Abstract
Conjugated linoleic acid (CLA) has garnered special attention as a food bioactive compound that prevents and attenuates obesity. Although most studies on the effects of CLA on obesity have focused on the reduction of body fat, a number of studies have demonstrated that CLA also increases lean body mass and enhances physical performances. It has been suggested that these effects may be due in part to physiological changes in the skeletal muscle, such as changes in the muscle fiber type transformation, alteration of the intracellular signaling pathways in muscle metabolism, or energy metabolism. However, the mode of action for CLA in muscle metabolism is not completely understood. The purpose of this review is to summarize the current knowledge of the effects of CLA on skeletal muscle metabolism. Given that CLA not only reduces body fat, but also improves lean mass, there is great potential for the use of CLA to improve muscle metabolism, which would have a significant health impact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of conjugated linoleic acid (CLA, conjugated octadecadienoic acid) in milk was first reported in the 1930s, but it was not until the 1980s that CLA was shown to be a bioactive food component [1]. CLA is formed during the biohydrogenation of linoleic acid to stearic acid by rumen bacteria [2]. In addition, trans-11 vaccenic acid (another metabolite of biohydrogenation) is known to be converted to cis-9,trans-11 CLA by Δ9-desaturase in the tissues [3]. Thus, the primary dietary sources of CLA are meats and dairy products from ruminants, although the overall CLA intake from food is not considered substantial [4]. It has been reported that CLA content ranges from 0.34 to 1.07 % of the total fat in dairy products, and 0.12 to 0.68 % in raw or processed beef [4]. In the United States, the average daily intake of CLA from food sources is 104–151 mg and 176–212 mg for women and men, respectively [5]. Accordingly, studies have reported serum CLA levels of approximately 20 μM, or 0.1 % of total fatty acids, in subjects with low dairy or meat consumption [6, 7], and approximately 50 to 180 μM with CLA supplementation of 0.8–3.2 g per day for 2 months [7].
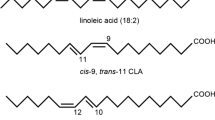
There are at least 28 known CLA isomers. Among them, the cis-9,trans-11 and trans-10,cis-12 isomers have been the focus of studies on various biological effects of CLA [8]. The cis-9,trans-11 isomer is a naturally predominant isomer, accounting for over 80 % of naturally occurring CLA [4]. In addition to the cis-9,trans-11 isomer, the trans-10,cis-12 isomer is found at very low levels in natural foods, but, when CLA is produced by chemical synthesis, this isomer is formed in significant amounts [8–10]. Currently, most commercial CLA preparations comprise almost equal amounts of cis-9,trans-11 and trans-10,cis-12 isomers, up to >90 % of total CLA, and these preparations are referred to as CLA mixtures or 50:50 mixtures.
CLA contains a trans configuration, and as there are known negative health issues associated with trans fat, some clarification with regard to CLA and trans fatty acids is warranted. The definition of trans fat labeling by the US Food and Drug Administration (FDA) is “all unsaturated fatty acids that contain one or more isolated double bonds in a trans configuration” [11]. It is clear, therefore, that CLA is excluded from "trans" fat product labeling, as it has a trans double bond that is conjugated, not isolated. Furthermore, in July 2008, the US FDA approved CLA mixtures for GRAS (generally recognized as safe) status in specific food categories, including fluid milk, yogurt, meal-replacement shakes, nutritional bars, fruit juices, and soy milk. Thus, it is expected that there will be an increase in CLA in foodstuffs, resulting in increased CLA intake for human health benefits.
CLA and Body Composition
Since 1997, with the discovery of the effects of CLA on body composition in a mouse model [12], numerous studies in various mammalian models have reported the effects of CLA supplementation on the modulation of body composition by reducing body fat and/or increasing lean body mass [8–10, 13–16]. While most studies in CLA have focused on the reduction of body fat, there is significant evidence supporting a concurrent increase in lean body mass, body proteins, or specific skeletal muscle mass [5, 8, 16, 17]. CLA was also confirmed to increase total protein content (not only %) as a representation of lean mass in animals [12]. Tables 1 and 2 summarize studies that have investigated changes in body composition in rodents. Of the two major isomers, the trans-10,cis-12 CLA isomer significantly correlates with this effect [18–21]. Some researchers have suggested that CLA supplementation causes re-partitioning of the body composition, with fewer adipose depots and greater lean mass [22]. This observation was further supported in a pig model, where a CLA mixture fed to pigs at levels between 0.25 and 2 % of their diet acted as a re-partitioning agent to induce a reduction in back fat and an increase in lean body mass [23–27].
To date, there have been approximately 100 human studies investigating the regulation of body fat by CLA, and Table 3 summarizes only those in which changes in both body fat and lean body mass were reported. Compared to the results observed in animal models, CLA intervention studies in humans has yielded less substantial and more inconsistent results (Table 3). Among the clinical trials investigating the effects of CLA on both body fat and lean mass, five publications reported changes in both [28–32], while two studies reported increases in lean body mass with no effect on body fat [33, 34]. Schoeller et al. [35] performed a meta-analysis of 18 independent clinical studies assessing the effect of CLA on lean body mass, and concluded that CLA supplementation led to a relatively rapid onset of increased lean body mass, although the total increase was not remarkable (less than 1 %). This conclusion is further supported by a study of CLA in a mouse model [36], in which an increase in lean muscle mass preceded a reduction in fat mass. These observations suggest a potentially significant role of the muscle in the effects of CLA on body composition.
Mechanism of CLA-Mediated Change in Body Composition
Multiple mechanisms have been suggested to explain the effects of CLA on body composition [16, 17, 37]. These include CLA-mediated energy modulation, including reduced energy intake and enhanced energy expenditure, along with the inhibition of fat accumulation in adipose tissue.
The balance between energy intake and energy expenditure is important for proper weight regulation. Energy intake is from the food consumed, while energy expenditure is the sum of the basal metabolic rate (BMR), thermogenesis, and physical activity. First, with regard to CLA and energy intake, some studies have demonstrated that CLA-fed mice ate less food, whereas other studies have reported inconsistent results (Tables 1, 2) [38–43]. However, some have suggested that the temporary reduction in food intake as seen with a CLA-containing diet may be due to its palatability when CLA is used as a free fatty acid. Moreover, in a study using a pair-feeding comparison, changes in body composition occurring with CLA were shown to be independent of reduced food intake [44], and human clinical trials showed no effect of CLA supplementation on food intake [29, 30, 45–49]. These human studies all used self-reported food intake methods, which calls into question their validity [50]. Nevertheless, despite the lack of conclusive evidence regarding the relationship between CLA and dietary intake in humans, it is unlikely that the reduction in food intake is the main mechanism of action for the change in body composition seen with CLA.
Enhanced energy expenditure is one key to controlling body composition. Several animal studies have suggested that CLA increases overall energy expended [43, 51–58]. In clinical trials, CLA supplementation was shown to increase BMR (as resting metabolic rate, RMR) [28, 48, 59, 60], although other studies found no influence of CLA on BMR, regardless of changes in body composition [33, 61–63].
As part of the increased expenditure of energy, CLA supplementation may increase thermogenesis, as evidenced by the upregulation of uncoupling proteins (UCPs) expressed in various tissues, such as the adipose, liver, and the skeletal muscle in mice and rats [38, 40, 55, 56, 64–66]. UCP1 through UCP5 are mitochondrial proteins involved in the combustion of stored or excess energy into heat. They are expressed in distinct tissues in the body, and are responsible for adaptive thermogenesis. Thus, an increase in UCPs by CLA suggests that CLA may increase energy expenditure by enhancing thermogenesis [67]. Likewise, physical activity also contributes to the overall expenditure of energy. Studies in rodents have reported that CLA supplementation increased energy expenditure in part by increasing the level of physical activity [43, 56, 68], although human studies are inconsistent in this regard [59, 61, 69].
In addition, fatty acid β-oxidation may contribute to reducing body fat mass by using fat as an energy source, rather than storing it in the body. Increased fat oxidation in CLA-fed animals has been reported, as measured either by reduced respiratory quotient or by increased activity and/or the expression of carnitine palmitoyltransferase 1 (CPT-1) in the skeletal muscle [12, 21, 41, 55, 56, 68, 70–74]. Intriguingly, Close et al. [60] reported that human subjects who received supplements with 4 g of CLA mixture for 6 months had significantly increased fat oxidation and energy expenditure during sleep. In another study, CLA was found to potentiate adipocyte apoptosis, reduce fat uptake, and/or modulate adipokine production, all of which collectively contributed to the effective reduction of fat accumulation [17]. At the same time, CLA increased lean mass, which is an important observation, suggesting that CLA targets skeletal muscle metabolism. The potential effects of CLA on skeletal muscle metabolism, however, have been less investigated.
CLA and Skeletal Muscle Metabolism
Skeletal muscle typically accounts for nearly 40 % of total body mass, and acts as a significant regulator in overall energy metabolism [75]. Muscle metabolism is a term used to describe the complex biochemical reactions associated with skeletal muscle function and development.
Overview of Muscle Energy Metabolism
The process of energy production for skeletal muscle is tightly regulated by the type, intensity, and duration of muscle exercise [76, 77]. Glycolysis is the catabolic pathway for glucose in the cytosol under both anaerobic (absence of oxygen) and aerobic (presence of oxygen) conditions. Aerobic glycolysis is an efficient means of producing adenosine triphosphate (ATP) through mitochondrial oxidative phosphorylation, while anaerobic glycolysis produces an energy supply with a much lower yield (36–38 ATPs produced by aerobic glycolysis vs. 2 ATPs by anaerobic glycolysis). During high-intensity exercise, anaerobic metabolic pathways are important, as aerobic metabolism alone may not be adequate to meet energy demands, especially when there is insufficient oxygen supply [78–80]. In contrast, low-intensity endurance exercise (requiring less than 60 % of maximal oxygen uptake) such as jogging and swimming consumes glucose and fatty acids as the primary energy sources during the first hour, and then relies on stored intramuscular and adipose tissue triglycerides for energy [81]. Thus it is believed that prolonged endurance exercise is the more efficient way to consume stored body fat.
Adaptive Responses of Skeletal Muscle
The skeletal muscle tissue also demonstrates metabolic plasticity in response to altered external and internal conditions, such as nutrient deprivation during fasting or calorie restriction and contractile activity including exercise [82]. One of the adaptive responses of the muscle is the ability to change the fiber type to meet energy demands. Muscle fiber in humans is composed of three myosin heavy chain (MHC) isoforms: MHC I, MHC IIa, and MHC IIx/d or IIb. MHC I are slow-twitch type I fibers, which have greater mitochondrial content, oxidative capacity, and resistance to fatigue, using fatty acids as a main energy source. Fast-twitch type II fibers (especially type IIb) are classified as glycolytic fibers, since they use glucose and phosphocreatine as primary energy sources. Type IIa is an intermediate type between type I and type IIb [83]. In response to exercise, the skeletal muscle remodels its fiber type between oxidative slow-twitch and glycolytic fast-twitch [84] in correlation with the contractile properties and the physiological and metabolic characteristics [85]. For example, an endurance exercise triggers fiber type remodeling from glycolytic fast-twitch to oxidative slow-twitch [84]. These adaptations in the skeletal muscle are accompanied by an increase in mitochondrial biogenesis, with the alteration of mitochondrial volume (content per gram of tissue) and composition (protein-to-lipid ratio in the inner mitochondrial membrane) [86].
Molecular Responses of Skeletal Muscle Metabolism
A number of regulators participate in the above-described adaptive responses in skeletal muscle. Among them, AMP-activated protein kinase (AMPK) is the prime initial sensor of fuel and energy status in the skeletal muscle (Scheme 1) [87]. An increase in intracellular AMP concentration causes a shift to an increased AMP/ATP ratio, and AMPK is then activated to provide the needed energy in the cell. An activated AMPK deactivates acetyl-CoA carboxylase (ACC) by phosphorylation, inhibits the synthesis of malonyl-CoA from two acetyl-CoAs, and results in the activation of carnitine palmitoyltransferase 1 (CPT1), a rate-limiting enzyme for fatty acid β-oxidation in mitochondria. AMPK also induces metabolic changes including an increase in glucose uptake by the induction of glucose transporter type 4 (GLUT4), translocation in the skeletal muscle, and a decrease in the rate of glycogen synthesis through the phosphorylation of glycogen synthase [82]. Similar to AMPK, sirtuin 1 (SIRT1, a conserved nicotinamide adenine dinucleotide [NAD]+-dependent deacetylase) acts as a sensor of metabolic stimuli (such as stress, starvation, or calorie restriction) [88]. SIRT1 also regulates several transcriptional factors (including protein 53, forkhead box O, and nuclear factor κ-light-chain-enhancer of activated B cells, NFκB), and is known to be involved in longevity [88]. Both AMPK and SIRT1 may coherently mediate the response at the cellular level to the metabolic stimuli in the skeletal muscle [89].
Proposed mechanism of CLA on muscle metabolism. AMPK AMP-activated protein kinase, CLA conjugated linoleic acid, SIRT1 silent information regulator two protein 1, PGC-1α peroxisome proliferator-activated receptor γ coactivator 1α, PPARδ peroxisome proliferator-activated receptor δ (Used with permission of UMass Amherst)
Peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), a downstream target of AMPK and SIRT1, regulates several downstream transcription factors, including peroxisome proliferator-activated receptor δ (PPARδ), nuclear respiratory factor-1 and -2 (NRF), estrogen-related receptor α (ERRα), and myocyte enhancer factor 2 (MEF2). These factors are important in initiating mitochondrial biogenesis and inducing fiber type transformation in the skeletal muscle [90, 91]. Further support for the significance of PGC-1α was provided in a study reporting that ectopically expressing PGC-1α in the skeletal muscle of transgenic mice induced the muscle fiber conversion of glycolytic fast-twitch type II fibers into oxidative slow-twitch type I fibers [92]. In a similar manner, the overexpression of PPARδ (a downstream regulator of PGC-1α) resulted in the development of slow-twitch type I fibers in skeletal muscle [93, 94]. The signaling cascade AMPK to PPARδ via PGC-1α is an important metabolic pathway involved in adaptive metabolism in the skeletal muscle. As such, we have focused primarily on this pathway to uncover the potential mechanism of CLA in skeletal muscle metabolism.
Overall Effects of CLA on Skeletal Muscle Metabolism
Previous studies using mouse models have clearly suggested that CLA is associated with a significant quantitative increase in lean mass [12, 95]. In addition, CLA supplementation up-regulates CPT1 and UCP2 from the skeletal muscle, suggesting that an overall increase in energy expenditure and fatty acid oxidation with CLA may contribute to the reduction in fat accumulation [52, 56, 95, 96]. CLA has also been reported to prevent age-associated skeletal muscle loss in aged rodents [19, 97]. The preventive role of CLA in muscle is further supported by our results in Fig. 1 with animals known to develop inactivity-induced obesity with muscle dystrophy. When a cognate of CLA (conjugated nonadecadienoic acid, known to have biological effects similar to those of CLA) was given to these animals, we observed an increase in voluntary activity and a reduction in body fat, as well as an increase in muscle size, suggesting that this treatment may have prevented muscle dystrophy typically associated with these animals [56, 98].
A cognate of CLA, conjugated nonadecadienoic acid (CNA), significantly prevented muscle dystrophy in animals with inactivity-induced obesity. a The data show that CNA supplementation (light gray bars) resulted in a reduced number of smaller muscles (less than 700 μm) and increased number of medium-sized muscles (between 1500 and 2100 μm) compared to controls (black bars). b CNA-fed animals had significantly increased average muscle size compared to Nhlh-2 knockout controls. *Significantly different at P < 0.05. Six-week-old female Nhlh-2 KO mice were fed either a control or CNA-containing diet (0.1 % w/w of diet) for 8 weeks [semi-purified powder diet, TD07518 (Teklad; Harlan Laboratories/Envigo, Madison, WI, USA) with "vitamin-free" tested casein to avoid the naturally occurring CLA in casein was used]. The diet consisted of an AIN-93-based diet with 20 % fat total as soybean oil. The thigh muscle, vastus lateralis, was frozen in liquid nitrogen, and frozen muscles were cut into 10-μm section using a Cryotome. The sections were stained with hematoxylin and eosin in order to visualize the muscle, and muscle size was measured (>500 fibers) with ImageJ software (NIH). Numbers are mean ± S. E (n = 3)
Effects of CLA on Adaptive Muscle Responses
There is currently limited evidence demonstrating the role of CLA in skeletal muscle metabolism [19, 21, 66, 99–102]. Supplementation of 1.2–2.0 % CLA in the diet of pigs was found to significantly increase expression levels of oxidative slow-twitch type I fiber, but did not increase the expression of glycolytic fast-twitch type IIb and IIx fibers in the pig’s skeletal muscle [103]. However, fiber type changes are dependent on the growth phase in pigs [104]. Similarly, Parra et al. [100] observed no CLA effect on PPARδ and muscle fiber change in mice. Given these limited studies, it cannot be conclusively stated that CLA promotes muscle fiber type transformation. However, along with the observation that CLA is linked to improved maximum endurance capacity in mice, it is highly likely that CLA influences muscle fiber type transformation [21, 68, 105, 106]. The effect of CLA on physical activity is further discussed below.
Effects of CLA on Molecular Responses of Muscle Metabolism
Several studies have reported the effects of CLA on the biochemical alteration of several molecular markers of muscle metabolism [19, 21, 66, 99–102]. CLA treatment was shown to activate AMPK in murine skeletal muscle cells [107–110], which negatively regulated ACC and enhanced fatty acid β-oxidation [107, 110]. One study reported that the cis-9,trans-11 CLA isomer activated AMPK at lower concentrations (~50 μM), while the trans-10,cis-12 isomer gradually activated AMPK in a dose-dependent manner up to 120 μM, and then plateaued [108]. However, the effect of CLA on SIRT1 activity in the skeletal muscle is currently not known [109].
CLA treatment did not affect the activity of PGC-1α, a primary regulator in mitochondrial biogenesis, even when the mitochondrial content in the human skeletal muscle cells was increased by CLA [101]. Similarly, CLA-fed mice and rats demonstrated no significant differences in PGC-1α compared to control groups [66, 100]. On the other hand, CLA treatment significantly up-regulated PGC-1α in murine skeletal muscle cells [109], supporting the contention that CLA supplementation significantly up-regulates molecular biomarkers such as succinate dehydrogenase, cytochrome c oxidase, superoxide dismutase 2, catalase, and glutathione peroxidase in the skeletal muscle, which is related to increased ATP production and thermogenesis via improved oxidative phosphorylation and anti-oxidative capacity in the rodent models [19, 66]. These results suggest that further confirmation is needed as to whether CLA treatment is associated with mitochondrial biogenesis through PGC-1α. Thus, further investigation is required, particularly in humans, for a better understanding of the correlation between CLA supplementation and muscle fiber type transformation. In addition, CLA—in particular, trans-10,cis-12—increased PPARδ expression in murine muscle cells and mice [21, 102]. While these results suggest that CLA may target muscle metabolism, no mechanistic studies have been completed to determine whether CLA directly or indirectly influences any of these molecular targets.
Effect of CLA on Physical Activity
Animal studies using CLA and exercise are summarized in Table 4. Studies using mice showed consistent effects of reduced body fat or increased lean mass. Moreover, there was a significant improvement in the exercise outcome with CLA treatment (Table 4) [21, 68, 105, 106, 111]. Specifically, Kim et al. [21] reported that the trans-10,cis-12 isomer was responsible for this effect, but not the cis-9,trans-11 isomer. This is consistent with the role of the trans-10,cis-12 isomer as the active isomer in body fat reduction [18]. In contrast, studies in rats observed no additional or synergistic effects of CLA treatment and exercise training on endurance capacity and lean body mass [43, 112]. This discrepancy was previously recognized as a consequence of the greater sensitivity of mice than of rats to CLA, partly due to differences in the administered CLA dose on a weight basis and/or differences in the physiology of animals (in particular, male rats continuously gain weight, and no significant effects of CLA have been reported for body fat) [113].
There are currently 17 CLA human intervention studies reporting on CLA with exercise, as summarized in Table 5. Among them, ten studies tested exercise outcomes [46, 61, 114–122]. Overall, the effect of CLA supplementation on exercise outcome varied across studies; six reported positive results [46, 61, 114, 116, 117, 120], while others reported no difference [115, 118, 119, 121, 122]. Four clinical trials evaluated the effect of CLA supplementation on physical activity, without a regular exercise regime [30, 48, 123, 124]. Among them, one study reported improved physical activity with CLA treatment over a period of 3 months [123]. In general, the studies were relatively short-term in nature (with the exception of two, they were less than 12 weeks in length), and thus no conclusion can be drawn as to whether the lack of effect was due to the limited supplementation periods or the ineffectiveness of CLA.
Four studies in humans evaluated the effects of co-supplementation of CLA with other supplements, such as creatine monohydrate, chromium picolinate, whey protein, or amino acids, along with exercise training [46, 117, 118, 125]. Two of these studies used CLA and creatine monohydrate for short- (5 weeks) or long-term (6 months) durations, accompanied by resistance training, and reported increased lean body mass and improved strength compared to the control group [46, 117].
Interestingly, Macaluso et al. [126] conducted a clinical trial to investigate the effect of CLA with resistance training on serum testosterone levels. The authors reported significantly increased serum testosterone and resistance exercise capability with CLA supplementation, with no significant change in body weight, fat mass, or lean body mass. Others have reported that testosterone can improve mitochondrial biogenesis and total energy expenditure, and that CLA supplementation was found to promote endurance capacity in trained mice via the upregulation of testosterone biosynthesis [106, 127]. Thus, it is possible that CLA improves exercise outcome by modulating testosterone; however, the Macaluso et al. [126] study may have been too short to have observed changes in body composition due to CLA. Generally, indications are that CLA may influence muscle metabolism, but mechanistic studies are currently lacking.
Potential Health Concerns of CLA
Based on the results of animal and human studies, four aspects of CLA supplementation are of concern: insulin sensitivity, oxidative stress, maternal milk fat, and liver function. These topics have been previously reviewed in detail [5, 14, 15]. Among these potential health concerns, the effects of CLA on glucose metabolism may affect the potential role of CLA in skeletal muscle metabolism, and effects are inconsistent in both animal and human studies. However, evidence suggests that the long-term use of CLA, particularly as a mixture of the two main isomers, will likely have no adverse influence on glucose metabolism [5, 45, 128].
Other health concerns associated with CLA do not directly involve the skeletal muscle metabolism, although this aspect is important in understanding the health impact of CLA. Reports of human studies have consistently linked CLA supplements to increased oxidative markers, particularly isoprostanes, but not to other biomarkers [5, 129, 130]. It has been suggested that CLA itself might be metabolized to structurally similar isoprostanes that cannot be distinguished from the isoprostanes used as oxidative markers [131, 132].
CLA is known to reduce body fat, and CLA supplementation has been reported to significantly reduce milk fat, particularly in cows [133, 134]. A limited number of human studies have reported none or minimal change in milk fat content after short-term CLA supplementation (less than 5 days) [135–137], and in light of the primary difference in milk fat origin between ruminants and humans, CLA is expected to have minimal effects on human milk fat [133, 134]. The long-term effects of CLA on human milk fat have yet to be determined.
In animal studies, there have been consistent observations of an enlarged liver with CLA feeding, but minimal changes have been reported in human studies [8, 65, 138–142]. While it is likely that the effect of CLA on the enlarged liver is specific to rodents, three human cases of hepatitis have been associated with CLA [143–145]. Thus, close monitoring of CLA supplementation with regard to the health of the liver will be important, particularly with long-term use.
Conclusion
To date, most mechanistic studies of the effects of CLA on body composition have focused on lipid metabolism in the adipose tissue. At the same time, a growing number of studies have highlighted the importance of CLA with respect to skeletal muscle metabolism, with effects including increased energy expenditure and enhanced physical activity. However, mechanistic studies investigating the mechanism by which CLA modulates skeletal muscle metabolism are very preliminary, and further investigation of the mechanistic effects of CLA on the skeletal muscle metabolism, including mitochondrial biogenesis and muscle fiber type transformation, is needed. We expect that knowledge of the effect of CLA on muscle metabolism will help to elucidate the preventive effects of CLA on obesity, along with current knowledge of its effects on adipose tissue. This knowledge will also support the potential application of CLA in the prevention of age-associated muscle loss, such as sarcopenia.
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- BMR:
-
Basic metabolic rate
- CLA:
-
Conjugated linoleic acid
- CPT:
-
Carnitine palmitoyltransferase
- ERR:
-
Estrogen-related receptor
- FOXO:
-
Forkhead box O
- GLUT4:
-
Glucose transporter type 4
- IL-6:
-
Interleukin 6
- LPL:
-
Lipoprotein lipase
- MEF2:
-
Myocyte enhancer factor 2
- MHC:
-
Myosin heavy chain
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NRF:
-
Nuclear respiratory factor
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ coactivator 1α
- PPARδ:
-
Peroxisome proliferator-activated receptor δ
- RMR:
-
Resting metabolic rate
- SIRT1:
-
Silent information regulator two protein 1
- TAG:
-
Triglyceride
- TNF-α:
-
Tumor necrosis factor α
- UCP:
-
Uncoupling protein
References
Parodi PW (1999) Conjugated linoleic acid: the early years. In: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ (eds) Advances in conjugated linoleic acid research. AOCS Press, Urbana
Kepler CR, Hirons KP, McNeill JJ, Tove SB (1966) Intermediates and products of the biohydrogenation of linoleic acid by Butyrinvibrio fibrisolvens. J Biol Chem 241(6):1350–1354
Kay JK, Mackle TR, Auldist MJ, Thomson NA, Bauman DE (2004) Endogenous synthesis of cis-9, trans-11 conjugated linoleic acid in dairy cows fed fresh pasture. J Dairy Sci 87(2):369–378
Chin S, Liu W, Storkson J, Ha Y, Pariza M (1992) Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J Food Compos Anal 5(3):185–197
Park Y (2014) Chapter 37—Conjugated linoleic acid in human health effects on weight control. In: Watson RR (ed) Nutrition in the prevention and treatment of abdominal obesity. Academic Press, Dublin
Zlatanos SN, Laskaridis K, Sagredos A (2008) Conjugated linoleic acid content of human plasma. Lipids Health Dis 7:34. doi:10.1186/1476-511X-7-34
Mele MC, Cannelli G, Carta G, Cordeddu L, Melis MP, Murru E, Stanton C, Banni S (2013) Metabolism of c9 t11-conjugated linoleic acid (CLA) in humans. Prostaglandins Leukot Essent Fat Acids (PLEFA) 89(2):115–119
Dilzer A, Park Y (2012) Implication of conjugated linoleic acid (CLA) in human health. Crit Rev Food Sci Nutr 52(6):488–513
Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G (2006) Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem 17(12):789–810
Wahle KW, Heys SD, Rotondo D (2004) Conjugated linoleic acids: are they beneficial or detrimental to health? Prog Lipid Res 43(6):553–587
US Food and Drug Administration (2015) Nutrition labeling: Trans Fat Labeling. http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm064904.htm#transfat. Accessed 26 Aug 2015
Park Y, Albright K, Liu W, Storkson J, Cook M, Pariza M (1997) Effect of conjugated linoleic acid on body composition in mice. Lipids 32(8):853–858
McCrorie TA, Keaveney EM, Wallace JM, Binns N, Livingstone MBE (2011) Human health effects of conjugated linoleic acid from milk and supplements. Nutr Res Rev 24(02):206–227
Park Y (2009) Conjugated linoleic acid (CLA): good or bad trans fat? J Food Compos Anal 22:S4–S12
Pariza M (2004) Perspective on the safety and effectiveness of conjugated linoleic acid. Am J Clin Nutr 79(6):1132S–1136S
Park Y, Pariza MW (2007) Mechanisms of body fat modulation by conjugated linoleic acid (CLA). Food Res Int 40(3):311–323
Kennedy A, Martinez K, Schmidt S, Mandrup S, LaPoint K, McIntosh M (2010) Antiobesity mechanisms of action of conjugated linoleic acid. J Nutr Biochem 21(3):171–179
Park Y, Storkson J, Albright K, Liu W, Pariza M (1999) Evidence that the trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids 34(3):235–241
Rahman MM, Halade GV, El Jamali A, Fernandes G (2009) Conjugated linoleic acid (CLA) prevents age-associated skeletal muscle loss. Biochem Biophys Res Commun 383(4):513–518
Halade GV, Rahman MM, Fernandes G (2010) Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57Bl/6J mice. J Nutr Biochem 21(4):332–337
Kim JH, Kim J, Park Y (2012) trans-10, cis-12 conjugated linoleic acid enhances endurance capacity by increasing fatty acid oxidation and reducing glycogen utilization in mice. Lipids 47(9):855–863
Stangl GI (2000) Conjugated linoleic acids exhibit a strong fat-to-lean partitioning effect, reduce serum VLDL lipids and redistribute tissue lipids in food-restricted rats. J Nutr 130(5):1140–1146
Dugan M, Aalhus J, Schaefer A, Kramer J (1997) The effect of conjugated linoleic acid on fat to lean repartitioning and feed conversion in pigs 77(4):723–725
Dugan M, Aalhus J, Jeremiah L, Kramer J, Schaefer A (1999) The effects of feeding conjugated linoleic acid on subsequent pork quality 79(1):45–51
Dunshea F, Ostrowska E, Muralitharan M, Cross R, Bauman D, Pariza M, Skarie C (1998) Dietary conjugated linoleic acid decreases back fat in finisher gilts. J Anim Sci 76(Suppl 1):131
Tischendorf F, Schöne F, Möckel P, Jahreis G (1999) The effect of conjugated linoleic acid on porcine growth, body composition, and fatty acids distribution in backfat, muscle and liver. In: Symposium Vitamine und Zusatzstoffe in der Ernährung von Mensch und Tier, pp 244–249
Ostrowska E, Muralitharan M, Cross RF, Bauman DE, Dunshea FR (1999) Dietary conjugated linoleic acids increase lean tissue and decrease fat deposition in growing pigs. J Nutr 129(11):2037–2042
Kamphuis MM, Lejeune MP, Saris WH, Westerterp-Plantenga MS (2003) The effect of conjugated linoleic acid supplementation after weight loss on body weight regain, body composition, and resting metabolic rate in overweight subjects. Int J Obes 27(7):840–847
Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O (2004) Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am J Clin Nutr 79(6):1118–1125
Gaullier J, Halse J, Høivik HO, Høye K, Syvertsen C, Nurminiemi M, Hassfeld C, Einerhand A, O’Shea M, Gudmundsen O (2007) Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr 97(03):550–560
Raff M, Tholstrup T, Toubro S, Bruun JM, Lund P, Straarup EM, Christensen R, Sandberg MB, Mandrup S (2009) Conjugated linoleic acids reduce body fat in healthy postmenopausal women. J Nutr 139(7):1347–1352
Racine NM, Watras AC, Carrel AL, Allen DB, McVean JJ, Clark RR, O’Brien AR, O’Shea M, Scott CE, Schoeller DA (2010) Effect of conjugated linoleic acid on body fat accretion in overweight or obese children. Am J Clin Nutr 91(5):1157–1164
Steck SE, Chalecki AM, Miller P, Conway J, Austin GL, Hardin JW, Albright CD, Thuillier P (2007) Conjugated linoleic acid supplementation for twelve weeks increases lean body mass in obese humans. J Nutr 137(5):1188–1193
Sneddon AA, Tsofliou F, Fyfe CL, Matheson I, Jackson DM, Horgan G, Winzell MS, Wahle KWJ, Ahren B, Williams LM (2008) Effect of a conjugated linoleic acid and omega-3 fatty acid mixture on body composition and adiponectin. Obesity 16(5):1019–1024
Schoeller DA, Watras AC, Whigham LD (2009) A meta-analysis of the effects of conjugated linoleic acid on fat-free mass in humans. Appl Physiol, Nutr, Metab 34(5):975–978
Park Y, Albright KJ, Storkson JM, Liu W, Cook ME, Pariza MW (1999) Changes in body composition in mice during feeding and withdrawal of conjugated linoleic acid. Lipids 34(3):243–248
Pariza M, Park Y, Cook M (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40(4):283–298
Ryder JW, Portocarrero CP, Song XM, Cui L, Yu M, Combatsiaris T, Galuska D, Bauman DE, Barbano DM, Charron MJ, Zierath JR, Houseknecht KL (2001) Isomer-specific antidiabetic properties of conjugated linoleic acid—improved glucose tolerance, skeletal muscle insulin action, and UCP-2 gene expression. Diabetes 50(5):1149–1157
Gudbrandsen OA, Rodriguez E, Wergedahl H, Mørk S, Reseland JE, Skorve J, Palou A, Berge RK (2009) Trans-10, cis-12-conjugated linoleic acid reduces the hepatic triacylglycerol content and the leptin mRNA level in adipose tissue in obese Zucker fa/fa rats. Br J Nutr 102(06):803–815
Choi JS, Jung MH, Park HS, Song J (2004) Effect of conjugated linoleic acid isomers on insulin resistance and mRNA levels of genes regulating energy metabolism in high-fat–fed rats. Nutrition 20(11):1008–1017
Inoue N, Nagao K, Wang Y, Noguchi H, Shirouchi B, Yanagita T (2006) Dietary conjugated linoleic acid lowered tumor necrosis factor-α content and altered expression of genes related to lipid metabolism and insulin sensitivity in the skeletal muscle of Zucker rats. J Agric Food Chem 54(20):7935–7939
Park Y, Albright KJ, Storkson JM, Liu W, Pariza MW (2010) Effects of dietary conjugated linoleic acid (CLA) on spontaneously hypertensive rats. J Funct Foods 2(1):54–59
Mirand PP, Mosoni L, Arnal-Bagnard M, Faulconnier Y, Chardigny J, Chilliard Y (2006) Dietary conjugated linoleic acid has limited effects on tissue protein anabolism in sedentary and exercising adult rats 46(6):621–632
Park Y, Albright K, Storkson J, Liu W, Pariza M (2007) Conjugated linoleic acid (CLA) prevents body fat accumulation and weight gain in an animal model. J Food Sci 72(8):S612–S617
Gaullier JM, Halse J, Hoye K, Kristiansen K, Fagertun H, Vik H, Gudmundsen O (2005) Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr 135(4):778–784
Cornish SM, Candow DG, Jantz NT, Chilibeck PD, Little JP, Forbes S, Abeysekara S, Zello GA (2009) Conjugated linoleic acid combined with creatine monohydrate and whey protein supplementation during strength training. Int J Sport Nutr 19(1):79
Malpuech-Brugère C, de Venne Verboeket-van, Wilhelmine PHG, Mensink RP, Arnal M, Morio B, Brandolini M, Saebo A, Lassel TS, Chardigny JM, Sébédio JL (2004) Effects of two conjugated linoleic acid isomers on body fat mass in overweight humans. Obes Res 12(4):591–598
Watras A, Buchholz A, Close R, Zhang Z, Schoeller D (2006) The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes 31(3):481–487
Thrush AB, Chabowski A, Heigenhauser GJ, McBride BW, Or-Rashid M, Dyck DJ (2007) Conjugated linoleic acid increases skeletal muscle ceramide content and decreases insulin sensitivity in overweight, non-diabetic humans. Appl Physiol, Nutr, Metab 32(3):372–382
Dhurandhar NV, Schoeller DA, Brown AW, Heymsfield SB, Thomas D, Sorensen TI, Speakman JR, Jeansonne M, Allison DB, Energy Balance Measurement Working Group (2015) Response to ‘Energy balance measurement: when something is not better than nothing’. Int J Obes (Lond) 39(7):1175–1176
West DB, Delany JP, Camet PM, Blohm F, Truett AA, Scimeca J (1998) Effects of conjugated linoleic acid on body fat and energy metabolism in the mouse. Am J Physiol 275(3 Pt 2):R667–R672
West DB, Blohm FY, Truett AA, DeLany JP (2000) Conjugated linoleic acid persistently increases total energy expenditure in AKR/J mice without increasing uncoupling protein gene expression. J Nutr 130(10):2471–2477
Ohnuki K, Haramizu S, Ishihara K, Fushiki T (2001) Increased energy metabolism and suppressed body fat accumulation in mice by a low concentration of conjugated linoleic acid. Biosci Biotechnol Biochem 65(10):2200–2204
Ohnuki K, Haramizu S, Oki K, Ishihara K, Fushiki T (2001) A single oral administration of conjugated linoleic acid enhanced energy metabolism in mice. Lipids 36(6):583–587
Park Y, Park Y (2010) Conjugated nonadecadienoic acid is more potent than conjugated linoleic acid on body fat reduction. J Nutr Biochem 21(8):764–773
Park Y, Park Y (2012) Conjugated fatty acids increase energy expenditure in part by increasing voluntary movement in mice. Food Chem 133(2):400–409
Bhattacharya A, Rahman MM, Sun D, Lawrence R, Mejia W, McCarter R, O’Shea M, Fernandes G (2005) The combination of dietary conjugated linoleic acid and treadmill exercise lowers gain in body fat mass and enhances lean body mass in high fat-fed male Balb/C mice. J Nutr 135(5):1124–1130
Nagao K, Wang Y, Inoue N, Han S, Buang Y, Noda T, Kouda N, Okamatsu H, Yanagita T (2003) The 10trans, 12cis isomer of conjugated linoleic acid promotes energy metabolism in OLETF rats. Nutrition 19(7):652–656
Nazare J, Perrière, de la AB, Bonnet F, Desage M, Peyrat J, Maitrepierre C, Louche-Pelissier C, Bruzeau J, Goudable J, Lassel T (2007) Daily intake of conjugated linoleic acid-enriched yoghurts: effects on energy metabolism and adipose tissue gene expression in healthy subjects. Br J Nutr 97(02):273–280
Close RN, Schoeller DA, Watras AC, Nora EH (2007) Conjugated linoleic acid supplementation alters the 6-mo change in fat oxidation during sleep. Am J Clin Nutr 86(3):797–804
Pinkoski C, Chilibeck PD, Candow DG, Esliger D, Ewaschuk JB, Facci M, Farthing JP, Zello GA (2006) The effects of conjugated linoleic acid supplementation during resistance training. Med Sci Sports Exerc 38(2):339
Lambert EV, Goedecke JH, Bluett K, Heggie K, Claassen A, Rae DE, West S, Dugas J, Dugas L, Meltzer S (2007) Conjugated linoleic acid versus high-oleic acid sunflower oil: effects on energy metabolism, glucose tolerance, blood lipids, appetite and body composition in regularly exercising individuals. Br J Nutr 97(05):1001–1011
Zambell KL, Keim NL, Van Loan MD, Gale B, Benito P, Kelley DS, Nelson GJ (2000) Conjugated linoleic acid supplementation in humans: effects on body composition and energy expenditure. Lipids 35(7):777–782
Ealey K, El-Sohemy A, Archer M (2002) Effects of dietary conjugated linoleic acid on the expression of uncoupling proteins in mice and rats. Lipids 37(9):853–861
Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, Kim HJ, Tange T, Okuyama H, Kasai M, Ikemoto S, Ezaki O (2000) Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes 49(9):1534–1542
Choi JS, Koh I, Jung MH, Song J (2007) Effects of three different conjugated linoleic acid preparations on insulin signalling, fat oxidation and mitochondrial function in rats fed a high-fat diet. Br J Nutr 98(2):264–275
Adams S, Pan G, Yu X (2001) Perspectives on the biology of uncoupling protein (UCP) homologues. Biochem Soc Trans 29(5):100
Mizunoya W, Haramizu S, Shibakusa T, Okabe Y, Fushiki T (2005) Dietary conjugated linoleic acid increases endurance capacity and fat oxidation in mice during exercise. Lipids 40(3):265–271
Tsao J, Liao S, Korivi M, Hou C, Kuo C, Wang H, Cheng I (2014) Oral conjugated linoleic acid supplementation enhanced glycogen resynthesis in exercised human skeletal muscle. J Sports Sci (ahead-of-print):1–9
Bouthegourd JC, Even PC, Gripois D, Tiffon B, Blouquit MF, Roseau S, Lutton C, Tome D, Martin JC (2002) A CLA mixture prevents body triglyceride accumulation without affecting energy expenditure in Syrian hamsters. J Nutr 132(9):2682–2689
Degrace P, Demizieux L, Gresti J, Chardigny JM, Sebedio JL, Clouet P (2004) Hepatic steatosis is not due to impaired fatty acid oxidation capacities in C57BL/6J mice fed the conjugated trans-10, cis-12-isomer of linoleic acid. J Nutr 134(4):861–867
Nagao K, Inoue N, Wang YM, Shirouchi B, Yanagita T (2005) Dietary conjugated linoleic acid alleviates nonalcoholic fatty liver disease in Zucker (fa/fa) rats. J Nutr 135(1):9–13
Peters JM, Park Y, Gonzalez FJ, Pariza MW (2001) Influence of conjugated linoleic acid on body composition and target gene expression in peroxisome proliferator-activated receptor α-null mice 1533(3):233–242
Ribot J, Portillo MP, Picó C, Teresa Macarulla M, Palou A (2007) Effects of trans-10, cis-12 conjugated linoleic acid on the expression of uncoupling proteins in hamsters fed an atherogenic diet. Br J Nutr 97(06):1074–1082
Salmons S, Henriksson J (1981) The adaptive response of skeletal muscle to increased use. Muscle Nerve 4(2):94–105
Hänninen O, Atalay M (1998) Oxidative metabolism in skeletal muscle. In: Anonymous oxidative stress in skeletal muscle, Springer
Westerblad H, Bruton JD, Katz A (2010) Skeletal muscle: energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res 316(18):3093–3099
Felig P, Wahren J (1975) Fuel homeostasis in exercise. N Engl J Med 293(21):1078–1084
Wahren J (1977) Glucose turnover during exercise in man*. Ann NY Acad Sci 301(1):45–55
Esséen B (1977) Intramuscular substrate utilization during prolonged exercise*. Ann NY Acad Sci 301(1):30–44
Sahlin K, Tonkonogi M, Söderlund K (1998) Energy supply and muscle fatigue in humans. Acta Physiol Scand 162(3):261–266
Cantó C, Auwerx J (2010) AMP-activated protein kinase and its downstream transcriptional pathways 67(20):3407–3423
Spangenburg E, Booth F (2003) Molecular regulation of individual skeletal muscle fibre types. Acta Physiol Scand 178(4):413–424
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91(4):1447–1531
Brooke MH, Kaiser KK (1970) Muscle fiber types: how many and what kind? Arch Neurol 23(4):369–379
Hood DA, Irrcher I, Ljubicic V, Joseph A (2006) Coordination of metabolic plasticity in skeletal muscle. J Exp Biol 209(12):2265–2275
Hardie DG, Sakamoto K (2006) AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda) 21:48–60
Kwon H, Ott M (2008) The ups and downs of SIRT1. Trends Biochem Sci 33(11):517–525
Ruderman NB, Xu XJ, Nelson L, Cacicedo JM, Saha AK, Lan F, Ido Y (2010) AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab 298(4):E751–E760
Lin J, Handschin C, Spiegelman BM (2005) Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1(6):361–370
Olesen J, Kiilerich K, Pilegaard H (2010) PGC-1α-mediated adaptations in skeletal muscle. Pflüg Arch-Eur J Physiol 460(1):153–162
Lin J, Wu H, Tarr PT, Zhang CY, Wu ZD, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418(6899):797–801
Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA (2003) Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 17(15):2299–2301
Wang Y, Zhang C, Yu R, Cho H, Nelson M, Bayuga-Ocampo C, Ham J, Kang H, Evans R (2004) Regulation of muscle fiber type and running endurance by PPAR delta. PLoS Biol 2(10):1532–1539
Terpstra AH, Beynen AC, Everts H, Kocsis S, Katan MB, Zock PL (2002) The decrease in body fat in mice fed conjugated linoleic acid is due to increases in energy expenditure and energy loss in the excreta. J Nutr 132(5):940–945
Kim JH, Gilliard D, Good DJ, Park Y (2012) Preventive effects of conjugated linoleic acid on obesity by improved physical activity in nescient basic helix-loop-helix 2 knockout mice during growth period. Food Funct 3(12):1280–1285
Halade GV, Rahman MM, Fernandes G (2009) Effect of CLA isomers and their mixture on aging C57Bl/6J mice. Eur J Nutr 48(7):409–418
Kim JH, Park Y, Kim D, Good DJ, Park Y (2013) Dietary conjugated nonadecadienoic acid prevents adult-onset obesity in nescient basic helix-loop-helix 2 knockout mice. J Nutr Biochem 24(3):556–566
Kim Y, Kim D, Good DJ, Park Y (2015) Effects of post-weaning administration of conjugated linoleic acid on development of obesity in nescient basic helix-loop-helix 2 knockout mice. J Agric Food Chem 63:5212–5223
Parra P, Serra F, Palou A (2012) Transcriptional analysis reveals a high impact of conjugated linoleic acid on stearoyl-Coenzyme A desaturase 1 mRNA expression in mice gastrocnemius muscle. Genes Nutr 7(4):537–548
Vaughan RA, Garcia-Smith R, Bisoffi M, Conn CA, Trujillo KA (2012) Conjugated linoleic acid or omega 3 fatty acids increase mitochondrial biosynthesis and metabolism in skeletal muscle cells. Lipids Health Dis 11:142
Oraldi M, Maggiora M, Paiuzzi E, Canuto RA, Muzio G (2013) CLA Reduces inflammatory mediators from A427 human lung cancer cells and A427 conditioned medium promotes differentiation of C2C12 murine muscle cells. Lipids 48(1):29–38
Huang J, Qi R, Chen X, You X, Liu X, Yang F, Liu Z (2014) Improvement in the carcass traits and meat quality of growing-finishing Rongchang pigs by conjugated linoleic acid through altered gene expression of muscle fiber types. Genet Mol Res: GMR 13(3):7061–7069
Men X, Deng B, Xu Z, Tao X, Qi K (2013) Age-related changes and nutritional regulation of myosin heavy-chain composition in longissimus dorsi of commercial pigs. Animal 7(09):1486–1492
Kim JH, Park HG, Pan JH, Kim SH, Yoon HG, Bae GS, Lee H, Eom S, Kim YJ (2010) Dietary conjugated linoleic acid increases endurance capacity of mice during treadmill exercise 13(5):1057–1060
Barone R, Macaluso F, Catanese P, Gammazza AM, Rizzuto L, Marozzi P, Giudice GL, Stampone T, Cappello F, Morici G (2013) Endurance exercise and Conjugated Linoleic Acid (CLA) supplementation up-regulate CYP17A1 and stimulate testosterone biosynthesis 8(11):e79686
Mohankumar SK, Taylor CG, Siemens L, Zahradka P (2013) Activation of phosphatidylinositol-3 kinase, AMP-activated kinase and Akt substrate-160 kDa by trans-10, cis-12 conjugated linoleic acid mediates skeletal muscle glucose uptake. J Nutr Biochem 24(2):445–456
Mohankumar SK, Taylor CG, Siemens L, Zahradka P (2012) Acute exposure of L6 myotubes to cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid isomers stimulates glucose uptake by modulating Ca2+/calmodulin-dependent protein kinase II. Int J Biochem Cell Biol 44(8):1321–1330
Kim Y, Park Y (2015) Conjugated linoleic acid (CLA) stimulates mitochondrial biogenesis signaling by the upregulation of PPARγ coactivator 1α (PGC-1α) in C2C12 cells. Lipids 50(4):329–338
Qin H, Liu Y, Lu N, Li Y, Sun C (2009) cis-9, trans-11-conjugated linoleic acid activates AMP-activated protein kinase in attenuation of insulin resistance in C2C12 myotubes. J Agric Food Chem 57(10):4452–4458
Hur SJ, Kim DH, Chun SC, Lee SK (2013) Effects of dietary conjugated linoleic acid and biopolymer encapsulation on lipid metabolism in mice. Int J Mol Sci 14(4):6848–6862
Salgado JM, Ferreira TRB, Donado-Pestana CM, de Almeida OC, Das Neves, Aline Mouro Ribeiro, Mansi DN, Dias, dos Santos CT (2012) Conjugated linoleic acid combined with physical activity reduces body fat accumulation but does not modify lean body mass in male and female Wistar rats. J Med Food 15(4):406–412
Park Y (1996) Regulation of energy metabolism and the catabolic effects of immune stimulation by conjugated linocleic acid
Blankson H, Stakkestad JA, Fagertun H, Thom E, Wadstein J, Gudmundsen O (2000) Conjugated linoleic acid reduces body fat mass in overweight and obese humans. J Nutr 130(12):2943–2948
Kreider RB, Ferreira MP, Greenwood M, Wilson M, Almada AL (2002) Effects of conjugated linoleic acid supplementation during resistance training on body composition, bone density, strength, and selected hematological markers. J Strength Cond Res 16(3):325–334
Colakoglu S, Colakoglu M, Taneli F, Cetinoz F, Turkmen M (2006) Cumulative effects of conjugated linoleic acid and exercise on endurance development, body composition, serum leptin and insulin levels. J Sports Med Phys Fit 46(4):570–577
Tarnopolsky M, Zimmer A, Paikin J, Safdar A, Aboud A, Pearce E, Roy B, Doherty T (2007) Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS One 2(10):e991
Diaz ML, Watkins BA, Li Y, Anderson RA, Campbell WW (2008) Chromium picolinate and conjugated linoleic acid do not synergistically influence diet-and exercise-induced changes in body composition and health indexes in overweight women. J Nutr Biochem 19(1):61–68
Chen S, Lin Y, Huang H, Hsu W, Houng J, Huang C (2012) Effect of conjugated linoleic acid supplementation on weight loss and body fat composition in a Chinese population. Nutrition 28(5):559–565
Macaluso F, Barone R, Catanese P, Carini F, Rizzuto L, Farina F, Di Felice V (2013) Do fat supplements increase physical performance? Nutrients 5(2):509–524
Jenkins NDM, Buckner SL, Baker RB, Bergstrom HC, Cochrane KC, Weir JP, Housh TJ, Cramer JT (2014) Effects of 6 weeks of aerobic exercise combined with conjugated linoleic acid on the physical working capacity at fatigue threshold. J Strength Cond Res 28(8):2127–2135
Jenkins NDM, Buckner SL, Cochrane KC, Bergstrom HC, Goldsmith JA, Weir JP, Housh TJ, Cramer JT (2014) CLA supplementation and aerobic exercise lower blood triacylglycerol, but have no effect on peak oxygen uptake or cardiorespiratory fatigue thresholds. Lipids 49(9):871–880
Ha YL, Jeong SB (2010) Effects of conjugated linoleic acid on body fat reduction and physical exercise enhancement of obese male middle school students. J Life Sci 20:1844–1850
Tajmanesh M, Aryaeian N, Hosseini M, Mazaheri R, Kordi R (2015) Conjugated linoleic acid supplementation has no impact on aerobic capacity of healthy young men. Lipids:1–5
Michishita T, Kobayashi S, Katsuya T, Ogihara T, Kawabuchi K (2010) Evaluation of the antiobesity effects of an amino acid mixture and conjugated linoleic acid on exercising healthy overweight humans: a randomized, double-blind, placebo-controlled trial. J Int Med Res 38(3):844–859
Macaluso F, Morici G, Catanese P, Ardizzone NM, Marino Gammazza A, Bonsignore G, Lo Giudice G, Stampone T, Barone R, Farina F, Di Felice V (2012) Effect of conjugated linoleic acid on testosterone levels in vitro and in vivo after an acute bout of resistance exercise. J Strength Cond Res 26(6):1667–1674
Usui T, Kajita K, Kajita T, Mori I, Hanamoto T, Ikeda T, Okada H, Taguchi K, Kitada Y, Morita H (2014) Elevated mitochondrial biogenesis in skeletal muscle is associated with testosterone-induced body weight loss in male mice. FEBS Lett 588(10):1935–1941
Whigham L, O’shea M, Mohede I, Walaski H, Atkinson R (2004) Safety profile of conjugated linoleic acid in a 12-month trial in obese humans. Food Chem Toxicol 42(10):1701–1709
Pfeuffer M, Fielitz K, Laue C, Winkler P, Rubin D, Helwig U, Giller K, Kammann J, Schwedhelm E, Boeger RH, Bub A, Bell D, Schrezenmeir J (2011) CLA does not impair endothelial function and decreases body weight as compared with safflower oil in overweight and obese male subjects. J Am Coll Nutr 30(1):19–28
Kim J, Paik H, Shin M, Park E (2012) Eight weeks of conjugated linoleic acid supplementation has no effect on antioxidant status in healthy overweight/obese Korean individuals. Eur J Nutr 51(2):135–141
Banni S, Petroni A, Blasevich M, Carta G, Cordeddu L, Murru E, Melis M, Mahon A, Belury M (2004) Conjugated linoleic acids (CLA) as precursors of a distinct family of PUFA. Lipids 39(11):1143–1146
Turpeinen AM, Mutanen M, Aro A, Salminen I, Basu S, Palmquist DL, Griinari JM (2002) Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am J Clin Nutr 76(3):504–510
Bernard L, Leroux C, Chilliard Y (2008) Expression and nutritional regulation of lipogenic genes in the ruminant lactating mammary gland. Bioact Compon Milk 606:67–108
Bauman D, Griinari J (2003) Nutritional regulation of milk fat synthesis. Annu Rev Nutr 23:203–227
Hasin A, Griinari JM, Williams JE, Shahin AM, McGuire MA, McGuire MK (2007) Consumption of c9, t11–18: 2 or t10, c12–18: 2 enriched dietary supplements does not influence milk macronutrients in healthy, lactating women. Lipids 42(9):835–843
Mosley SA, Shahin AM, Williams J, McGuire MA, McGuire MK (2007) Supplemental conjugated linoleic acid consumption does not influence milk macronutrient contents in all healthy lactating women. Lipids 42(8):723–729
Masters N, McGuire MA, Beerman KA, Dasgupta N, McGuire MK (2002) Maternal supplementation with CLA decreases milk fat in humans. Lipids 37(2):133–138
Belury MA, Kempa-Steczko A (1997) Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids 32(2):199–204
Jaudszus A, Moeckel P, Hamelmann E, Jahreis G (2010) Trans-10, cis-12-CLA-caused lipodystrophy is associated with profound changes of fatty acid profiles of liver, white adipose tissue and erythrocytes in mice: possible link to tissue-specific alterations of fatty acid desaturation. Ann Nutr Metab 57(2):103–111
Larsen TM, Toubro S, Astrup A (2003) Efficacy and safety of dietary supplements containing CLA for the treatment of obesity: evidence from animal and human studies. J Lipid Res 44(12):2234–2241
Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, Ernst E (2012) The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials. Eur J Nutr 51(2):127–134
Wanders AJ, Leder L, Banga JD, Katan MB, Brouwer IA (2010) A high intake of conjugated linoleic acid does not affect liver and kidney function tests in healthy human subjects. Food Chem Toxicol 48(2):587–590
Ramos R, Mascarenhas J, Duarte P, Vicente C, Casteleiro C (2009) Conjugated linoleic acid-induced toxic hepatitis: first case report. Dig Dis Sci 54(5):1141–1143
Nortadas R, Barata J (2012) Fulminant hepatitis during self-medication with conjugated linoleic acid. Ann Hepatol 11(2):265–267
Bilal M, Patel Y, Burkitt M, Babich M (2015) Linoleic acid induced acute hepatitis: a case report and review of the literature. Case Reports Hepatol 2015:807354
DeLany JP, Blohm F, Truett AA, Scimeca JA, West DB (1999) Conjugated linoleic acid rapidly reduces body fat content in mice without affecting energy intake. Am J Physiol 276(4 Pt 2):R1172–R1179
Park Y, Pariza MW (2001) Lipoxygenase inhibitors inhibit heparin-releasable lipoprotein lipase activity in 3T3-L1 adipocytes and enhance body fat reduction in mice by conjugated linoleic acid. Biochim et Biophys Acta (BBA)-Mol Cell Biol Lipids 1534(1):27–33
Park Y, Pariza MW (2001) The effects of dietary conjugated nonadecadienoic acid on body composition in mice. Biochim et Biophys Acta (BBA)-Mol Cell Biol Lipids 1533(3):171–174
Ntambi JM, Choi Y, Park Y, Peters JM, Pariza MW (2002) Effects of conjugated linoleic acid (CLA) on immune responses, body composition and stearoyl-CoA desaturase. Can J Appl Physiol 27(6):617–627
Hayman A, MacGibbon A, Pack RJ, Rutherfurd K, Green JH (2002) High intake, but not low intake, of CLA impairs weight gain in growing mice. Lipids 37(7):689–692
Warren JM, Simon VA, Bartolini G, Erickson KL, Mackey BE, Kelley DS (2003) Trans-10, cis-12 CLA increases liver and decreases adipose tissue lipids in mice: possible roles of specific lipid metabolism genes. Lipids 38(5):497–504
Chardigny JM, Hasselwander O, Genty M, Kraemer K, Ptock A, Sededio JL (2003) Effect of conjugated FA on feed intake, body composition, and liver FA in mice. Lipids 38(9):895–902
Terpstra AHM, Javadi M, Beynen AC, Kocsis S, Lankhorst AE, Lemmens AG, Mohede ICM (2003) Dietary conjugated linoleic acids as free fatty acids and triacylglycerols similarly affect body composition and energy balance in mice. J Nutr 133(10):3181–3186
Hargrave KM, Meyer BJ, Li C, Azain MJ, Baile CA, Miner JL (2004) Influence of dietary conjugated linoleic acid and fat source on body fat and apoptosis in mice*. Obes Res 12(9):1435–1444
Park Y, Storkson JM, Liu W, Albright KJ, Cook ME, Pariza MW (2004) Structure–activity relationship of conjugated linoleic acid and its cognates in inhibiting heparin-releasable lipoprotein lipase and glycerol release from fully differentiated 3T3-L1 adipocytes. J Nutr Biochem 15(9):561–568
Javadi M, Beynen AC, Hovenier R, Lankhorst AE, Lemmens AG, Terpstra AHM, Geelen MJH (2004) Prolonged feeding of mice with conjugated linoleic acid increases hepatic fatty acid synthesis relative to oxidation. J Nutr Biochem 15(11):680–687
Ohashi A, Matsushita Y, Shibata H, Kimura K, Miyashita K, Saito M (2004) Conjugated linoleic acid deteriorates insulin resistance in obese/diabetic mice in association with decreased production of adiponectin and leptin. J Nutr Sci Vitaminol 50(6):416–421
Javadi M, Everts H, Hovenier R, Kocsis S, Lankhorst AE, Lemmens AG, Schonewille JT, Terpstra AHM, Beynen AC (2004) The effect of six different C18 fatty acids on body fat and energy metabolism in mice. Br J Nutr 92(3):391–399
Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW (2005) Biological activities of conjugated fatty acids: conjugated eicosadienoic (conj. 20: 2 Delta (c11, t13/t12, c14)) eicosatrienoic (conj. 20: 3 Delta (c8, t12, c14)) and heneicosadienoic (conj. 21: 2 Delta (c12, t144/c13, t15)) acids and other metabolites of conjugated linoleic acid. Biochim et Biophys Acta (BBA)-Mol Cell Biol Lipids 1687(1–3):120–129
de Roos B, Rucklidge G, Reid M, Ross K, Duncan G, Navarro MA, Arbones-Mainar JM, Guzman-Garcia MA, Osada J, Browne J, Loscher CE, Roche HM (2005) Divergent mechanisms of cis9, trans11-and trans10, cis12-conjugated linoleic acid affecting insulin resistance and inflammation in apolipoprotein E knockout mice: a proteomics approach. FASEB J 19(9):1746
Hargrave KM, Azain MJ, Miner JL (2005) Dietary coconut oil increases conjugated linoleic acid-induced body fat loss in mice independent of essential fatty acid deficiency. Biochim et Biophys Acta (BBA)-Mol Cell Biol Lipids 1737(1):52–60
Winzell MS, Pacini G, Ahren B (2006) Insulin secretion after dietary supplementation with conjugated linoleic acids and n-3 polyunsaturated fatty acids in normal and insulin-resistant mice. Am J Physiol-Endocrinol Metab 290(2):E347–E354
Bhattacharya A, Rahman MM, McCarter R, O’Shea M, Fernandes G (2006) Conjugated linoleic acid and chromium lower body weight and visceral fat mass in high-fat-diet-fed mice. Lipids 41(5):437–444
Viswanadha S, McGilliard ML, Herbein JH (2006) Desaturation indices in liver, muscle, and bone of growing male and female mice fed trans-10, cis-12 conjugated linoleic acid. Lipids 41(8):763–770
Rahman MM, Bhattacharya A, Banu J, Fernandes G (2007) Conjugated linoleic acid protects against age-associated bone loss in C57BL/6 female mice. J Nutr Biochem 18(7):467–474
Javadi M, Geelen MJH, Everts H, Lemmens AG, Beynen AC (2007) Body composition and selected blood parameters in mice fed a combination of fibre and conjugated linoleic acid. J Anim Physiol Anim Nutr 91(11–12):492–497
Hur S, Whitcomb F, Rhee S, Park Y, Good DJ, Park Y (2009) Effects of trans-10, cis-12 conjugated linoleic acid on body composition in genetically obese mice. J Med Food 12(1):56–63
Andreoli MF, Gonzalez MA, Martinelli MI, Mocchiutti NO, Bernal CA (2009) Effects of dietary conjugated linoleic acid at high-fat levels on triacylglycerol regulation in mice. Nutrition 25(4):445–452
Moon H, Lee H, Seo J, Chung C, Kim T, Choi Y, Cho C (2009) Antiobesity effect of PEGylated conjugated linoleic acid on high-fat diet-induced obese C57BL/6J (ob/ob) mice: attenuation of insulin resistance and enhancement of antioxidant defenses. J Nutr Biochem 20(3):187–194
Parra P, Serra F, Palou A (2010) Moderate doses of conjugated linoleic acid isomers mix contribute to lowering body fat content maintaining insulin sensitivity and a noninflammatory pattern in adipose tissue in mice. J Nutr Biochem 21(2):107–115
Halade GV, Rahman MM, Williams PJ, Fernandes G (2011) Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6 J mice. J Nutr Biochem 22(5):459–469
Park Y, Terk M, Park Y (2011) Interaction between dietary conjugated linoleic acid and calcium supplementation affecting bone and fat mass. J Bone Miner Metab 29(3):268–278
Fedor DM, Adkins Y, Newman JW, Mackey BE, Kelley DS (2013) The Effect of docosahexaenoic acid on t 10, c 12-conjugated linoleic acid-induced changes in fatty acid composition of mouse liver, Adipose, and Muscle. Metab Syndr Relat Disord 11(1):63–70
Scalerandi MV, Gonzalez MA, Saín J, Fariña AC, Bernal CA (2014) Effect of conjugated linoleic acid mixtures and different edible oils on body composition and lipid regulation in mice. Nutr Hosp 3(29):591–601
Azain MJ, Hausman DB, Sisk MB, Flatt WP, Jewell DE (2000) Dietary conjugated linoleic acid reduces rat adipose tissue cell size rather than cell number. J Nutr 130(6):1548–1554
Sisk MB, Hausman DB, Martin RJ, Azain MJ (2001) Dietary conjugated linoleic acid reduces adiposity in lean but not obese Zucker rats. J Nutr 131(6):1668–1674
Kim MR, Park Y, Albright KJ, Pariza MW (2002) Differential responses of hamsters and rats fed high-fat or low-fat diets supplemented with conjugated linoleic acid. Nutr Res 22(6):715–722
Yamazaki N, Yamanaka Y, Hashimoto Y, Shinohara Y, Shima A, Terada H (1997) Structural features of the gene encoding human muscle type carnitine palmitoyltransferase I. FEBS Lett 409(3):401–406
Henriksen EJ, Teachey MK, Taylor ZC, Jacob S, Ptock A, Kramer K, Hasselwander O (2003) Isomer-specific actions of conjugated linoleic acid on muscle glucose transport in the obese Zucker rat. Am J Physiol-Endocrinol Metabol 285(1):E98–E105
Sanders SR, Teachey MK, Ptock A, Kraemer K, Hasselwander O, Henriksen EJ, Baumgard LH (2004) Effects of specific conjugated linoleic acid isomers on growth characteristics in obese Zucker rats. Lipids 39(6):537–543
Botelho AP, Santos-Zago LF, de Oliveira AC (2008) Conjugated linoleic acid supplementation modified the body composition and serum leptin levels in weaning rats. Arch Latinoam Nutr 58(2):156–163
Ogborn MR, Nitschmann E, Goldberg A, Bankovic-Calic N, Weiler HA, Aukema HM (2008) Dietary conjugated linoleic acid renal benefits and possible toxicity vary with isomer, dose and gender in rat polycystic kidney disease. Lipids 43(9):783–791
Roy BD, Bourgeois J, Rodriguez C, Payne E, Young K, Shaughnessy SG, Tarnopolosky MA (2008) Conjugated linoleic acid prevents growth attenuation induced by corticosteroid administration and increases bone mineral content in young rats. Appl Physiol, Nutr, Metab 33(6):1096–1104
DeGuire JR, Weiler HA (2013) Free fatty acid and triacylglycerol forms of CLA isomers are not incorporated equally in the liver but do not lead to differences in bone density and biomarkers of bone metabolism. Prostaglandins Leukot Essent Fat Acids 88(5):399–403
de Almeida MM, de Souza YO, Potente Dutra Luquetti SC, Sabarense CM, do Amaral Correa JO, Santos da Conceicao EP, Lisboa PC, de Moura EG, Andrade Soares SM, Moura Gualberto AC, Gameiro J, Sundfeld da Gama MA, Ferraz Lopes FC, Gonzalez Garcia RM (2015) Cis-9, trans-11 and trans-10, cis-12 CLA mixture does not change body composition, induces insulin resistance and increases serum HDL cholesterol level in rats. J Oleo Sci 64(5):539–551
Berven G, Bye A, Hals O, Blankson H, Fagertun H, Thom E, Wadstein J, Gudmundsen O (2000) Safety of conjugated linoleic acid (CLA) in overweight or obese human volunteers. Eur J Lipid Sci Technol 102(7):455–462
Riserus U, Arner P, Brismar K, Vessby B (2002) Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes Care 25(9):1516–1521
Riserus U, Vessby B, Arnlov J, Basu S (2004) Effects of cis-9, trans-11 conjugated linoleic acid supplementation on insulin sensitivity, lipid peroxidation, and proinflammatory markers in obese men. Am J Clin Nutr 80(2):279–283
Larsen TM, Toubro S, Gudmundsen O, Astrup A (2006) Conjugated linoleic acid supplementation for 1 y does not prevent weight or body fat regain. Am J Clin Nutr 83(3):606–612
Laso N, Brugué E, Vidal J, Ros E, Arnaiz JA, Carné X, Vidal S, Mas S, Deulofeu R, Lafuente A (2007) Effects of milk supplementation with conjugated linoleic acid (isomers cis-9, trans-11 and trans-10, cis-12) on body composition and metabolic syndrome components. Br J Nutr 98(04):860–867
Park E, Kim J, Kim K, Paik H (2008) Conjugated linoleic acid (CLA) supplementation for 8 weeks reduces body weight in healthy overweight/obese Korean subjects. Food Sci Biotechnol 17(6):1261–1264
Norris LE, Collene AL, Asp ML, Hsu JC, Liu LF, Richardson JR, Li D, Bell D, Osei K, Jackson RD, Belury MA (2009) Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am J Clin Nutr 90(3):468–476
Joseph SV, Jacques H, Plourde M, Mitchell PL, McLeod RS, Jones PJ (2011) Conjugated linoleic acid supplementation for 8 weeks does not affect body composition, lipid profile, or safety biomarkers in overweight, hyperlipidemic men. J Nutr 141(7):1286–1291
López-Plaza B, Bermejo LM, Weber TK, Parra P, Serra F, Hernández M, Milla SP, Gómez-Candela C (2013) Effects of milk supplementation with conjugated linoleic acid on weight control and body composition in healthy overweight people. Nutr Hosp 28(6):2090–2098
Shadman Z, Taleban FA, Saadat N, Hedayati M (2013) Effect of conjugated linoleic acid and vitamin E on glycemic control, body composition, and inflammatory markers in overweight type2 diabetics. J diabetes metab disord 12(1):1–9
Ormsbee MJ, Rawal SR, Baur DA, Kinsey AW, Elam ML, Spicer MT, Fischer NT, Madzima TA, Thomas DD (2014) The effects of a multi-ingredient dietary supplement on body composition, adipokines, blood lipids, and metabolic health in overweight and obese men and women: a randomized controlled trial. J Int Soc Sports Nutr 11:37
Di Felice V, Macaluso F, Montalbano A, Gammazza AM, Palumbo D, Angelone T, Bellafiore M, Farina F (2007) Effects of conjugated linoleic acid and endurance training on peripheral blood and bone marrow of trained mice. J Strength Cond Res 21(1):193–198
Banu J, Bhattacharya A, Rahman M, Fernandes G (2008) Beneficial effects of conjugated linoleic acid and exercise on bone of middle-aged female mice. J Bone Miner Metab 26(5):436–445
Zhang G, Shirai N, Higuchi T, Suzuki H, Shimizu E (2009) A comparative study of the effects of Erabu sea snake (Laticauda semifasciata) lipids, green tea extract and conjugated linoleic acid on the swimming endurance of mice. Int J Vitam Nutr Res 79(56):362–374
Shen W, Baldwin J, Collins B, Hixson L, Lee K, Herberg T, Starnes J, Cooney P, Chuang C, Hopkins R, Reid T, Gupta S, McIntosh M (2015) Low level of trans-10, cis-12 conjugated linoleic acid decreases adiposity and increases browning independent of inflammatory signaling in overweight Sv129 mice. J Nutr Biochem 26(6):616–625
Mirand PP, Arnal-Bagnard MA, Mosoni L, Faulconnier Y, Chardigny JM, Chilliard Y (2004) Cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid isomers do not modify body composition in adult sedentary or exercised rats. J Nutr 134(9):2263–2269
Faulconnier Y, Arnal MA, Mirand PP, Chardigny JM, Chilliard Y (2004) Isomers of conjugated linoleic acid decrease plasma lipids and stimulate adipose tissue lipogenesis without changing adipose weight in post-prandial adult sedentary or trained Wistar rat. J Nutr Biochem 15(12):741–748
Thom E, Wadstein J, Gudmundsen O (2001) Conjugated linoleic acid reduces body fat in healthy exercising humans. J Int Med Res 29(5):392–396
Adams RE, Hsueh A, Alford B, King C, Mo H, Wildman R (2006) Conjugated linoleic acid supplementation does not reduce visceral adipose tissue in middle-aged men engaged in a resistance-training program. J Int Soc Sports Nutr 3(2):28–36
Bulut S, Bodur E, Colak R, Turnagol H (2013) Effects of conjugated linoleic acid supplementation and exercise on post-heparin lipoprotein lipase, butyrylcholinesterase, blood lipid profile and glucose metabolism in young men. Chem Biol Interact 203(1):323–329
Acknowledgments
The authors thank Ms. Jayne M. Storkson for help preparing this manuscript and Dr. Deborah J. Good at the Virginia Polytechnic Institute and State University for providing Nhlh-2 knockout animals. This material is based on work supported in part by the National Institute of Food and Agriculture, U.S. Department of Agriculture, the Massachusetts Agricultural Experimental Station, and the Department of Food Science under Project No. MAS00998 and MAS00450. Yoo Kim was supported in part by the Charm Sciences scholarship from the Department of Food Science, University of Massachusetts, Amherst. Dr. Yeonhwa Park is one of the inventors of CLA use patents assigned to the Wisconsin Alumni Research Foundation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kim, Y., Kim, J., Whang, KY. et al. Impact of Conjugated Linoleic Acid (CLA) on Skeletal Muscle Metabolism. Lipids 51, 159–178 (2016). https://doi.org/10.1007/s11745-015-4115-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4115-8