Abstract
Purpose of Review
Management of chronic daily headaches (CDH) remains challenging due to the limited efficacy of standard prophylactic pharmacological measures. Several studies have reported that repetitive transcranial magnetic stimulation (rTMS) can effectively treat chronic headaches. The objective was to determine the utility of rTMS for immediate post-treatment and sustained CDH prophylaxis.
Recent Findings
All procedures were conducted per PRISMA guidelines. PubMed, Scopus, Web of Science, and ProQuest databases were searched for controlled clinical trials that have tested the efficacy of rTMS on populations with CDH. DerSimonian-Laird random-effects meta-analyses were performed using the ‘meta’ package in R to examine the post- vs. pre-rTMS changes in standardized headache intensity and frequency compared to sham-control conditions. Thirteen trials were included with a combined study population of N = 538 patients with CDH (rTMS, N = 284; Sham, N = 254). Patients exposed to rTMS had significantly reduced standardized CDH intensity and frequency in the immediate post-treatment period (Hedges’ g = -1.16 [-1.89, -0.43], p = 0.002 and Δ = -5.07 [-10.05, -0.11], p = 0.045 respectively). However, these effects were sustained marginally in the follow-up period (Hedges’ g = -0.43 [-0.76, -0.09], p = 0.012 and Δ = -3.33 [-5.52, -1.14], p = 0.003). Significant between-study heterogeneity was observed, at least partially driven by variations in rTMS protocols.
Summary
Despite the observed clinically meaningful and statistically significant benefits in the immediate post-treatment period, the prophylactic effects of rTMS on CDH do not seem to sustain with discontinuation. Thus, the cost-effectiveness of the routine use of rTMS for CDH prophylaxis remains questionable.
Registration
Protocol preregistered in PROSPERO International Prospective Register of Systematic Reviews (CRD42021250100)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic daily headaches (CDH) are a disabling condition affecting approximately 4–5% of the global population, significantly affecting quality-of-life [1,2,3]. Resistance of CDH to standard therapies results in substantial social and financial burdens [1, 4, 5]. For instance, chronic migraine (CM) patients incur higher healthcare costs than patients with episodic headaches due to more consultations, psychiatric interventions, hospitalizations, and medication costs [5]. Furthermore, 90% of patients with CDH have psychiatric comorbidities, such as depression, anxiety, or disordered sleep [2, 4], which worsen outcomes [6]. CDH, by definition, lasts ≥ 15 days/month for at least three months, for ≥ 4 h a day when untreated, which was initially defined by Silberstein and Lipton in 1996 [7]. However, later on, CDH became more of a category of headaches rather than a diagnosis, and the International Classification of Headache Disorders, 3rd edition (ICHD-3) does not define CDH [4, 8]. Chronic tension-type headaches (CTTH) and chronic migraine (CM) comprise most cases of primary CDH and frequently evolve from episodic headaches [1, 4]. Less common types include hemicrania continua and new daily persistent headaches [1]. Secondary CDH may be post-traumatic brain injury (TBI) or medication-induced, and these causes must be identified before diagnosing and treating primary CDH [9].
CDH is often managed prophylactically using antidepressants, anticonvulsants, and antihypertensives [1]. While tricyclic antidepressants (TCA) have shown the greatest potential for reducing headache symptoms, they are poorly tolerated (> 30% experience side effects) [10]. Whereas selective serotonin and norepinephrine-reuptake inhibitors may be more tolerable, evidence regarding efficacy is inconsistent [10]. Similarly, the effectiveness of anticonvulsants is not entirely clear, except for topiramate [11, 12]. Recently, botulinum toxin A (BTX-A) and monoclonal calcitonin gene-related peptide (CGRP) antibodies have shown promise, as these agents directly target the pathological processes of CDH [1, 9, 13, 14]. BTX-A is deemed safe and effective in preventing CM; however, patient response varies, and sustained effects are not consistently observed in patients with severe, refractory CDH [15]. Furthermore, few studies have documented CGRP antibody use in CDH [13, 14], particularly for CM [16]. Moreover, abortive drugs like non-steroidal anti-inflammatory drugs can induce medication overuse headaches, complicating management [1]. Collectively, pharmacologic treatments have limited efficacy—improving headache duration and frequency in only 10% of CDH patients—and are associated with adverse effects that limit compliance by 25% [17]. Thus, a lack of reliable abortive and prophylactic treatments necessitates establishing clinically effective, evidence-based solutions for CDH.
Repetitive transcranial magnetic stimulation (rTMS) temporally alters cortical neuron excitability through non-invasive neurostimulation [18•]. rTMS has utility in treating psychiatric, movement, and chronic pain disorders and has recently gained attention as a potential treatment for headaches [19,20,21]. High-frequency rTMS (e.g., 10–20 Hz) results in excitatory effects, which increase cortical excitability and neuronal firing, while low-frequency rTMS (e.g., < 1 Hz) is generally inhibitory, suppressing cortical excitability and neuronal activity in target brain regions [22, 23]. Furthermore, the effects of rTMS extend beyond the target area to interconnected brain networks, altering functional connectivity and neuroplasticity within and between networks [21, 24].
The mechanism underlying rTMS for reducing CDH remains unclear. rTMS can influence cortical areas involved in pain processing by modulating cortical excitability [23, 25, 26]. Additionally, rTMS may modulate pain pathways, including descending inhibitory pathways, which influence supraspinal pain tracts and social-affective regions of the brain, like the right temporal lobe [27, 28]. Particularly, rTMS demonstrated analgesic effects in migraine and tension headaches by modulating the dorsolateral prefrontal cortex (dlPFC), which regulates pain perception and pain-related emotional/cognitive processes [18•, 25, 26, 29,30,31]. Moreover, rTMS may induce analgesia by increasing endogenous opioids such as β endorphin in the anterior cingulate cortex, hypothalamus, and peri-aqueductal gray matter [32, 33], dopamine in the hippocampus and caudate nucleus [34, 35] and glutamate levels in the neocortex [36]. Finally, rTMS may promote neurogenesis and modulate synaptic plasticity in cortex and diencephalon [37]. These neuroplastic changes may underlie the therapeutic effects of rTMS on CDH.
Several studies have compared CDH patients receiving high-frequency rTMS in multiple sessions for several weeks with placebo controls [18•, 29, 38, 39]. Here, we aimed to conduct a systematic review and meta-analysis of the literature to examine the effectiveness of rTMS in managing CDH, with a focus on its impact on headache intensity and frequency, its effects on CDH subtypes, and factors influencing its efficacy through subgroup and meta-regression analyses. We specifically hypothesized that exposure to regular treatment with rTMS will not only decrease the intensity and frequency of acute headache episodes in the immediate post-intervention period but will also elicit a sustained prophylactic effect among patients with CDH.
Methods
All procedures followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [40]. The study protocol and analysis plan were pre-registered in the PROSPERO registry (CRD42021250100).
Search Strategy
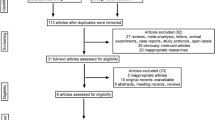
PubMed, Scopus, ProQuest, and Web-of-Science databases were searched on June 6, 2021, for peer-reviewed interventional studies using pre-defined keyword combinations (Table S1). The search was not limited by language or year of publication. The records identified from the initial database search were pooled, and the duplicate records were removed using an in-house pipeline used for several prior systematic reviews and meta-analyses. Titles and abstracts of all records were screened by one reviewer (ES) based on pre-defined eligibility criteria (Fig. 1, Table S2). All records were randomly divided among three additional reviewers (AB, AH, or RH) to ensure that at least two reviewers screened each record. Agreements between the reviewers were examined, and a senior tie-breaker (CNK) resolved discrepancies in judgment. Full-text articles of the records that were deemed eligible during initial screening were thoroughly examined for eligibility by two study personnel (ES and CSD), and the discrepancies were resolved by a senior tie-breaker (CNK) (Table S3). An additional systematic search was performed through June 26, 2023, broadening the search to include Cochrane database. A manual search was conducted on the reference lists and citations of eligible articles; 11 additional records were added to the pool. Records published in other languages were translated into English using Google Translate®. The full-text articles that met eligibility criteria were included for quality assessment, review, and data extraction for meta-analyses.
Data Extraction
Data were extracted from the eligible manuscripts into pre-defined data fields in a spreadsheet. Immediate post-intervention (i.e., soon after discontinuation of rTMS or control intervention) versus pre-intervention changes in headache intensity and frequency were extracted as primary outcomes from the intervention and control groups in the included studies. When available, post-follow-up (i.e., re-examining headache at least > 2 weeks after the discontinuation of the rTMS or control intervention) versus pre-intervention changes in headache intensity and frequency of intervention and control groups were also extracted. Quality of life measures were extracted as secondary outcomes. Migraine Disability Assessment (MIDAS) and Headache Impact Test–6 (HIT-6) were extracted as measures of quality of life [41]. Year of publication, mean age, percentage of females in the intervention group, types of CDH observed, mean duration of headache, duration of intervention, frequency of rTMS sessions per week, length of follow-up, and total number of sessions (i.e., exposure to rTMS) in a given study were extracted. Additional data regarding the rTMS protocol (i.e., rTMS pulse frequency, total rTMS pulses delivered per session, the intensity of magnetic impulses, the anatomical site used for rTMS, type of coil used for rTMS pulse delivery, and the equipment used) were also extracted to include in meta-regression analyses.
Data Analysis
Four separate DerSimonian-Laird random-effects meta-analyses were performed using the ‘meta’ package (version 4.11–0) in R software (version 4.0.3) to examine the immediate post- versus pre-intervention changes and post-follow-up versus pre-intervention changes in standardized headache intensity and frequency of CDH following rTMS. When interpreting the findings, a conservative estimate of the standardized between-group difference of 0.5 was considered the threshold for minimal clinically important difference (MCID) [42, 43]. In addition, a decrease in headache frequency by at least 1 day/month was considered the MCID for interpretation of outcomes regarding headache frequency [44, 45]. When sufficient data were available, subgroup meta-analyses were performed to explore the effects of rTMS on each primary outcome variable within the patient populations of subtypes of CDH (i.e., CM, TBI, or CTTH). Leave-one-out sensitivity analyses confirmed the consistency of findings. Additionally, a series of subgroup analyses were conducted to explore the effects of frequency subtypes and anatomical site of rTMS application on primary outcomes. The likelihood of publication bias was explored using funnel plots, and symmetry was assessed using Egger’s tests [46]. Effect-sizes of missing (i.e., unpublished/unreported) studies were imputed via the trim-and-fill method. Heterogeneity of effect-sizes was quantified with the Higgins’ I2 statistic [47, 48]. Exploratory univariate random-effects meta-regression analyses were performed to explain heterogeneity using potential moderator variables described above [49].
Quality Check and Grading Quality of Evidence
The risk of bias was assessed within the individual studies using the Cochrane Collaboration’s Tool RoB 2: A revised Cochrane risk-of-bias tool for randomized trials [50]. The quality of the evidence of the short-term and long-term outcomes was assessed according to the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) methodology for risk of bias, inconsistency, indirectness, imprecision, and publication bias [51]. Each quality criterion was rated very low, low, moderate, or high. Summary tables were constructed using the GRADE Profiler (GRADEpro, version 3.6) [52].
Results
Study Characteristics
The PRISMA flow diagram depicting the outcomes of the database search and screening of the literature based on the eligibility criteria is shown in Fig. 1. Fourteen randomized controlled trials (15 study arms) examining the effects of rTMS on the intensity and frequency of headache episodes among a total of 538 patients with CDH (284 exposed to rTMS; 254 exposed to sham control interventions) were included in the systematic review (Table 1) [18•, 29, 30, 38, 39, 53,54,55,56,57,58,59,60, 61••]. The sample sizes of rTMS intervention groups of the included studies ranged from 6 to 52, and the sample sizes of the control groups ranged from 6 to 46. The percentage of female participants in the rTMS groups of the included studies ranged from 17–87%, and the mean age of the participants of the rTMS groups was 40.19 years (range 32.93–53.28 years).
rTMS Protocols
When the protocols of rTMS were considered, only three studies used low-frequency rTMS, whereas the rest used high-frequency rTMS. The frequency of rTMS sessions per week ranged from 3–5 sessions per week, with a total duration of treatment ranging from 1–8 weeks. Therefore, the mean total number of sessions was 12.4 (range 3–24 rTMS sessions), and the mean rTMS pulses per session was 989.3 (range 100–2000). The intensity of magnetic impulses was ranging from 60–110%RMT. Nine study arms used left dlPFC, three studies used right dlPFC, and three study arms used left motor cortex (L/MC) as sites of stimulation. Out of all the included studies, ten studies used angle manipulation as a sham, whereas only three studies used a sham coil stimulator.
Quality of Studies
The quality of the included RCTs was evaluated using the Cochrane risk of bias assessment tool (Fig. S1). Six out of the 14 studies included were considered high quality, three were considered to be of moderate quality, and five were low quality. Quality ratings and the risks of bias of included studies are summarized in Fig. S1. The GRADE evidence profiles for the considered outcomes are summarized in Table S4. The GRADE Working Group grades of the level of evidence were low for all considered variables.
Effects of rTMS on the Intensity of Headaches in the Immediate Post-intervention Period
Compared to sham control interventions, exposure to rTMS significantly decreased the standardized headache intensity in the immediate post-intervention period versus pre-intervention state among patients with CDH (10 studies [18•, 38, 53,54,55,56,57,58,59,60], pooled Hedges’ g = -1.16 [-1.89, -0.43], p = 0.002; Fig. 2A), which exceeded the MCID threshold of 0.5. Subgroup analyses revealed a significant reduction in standardized headache intensity following rTMS in patients with TBI and CTTH (pooled Hedges’ g = -0.58 [-1.05, -0.11], p = 0.016 and pooled Hedges’ g = -1.73 [-2.63; -0.82], p < 0.001, respectively). However, there was no significant difference in post-intervention standardized headache intensity between patients with CM who received rTMS and those who received a sham control intervention (pooled Hedges’ g = -0.90 [-3.37, 1.57], p = 0.475).
Forest plots depicting results of the random-effects meta-analyses examining the effects of rTMS on A. the intensity of headaches in the immediate post-intervention period and B. the intensity of headaches after a standard-care follow-up period. Black dots and the horizontal line indicate individual trial-specific estimates and their 95% CIs; the size of the grey squares denotes the weight of the trials in the meta-analysis. The pooled estimates and the corresponding 95% CI are denoted by the center of diamonds and the width of the diamonds, respectively
Additional subgroup analyses were performed, separating studies conducted using high-frequency rTMS and low-frequency rTMS. There was a clinically and statistically significant reduction in headache intensity following exposure to low-frequency rTMS, while no significant difference was noted with the implementation of high-frequency rTMS (pooled Hedges’ g = -1.73 [-2.63, -0.82], p < 0.001 and pooled Hedges’ g = -0.87 [-1.81, 0.06], p = 0.067, respectively; Fig. S2). When the site of rTMS application was considered, right dlPFC and L/MC had statistically significant reductions in headache intensity (pooled Hedges’ g = -1.73 [-2.63, -0.82], p < 0.001 and pooled Hedges’ g = -1.11 [-1.76, -0.47], p < 0.001, respectively; Fig. S3), whereas left dlPFC did not show a difference (pooled Hedges’ g = -0.78 [-2.18, 0.61], p = 0.272).
Leave-one-out sensitivity analyses did not significantly change the pooled estimates (Table S5). A funnel plot of the effect-sizes indicated minimal publication bias (Fig. 3A, Table 2). Imputing one effect-size using the trim-and-fill method to adjust for publication bias and re-analyzing the data using the imputed effect-size revealed a significant decrease in immediate post-verses pre-intervention headache intensity following exposure to rTMS versus control conditions (pooled Hedges’ g = -1.00 [-1.75, -0.24], p = 0.010, Table 2). Significant heterogeneity was observed among the standardized mean differences pooled in the random-effects model (τ2 = 1.121; I2 = 77.5%, p < 0.001). None of the considered covariates showed significant moderator effects in a series of univariate meta-regression analyses that attempted to explain between-study heterogeneity (Table S6).
Funnel plots depicting publication bias in the literature included in the random-effects meta-analysis examining the effects of rTMS on A. the intensity of headaches in the immediate post-intervention period, B. the frequency of headaches in the immediate post-intervention period, C. the intensity of headaches after a standard-care follow-up period, and D. the frequency of headaches after a standard-care follow-up period. Effect-sizes of studies included in the literature are shown in gray-colored circles. Effect-sizes imputed using the trim-and-fill method to maintain funnel plot symmetry to correct for publication bias are shown in white-colored circles
Effects of rTMS on the Frequency of Headache Episodes in the Immediate Post-intervention Period
Exposure to rTMS decreased the frequency of headaches by approximately 5 days/month during the immediate post-intervention period compared with a sham control intervention. This difference was statistically and clinically significant (8 studies—9 study arms [30, 53,54,55, 57, 58, 60, 61••], Δ = -5.07 [-10.05, -0.11], p = 0.045, MCID = 1 day/month, Hedges’ g = -0.85 [-1.53 -0.17], p = 0.014, MCID = 0.5; Fig. 4A). On subgroup analyses, this rTMS-associated reduction in the frequency of headaches was more marked among patients with CM (Δ = -6.51 [-12.93, -0.10], p = 0.047) but was not significant among patients with TBI (Δ = -0.89 [-2.37, 0.58], p = 0.234). Leave-one-out sensitivity analyses did not significantly change the pooled estimates (Table S5). The funnel plot indicated possible publication bias (Fig. 3B, Table 2). When the data were re-analyzed, including two effect-sized imputed using the Trim-and-fill method to correct for funnel plot asymmetry, the previously observed significant post-versus pre-intervention decrease in headache frequency was not observed (Δ = -1.64 [-7.84, 4.56], p = 0.604). Significant between-study heterogeneity remained a concern (τ2 = 37.33; I2 = 86%, p < 0.001). Attempts to explain the significant between-study heterogeneity in a series of univariate meta-regression analyses remained unfruitful (Table S6). Subgroup analyses were not conducted for rTMS frequency and site of application due to a limited number of studies.
Forest plots depicting results of the random-effects meta-analyses examining the effects of rTMS on A. the frequency of headaches in the immediate post-intervention period and B. the frequency of headaches after a standard-care follow-up period. Black dots and the horizontal line indicate individual trial-specific estimates and their 95% CIs; the size of the grey squares denotes the weight of the trials in the meta-analysis. The pooled estimates and the corresponding 95% CI are denoted by the center of diamonds and the width of the diamonds, respectively
Effects of rTMS on the Intensity of Headaches After a Routine Care Follow-up
The meta-analysis that compared the standardized post-follow-up versus pre-intervention changes in headache intensity between rTMS versus sham control groups revealed a statistically significant yet clinically not significant (less than MCID = 0.5) beneficial effect (7 studies [18•, 29, 30, 38, 55, 57, 58], pooled Hedges’ g = -0.43 [-0.76, -0.09], p = 0.012; Fig. 2B). Subgroup analyses performed within patient populations with CM had significant rTMS versus control differences, whereas CDH due to TBI also did not yield significant rTMS versus control differences (pooled Hedges’ g = -0.62 [-1.15, -0.07], p = 0.028 and pooled Hedges’ g = -0.32 [-0.79, 0.14], p = 0.173 respectively). Leave-one-out sensitivity analyses did not change the observed pooled effect-size (Table S5). The funnel plot suggested minimal publication bias (Fig. 3C, Table 2). Statistical significance in the follow-up versus pre-intervention difference in headache intensity was no longer observed after adjusting for publication bias by imputing two effect-sizes to restore funnel plot symmetry (pooled Hedges’ g = -0.29 [-0.64, 0.06], p = 0.100). Significant between-study heterogeneity remained a concern (τ2 = 0.14, I2 = 63.1%, p = 0.006). The duration of intervention and total number of sessions had negative moderator effects (β = -0.273, SE = 0.112, p = 0.015 and β = -0.049, SE = 0.022, p = 0.027, respectively), indicating that an increased duration of rTMS and increased number of sessions seem to result in a long-lasting decrease in headache intensity after the cessation of the rTMS intervention. Furthermore, including either the duration of intervention or the total number of sessions in the model decreased the residual heterogeneity (τ2 = 0.054, I2 = 34%, p = 0.152 and τ2 = 0.053, I2 = 33%, p = 0.12, respectively) (Table S6).
Effects of rTMS on the Frequency of Headache Episodes After a Routine Care Follow-up
The frequency of headaches after a routine care follow-up period following an rTMS intervention decreased by approximately 3 days/month compared to exposure to a sham control condition (8 studies—9 study arms [29, 30, 39, 53, 55, 57, 58, 61••], Δ = -3.33 [-5.52, -1.14], p = 0.003, MCID = 1 day/month, Hedges’ g = -0.54 [-0.91, -0.17], p = 0.004, MCID = 0.5; Fig. 4B). The post-follow-up versus pre-intervention changes between rTMS versus control conditions were statistically significant within the subgroups of patients with CM but not significant for TBI (Δ = -3.90 [-6.47, -1.33], p = 0.003 and Δ = -1.13 [-2.62, 0.36], p = 0.136, respectively). Leave-one-out sensitivity analyses did not significantly change the pooled outcome (Table S5). The funnel plot appeared symmetrical and did not suggest publication bias (Fig. 3D, Table 2). Nevertheless, the statistical significance of follow-up versus pre-intervention difference in headache frequency was lost with the addition of two effect sizes imputed based on trim-and-fill analysis (Δ = -2.16 [-4.68, 0.35], p = 0.092). Significant between-study heterogeneity was a concern for this meta-analysis as well (τ2 = 6.110; I2 = 62.4%, p = 0.007). Meta-regression analyses revealed that the anatomical site (i.e., L/MC versus L/dlPFC) was a negative moderator, indicating that the frequency of headache episodes seems to be significantly lower with an application of rTMS over the L/MC as compared to the L/dlPFC (β = -4.829, SE = 2.018, p = 0.017). The anatomical site also decreased residual heterogeneity (τ2 = 1.831; I2 = 39%, p = 0.110). On the contrary, having a sham coil instead of an angle manipulation as a sham had a positive moderator effect (β = 5.853, SE = 2.80, p = 0.037), indicating that the frequency of headache episodes seems to be significantly higher in the group with sham coil compared to the group with angle manipulation as a sham. Furthermore, an increase in the duration of the follow-up period decreased the frequency of headaches (β = -0.874, SE = 0.441, p = 0.048), which may indicate a long-term effect. However, we did not explore it further due to a limited number of studies (Table S6).
Side Effects and Tolerability
Only a few studies reported side effects, which included a short-lasting increase in headache intensity, mild localized pain, dizziness, sleepiness, and toothache [38, 58, 61••, 62]. rTMS was well tolerated among the patients except for one report by Granato et al. [39] describing an episode of migraine with complex aura (visual, sensitive, and aphasic) in a patient. A summary of all the side effects is included in Table 1.
Effects of rTMS on Quality of Life
Compared to sham control interventions, exposure to rTMS significantly decreased the standardized quality of life measures in the post-intervention period versus pre-intervention state among patients with CDH (5 studies—6 study arms [38, 53, 56, 58, 61••], pooled Hedges’ g = -0.73 [-1.36, -0.10], p = 0.024; Fig. S4), which exceeded the MCID threshold of 0.5. Leave-one-out sensitivity analyses did not significantly change the pooled estimates (Table S5). A funnel plot of the effect-sizes indicated minimal publication bias (Fig. S5, Table 2). Imputing two effect-size using the trim-and-fill method to adjust for publication bias and re-analyzing the data using the imputed effect-size revealed a significant decrease in post-versus pre-intervention headache intensity following exposure to rTMS versus control conditions (pooled Hedges’ g = -1.04 [-1.69, -0.39], p = 0.002, Table 2). Significant heterogeneity was observed among the standardized mean differences pooled in the random-effects model (τ2 = 0.459; I2 = 69.3%, p = 0.006). Subgroup and meta-regression analyses were not conducted as there were only a limited number of studies.
Discussion
While meta-analyses exist on rTMS for CM [63•, 64, 65], to our knowledge, this is the first comprehensive systematic review and meta-analysis examining the effects of rTMS on immediate and long-term outcomes for CDH. Our meta-analyses showed rTMS has a statistically and clinically meaningful impact on improving CDH intensity and frequency post-treatment, though effects tend to wear off after discontinuation.
rTMS is well documented to treat neuropathic pain and headaches like migraine, cluster headaches, and trigeminal neuralgia by transiently suppressing central pain perception and increasing pain stimulus thresholds [66, 67]. Several meta-analyses have shown that rTMS is beneficial in treating migraine prophylactically and therapeutically [63•, 64, 65]. Lan et al. [65] found that rTMS was only beneficial for treating migraine attacks, not CM symptoms. In contrast, Zhong et al. [63•] found that headache frequencies were reduced in episodic and CM types. Furthermore, Mohamad Safiai et al. [64] found that high-frequency rTMS reduces acute medication intake and functional disability associated with migraine but not headache days or pain intensity. All these meta-analyses have focused primarily on episodic migraine or a combination of episodic and CM, not purely on CM. According to our subgroup analyses, rTMS seems to have limited short-term and long-term impact in improving CM headache frequency, and data regarding improvement of headache intensity is abstruse.
One possible reason could be that CM is more of a problem of threshold where certain predisposing factors combined with frequent headache pain lower the threshold of migraine attacks and increase central and peripheral sensitization [68, 69]. For instance, genetic, anatomical, functional, and inflammatory factors change during the progression of a migraine attack or the transformation of episodic to CM [70,71,72,73]. As a result, CM becomes independent of triggers and depends more on fronto-limbic sensitization. Therefore, the application of rTMS should reset or reduce fronto-limbic dysfunction and cortical plasticity, which requires repeated exposure and long-term follow-up [17, 29, 74]. For instance, Fumal et al. [75] showed that daily rTMS induces cortical excitability and habituation patterns in migraine patients, which may contribute to its long-term efficacy in controlling headaches. Furthermore, it is essential to note that a small number of studies drove our results of the CM subgroup, and one study showed a strong placebo effect, which affected the overall estimates. Hence, further studies are needed to conclude whether this observation is generalizable.
CDH secondary to TBI is a constellation of debilitating chronic neuropathic pain, also called post-traumatic headaches [54, 55]. These patients are prone to adverse effects from chronic use of analgesics [76]. Therefore, a non-systemic, targeted therapy is ideal for these patients. Our subgroup analysis showed that TBI patients may benefit from rTMS by immediately lowering headache intensity. However, the number of studies and the sample size were limited, restricting our ability to confirm the effect [54, 55, 58]. Therefore, more research is needed on the applicability of rTMS. Similarly, rTMS showed limited evidence for reducing headache intensity for CTTH immediately post-intervention; a limited number of studies conducted on patients with CTTH was the primary limiting factor [59, 60].
Our meta-analysis showed that with rTMS, there is an immediate effect of reducing headache frequency by 5 days/month, and after one month, it remained reduced up to 3 days/month. Despite these effect-sizes being derived from a moderately heterogeneous limited number of small studies, our results substantiated the utility of rTMS in CDH. In contrast, current literature indicates that the therapeutic effects of rTMS for CDH are limited, with most studies being on CM [77,78,79,80,81]. Even for CM, BTX-A and anti-CGRPs are more promising, significantly reducing headache days and intensity short and long-term, unlike rTMS [82]. For instance, Sacco et al. [83] showed that anti-CGRPs reduce CM headache frequency by 2.39 days/month [-2.69, -2.08, n = 8902]. Similarly, BTX-A reduced headache frequency by 2.0 days/month [− 2.8, − 1.1, n = 1384] [79]. Although we observed that rTMS may decrease headache frequency by days/month, the effect-sizes regarding BTX-A and anti-CGRP effect sizes were more robust and were derived from larger samples [77, 84].
Our study found that the effects of rTMS on CDH wears off over time. Thus, while rTMS may help as an abortive strategy for refractory CDH, its utility for CDH prophylaxis seems limited. On the contrary, BTX-A and anti-CGRPs demonstrated persistent effects in CM patients [82]. For instance, Lanteri-Minet et al. [84] showed that BTX-A has a lasting effect on headache frequency even at 24 weeks and 52 weeks based on long-term follow-up studies. Shehatha et al. [85] showed that compared to rTMS, BTX-A had sustained improvement in CM symptoms even after three months. However, some studies observed a lack of sustained efficacy for CDH, indicating a need for further investigations [15].
Our meta-regression analyses also showed that a longer duration of treatment and a higher number of sessions have better long-term effects in terms of sustained reduction in headache intensity. Furthermore, our meta-regression analyses showed a profound and sustained effect with increased duration and number of sessions. This dose-dependent effect—a notable observation—needs further exploration. Zhan et al. [86] showed that at least five sessions of rTMS treatment are required for long-lasting motor functional recovery in the injured upper limb of stroke patients. Similarly, the motor evoked potentials last longer with an increased number of pulses of rTMS [23, 87]. Furthermore, the temporal pattern of rTMS induction (simple protocols versus patterned rTMS protocols) also seems to have a substantial impact [88••]. Long-lasting effects of rTMS are thought to originate from synaptic plasticity, which produces either potentiation or depression of synaptic strength. Long-term potentiation involves an increase in synaptic strength that can last for days or even weeks and months. Conversely, long-term depression encompasses a long-lasting weakening of synaptic connections. Studies have shown that rTMS-induced changes can last for at least eight days, as evidenced by altered uptake of F-fluorodeoxyglucose, indicating changes in neuronal excitability [89]. rTMS is also capable of increasing the expression of genes involved in synaptic plasticity, such as c-Fos and zif268, which play a role in the induction of long-term potentiation [90]. Similarly, activation of the NMDA receptor leads to a post-synaptic influx of calcium ions, playing a pivotal role in these long-lasting changes [91]. Furthermore, dopamine receptor activation was found to be involved in the maintenance of plasticity [92].
Of note, the treatment protocols included in the meta-regressions were highly heterogeneous—for instance, the majority of studies used coil perpendicular as a sham instead of a sham stimulator. A sham stimulator mimics the sensation of active stimulation without inducing the actual neurophysiological effects [93]. However, recent evidence suggests that sham stimulation may introduce variability, confound results, and pose challenges in blinding [94]. Using angle manipulation as a sham may provide more effective control conditions, as it minimizes the direct neurophysiological effects while maintaining the sensation of stimulation [94, 95]. Nevertheless, accurate placement and orientation of the coil require precision and understanding of the orientation of the induced electric field, adding complexity to the methodology [96]. Therefore, we cannot avoid the bias endorsed by different sham methods in this meta-analysis. We have tried to explore the moderator effect by doing a meta-regression analysis, and we found that angle manipulation seems to be the better option.
There were variations in the target region in the protocol. How the mechanism of action differs according to the target region in the brain is an important question worth addressing. A strong focal activation was observed in the thalamus, insula, cingulate-orbitofrontal junction, and a periaqueductal gray area in the brainstem following rTMS to MC, suggesting that a direct top–down activation of descending pain control system mediating via motor cortex, thalamus, insula, anterior cingulate cortex, and periaqueductal gray matter, which are components of pain modulation pathways [25, 97, 98]. Accordingly, rTMS applied to the MC reduces CM headache frequency [30, 99]. Conversely, rTMS to dlPFC exerts a top-down inhibitory neural circuit along the ascending midbrain-thalamic-cingulate pathway through the descending fibers from the PFC. dlPFC is involved in cognitive components of the pain experience, such as pain inhibition, perceived control of pain, and pain anticipation [98]. dlPFC and limbic cortex have been proposed to be extremely important in the pathophysiology of many chronic neurobehavioral conditions, such as addiction, depression, bipolar disorder, and migraine [29, 100]. Stimulation of dlPFC could reset or reduce fronto-limbic dysfunction in CM, leading to pain reduction [18•]. Todorov et al. [61••] compared rTMS to dlPFC and MC in CM and showed comparative results for both regions. Interestingly, our subgroup analyses showed that applying rTMS to MC or right dlPFC has a better outcome in reducing headache intensity, and our meta-regression analysis found rTMS to MC more effective versus dlPFC in reducing headache frequency. However, this analysis had < 10 studies, so more studies are needed to verify our dlPFC versus MC findings. Since our results highlighted several factors representing treatment protocols (e.g., anatomical location of stimulation, stimulation frequency, duration of stimulation), further exploration is needed to identify potential factors/parameters.
Guidelines have reiterated the high safety and tolerability profile of rTMS [101]. The most common side effect of rTMS is scalp discomfort or pain during treatment (~ 40%) [102], followed by headaches after treatment (20–30%) [103] and fatigue (15–20%) [104]. rTMS has also been associated, albeit rarely, with more severe adverse events such as seizures [105]. However, seizure risk is currently estimated to be minuscule overall < 1%. A large population-based study reported 24 seizures in 300,000 rTMS sessions (standardized risk of 7/100,000 sessions). Of those, 79% (n = 19) of seizures have occurred in patients with pre-existing risk factors (medication, neurological condition, epilepsy) [106]. This study estimated that rTMS delivered within published guidelines to individuals without risk factors appears to cause fewer than one seizure per 60,000 sessions. Apart from that, hearing impairment necessitates the use of hearing protection during treatment, EEG after-effects or abnormalities without overt clinical symptoms, and syncope or fainting episodes are some of the other side effects related to rTMS [101]. In conclusion, rTMS is an acceptable treatment modality with overall safety and tolerability. The studies included in this meta-analysis reported minimal number of side effects with good tolerability.
This meta-analysis has notable limitations. First, only a few eligible studies met inclusion, with several excluded due to unavailable variance estimates or combining episodic and CM. Second, we initially intended to focus only on CTTH, but the number of studies was limited, so we combined all CDH subtypes. Therefore, the studies included were heterogeneous in various aspects. Variability in treatment response and potential heterogeneity of CDH in terms of neuroanatomical and neurophysiological differences limit our ability to draw solid conclusions. Furthermore, the lack of consensus regarding brain targets and variation in stimulation parameters caused difficulties in comparing and combining all the studies. However, we attempted to explore these variations using meta-regression analyses that partially explained the heterogeneity. Third, only a limited number of studies focused specifically on the effect of rTMS on CDH sub-types such as CTTH, limiting the ability to make inferences on subgroups of CDH. Finally, outcomes such as the impact on disability, absolute or relative risk, and number needed to treat could not be determined due to limited studies reporting these outcomes. Nevertheless, the efficacy of rTMS may vary depending on individual patient characteristics and the specific parameters of the treatment.
In conclusion, rTMS demonstrates an immediate effect on reducing CDH intensity and frequency, indicating a potential for CDH symptom control. Importantly, rTMS is non-invasive, targeted, and safe, making it favorable for patients who cannot tolerate medications. While beneficial for short-term headache control, its effect does not seem to persist; thus, the cost-effectiveness of rTMS as a primary treatment is questionable. As the synthesized evidence stems from small, low-quality studies, adequately powered randomized controlled trials (especially for CTTH) are necessary to establish the effects of rTMS on CDH. Furthermore, identifying the best treatment protocols (in terms of frequency, motor threshold, anatomical site, and a minimum number of interventions) and developing a consensus statement/guideline is essential. Based on the limited available evidence, rTMS appears to be safe and has the potential utility as part of a comprehensive treatment approach that may include other interventions such as lifestyle modifications, oral medications, BTX-A, and anti-CGRP antibodies in the management of particularly refractory CDH, potentially leading to better patient outcomes.
Data Availability
The datasets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- CDH :
-
Chronic daily headaches
- CM:
-
Chronic migraine
- CTTH:
-
Chronic tension-type headaches
- TCA:
-
Tricyclic antidepressants
- BTX-A:
-
Botulinum toxin A
- CGRP :
-
Calcitonin gene-related peptide
- rTMS :
-
Repetitive transcranial magnetic stimulation
- dlPFC :
-
Dorsolateral prefrontal cortex
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- MCID:
-
Minimal clinically important difference
- GRADE:
-
Grading of Recommendations, Assessment, Development, and Evaluation
- MC:
-
Motor cortex
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Garza I, Schwedt TJ. Diagnosis and Management of Chronic Daily Headache. Semin Neurol. 2010;30(02):154–66.
Sheikh HU. Approach to chronic daily headache. Curr Neurol Neurosci Rep. 2015;15:1–6.
Pascual J, Colás R, Castillo J. Epidemiology of chronic daily headache. Curr Pain Headache Rep. 2001;5(6):529–36.
Dodick DW. Chronic daily headache. N Engl J Med. 2006;354(2):158–65.
Lantéri-Minet M, et al. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia. 2011;31(7):837–50.
Silberstein SD, Lipton RB. Chronic daily headache. Curr Opin Neurol. 2000;13(3):277–83.
Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near-daily headaches: field trial of revised IHS criteria. Neurology. 1996;47(4):871–5.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33(9):629-808.
Yancey JR, Sheridan R, Koren KG. Chronic daily headache: diagnosis and management. Am Fam Physician. 2014;89(8):642–8.
Dharmshaktu P, Tayal V, Kalra BS. Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol. 2012;52(1):6–17.
Silberstein SD, et al. Efficacy and safety of topiramate for the treatment of chronic migraine: A randomized, double‐blind, placebo‐controlled trial. Headache J Head Face Pain. 2007;47(2):170–80.
Rothrock JF, et al. Predictors of a negative response to topiramate therapy in patients with chronic migraine. Headache J Head Face Pain. 2005;45(7):932–5.
Scheffler A, et al. CGRP antibody therapy in patients with drug resistant migraine and chronic daily headache: a real-world experience. J Headache Pain. 2021;22(1):1–6.
Lipton RB, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology. 2020;94(13):e1365–77.
Ornello R, et al. Early management of onabotulinumtoxinA treatment in chronic migraine: insights from a Real-Life European Multicenter Study. Pain Ther. 2021;10:637–50.
Urits I, et al. CGRP antagonists for the treatment of chronic migraines: a comprehensive review. Curr Pain Headache Rep. 2019;23:1–10.
Coppola G, et al. Neuromodulation for chronic daily headache. Curr Pain Headache Rep. 2022;26(3):267–78.
• AbdElkader AA, et al. The efficacy of repetitive transcranial magnetic stimulation in treating patients with chronic daily headache. Egypt J Neurol Psychiatr Neurosurg. 2021;57:1–7. This is the only RCT conducted on chronic daily headaches as a whole. All the other included studies considered a particular type of chronic daily headache.
Chail A, et al. Transcranial magnetic stimulation: a review of its evolution and current applications. Ind Psychiatry J. 2018;27(2):172.
Lefaucheur JP, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol. 2020;131(2):474–528.
Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. 2012;37(1):102–16.
Mansur C, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64(10):1802–4.
Peinemann A, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115(7):1519–26.
Bestmann S, et al. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19(7):1950–62.
Graff-Guerrero A, et al. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Cogn Brain Res. 2005;25(1):153–60.
Fierro B, et al. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res. 2010;203:31–8.
Yoo W-K, et al. High frequency rTMS modulation of the sensorimotor networks: behavioral changes and fMRI correlates. Neuroimage. 2008;39(4):1886–95.
Boyer L, et al. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology. 2014;82(14):1231–8.
Brighina F, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci. 2004;227(1):67–71.
Kalita J, et al. Efficacy of single versus three sessions of high rate repetitive transcranial magnetic stimulation in chronic migraine and tension-type headache. J Neurol. 2016;263(11):2238–46.
Seminowicz DA, Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain. 2017;18(9):1027–35.
Moisset X, de Andrade DC, Bouhassira D. From pulses to pain relief: An update on the mechanisms of rTMS-induced analgesic effects. Eur J Pain. 2016;20(5):689–700.
Misra UK, et al. Role of β endorphin in pain relief following high rate repetitive transcranial magnetic stimulation in migraine. Brain Stimul. 2017;10(3):618–23.
Keck M, et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology. 2002;43(1):101–9.
Strafella AP, et al. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):RC157.
Michael N, et al. Metabolic changes after repetitive transcranial magnetic stimulation (rTMS) of the left prefrontal cortex: a sham-controlled proton magnetic resonance spectroscopy (1H MRS) study of healthy brain. Eur J Neurosci. 2003;17(11):2462–8.
Ueyama E, et al. Chronic repetitive transcranial magnetic stimulation increases hippocampal neurogenesis in rats. Psychiatry Clin Neurosci. 2011;65(1):77–81.
Conforto AB, et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia. 2014;34(6):464–72.
Granato A, et al. Dramatic placebo effect of high frequency repetitive TMS in treatment of chronic migraine and medication overuse headache. J Clin Neurosci. 2019;60:96–100.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7): e1000097.
Sauro KM, et al. HIT‐6 and MIDAS as measures of headache disability in a headache referral population. Headache J Head Face Pain. 2010;50(3):383–95.
Norman GR, Sloan JA, and Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582-592.
Lemieux J, et al. Three methods for minimally important difference: no relationship was found with the net proportion of patients improving. J Clin Epidemiol. 2007;60(5):448–55.
Silberstein SD, et al. Headache prophylaxis with BoNTA: patient characteristics. Headache J Head Face Pain. 2010;50(1):53–70.
Evans AG, et al. Outcomes of Surgical Treatment of Migraines: A Systematic Review & Meta-Analysis. Plast Surg (Oakv). 2023;31(2):192-205.
Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58.
Higgins JP, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from https://www.training.cochrane.org/handbook.
Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–708.
Sterne JA, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ, 2019. 366: l4898.
Schünemann H, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from https://guidelinedevelopment.org/handbook.
GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2024. Available from https://www.gradepro.org/.
Kumar A, et al. Neuronavigation based 10 sessions of repetitive transcranial magnetic stimulation therapy in chronic migraine: an exploratory study. Neurol Sci. 2021;42(1):131–9.
Leung A, et al. Repetitive transcranial magnetic stimulation in managing mild traumatic brain injury-related headaches. Neuromodulation: Technology at the Neural Interface. 2016;19(2): 133–41.
Leung A, et al. Left dorsolateral prefrontal cortex rTMS in alleviating MTBI related headaches and depressive symptoms. Neuromodulation: Technology at the Neural Interface. 2018;21(4): 390–401.
Mattoo B, et al. Repetitive transcranial magnetic stimulation in chronic tension-type headache: A pilot study. Indian J Med Res. 2019;150(1):73–80.
Rapinesi C, et al. Add-on deep Transcranial Magnetic Stimulation (dTMS) for the treatment of chronic migraine: A preliminary study. Neurosci Lett. 2016;623:7–12.
Stilling J, et al. Treatment of Persistent Post-Traumatic Headache and Post-Concussion Symptoms Using Repetitive Transcranial Magnetic Stimulation: A Pilot, Double-Blind. Randomized Controlled Trial J Neurotrauma. 2020;37(2):312–23.
Rajain M, et al. Low-Frequency Repetitive Transcranial Magnetic Stimulation for Chronic Tension-Type Headache: A Randomized Controlled Study. Cureus. 2023;15(2):e34922.
Wei J, et al. Clinical efficacy of Deanxit combined with low-frequency repetitive transcranial magnetic stimulation in the treatment of chronic tension-type headache. J Clin Intern Med. 2023;40(4):277–8.
•• Todorov V, et al. Repetitive transcranial stimulation over two target areas, sham stimulation and topiramate in the treatment of chronic migraine. Proceedings of the Bulgarian Academy of Sciences. 2020;73(9):1298–305. The only study that tested the efficacy of applying rTMS on different regions of the brain.
Leung A, et al. rTMS in Alleviating Mild TBI Related Headaches–A Case Series. Pain Physician. 2016;19(2):E347–54.
• Zhong J, et al. Efficacy of repetitive transcranial magnetic stimulation on chronic migraine: A meta-analysis. Front Neurol. 2022;13:1050090. This a meta-analysis conducted recently on the application of rTMS for treating chronic migraine. However, they have included several studies on episodic migraine as well.
Mohamad Safiai NI, et al. High-frequency repetitive transcranial magnetic stimulation at dorsolateral prefrontal cortex for migraine prevention: A systematic review and meta-analysis. Cephalalgia. 2022;42(10):1071–85.
Lan L, et al. The efficacy of transcranial magnetic stimulation on migraine: a meta-analysis of randomized controlled trails. J Headache Pain. 2017;18:1–7.
Sevel LS, et al. Interhemispheric dorsolateral prefrontal cortex connectivity is associated with individual differences in pain sensitivity in healthy controls. Brain Connect. 2016;6(5):357–64.
Kanda M, et al. Transcranial magnetic stimulation (TMS) of the sensorimotor cortex and medial frontal cortex modifies human pain perception. Clin Neurophysiol. 2003;114(5):860–6.
May A, Schulte LH. Chronic migraine: risk factors, mechanisms and treatment. Nat Rev Neurol. 2016;12(8):455–64.
Boyer N, et al. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. PAIN®. 2014;155(7):1196–205.
Filippi M, Messina R. The chronic migraine brain: what have we learned from neuroimaging? Front Neurol. 2020;10:1356.
Vieira DSS, et al. Glutamate levels in cerebrospinal fluid and triptans overuse in chronic migraine. Headache J Head Face Pain. 2007;47(6):842–7.
Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. 2019;20(1):1–17.
Bigal ME, et al. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology. 2007;68(21):1851–61.
Siebner H, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16.
Fumal A, et al. Induction of long-lasting changes of visual cortex excitability by five daily sessions of repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers and migraine patients. Cephalalgia. 2006;26(2):143–9.
Choi G-S, et al. Effect of high-frequency repetitive transcranial magnetic stimulation on chronic central pain after mild traumatic brain injury: a pilot study. J Rehabil Med. 2018;50(3):246–52.
Giri S, et al. Randomized controlled studies evaluating Topiramate, Botulinum toxin type A, and mABs targeting CGRP in patients with chronic migraine and medication overuse headache: A systematic review and meta-analysis. Cephalalgia. 2023;43(4):03331024231156922.
Zheng H, et al. Topiramate, acupuncture, and BoNT-A for chronic migraine: a network meta-analysis. Acta Neurol Scand. 2021;143(5):558–68.
Herd CP, et al. Cochrane systematic review and meta-analysis of botulinum toxin for the prevention of migraine. BMJ Open. 2019;9(7): e027953.
Frank F, et al. CGRP-antibodies, topiramate and botulinum toxin type A in episodic and chronic migraine: a systematic review and meta-analysis. Cephalalgia. 2021;41(11–12):1222–39.
Jackson JL, et al. A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache. PLoS ONE. 2015;10(7): e0130733.
Yang CP, et al. Comparative Effectiveness and Tolerability of the Pharmacology of Monoclonal Antibodies Targeting the Calcitonin Gene-Related Peptide and Its Receptor for the Prevention of Chronic Migraine: a Network Meta-analysis of Randomized Controlled Trials [published correction appears in Neurotherapeutics. 2021 Oct 6]. Neurotherapeutics. 2021;18(4):2639-2650.
Sacco S, et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention–2022 update. J Headache Pain. 2022;23(1):1–19.
Lanteri-Minet M, et al. Effectiveness of onabotulinumtoxinA (BOTOX®) for the preventive treatment of chronic migraine: A meta-analysis on 10 years of real-world data. Cephalalgia. 2022;42(14):1543–64.
Shehata HS, et al. Repetitive transcranial magnetic stimulation versus botulinum toxin injection in chronic migraine prophylaxis: a pilot randomized trial. J Pain Res. 2016;9:771–7.
Zhang L, et al. Short-and long-term effects of repetitive transcranial magnetic stimulation on upper limb motor function after stroke: a systematic review and meta-analysis. Clin Rehabil. 2017;31(9):1137–53.
Nyffeler T, et al. Repetitive TMS over the human oculomotor cortex: comparison of 1-Hz and theta burst stimulation. Neurosci Lett. 2006;409(1):57–60.
•• Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3(2):95–118. A comprehensive review of the mechanism of action of rTMS.
Hayashi T, et al. Long-term effect of motor cortical repetitive transcranial magnetic stimulation induces. Ann Neurol. 2004;56(1):77–85.
Aydin-Abidin S, et al. High-and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res. 2008;188:249–61.
Huang Y-Z, et al. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118(5):1028–32.
Huang Y-Y, et al. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci. 2004;101(9):3236–41.
Mennemeier MS, et al. Sham transcranial magnetic stimulation using electrical stimulation of the scalp. Brain Stimul. 2009;2(3):168–73.
Duecker F, Sack AT. Rethinking the role of sham TMS. Front Psychol. 2015;6:210.
Lisanby SH, et al. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiat. 2001;49(5):460–3.
Loo CK, et al. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some “sham” forms active? Biol Psychiat. 2000;47(4):325–31.
García-Larrea L, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83(2):259–73.
Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126(Pt 5):1079–91.
Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: a randomized, placebo-controlled study. J Neurol. 2013;260(11):2793–801.
Avery DH, et al. Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry. 2008;69(3):441–51.
Rossi S, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–39.
Machii K, et al. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol. 2006;117(2):455–71.
Loo CK, McFarquhar TF, Mitchell PB. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int J Neuropsychopharmacol. 2008;11(1):131–47.
Miron J-P, et al. Repetitive transcranial magnetic stimulation for major depressive disorder: basic principles and future directions. Ther Adv Psychopharmacol. 2021;11:20451253211042696.
Stultz DJ, et al. Transcranial Magnetic Stimulation (TMS) Safety with Respect to Seizures: A Literature Review. Neuropsychiatr Dis Treat. 2020;16:2989-3000.
Lerner AJ, Wassermann EM, Tamir DI. Seizures from transcranial magnetic stimulation 2012–2016: results of a survey of active laboratories and clinics. Clin Neurophysiol. 2019;130(8):1409–16.
Funding
The study was funded in part by the Medical Student Summer Research Project of the School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX, 2021.
Author information
Authors and Affiliations
Contributions
CSD and CNK have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. CSD, CLS, CRL, and CNK designed the study; ES, AB, RH, AR and CSD collected the data; CLS, CRL and CNK supervised data collection; CSD and CNK extracted and analyzed the data; ES, CSD and CNK wrote the manuscript; all authors read, revised and helped finalize the manuscript. All authors accept full responsibility for all aspects of to the work described.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors have no potential conflicts of interest to declare pertinent to the content in the manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stephens, E., Dhanasekara, C.S., Montalvan, V. et al. Utility of Repetitive Transcranial Magnetic Stimulation for Chronic Daily Headache Prophylaxis: A Systematic Review and Meta-Analysis. Curr Pain Headache Rep 28, 149–167 (2024). https://doi.org/10.1007/s11916-024-01210-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-024-01210-0