Abstract
Evidence by functional imaging studies suggests the role of left DLPFC in the inhibitory control of nociceptive transmission system. Pain exerts an inhibitory modulation on motor cortex, reducing MEP amplitude, while the effect of pain on motor intracortical excitability has not been studied so far. In the present study, we explored in healthy subjects the effect of capsaicin-induced pain and the modulatory influences of left DLPFC stimulation on motor corticospinal and intracortical excitability. Capsaicin was applied on the dorsal surface of the right hand, and measures of motor corticospinal excitability (test-MEP) and short intracortical inhibition (SICI) and facilitation (ICF) were obtained by paired-pulse TMS on left motor cortex. Evaluations were made before and at different times after capsaicin application in two separate sessions: without and with high-frequency rTMS of left DLPF cortex, delivered 10 min. after capsaicin application. We performed also two control experiments to explore: 1: the effects of Left DLPFC rTMS on capsaicin-induced pain; 2: the modulatory influence of left DLPFC rTMS on motor cortex without capsaicin application. Capsaicin-induced pain significantly reduced test MEP amplitude and decreased SICI leaving ICF unchanged. Left DLPFC rTMS, together with the analgesic effect, was able to revert the effects of capsaicin-induced pain on motor cortex restoring normal MEP and SICI levels. These data support the notion that that tonic pain exerts modulatory influence on motor intracortical excitability; the activation of left DLPFC by hf rTMS could have analgesic effects, reverting also the motor cortex excitability changes induced by pain stimulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1991, Tsubokawa et al. reported efficacy of motor cortex stimulation (MCS) by dural-implanted electrodes for treatment of chronic, central, drug-resistant neuropathic pain on twelve patients. Since then, a consistent bulk of evidence showed this approach as effective for pain control in several patients (García-Larrea et al. 1999; Katayama et al. 1998; Nguyen et al. 1997; Peyron et al. 1995).
The introduction of repetitive transcranial magnetic stimulation (rTMS) has increased the opportunities to easily and painlessly perform effective human cortex stimulation (Pascual-Leone et al. 1998). On the basis of MCS results, Migita et al. (1995) showed pain reduction in two patients treated by low-frequency rTMS of motor cortex. To date, on the basis of literature data, motor cortex is considered the TMS target area for neuropathic pain (Fregni et al. 2007; Lefaucheur et al. 2001; Leo and Latif 2007) also by the EFNS Guideline on neurostimulation therapy (Cruccu et al. 2007).
The reason why stimulation of motor cortex is effective in treatment of pain is not yet completely understood. PET study showed a regional increase in cortical blood flow in the contralateral M1 after tonic stimulation of the skin (Casey et al. 1994; Iadarola et al. 1998). The detail of this connection together with its functional relevance remains yet to be clarified. The effects of tonic cutaneous pain on motor cortex excitability have been few studied. First, Farina et al. (2001) demonstrated that the activation of the C-fibers induced motor cortex inhibition close to the painful area from 20 to 30 min after the capsaicin application. More recently, defective intracortical inhibition was observed in patients with chronic neuropathic pain (Lefaucheur et al. 2006). But so far, changes in motor intracortical excitability of facilitatory and inhibitory circuits during experimental tonic pain have never been explored. Motor cortex participates to a neural network involved in pain experience including thalamus, anterior cingulate cortex (ACC), dorsolateral prefrontal cortex (DLPFC), cerebellum, and hippocampus (Pridmore and Samilowitz 2003; O’Reardon et al. 2007).
In a study by Tamura et al. (2004a, b), under the condition of 1 Hz-rTMS of right motor cortex (M1), SPECT analysis demonstrated a significant relative rCBF decrease in the right medial prefrontal cortex (MPFC) and a significant increase in the caudal part of the right-ACC both correlating with pain reduction. This could mean that motor cortex stimulation could indirectly act on pain through the deactivation of the right MPFC and activation of ACC.
However, only a few studies explored other potential target areas for pain treatment. Among the brain areas of the pain neural network, the DLPFC is considered to play an important role in nociceptive control (Mylius et al. 2006). Functional imaging studies (Lorenz et al. 2002, 2003) showed that left DLPFC activation is temporally related to amelioration of pain sensation in a model of acute pain induced by capsaicin. Recently, interesting results on relief of pain syndromes came also by stimulation of DLPFC in patients with migraine (Brighina et al. 2004; O’Reardon et al. 2007) and fibromyalgia (Sampson et al. 2006).
In agreement with these results, we recently observed the bilateral analgesic effects of the high-frequency stimulation of the left DLPFC on capsaicin-induced pain in normal subjects (Giglia et al. 2008).
DLPFC has reciprocal connections with brain regions that are associated with motor control (Fuster 1997; Wood and Grafman 2003). This together with the evidence that M1 stimulation for pain control modulates activation of PFC (Salako 2006) makes it reasonable to suppose that DLPFC stimulation during acute-tonic pain might modulate motor intracortical excitability. The question is if the analgesic effect of DLPFC stimulation involves in some way the motor cortex. In the present study, acute pain was induced by topical capsaicin application in healthy subjects (Baumann et al. 1991; Schmidt et al. 1995).
On such grounds, the first aim of the study was to explore in normal subjects the effects of topic capsaicin application on motor cortex excitability evaluated by paired-pulse (pp) TMS technique. The second aim was to investigate if the analgesic effect induced by left DLPFC activation by rTMS on capsaicin-related tonic pain could concur with the reversion of motor cortical excitability.
Methods
Seven healthy patients (four men/three women, mean age 32.4 ± 8.79) participated in the study. All subjects gave their informed consent and the study was conducted in accordance with the Declaration of Helsinki (Salako 2006).
Experimental procedure and magnetic stimulation
All subjects were comfortably seated in a chair and instructed to be as relaxed as possible. They wore a tight-fitting plastic swimmer’s cap to mark the optimum site of stimulation and coil placement. Electromyography (EMG) signals were recorded from the right abductor pollicis brevis (APB) muscle using 0.9-cm diameter Ag–AgCl surface electrodes placed 3 cm apart over the belly and tendon of the muscle. The EMG activity was recorded with a band-pass between 10 and 1,000 Hz and a display gain ranging from 50 to 200 μV/cm. EMG signals were collected, averaged, and analyzed off-line.
Focal TMS was applied over the hand motor cortex of the left hemisphere by using a figure-of-eight coil connected to two Magstim 200 stimulators through a Bistim module (Magstim Co., Dyfed, UK). The stimulating coil was placed over the optimal site for eliciting responses in the contralateral target muscle, paying attention to keep orientation and position constant. The resting motor threshold (MT) for eliciting responses in the relaxed APB muscle was defined as the intensity of stimulation needed to produce responses of 50 μV in at least 50% of trials. Subjects were given audiovisual feedback of EMG activity to assist in maintaining complete relaxation. Recordings contaminated by muscle activity were discharged from the analysis.
Intracortical facilitation (ICF) and short intracortical inhibition (SICI) of motor cortex were assessed by means of a paired-pulse paradigm with a sub-threshold conditioning stimulus (CS) set to 80% of the MT followed by a testing stimulus (TS) at an intensity of 120% of the resting MT. Two different interstimulus intervals (ISIs) were used: 2 and 10 ms representing, respectively the inhibitory and facilitatory limbs of the paired-pulse paradigm.
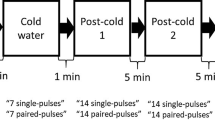
Three conditions (test stimulus alone and paired stimulation at ISIs of 2 and 10 ms) were applied ten times each, intermixed in a pseudo-randomized order based on single trials. The inter-trial interval was 10 s for all measurements.
Before topical application of capsaicin, two recordings each consisting of 30 conditioned and unconditioned MEPs were performed. The peak-to-peak amplitude of MEPs recorded at 2 and 10 ms ISIs were averaged in each subject and expressed as percentage of change from the respective mean TS alone. Amplitude of MEP measured for each condition (n = 20) was averaged and used as baseline values.
Main experiment
Capsaicin (Dolpyc Teofarma 3%) was applied over the dorsal surface of the right hand on square area of 2 × 2 cm for 60 min.
After capsaicin application, 30 trials, 10 for each condition (test stimulus alone and paired stimulation at ISIs of 2 and 10 ms), were recorded every 10 min from the application up to 80 min after. Two sessions with and without DLPFC rTMS were performed with at least one-week interval; each session, the order of which was randomly selected across subjects, followed the same protocol.
DLPFC rTMS was delivered after 10 min of capsaicin application, through a water-cooled figure-of-eight coil powered by a Cadwell High Speed Magnetic Stimulator (Cadwell Laboratories, Kennewick, Wash. USA). rTMS session consisted of 1,800 stimuli, divided in 12 trains, at 5 Hz frequency, 90% MT intensity, and separated by 10-sec pause. The site of stimulation was set on the scalp 5 cm anterior to the hot-spot for the Abductor Pollicis Brevis (APB) muscle (Brighina et al. 2004).
Constant coil position was continuously monitored during the experiment. Peak-to-peak amplitude of MEPs was measured in the single trial and averages were calculated for each condition within each session (with and without rTMS) in each subject. Amplitude of conditioned and unconditioned MEPs collected after capsaicin application was expressed as percentage changes from baseline.
Control experiment 1
In five subjects, measurement of spontaneous pain was performed by using a 0–100 point visuo-analogic scale (VAS) every 10 min from the time of capsaicin application up to its removal (60 min). Two sessions with and without DLPFC rTMS were performed with at least one-week interval; each session, the order of which was randomly selected across subjects, followed the same protocol. In the session with DLPFC stimulation, rTMS was delivered 10 min after capsaicin application with the same stimulation parameters as the main experiment.
Control experiment 2
Because of the lack of data about the effects of hf DLPFC rTMS on motor cortex excitability, three of the seven subjects underwent the same experimental procedure of the main experiment without capsaicin application. DLPFC rTMS was carried out by using the same parameters as above. A paired-pulse paradigm with three conditions (test stimulus alone and paired stimulation at ISIs of 2 and 10 ms) was applied on the left motor cortex; 30 trials, 10 for each condition, were recorded and motor cortex excitability (MEP amplitude, SICI and ICF) was evaluated every ten minutes from 10 up to 30 min after rTMS.
Statistical analysis
Three separate repeated measures analysis of variance (ANOVA) were used to compare amplitude of unconditioned (TS) and conditioned MEP at 2 ms (SICI) and 10 ms ISI (ICF) for main and control 2 experiments.
A repeated measures ANOVA was also used to compare the intensity of capsaicin-induced pain in control 1 experiment.
A 5% level of significance was used for all measurements.
Results
No serious negative consequences of left prefrontal rTMS were observed, except only a mild headache in few cases.
Main experiment
Mean MT before capsaicin application was 47.6 ± 10.69.
For analysis of each measure (TS MEP, SICI, ICF), ANOVA was performed with Condition (two levels: with and without rTMS) and Time (seven levels: 20, 30, 40, 50, 60, 70, 80 min) as within subject factors. The ANOVA employed to compare amplitude of unconditioned TS MEP showed a significant main effect of the two-way interaction Condition X Time [F(6, 36) = 3,38, P < .005]. The main effect of Condition was only marginally significant [(F(1,6) = 4,50, P = .08]; while the main effect of Time was not significant [F(6,36) = 1,31, P = .28].
Duncan’s post hoc analysis showed that in the control condition (without rTMS), MEP inhibition occurred as early as 10 min after application of capsaicin but became significant (P < .005) 20 min after capsaicin application, then progressively returned to the basal value up to 50-80 min. In the rTMS condition, no significant differences with respect to the baseline values were found throughout the experiment. (Fig. 1).
ANOVA analysis to compare SICI values with and without rTMS showed a significant main effect of Condition [F(1, 6) = 6,7512, P = .04]. No significant effects of Time [F(6,36) = 1,003, P = .44] and of the interaction Condition with Time [F(6,36) = 0,69, P = .66] were found.
Post hoc analysis showed that in control condition (without rTMS) SICI was significantly decreased (P < .01) 20 min after capsaicin application and remained almost unchanged throughout the experiment, tending to return to the basal values after capsaicin removal.
In rTMS condition, SICI approximated baseline values at the first evaluation (10 min) after rTMS and remained stable for all the time. (Fig. 2) The ANOVA comparing ICF in control and rTMS conditions did not show any significance. Main effect of Condition [F(1, 6) = 0,043, P = .84]; Time[F(6, 36) = 0,51, P = .79]; Interaction between Condition X Time [F(6, 36) = 1,94, P = .1]. In both conditions, ICF maintained closed to baseline values. (Fig. 3).
Traces of a representative subject concerning the significant changes observed (unconditioned MEP and SICI) are reported in Fig. 4.
Control experiment 1
Two-way ANOVA was used to compare the time course of capsaicin-induced pain with Condition (two levels: with and without rTMS) and Time (seven levels: 0, 10, 20, 30, 40, 50, 60 min) as within subject factors. The main effects of Time (F(6,24) = 4,03, P < .01) and the interaction Condition x Time [F(6,24) = 4,38, P < .005] were significant.
Duncan’s post hoc analysis showed that without rTMS the intensity of pain reached a significant level after 20 min and persisted high through all its application. In the session with DLPFC rTMS, pain did not significantly increase throughout the experiment. (Fig. 5).
Control experiment 2
For analysis of each measure (TS, SICI, ICF), three separate ANOVAs were performed with Time (four levels: baseline pre-rTMS, and 10, 20, 30 min after rTMS) as within subject factor. No significant effects were found for all measures: ICF [F(4, 8) = ,85, P = .53]; SICI [F(4, 8) = ,64, P = .65]; MEP [F(4, 8) = 1,12, P = .411]. (Fig. 6).
Discussion
The main findings of the study were the following: (1) pain induced by topical capsaicin application decreases cortico-spinal pathway activation with reduced MEP amplitude and modulates intracortical excitability lowering intracortical inhibition on the contralateral motor cortex; (2) hf rTMS on the left DLPFC reverts motor intracortical excitability changes due to experimentally induced pain in healthy subjects. Concerning motor cortex inhibition, in agreement with Farina’s et al. study (2001), we found that it occurred simultaneously to the onset of pain and became significant as pain intensity progressed (20 min after capsaicin application). However, motor cortex inhibition started to fade when pain perception was still high thus indicating that changes in M1 excitability are not tightly temporally related to the intensity of pain. As suggested by Farina et al. (2001), one can argue that M1 area inhibition during acute painful stimulation might lead to a “disinhibition” of the protective nociceptive spinal reflexes. So that inhibition of motor cortex is no longer necessary when levels of perceived pain become stable and no further increase in pain perception is expected. Indeed a central facilitation of the nociceptive flexor reflex (due to C-fibers-induced pain) was previously demonstrated by Gronroos and Pertovaara (1993) in humans. In our study, hf rTMS reverts M1 area inhibition probably not influencing nociceptive reflexes. This is consistent with a recent work by Mylius et al. (2007) showing no effects of hf rTMS of motor cortex on A delta fiber–mediated spinal nociceptive reflexes.
Here, we did not test spinal and peripheral nerve excitability, since in a previous study it has been demonstrated that the F- H and the M-waves were not modified by capsaicin-induced pain, suggesting that the transient inhibition of MEP amplitude to contralateral motor cortex was of cortical origin (Farina et al. 2001).
Our results showed also that during capsaicin-induced pain SICI was reduced, while no effects on ICF were observed. The decreasing of SICI maintained in the course of capsaicin application and started to increase toward the baseline values after capsaicin removal, suggesting that modulation of intracortical excitability depends upon intensity or variation of pain perception.
During the application of capsaicin, we did not explore motor threshold whose changes could have conditioned test stimulus intensity and consequently SICI and ICF (Ili’c et al. 2002), but it was previously found that the resting motor threshold remained unchanged during the course of experiment (Farina et al. 2001). To our knowledge, this is the first evidence of a modulatory effect of acute experimental cutaneous tonic pain on motor intracortical excitability. Interestingly, similar effects concerning reduced SICI were found by Lefaucheur et al. (2006) in patients with chronic neuropathic pain. This is presumed to reflect disruptions in the balance between inhibitory gamma-aminobutyric acid (GABA) and excitatory glutamate neurotransmission. In a model of acute experimentally induced pain, as here employed, the reduced inhibition could be a sort of transient “alert mechanism” that allows motor cortex to promptly adapt itself to ongoing requests.
In our study, the DLPFC rTMS delivered 10 min after capsaicin application reduced pain and concurrently changed motor intracortical excitability. Pain was significantly reduced 10 min after DLPFC rTMS, in the same time when MEP amplitude and SICI approached baseline values, remaining almost stable through the time of experiment. This could suggest that the effects of DLPFC stimulation on motor intracortical excitability contributed to or were partly dependent upon the analgesic effect.
To date, few studies have demonstrated analgesic effects on experimental pain induced by prefrontal cortex TMS (Borckardt et al. 2007; Graff-Guerrero et al. 2005; Kanda et al. 2003).
Graff-Guerrero et al. (2005) evaluated whether rTMS of DLPFC modifies experimental pain threshold and tolerance by using the cold pressor test as the painful stimulus. Right DLPFC low-frequency rTMS increased pain tolerance even if did not influence other measures of pain. Based on the evidence of inter-hemispheric rivalry, these findings are not in contrast with the present results. The low-frequency stimulation on the right cortex or the high frequency on the left homologous cortex have been indifferently used in the treatment of psychiatric (Avery et al. 2008; Brunelin et al. 2007) or neurologic disorders (Finocchiaro et al. 2006; Naeser et al. 2005). Indeed, more recently, Borckardt et al. (2007) demonstrated that 15 min of high-frequency left prefrontal TMS acutely increases thermal pain threshold in healthy subjects.
However, it is also to be considered that the varied effects of DLPFC rTMS on acute pain may be influenced by the type of experimentally induced pain. It is known that motor cortex rTMS can differently influence A delta and C-fibers mediating pain (Tamura et al. 2004a, b). Specifically, pain elicited by capsaicin and mediated by activation of C-fiber pathways was reduced by slow motor cortex rTMS (Tamura et al. 2004a); by contrast, acute laser-implemented pain primarily involving A delta fibers increased by slow motor cortex rTMS (Tamura et al. 2004b). Pain induced by CO2-laser stimuli was attenuated by paired-pulse (pp) TMS over the medial frontal cortex (MFC) (Kanda et al. 2003), whereas ppTMS over MFC can enhance pain perception of acute Ad fiber-mediated electrically induced pain (Mylius et al. 2006).
In our study, cutaneous tonic pain was experimentally induced by topical application of capsaicin on the skin, which predominantly activates polymodal C-fibers (Borckardt et al. 2007, Schmidt et al. 1995). Therefore, the observed motor cortical effects as well as the effects of DLPFC stimulation would be specifically regarded to C-fiber activation.
It has been hypothesized that prefrontal cortices may exert a ‘‘top–down’’ modulation of pain inhibitory system (Hadjipavlou et al. 2006) through anatomical circuitry connecting the prefrontal cortex with both the nucleus cuneiformis and periaquaductal gray. Pain processing might be modulated by direct effects of stimulation on PFC or indirect effects on ACC or on spinal nociception (Lorenz et al. 2003).
An early direct activation of the ACC by TMS is unlikely, since the TMS mainly affects the cortex to a depth of 20 mm (Peyron et al. 2000; Rudiak and Marg 1994), but a contribution from indirect modulating effects on ACC cannot be excluded. Indeed, a functional connectivity within the frontal lobe has been demonstrated by modulation of blood flow changes within the ACC induced by rTMS over the left mid-dorsolateral frontal cortex (Paus et al. 2001).
Based on this evidence, it is also conceivable that DLPFC rTMS can influence pain indirectly by modulating the emotional valences associated with pain through the activation of deeper limbic structures likely involved in the affective dimension of pain experience.
In this study, also to reduce the number of experimental sessions, we did not perform a sham stimulation, but the ability of DLPFC rTMS to revert capsaicin-induced effects on motor cortex excitability (the “null” effects of capsaicin cutaneous stimulation on motor cortex following DLPFC rTMS) seems reasonably to exclude a placebo or unspecific effect of the stimulation. Moreover, it is to be considered that some of the same subjects underwent a previous experiment where sham DLPFC rTMS showed no effect on capsaicin-induced pain (Giglia et al. 2008).
In the control 2 experiment (without capsaicin application), hf rTMS of DLPFC did not modify the excitability of the motor cortex. This finding could imply that activation of pain circuits could allow DLPFC to access the motor system to escape or defense.
Specular connections between motor and prefrontal cortices via direct or indirect (through cingulate or premotor areas), activated by anti-nociceptive mechanisms was evidenced by Tamura et al. (2004a, b) that suggested that these pathways might contribute to the mechanisms of pain relief by rTMS over M1.
Therefore, our data seem to reasonably support the notion that the activation of left DLPFC by hf rTMS during experimental cutaneous pain had analgesic effects, reverting the motor cortex excitability changes induced by pain stimulation.
Based on our previous observation (Giglia et al. 2008) of the bilateral analgesic effect of the left DLPFC stimulation, one limit of our study is to not have investigated the possible effect of the left DLPFC rTMS on the contralateral motor cortex excitabiity.
Since the pathophysiology of acute pain differs from chronic pain, in light of the present results, we could not fully speculate upon the possible modulatory effects on chronic pain by DLPFC rTMS.
Further studies in larger series should shed light on the physiological mechanisms underlying relief of acute-tonic pain perception through the use of rTMS in sites other than motor areas, providing a rationale for alternative therapeutic options of pain syndromes.
References
Avery DH, Isenberg KE, Sampson SM, Janicak PG, Lisanby SH, Maixner DF, Loo C, Thase ME, Demitrack MA, George MS (2008) Transcranial magnetic stimulation in the acute treatment of major depressive disorder: clinical response in an open-label extension trial. J Clin Psychiatry 69:441–451
Baumann TK, Simone DA, Shain CN, LaMotte RH (1991) Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol 66:212–227
Borckardt JJ, Smith AR, Reeves ST, Weinstein M, Kozel FA, Nahas Z, Shelley N, Branham RK, Thomas KJ, George MS (2007) Fifteen minutes of left prefrontal repetitive transcranial magnetic stimulation acutely increases thermal pain thresholds in healthy adults. Pain Res Manag 12:287–290
Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, Fierro B (2004) rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci 227:67–71
Brunelin J, Poulet E, Boeuve C, Zeroug-vial H, d’Amato T, Saoud M (2007) Efficacy of repetitive transcranial magnetic stimulation (rTMS) in major depression: a review. Encephale 33:126–134
Casey KL, Minoshima S, Berger KL, Koeppe RA, Morrow TJ, Frey KA (1994) Positron emission tomographic analysis of cerebral structures activated specifically by repetitive noxious heat stimuli. J Neurophysiol 71:802–807
Cruccu G, Aziz TZ, Garcia-Larrea L, Hansson P, Jensen TS, Lefaucheur JP, Simpson BA, Taylor RS (2007) EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol 14:952–970
Farina S, Valeriani M, Rosso T, Aglioti S, Tamburin S, Fiaschi A, Tinazzi M (2001) Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. Neurosci Lett 314:97–101
Finocchiaro C, Maimone M, Brighina F, Piccoli T, Giglia G, Fierro B (2006) A case study of primary progressive aphasia: improvement on verbs after rTMS treatment. Neurocase 12:317–321
Fregni F, Freedman S, Pascual-Leone A (2007) Recent advances in the treatment of chronic pain with non-invasive brain stimulation techniques. Lancet Neurol 6:188–191
Fuster JM (1997) The prefrontal cortex: anatomy, physiology, and neuropsychology of the frontal lobe. Raven, New York
García-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguière F, Sindou M, Laurent B (1999) Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain 83:259–273
Giglia F, De Tommaso M, Brighina F, Palermo A, Panetta ML, Puma A, Cosentino G, Giglia G, Fierro B (2008) Modulation of dorsolateral prefrontal cortex (DLPFC) through repetitive transcranial magnetic stimulation (rTMS) during capsaicin induced pain: effects on nociceptive control and motor cortical circuits. Eur J Neurol 15 (Suppl. 3)11: SC110
Graff-Guerrero A, González-Olvera J, Fresán A, Gómez-Martín D, Méndez-Núñez JC, Pellicer F (2005) Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Brain Res Cogn Brain Res 25:153–160
Grönroos M, Pertovaara A (1993) Capsaicin-induced central facilitation of a nociceptive flexion reflex in humans. Neurosci Lett 159:215–218
Hadjipavlou G, Dunckley P, Behrens TE, Tracey I (2006) Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain 123:169–178
Iadarola MJ, Berman KF, Zeffiro TA, Byas-Smith MG, Gracely RH, Max MB, Bennett GJ (1998) Neural activation during acute capsaicin-evoked pain and allodynia assessed with PET. Brain 121:931–947
Ili’c TV, Meintzschel F, CleV U, Ruge D, Kessler KR (2002) Ziemann U Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol 545:153–167
Kanda M, Mima T, Oga T, Matsuhashi M, Toma K, Hara H, Satow T, Nagamine T, Rothwell JC, Shibasaki H (2003) Transcranial magnetic stimulation (TMS) of the sensorimotor cortex and medial frontal cortex modifies human pain perception. Clin Neurophysiol 114(5):860–866
Katayama Y, Fukaya C, Yamamoto T (1998) Poststroke pain control by chronic motor cortex stimulation: neurological characteristics predicting a favorable response. J Neurosurg 89:585–591
Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP (2001) Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. 12:2963–2965
Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y, Nguyen JP (2006) Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 67:1568–1574
Leo RJ, Latif T (2007) Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: a review. J Pain 8:453–459
Lorenz J, Cross DJ, Minoshima S, Morrow TJ, Paulson PE, Casey KL (2002) A unique representation of heat allodynia in the human brain. Neuron 35:383–393
Lorenz J, Minoshima S, Casey KL (2003) Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126:1079–1091
Migita K, Uozumi T, Arita K, Monden S (1995) Transcranial magnetic coil stimulation of motor cortex in patients with central pain. Neurosurgery 36:1037–1039
Mylius V, Reis J, Kunz M, Beyer TF, Oertel WH, Rosenow F, Schepelmann K (2006) Modulation of electrically induced pain by paired pulse transcranial magnetic stimulation of the medial frontal cortex. Clin Neurophysiol 117:1814–1820
Mylius V, Reis J, Knaack A, Haag A, Oertel WH, Rosenow F, Schepelmann K (2007) High-frequency rTMS of the motor cortex does not influence the nociceptive flexion reflex but increases the unpleasantness of electrically induced pain. Neurosci Lett 415:49–54
Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A (2005) Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang 93:95–105
Nguyen JP, Keravel Y, Feve A, Uchiyama T, Cesaro P, Le Guerinel C (1997) Pollin B. Treatment of deafferentation pain by chronic stimulation of the motor cortex: report of a series of 20 cases. Acta Neurochir Suppl 68:54–60
O’Reardon JP, Fontecha JF, Cristancho MA, Newman S (2007) Unexpected reduction in migraine and psychogenic headaches following rTMS treatment for major depression: a report of two cases. CNS Spectr 12:921–925
Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD (1998) Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol 15:333–343
Paus T, Castro-Alamancos MA, Petrides M (2001) Cortico-cortical connectivity of the human mid-dorsolateral frontal cortex and its modulation by repetitive transcranial magnetic stimulation. Eur J Neurosci 14:1405–1411
Peyron R, Garcia-Larrea L, Deiber MP, Cinotti L, Convers P, Sindou M, Mauguière F, Laurent B (1995) Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain 62:275–286
Peyron R, Laurent B, García-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin 30:263–288
Pridmore S, Samilowitz H (2003) The brain and chronic pain. J Psychiatry 6:8–15
Rudiak D, Marg E (1994) Finding the depth of magnetic brain stimulation: a re-evaluation. Electroencephalogr Clin Neurophysiol 93:358–371
Salako SE (2006) The declaration of Helsinki 2000: ethical principles and the dignity of difference. Med Law 25:341–354
Sampson SM, Rome JD, Rummans TA (2006) Slow-frequency rTMS reduces fibromyalgia pain. Pain Med 7:115–118
Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjörk E, Handwerker H (1995) Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci 15:333–341
Tamura Y, Okabe S, Ohnishi T, Saito DN, Arai N, Mochio S, Inoue K, Ugawa Y (2004a) Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain 107:107–115
Tamura Y, Hoshiyama M, Inui K, Nakata H, Qiu Y, Ugawa Y, Inoue K, Kakigi R (2004b) Facilitation of A[delta]-fiber-mediated acute pain by repetitive transcranial magnetic stimulation. Neurology 62:2176–2181
Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S (1991) Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 52:137–139
Wood JN, Grafman J (2003) Human prefrontal cortex: processing and representational perspectives. Nat Rev Neurosci 4:139–147
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fierro, B., De Tommaso, M., Giglia, F. et al. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex (DLPFC) during capsaicin-induced pain: modulatory effects on motor cortex excitability. Exp Brain Res 203, 31–38 (2010). https://doi.org/10.1007/s00221-010-2206-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2206-6