit the formation of osteoclastic cells from bone marrow cells [17], and the metal acts on the later stage of differentiation from bone marrow cells to osteoclasts [23]. Zinc can inhibit osteoclastogenesis induced by receptor activator of NF-κB ligand (RANKL), which plays a pivotal role in differentiation from pre-osteoclast to osteoclasts in bone marrow culture systems [24, 25]. Thus zinc may play a role in the preservation of bone mass due to stimulating bone formation and inhibiting bone resorption [11].
The cellular mechanism of zinc action in osteoblastic cells has not been fully clarified. Zinc has been demonstrated to stimulate proliferation and differentiation in osteoblastic cells [26, 27]. Zinc has stimulatory effects on protein synthesis in osteoblastic cells due to activating aminoacyl-tRNA synthetase, which is a rate-limiting enzyme at transcriptional process [21, 28], and the metal can increase the production of insulin-like growth factor-I (IGF-I), transforming growth factor-β1 (TGF-β1), or osteocalcin in osteoblastic cells, and other protein components [14].
The effect of zinc on gene expression in osteoblastic cells has not been fully determined, however. This study was undertaken to determine whether zinc has a regulatory effect on mRNA expression of various proteins that are expressed in osteoblastic cells in vitro. We paid attention to the gene expressions of Runx2, a stimulator factor in differentiation of osteoblastic cells; osteocalcin and α1(I) collagen, a matrix protein in bone; osteoprotegerin (OPG), a suppressor of osteoclastogenesis; IGF-I and TGF-β1, growth factors of osteoblastic cells; ZIP1, a zinc transporter; or regucalcin, a stimulator of osteoclastogenesis and suppressor of osteoblastic bone mineralization in the cells.
We found that culture with zinc has stimulatory effects on the mRNA expressions of Runx2, OPG, or regucalcin expressions in osteoblastic MC3T3-E1 cells in vitro. Zinc may have a role in the regulation of gene expression in osteoblastic cells.
Materials and methods
Chemicals
α-Minimal essential medium (α-MEM) and penicillin-streptomycin (5,000 U/ml penicillin; 5,000 μg/ml streptomycin) were obtained from Gibco Laboratories. Fetal bovine serum (FBS) was obtained from Bioproducts, Inc. 5,6-Dichloro-1-β-d-ribofuranosyl-benzimidazole (DRB), cycloheximide were purchased from Sigma Chemicals (St. Louis, MO). β-Alanyl-l-histidinato zinc (AHZ) was supplied from Zeria Pharmaceutical Co., Ltd. (Tokyo, Japan). Zinc acexamate (ZA) was obtained from Nipro Corporation (Kusatsu, Japan). Zinc sulfate and other chemicals were of reagent grade and were obtained Wako Pure Chemical Industries (Osaka, Japan). All water used were glass distilled.
Cell culture
Osteoblastic MC3T3-E1 cells were cultured at 37°C in a CO2 incubator in plastic dishes containing α-MEM supplemented with 10% FBS. They were subcultured every 3 days using 0.2% trypsin plus 0.02% EDTA in Ca2+/Mg2+-free phosphate-buffered saline (PBS). For experiments, about 1.0 × 105 cells per dish were cultured for 72 h to obtain subconfluent monolayers in 35-mm plastic containing 2-ml α-MEM with 10% FBS. After the cells were rinsed with PBS, the medium was exchanged for medium without FBS containing either vehicle or zinc sulfate (10−6–10−4 M), and the cells were cultured further for 24–72 h. Cell viability was estimated by staining with trypan blue. Experiments were repeated using a separate batch of cells with four dishes to ensure reproducibility of results.
Cell counting
After trypsinization of each of the culture dishes using 0.2% trypsin plus 0.02% EDTA in Ca2+/Mg2+-free PBS for 2 min at 37°C, cells were collected and centrifuged in a PBS solution at 100g for 5 min. The cells were resuspended on PBS solution and stained with eosin. Cell numbers were counted under a microscope using a Hemacytometer plate. For each dish, we took the average of two countings.
Preparation of RNA
Total RNAs were prepared as described previously [29]. Osteoblastic MC3T3-E1 cells with subconfluency were cultured for 24, 48, or 72 h in a medium containing either vehicle or zinc sulfate (10−6–10−4 M). After culture, cells were washed three times ice-cold PBS, and then cells were homogenized in buffer solution containing 4 M guanidinium thiocyanate, 24 mM sodium citrate (pH 7.0), 0.5% sarcosyl, and isoamyl alcohol, and the phases were separated by centrifugation at 10,000g for 20 min at 4°C. RNA located in the aqueous phase was precipitated with isopropanol at −20°C. RNA precipitates were pelleted by centrifugation, and the pellets were redissolved in diethyl-pyrocarbonate-treated water.
RT-PCR analysis
Primers for amplification of mouse Runx2 cDNA were: 5′-GTATGAGAGTAGGTGTCCCG-3′ (sense strand, positions 992–1,011 of cDNA sequence) and 5′-ACATCCCATCCATCCACTC-3′ (antisense strand, positions 1,156–1,175) [30]. The pair of oligonucleotide primers was designed to amplify a 1,834-bp sequence from the mRNA of mouse Runx2.
Primers for amplification of mouse osteocalcin cDNA were: 5′-GGGGAAGGGACAACACATGA-3′ (sense strand, positions 188–207 of cDNA sequence) and 5′-TCCTGGACATGGGGATTGA-3′ (antisense strand, positions 580–599) [31]. The pair of oligonucleotide primers was designed to amplify a 412-bp sequence from the mRNA of osteocalcin.
Primers for amplification of mouse α1(I) collagen cDNA were: 5′-TTCTCCTGGTAAAGATGGTGC-3′ (sense strand, positions 2,232–2,252 of cDNA sequence) and 5′-GGACCAGCATCACCTTTAACA-3′ (antisense strand, positions 2,466–2,486) [32]. The pair of oligonucleotide primers was designed to amplify a 254-bp sequence from the mRNA of mouse α1(I) collagen.
Primers for amplification of mouse IGF-I cDNA were: 5′-GCAAGCTTCAGCCACCTTAC-3′ (sense strand, positions 955–974 of cDNA sequence) and 5′-GGGTCGTTTACACAGCAGGT-3′ (antisense strand, positions 1,466–1,485) [33]. The pair of oligonucleotide primers was designed to amplify a 531-bp sequence from the mRNA of mouse IGF-I.
Primers for amplification of mouse TGF-β1 cDNA were: 5′-CTCTCCACCTGCAAGACCAT-3′ (sense strand, positions 633–652 of cDNA sequence) and 5′-CTGCCGTACAACTCCAGTGA-3′ (antisense strand, positions 1,312–1,331) [34]. The pair of oligonucleotide primers was designed to amplify a 699-bp sequence from the mRNA of mouse TGF-β1.
Primers for amplification of decoy receptor OPG were: 5′-CGTTACCTGGAGAT-3′ (sense strand, positions 421–435 of cDNA sequence) and 5′-GTTCCTACCAAGATT-3′ (antisense strand, positions 721–725) [35]. The pair of oligonucleotide primers was designed to amplify a 305-bp sequence from the mRNA of mouse OPG.
Primers for amplification of ZIP1 cDNA were: 5′-GACGTGGTCAGGGACATTAG-3′ (sense strand, positions 1,270–1,289 of cDNA sequence) and 5′-AAAGGTGAGGACAGGAGAGG-3′ (antisense strand, positions 1,582–1,600) [36]. The pair of oligonucleotide primers was designed to amplify a 331-bp sequence from the mRNA of mouse ZIP1.
Primers for amplification of regucalcin cDNA were: 5′-AGATGAACAAATCCCAGAT-3′ (sense strand, positions 618–636 of cDNA sequence) and 5′-TCACCCTGCATAGGAATAT-3′ (antisense strand, positions 906–924) [37]. The pair of oligonucleotide primers was designed to amplify a 307-bp sequence from the mRNA of mouse regucalcin.
Glyceroaldehyde-3-phosphate dehydrogenase (G3PDH) was used as an internal control to evaluate total RNA input. Primers for amplification of G3PDH cDNA were 5′-GATTTGGCCGTATCGGACGC-3′ (sense strand) and 5′-CTCCTTGGAGGCCATGTAGG-3′ (antisense strand). The pair of oligonucleotide primers was designed to amplify a 977-bp sequence from the mRNA of rat G3PDH.
RT-PCR was performed using reaction mixture (20 μl) containing 2 or 4 μg of total RNAs, supplied RT-PCR buffer, Titan™ enzyme mix (AMV and Expand™ High Fidelity), 0.2 mM dNTP, 5 mM dithiothreitol, 5 U RNase inhibitor, and 0.3 μM primers. Samples were incubated at 50°C for 30 min, and then amplified for 30 cycles under the following conditions: denaturation for 30 s at 94°C, annealing for 30 s at 56°C, and extension for 60 s at 68°C. The conditions for all genes used in this experiment of the RT-PCR reaction showed the linearity. The amplified products were separated by electrophoresis on a 1.5% agarose gel and visualized by ethidium bromide staining. Image density was quantified with a FluoroImager SI (Amersham Pharmacia Biotech).
Statistical analysis
Data are expressed as the mean ± SEM. Statistical differences were analyzed using Student’s t-test. P values less than 0.05 were considered to indicate statistically significant differences. Also, we used an ANOVA multiple comparison test to compare the treatment groups.
Results
Effect of zinc sulfate on gene expression in osteoblastic cells
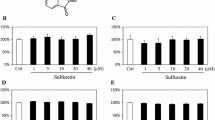
Osteoblastic MC3T3 cells with subconfluency were cultured for 48 h in a medium containing either vehicle or zinc sulfate (10−6–10−4 M), and the expression of various genes were examined (Fig. 1). Culture with zinc sulfate caused a significant increase in the mRNA expression of Runx2 (Fig. 1a), OPG (Fig. 2a), or regucalcin (Fig. 2c). Runx2 mRNA expression was significantly increased 25 or 45% after culture with 10−5 or 10−4 M zinc sulfate, respectively. OPG mRNA expression was significantly increased 25 or 30% after culture with 10−6 or 10−5 M zinc sulfate, respectively. Culture with zinc sulfate of 10−6–10−4 M caused a significant increase in about 30 to 50% of regucalcin mRNA expression. Meanwhile, culture with zinc sulfate (10−6–10−4 M) did not cause a significant increase in mRNA expression of osteocalcin (Fig. 1b), α1(I) collagen (Fig. 1c), IGF-I (Fig. 1d), TGF-β1 (Fig. 1e), G3PDH (Fig. 1f), or ZIP1 (Fig. 2b).
Effect of zinc sulfate on the expression of Runx2, osteocalcin, α1(I) collagen, IGF-I, TGF-β1, or G3PDH mRNAs in osteoblastic MC3T3-E1 cells. Osteoblastic cells were cultured for 72 h in a medium containing 10% FBS. Cells with subconfluency were changed to a medium without FBS in the presence or absence of zinc sulfate (10−6–10−4 M). After medium change, cells were cultured for 48 h. Total RNAs (2 or 4 μg) extracted from the cells were analyzed using RT-PCR with specific primers. The figure shows one of four experiments with separate culture. The densitometric data for each mRNA level in the cells cultured for 48 h in the presence of zinc sulfate were indicated as % of control (mean ± SEM of four experiments). * P < 0.025 compared with the control (none) value (Student’s t-test)
Effect of zinc sulfate on the expression of OPG, ZIP1, or regucalcin mRNAs in osteoblastic MC3T3-E1 cells. Osteoblastic cells were cultured for 72 h in a medium containing 10% FBS. Cells with subconfluency were changed to a medium without FBS in the presence or absence of zinc sulfate (10−6–10−4 M). After medium change, cells were cultured for 48 h. Total RNAs (2 or 4 μg) extracted from the cells were analyzed using RT-PCR with specific primers. The figure shows one of four experiments with separate culture. The densitometric data for each mRNA level in the cells cultured for 48 h in the presence of zinc sulfate were indicated as % of control (mean ± SEM of four experiments). * P < 0.025 or ** P < 0.01 compared with the control (none) value (Student’s t-test)
The time course of zinc sulfate effect on the mRNA expression of Runx2, OPG, ZIP1, regucalcin, or G3PDH in osteoblastic MC3T3-E1 cells with subconfluency was examined (Figs. 3, 4). Runx2 mRNA expression was significantly increased at 24, 48, or 72 h after culture with zinc sulfate (10−4 M) (Fig. 3a). OPG mRNA expression was significantly increased at 24 or 48 h after culture with 10−5 M zinc sulfate (Fig. 3b). A significant increase in regucalcin mRNA expression was seen at 48 or 72 h after culture with 10−4 M zinc sulfate (Fig. 4a). ZIP1 (Fig. 3c) or G3PDH (Fig. 4b) mRNA expressions were not significantly changed after culture with zinc sulfate (10−5 or 10−4 M) for 24, 48, or 72 h.
Effect of zinc sulfate on the expression of Runx2, OPG, or ZIP1 mRNAs in osteoblastic MC3T3-E1 cells. Osteoblastic cells were cultured for 72 h in a medium containing 10% FBS. Cells with subconfluency were changed to a medium without FBS in the presence or absence of zinc sulfate (10−5 or 10−4 M). After medium change, cells were cultured for 24, 48, or 72 h. Total RNAs (2 μg) extracted from the cells were analyzed using RT-PCR with specific primers. The figure shows one of four experiments with separate culture. The densitometric data for each mRNA level in the cells cultured for 48 h in the presence of zinc sulfate were indicated as % of control (mean ± SEM of four experiments). * P < 0.025 or ** P < 0.01 compared with the control (none) value (Student’s t-test)
Effect of zinc sulfate on the expression of regucalcin or G3PDH mRNAs in osteoblastic MC3T3-E1 cells. Osteoblastic cells were cultured for 72 h in a medium containing 10% FBS. Cells with subconfluency were changed to a medium without FBS in the presence or absence of zinc sulfate (10−5 or 10−4 M). After medium change, cells were cultured for 24, 48, or 72 h. Total RNAs (2 μg) extracted from the cells were analyzed using RT-PCR with specific primers. The figure shows one of four experiments with separate culture. The densitometric data for each mRNA level in the cells cultured for 48 h in the presence of zinc sulfate were indicated as % of control (mean ± SEM of four experiments). * P < 0.025 compared with the control (none) value (Student’s t-test)
Effect of cycloheximide or DRB on zinc sulfate-increased gene expression in osteoblastic cells
The effect of cycloheximide, an inhibitor of protein synthesis, or DRB, an inhibitor of transcription activity, on the zinc sulfate-increased mRNA expression of Runx2, OPG, or regucalcin was examined in osteoblastic cells with subconfluency cultured with zinc sulfate (10−5 M) for 48 h. The effect of zinc sulfate (10−5 M) in increasing Runx2 (Fig. 5a), OPG (Fig. 5b), or regucalcin (Fig. 6a) mRNA expressions was significantly inhibited in the presence of cycloheximide (10−7 M) or DRB (10−6 M).
Effect of cycloheximide or DRB on the zinc sulfate-induced increase in Runx2 or OPG mRNA expressions in osteoblastic MC3T3-E1 cells. Osteoblastic cells were cultured for 72 h in a medium containing 10% FBS. Cells with subconfluency were changed to a medium without FBS in the presence or absence of zinc sulfate (10−5 M) with or without cycloheximide (10−7 M) or DRB (10−6 M). After medium change, cells were cultured for 48 h. Total RNAs (2 μg) extracted from the cells were analyzed using RT-PCR with specific primers. The figure shows one of four experiments with separate culture. The densitometric data for each mRNA level in the cells cultured for 48 h in the presence of zinc sulfate were indicated as % of control (mean ± SEM of four experiments). * P < 0.025 or ** P < 0.01 compared with the control value without zinc addition (Student’s t-test or ANOVA). White bars, without zinc; black bars, with zinc
Effect of cycloheximide or DRB on the zinc sulfate-induced increase in regucalcin or G3PDH mRNA expressions in osteoblastic MC3T3-E1 cells. Osteoblastic cells were cultured as described in the legend of Fig. 5. Total RNAs (2 μg) extracted from the cells were analyzed using RT-PCR with specific primers. The figure shows one of four experiments with separate culture. The densitometric data for each mRNA level in the cells cultured for 48 h in the presence of zinc sulfate were indicated as % of control (mean ± SEM of four experiments). * P < 0.025 compared with the control value without zinc addition (Student’s t-test or ANOVA). White bars, without zinc; black bars, with zinc
Effect of various zinc compounds on gene expression in osteoblastic cells
The effect of zinc sulfate, AHZ, or ZA on the mRNA expression of Runx2, OPG, ZIP1, regucalcin, or G3PDH in osteoblastic MC3T3-E1 cells with subconfluency cultured with each zinc compound (10−5 M) for 48 h was compared (Fig. 7). Runx2 mRNA expression was significantly increased after culture with zinc sulfate or AHZ. OPG mRNA expression was significantly increased with zinc sulfate. Regucalcin mRNA expression was significantly increased with zinc sulfate or AHZ. Culture with zinc sulfate, AHZ, or ZA did not cause a significant change in ZIP1 or G3PDH mRNA expressions. The effect of zinc sulfate on the mRNA expression of Runx2, OPG, ZIP1, or regucalcin in osteoblastic MC3T3-E1 cells was potent in among zinc compounds used.
Effect of various zinc compounds on the expression of Runx2, OPG, ZIP1, regucalcin, or G3PDH mRNAs in osteoblastic MC3T3-E1 cells. Osteoblastic cells with subconfluency were cultured for 48 h in a medium containing either vehicle, zinc sulfate (10−5 M), β-alanyl-l-histidinato zinc (AHZ; 10−5 M), or zinc acexamate (ZA; 10−4 M) without FBS. Total RNAs (2 μg) extracted from the cells were analyzed using RT-PCR with specific primers. The figure shows one of four experiments with separate culture. The densitometric data for each mRNA level in the cells cultured for 48 h in the presence of zinc compound were indicated as % of control (mean ± SEM of four experiments). * P < 0.025 compared with the control (none) value (Student’s t-test)
Discussion
Zinc has been shown to have anabolic effects on osteoblastic MC3T3-E1 cells [14, 19–22, 26, 27]. Zinc can stimulate protein [14, 21] and DNA [22] syntheses in osteoblastic cells. The effect of zinc on gene expression in osteoblastic cells has not been fully clarified, however. This study was undertaken to determine the effect of zinc on the mRNA expressions of Runx2, osteocalcin, α1(I) collagen, OPG, IGF-I, TGF-β1, ZIP1, regucalcin, or G3PDH using RT-PCR. We found that culture with zinc sulfate caused a significant increase in Runx2, OPG, or regucalcin mRNA expressions in the cells, although it did not cause a significant increase in the mRNA expressions of osteocalcin, α1(I) collagen, IGF-I, TGF-β1, or ZIP1.
Runx2 is a member of the runt domain family of transcription factors, and it is involved in bone development and osteoblastic cell differentiation [38]. OPG involves in the inhibition of RANKL action that stimulates osteoclastogenesis [39]. Zinc has a role in the enhancement of these gene expressions in osteoblastic cells.
Zinc can stimulate differentiation in osteoblastic cells [27], and the metal has an inhibitory effect on RANKL-induced osteoclastogenesis in mouse marrow culture [25]. These effects of zinc may be partly mediated through the production of Runx2 or OPG in osteoblastic cells.
Regucalcin is a regulatory protein in intracellular signaling [40]. Regucalcin has been shown to suppress osteoblastic mineralization [41] and to stimulate osteoclastogenesis [42], thereby decreasing bone mass in regucalcin transgenic rats [43]. The physiologic significance which zinc stimulates regucalcin mRNA expression is unknown. However, it is possible that zinc has a role in the regulation of osteoblastic mineralization and osteoclastogenesis which is mediated through regucalcin expression in osteoblastic cells.
Culture with zinc stimulates protein production of IGF-I, TGF-β1, or osteocalcin in osteoblastic MC3T3-E1 cells using ELISA assay [14]. However, zinc did not enhance mRNA expressions of IGF-I, TGF-β1, or osteocalcin in osteoblastic MC3T3-E1 cells using RT-PCR analysis. Zinc has been shown to activate aminoacyl-tRNA synthetase, which is a rate-limiting enzyme in translational process of protein synthesis, in osteoblastic cells, and the metal could induce protein synthesis [21, 26]. Presumably, the effect of zinc in increasing IGF-I, TGF-β1, or osteocalcin in osteoblastic cells is mainly resulted from the stimulation of protein synthesis that acts on translational process. However, it cannot exclude the possibility that zinc has a partially stimulatory effect on transcriptional process in osteoblastic cells.
Culture with zinc sulfate, AHZ, or ZA, which are zinc compounds, increases bone components in rat femoral tissues in vitro [13, 44, 45]. Among these compounds, culture with zinc sulfate had a potent effect on the mRNA expressions of Runx2, OPG, or regucalcin in osteoblastic cells. However, AHZ has been shown to have potent effects on protein [14, 21] and DNA syntheses [22] in osteoblastic cells as compared with zinc sulfate. The effect of zinc in stimulating gene expression of transcriptional process may differ with chemical form of zinc compounds in osteoblastic cells. It is speculated that zinc ion in zinc sulfate is potentially translocated to the nucleus in osteoblastic cells and that the metal ion can bind to transcriptional proteins and/or DNA in the cells as compared with that of AHZ or ZA.
The effect of zinc sulfate in increasing the expression of Runx2, OPG, or regucalcin mRNAs in osteoblastic cells was completely prevented in the presence of cycloheximide, an inhibitor of protein synthesis, or DRB, an inhibitor of transcription activity. This result suggests that the zinc-increased gene expression was resulted from newly synthesized protein components in osteoblastic cells. Zinc is known to stimulate protein synthesis in osteoblastic cells in vitro [14, 21, 27]. It is speculated that zinc ion stimulates protein synthesis including transcription factors (proteins), that the synthesized proteins enhance gene expression in osteoblastic cells, and that zinc directly acts on gene expression due to activating transcription factors in the cells.
In conclusion, it has been demonstrated that culture with zinc sulfate has stimulatory effects on the mRNA expressions of Runx2, OPG, or regucalcin in osteoblastic MC3T3-E1 cells in vitro. Zinc may have a role in the regulation of gene expression in osteoblastic cells.
References
Prasad AS, Halsted JA, Nadimi M (1961) Syndrome of iron deficiency anemia, hepatosplenomegaly, hypoganadism, dwarfism and geophagia. Am J Med 31:532–546
Burt RE, Khan HK, Sullivan JF (1975) Newer aspects of the roles of zinc, manganese, and copper in human nutrition. Clin Chem 21:501–520
Hsieh HS, Navia JM (1980) Zinc deficiency and bone formation in guinea pig alveolar implants. J Nutr 110:1582–1588
Oner G, Bhaumick B, Bala RM (1984) Effect of zinc deficiency on serum somatomedin levels and skeletal growth in young rats. Endocrinology 114:1860–1863
Yamaguchi M, Ozaki K (1990) Aging affects cellular zinc and protein synthesis in the femoral diaphysis of rats. Res Exp Med 190:295–300
Yamaguchi M, Ehara Y (1995) Zinc decrease and bone metabolism in femoral-metaphyseal tissues of rats with skeletal unloading. Calcif Tissue Int 57:218–223
Reginster JY, Strause LG, Saltman P, Franchimont P (1998) Trace elements and postmenopausal osteoporosis. A preliminary study of decreased serum manganese. Med Sci Res 16:337–338
Herzberg M, Foldes J, Steinberg R, Menczel J (1990) Zinc excretion in osteoporotic woman. J Bone Miner Res 5:251–257
Kishi S, Segawa Y, Yamaguchi M (1994) Histomorphological confirmation of the preventive effect of β-alanyl-l-histidinato zinc on bone loss in ovariectomized rats. Biol Pharm Bull 17:862–865
Higashi A, Nakamura T, Nishiyama S, Matsukura M, Tomoeda S, Futagoshi Y, Shinohara M, Matsuda I (1993) Zinc kinetics in patients with bone demineralization due to physical immobilization. J Am Coll Nutr 12:61–65
Yamaguchi M (1998) Role of zinc in bone formation and bone resorption. J Trace Elem Exp Med 11:119–135
Yamaguchi M, Uchiyama S (2003) Preventive effect of zinc acexamate administration in streptozotocin-diabetic rats: restoration of bone loss. Int J Mol Med 12:755–761
Yamaguchi M, Oishi H, Suketa Y (1987) Stimulatory effect of zinc on bone formation in tissue culture. Biochem Pharmacol 36:4007–4012
Yamaguchi M, Hashizume M (1994) Effect of β-alanyl-l-histidinato zinc on protein components in osteoblastic MC3T3-E1 cells: increase in osteocalcin, insulin-like growth factor-land transforming growth factor-β. Mol Cell Biochem 136:163–169
Lutz W, Burritt MF, Nixon DE, Kao PC, Kumar R (2000) Zinc increases the activity of vitamin D-dependent promoters in osteoblasts. Biochem Biophys Res Commun 271:1–7
Yamaguchi M, Segawa Y, Shimokawa N, Tsuzuike N, Tagashira E (1992) Inhibitory effect of β-alanyl-l-histidinato zinc on bone resorption in tissue culture. Pharmacology 45:292–300
Kishi S, Yamaguchi M (1994) Inhibitory effect of zinc compounds on osteoclast-like cell formation in mouse marrow cultures. Biochem Pharmacol 48:1225–1230
Holloway WR, Collier FM, Herbst RE, Modge JM, Nicholson GC (1996) Osteoblast-mediated effects of zinc on isolated rat osteoclast: inhibition of bone resorption and enhancement of osteoclast number. Bone 19:137–142
Yamaguchi M, Ohtaki J (1991) Effect of beta-alanyl-l-histidinato zinc on osteoblastic MC3T3-E1 cells: Increase in alkaline phosphatase and proliferation. Pharmacology 43:225–232
Yamaguchi M, Fukagawa M (2005) Role of zinc in regulation of protein tyrosine phosphatase activity in osteoblastic MC3T3-E1 cells: Zinc modulation of insulin-like growth factor-1’s effect. Calcif Tissue Int 76:32–38
Yamaguchi M, Kishi S, Hashizume M (1994) Effect of zinc-chelating dipeptides on osteoblastic MC3T3-E1 cells: activation of aminoacyl-tRNA synthetase. Peptides 15:1367–1371
Yamaguchi M, Matsui T (1996) Stimulatory effect of zinc-chelating dipeptides on deoxyribonucleic acid synthesis in osteoblastic MC3T3-E1 cells. Peptides 17:1207–1211
Yamaguchi M, Kishi S (1996) Zinc compounds inhibit osteoclast-like cell formation at the earlier stage of rat marrow culture but not osteoclast formation. Mol Cell Biochem 158:171–177
Zaidi M, Blair HC, Moonga BS, Abe E, Huang CL-H (2003) Osteoclastogenesis, bone resorption, and osteoclast-based therapeutics. J Bone Miner Res 18:599–609
Yamaguchi M, Uchiyama S (2004) Receptor activator of NF-κB ligand-stimulated osteoclastogenesis in mouse marrow culture is suppressed by zinc in vitro. Int J Mol Med 14:81–85
Hashizume M, Yamaguchi M (1993) Stimulatory effect of β-alanyl-l-histidinato zinc on cell proliferation is dependent on protein synthesis in osteoblastic MC3T3-E1 cells. Mol Cell Biochem 122:59–64
Hashizume M, Yamaguchi M (1994) Effect of β-alanyl-l-histidinate zinc on differentiation of osteoblastic MC3T3-E1 cells: increases in alkaline phosphatase activity and protein concentration. Mol Cell Biochem 131:19–24
Yamaguchi M, Oishi H, Suketa Y (1988) Zinc stimulation of bone protein synthesis in tissue culture. Activation of aminoacyl-tRNA synthetase. Biochem Pharmacol 37:4075–4080
Chomczyshi P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Sohn KY, Maity SN, de Crombrugghe B (1994) Studies on the structure of CBF-A isoform generated from an alternatively spliced RNA. Gene 139:147–153
Desbois C, Hogue DA, Karsenty G (1994) The mouse osteocalcin gene culture contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem 269:1183–1190
Luppen CA, Smith E, Spevak L, Boskey AL, Frenkel B (2003) Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J Bone Miner Res 18:1186–1197
Bell GI, Stempien MM, Fong NW, Roll LB (1986) Sequences of liver cDNAs encoding two different mouse insulin-like growth factor I precursors. Nucleic Acids Res 14:7873–7882
Deryck R, Jarrett JA, Chen EY, Goeddel DV (1986) The murine transforming growth factor-beta precursor. J Biol Chem 261:4377–4379
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Lioumi M, Ferguson CA, Sharpe PT, Freeman T, Marenholz I, Mischke D, Heizmann C, Ragoussis J (1999) Isolation and characterization of human and mouse ZIRTL, a member of the IRT1 family of transporters, mapping within the epidermal differentiation complex. Genomics 62:272–8037
Misawa H, Yamaguchi M (2000) The gene of Ca2+-binding protein regucalcin is highly conserved in vertebrate species. Int J Mol Med 6:191–196
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764
Tanaka S, Nakajima I, Inoue J-I, Oda H, Nakamura K (2003) Signal transduction pathways regulating osteoclast differentiation and function. J Biol Miner Metab 21:123–133
Yamaguchi M (2005) Role of regucalcin in maintaining cell homeostasis and function. Int J Mol Med 15:372–389
Yamaguchi M, Kobayashi M, Uchiyama S (2005) Suppressive effect of regucalcin on cell differentiation and mineralization in osteoblastic MC3T3-E1 cells. J Cell Biochem 96:543–554
Yamaguchi M, Uchiyama S (2005) Regucalcin stimulates osteoclast-like cell formation in mouse marrow cultures. J Cell Biochem 94:794–803
Yamaguchi M, Misawa H, Uchiyama S, Morooka Y, Tsurusaki Y (2002) Role of endogenous regucalcin in bone metabolism: Bone loss is induced in regucalcin transgenic rats. Int J Mol Med 10:377–383
Yamaguchi M, Kishi S (1994) Effect of zinc-chelating dipeptides on bone metabolism in weanling rats: comparison with β-alanyl-l-histidinato zinc-related compounds. Peptides 15:671–673
Yamaguchi M, Gao YH (1998) Potent effect of zinc acexamate on bone components in the femoral-metaphyseal tissues of elderly female rats. Gen Pharmacol 30:423–427
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, M., Goto, M., Uchiyama, S. et al. Effect of zinc on gene expression in osteoblastic MC3T3-E1 cells: enhancement of Runx2, OPG, and regucalcin mRNA expressions. Mol Cell Biochem 312, 157–166 (2008). https://doi.org/10.1007/s11010-008-9731-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9731-7