Abstract
Introduction
Numerous supplements containing conjugated linoleic acid (CLA) are presently being promoted for body weight reduction. The aim of this systematic review is to evaluate the evidence for or against the long-term efficacy of CLA.
Methods
Electronic searches were conducted to identify relevant randomized clinical trials (RCTs). No restrictions in age, time, or language were imposed. Studies had to be at least 6 months in duration. Three reviewers independently determined the eligibility of studies. Two reviewers independently extracted data and assessed the reporting quality of all RCTs.
Results
Fifteen RCTs were identified, and seven were included. Four of the included RCTs had serious flaws in the reporting of their methodology. A meta-analysis revealed a statistically significant difference in weight loss favouring CLA over placebo (mean difference: −0.70 kg; 95% confidence interval: −1.09, −0.32). Our meta-analysis also revealed a small significant difference in fat loss favouring CLA over placebo (MD: −1.33 kg; 95% CI: −1.79, −0.86; I 2 = 54%). The magnitude of these effects is small, and the clinical relevance is uncertain. Adverse events included constipation, diarrhea, and soft stools.
Conclusion
The evidence from RCTs does not convincingly show that CLA intake generates any clinically relevant effects on body composition on the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of overweight and obesity has increased dramatically over the last few decades [1]. Different weight management options are available, and a variety of dietary supplements is being sold as slimming aids. However, the efficacy of some of these supplements is not proven. One such supplement is conjugated linoleic acid (CLA).
Conjugated linoleic acid is a group of isomers of linoleic acid which are linked by the presence of conjugated dienes [2]. Conjugated linoleic acid occurs naturally and can be found in the fat of ruminant animals. Meat and dairy products also contain moderate amounts of CLA [3]. Conjugated linoleic acid has been reported to possess biologic properties, including anticarcinogenic functions, as well as causes changes in human body composition [4]. Some authors have suggested that the ingestion of CLA results in weight reduction via decreasing the size of adipocytes, as well as modifying adipocyte differentiation [5]. Conjugated linoleic acid has also been purported to stimulate apoptotic mechanisms, as well as regulate lipid metabolism [6]. CLA has been demonstrated to have beneficial effects on body composition in animals, as well as in humans [7].
Several studies have linked CLA supplementation with reduction in body weight and fat mass in humans [4, 7, 8], and a previous systematic review report concluded that long-term randomized clinical trials evaluating the safety and efficacy of this dietary supplement are needed [8]. A number of long-term human trials involving investigating the effects of CLA supplementation on body composition have been conducted.
The objective of this systematic review is to critically evaluate the evidence from long-term RCTs investigating the efficacy of CLA supplementation for weight reduction.
Methods
We conducted electronic searches in the following databases: Medline, Embase, Amed, Cinahl, The Cochrane Library, Clinical Trials Database, and the ISI Web of Science. Each database was searched from inception up until October 2010. The search terms used included antiobesity agent, appetite depressant, overweight, obesity, weight loss, slimming, body weight, body fat, adiposity, BMI, Conjugated linoleic acid, CLA, Conjugated fatty acid, Bovinic acid, Rumenic acid, and derivatives of these. We also searched the Internet for relevant conference proceedings and hand-searched relevant medical journals, and our own files. The bibliographies of all located articles were also searched. No age, gender, or language restrictions were imposed.
Only randomized, double-blind, placebo-controlled trials (RCTs) were included in this review. To be considered for inclusion, RCTs had to test the efficacy of orally administered CLA or any of its salts for body weight reduction in overweight or obese human volunteers. Included studies also had to report body weight or body composition as an outcome measure. Trials testing CLA as part of a combination supplement, i.e., dietary interventions containing other supplements in addition to CLA, were excluded from the review. Studies also had to be at least 6 months in duration.
Three reviewers (IJO, LKW, and LAD) independently assessed the eligibility of studies. Data were extracted by two reviewers (IJO and PPP) according to patient characteristics, interventions, and results. The reporting quality of all included studies was assessed by the use of a quality assessment checklist adapted from the Consolidated Standard of Reporting Trials (CONSORT) guidelines [9, 10]. Disagreements were resolved through discussion.
Data were presented as means with standard deviations. Mean changes in body weight, body fat, waist circumference, and body mass index (BMI) were used as common endpoints to assess the differences between CLA and placebo groups. Using standard meta-analysis software [11], we calculated mean differences (MD) and 95% confidence intervals (CI) for studies with adequate data for statistical pooling. The I2 statistics were used to assess for statistical heterogeneity among studies, with values of 25, 50, and 75% indicating low, medium, and high statistical heterogeneity, respectively. We also carried out sensitivity and subgroup analyses to test the robustness of overall analysis.
Results
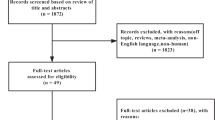
Our electronic searches returned 2,864 “hits”, of which 15 potentially relevant articles were identified (Fig. 1). One article was excluded because it was reported open trial [12], and another because it referred to single-blind study [13]. Three articles were excluded because they included normal-weight individuals [14–16], one because it was not randomized [17], and another because body weight was not reported as an outcome [18]. Finally, one study was excluded because it was a duplicate of an RCT already included [19]. Thus, 7 RCTs [20–26] including a total of 974 participants met our inclusion criteria and were included.
Three of the RCTs were conducted in two centers [20–22], while the remaining were single-centered studies [23–26]. The key characteristics of these RCTs are summarized in Tables 1 and 2. There was some variation in the reporting quality of the included RCTs (Table 1). Only 3 RCTs reported adequate randomization procedures [21, 22, 24], and only two reported appropriate allocation concealment [21, 22]. One RCT did not include a sample size calculation [26], 4 RCTs did not report appropriate blinding of care providers [20, 23, 25, 26], and 3 studies did not mention adequate blinding of patients [23, 25, 26]. Two RCTs did not report intention-to-treat (ITT) analyses [25, 26].
All the RCTs except one [20] incorporated some form of lifestyle adjustment into their treatment. One RCT [21] included exercise into the trial regimen, while 3 RCTs [22, 24, 25] reported caloric intakes for participants ranging from 1,750 to 2,500 kcal/day. Another RCT [26] reported daily caloric intakes of 25–30 kcal/kg/day for study participants, while participants in 1 RCT [23] were offered dietary advice. Overall, the RCTs reported that there were no significant differences between CLA and placebo groups in daily caloric intake.
Two RCTs [20, 21] measured the body weight of participants using digital scales, and another two [23, 25] used calibrated beam balance platform scale. One RCT [22] reported using a calibrated electronic weighing scale. In one RCT [24], the equipment used to determine body weight was not specified.
Six RCTs [20–23, 25, 26] reported the methods used to determine body fat composition. Five of these RCTs [20–23, 25] utilized dual-energy X-ray absorptiometry (DXA) to measure body fat, while one RCT [26] used bioelectric impedance analysis (BIA). Three of the RCTs [20–22] reported using the LUNAR PRODIGY software in addition to DXA. One RCT [24] did not report body fat as an outcome and hence did not report any method for measuring body fat.
One RCT did not provide information for computation of data for meta-analysis [26]. The investigators merely reported that there was no significant difference in body composition between CLA and placebo at the end of the trial. There was no documentation of p values, or of the participant final weight characteristics.
A forest plot (fixed effect model) of 6 RCTs which provided suitable data for statistical pooling (Fig. 2) revealed a small significant difference in weight loss favouring CLA over placebo (MD: −0.70 kg; 95% CI: −1.09, −0.32; I 2 = 0%). This equates to a weight loss of about 0.91% in the CLA group compared with placebo. A funnel plot of the 7 individual trials (Fig. 3) suggests symmetry as the studies are distributed around the mean difference for all the RCTs, but this does not necessarily rule out publication bias against the null hypothesis. A meta-analysis excluding the RCT which involved children [24] revealed a small significant difference in body weight favouring CLA over placebo (MD: −0.99 kg; 95% CI: −1.55, −0.43; I 2 = 0%). Further meta-analysis involving three RCTs with good reporting quality (based on adequate randomization and allocation concealment) [21, 22, 24] revealed a non-significant difference in weight loss between CLA and placebo (MD: −0.66 kg; 95% CI: −1.1, 0.21; I 2 = 19%).
A meta-analysis of 6 trials (Fig. 4) with 520 participants revealed a small significant difference in fat loss favouring CLA over placebo (MD: −1.33 kg; 95% CI: −1.79, −0.86; I 2 = 54%), while a forest plot of RCTs (Fig. 5) with 534 participants revealed a non-significant difference in waist circumference between CLA and placebo (MD: −0.12 cm; 95% CI: −0.82, 0.58; I 2 = 0%). Finally, a meta-analysis of 6 trials with 783 participants (Fig. 6) revealed a small significant difference in BMI favouring CLA over placebo (MD: −0.30 kg/m2; 95% CI: −0.44, −0.16; I 2 = 31%).
All the RCTs reported adverse events. These included diarrhea, soft stools, stomach pain, musculoskeletal symptoms, and myocardial infarction (MI). In one RCT, adverse events were reported to be less in the CLA group than in the placebo group [26]. In total, there were 170 dropouts/attrition, with 76 in the CLA group and 71 in the placebo. For the remaining 23 participants who dropped out from 3 RCTs [20, 23, 25], their treatment groups were not specified. All RCTs reported no significant difference in dropouts/attrition between the CLA and placebo groups.
There was a variation in the dosages and composition of the CLA used among the RCTs. The dosages ranged from 2.4 to 6 g daily [23, 26]. All RCTs except one [24] provided CLA in capsules. While the CLA used in 5 RCTs comprised of 2 isomers, namely cis-9,trans-11 and trans-10,cis-12 (c9,t11;t10,c12) [19–23], one RCT employed the single isomer, c9,t11 [20]. The composition of the CLA in one RCT was not specified, and in this same RCT, the CLA was conjugated to free fatty acids and triacylglycerol [21]. Of the 5 RCTs with 2 isomers, all had almost equal concentrations of both isomers except one RCT where the c9,t11 isomer was predominant [24]. A dose–response curve (Fig. 7) did not reveal a significant relationship between dosage and weight loss (p > 0.05).
All the RCTs also reported the composition of their placebos. Three RCTs [20–22] had olive oil as placebo, while another two [23, 25] used safflower oil. One RCT [24] reported their placebo as being composed of palm oil and soybean, and another [26] used high-oleic sunflower oil as placebo. In general, all the RCTs reported that there were no statistical differences in baseline indices in body weight between the participants in the CLA and placebo groups.
All included RCTs except one [26] provided information regarding compliance of the study participants. In two RCTs, compliance was ≥75% [23, 25]. The other 4 RCTs reported compliance rates ranging from 88 to 97% among completers [20–22, 24]. In general, the RCTs reported no significant difference in compliance rates between the CLA and placebo groups.
Discussion
The main objective of this review was to evaluate the evidence for or against the use of CLA as a weight-reducing agent. Our main meta-analysis suggests that CLA intake generates a statistically significant decrease in body weight when compared with placebo. The magnitude of the effect is, however, small and does not seem clinically relevant, as it does not equate to at least a 5% loss in body weight from baseline [27].
Our meta-analysis also suggested that CLA intake generates a small significant decrease in body fat and BMI, but no significant decrease in waist circumference. Our findings corroborate those of a previous review which suggested that CLA has modest effects on body composition [4]. Our results also confirm an earlier review which concluded that CLA intake has no clinically relevant effects on body composition [8]. In contrast to these previous reports [4, 8], however, our analyses involved only overweight and obese individuals, and we also assessed and accounted for the reporting quality of the included trials. Furthermore, this review involved only trials with at least 6-month duration, including 2 RCTs that were not available for the previous reviews [23, 24].
Conjugated linoleic acid is thought to influence body composition via a variety of mechanisms. These include reduction in lipid accumulation through its effect on lipoprotein lipase and stearoyl coenzyme A desaturase [4], as well as activation of PPAR receptors and stimulation of the production of proinflammatory cytokines [8]. However, the findings from our meta-analyses do not indicate that these effects cause any clinically relevant effects on human body composition.
A number of RCTs had serious flaws in the reporting of their methodology, such as randomization and allocation concealment (see Table 1). The failure of the authors to provide information regarding these important aspects of the RCTs limits the internal and external validity of the study results. Though 5 RCTs [20–24] reported performing ITT analysis, there was also some variation in how this analysis was conducted (and reported) among the RCTs. While 2 RCTs [21, 23] included all participants irrespective of when they dropped out from the trial in their analysis, subjects in the other RCTs were not included in the ITT analysis due to dropout halfway into the trial [20, 22], or failure to turn up at the last follow-up visit [24]. The lack of ITT analysis or failure to analyze all randomized participants in ITT analysis limits the robustness of the overall analyses of these RCTs [28, 29].
There were differences in the dosages and composition of CLA administered to participants in the included RCTs. It is not clear whether the differences in dosages influenced the effects of CLA on body composition. It is also unclear the extent to which the difference in isomer combination affected body weight. CLA is present in meat, dairy products, as well as in vegetable oil [3, 30, 31], and it is plausible to think that participants in RCTs could have ingested CLAs that were not prescribed as capsules. This could have blunted any differences in body composition which may have been observed between the CLA and placebo groups at the end of the studies. These uncertainties seem corroborated by our dose–response curve (see Fig. 7).
Reductions in daily caloric intake, as well as increased physical activity, are essential for effective weight loss, and there is sufficient evidence which shows that low-caloric diet facilitates long-term weight loss [32]. This was evident in one included RCT where the participants had an 8-week run-in trial period with restricted caloric intake [22]. The participants lost between at least 8% body weight from baseline during this period, but all regained weight during the intervention period when they had normal daily caloric intakes. This raises the possibility that the adjustment in lifestyle factors may have been responsible for the weight loss reported in some of the RCTs.
Though all included RCTs gave information relating to adverse events, there were variations in the frequency as well as the severity of these events. While gastrointestinal symptoms were reported to be more common in the CLA groups in 5 RCTs [20–24], one included RCT reported no adverse event relating to the intake of CLA [25], and the other RCT reported fewer adverse events in the CLA group compared with placebo [26]. Though MI relating to CLA intake was reported as an adverse event in one RCT [20], obesity itself has been described as a risk factor for MI [33]. Considering the inconsistencies in the reports of adverse events among the RCTs, it might be advisable for future trial investigators to incorporate surveillance strategies (extending beyond the duration of intervention itself) into their trial designs to monitor for adverse events over the long term.
This review has several limitations. Though we employed a robust strategy to search electronic and non-electronic sources, we may not have identified all long-term RCTs involving CLA supplementation for weight reduction, and the number of RCTs that were included in the review is few. Furthermore, there are discrepancies in the reporting quality of the included RCTs. These factors render our conclusions less secure than we had hoped them to be.
Conclusion
The evidence from RCTs fails to convincingly demonstrate that CLA supplementation generates any clinically relevant effects on body composition on the long term.
References
Ogden CL, Yanovski SZ, Carroll MD, Flegal KM (2007) The epidemiology of obesity. Gastroentorology 132:2087–2102
Belury M (2002) Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Ann Rev Nutr 22:505–531
Chin SF, Liu W, Storkson JM, Ha YL, Pariza MW (1992) Dietary sources of conjugated dienoic isomers of linoleic acid, a newly recognized class of anticarcinogens. J Food Compos Anal 5:185–197
Whigham LD, Watras AC, Schoeller DA (2007) Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr 85(5):1203–1211
Brown J, McIntosh M (2003) Conjugated linoleic acid in humans: regulation of adiposity and insulin sensitivity. J Nutr 133:3041–3046
Mirand PP, Arnal-Bagnard MA, Mosoni L et al (2004) Cis-9, trans-11 and trans-10, cis-12 conjugated linoleic acid isomers do not modify body composition in adult sedentary or exercised rats. J Nutr 134(9):2263–2269
Baddini FA, Fernandes PA, Ferreria da Costa N, Gonçalves RB (2009) Conjugated linoleic acid (CLA): effect modulation of body composition and lipid profile. Nutr Hosp 24(4):422–428
Salas-Salvadó J, Márquez-Sandoval F, Bulló M (2006) Conjugated linoleic acid intake in humans: a systematic review focussing on its effect on body composition, glucose, and lipid metabolism. Cri Rev Food Sci Nutr 46:479–488
Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. Lancet 357(9263):1191–1194
Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, Gøtzsche PC, Lang T (2001) The revised CONSORT statement for reporting randomised trials: explanation and elaboration. Ann Intern Med 134(8):663–694
Review Manager (RevMan) [Computer Program] (2008) Version 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen
Gaullier J-M, Halse J, Høye K, Kristiansen K, Fagertun H, Vik H et al (2005) Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr 135:778–784
Venkatramanan S, Joseph SV, Chouinard PY, Jacques H, Farnworth ER, Jones PJH (2010) Milk enriched with conjugated linoleic acid fails to alter blood lipids or body composition in moderately overweight, borderline hyperlipidemic individuals. J Am Coll Nutr 29(2):152–159
Von Loeffelholz C, von Loeffelholz B, Jahreis G (1999) Influence of CLA supplementation on body composition and strength in body builders. Vitamins and Additives in Human and Animal Nutrition 238–243
Von Loeffelholz C, Kratzsch J, Jahreis G (2003) Influence of conjugated linoleic acids on body composition and selected serum and endocrine parameters in resistance-trained athletes. Eur J Lipid Sci 105:251–259
Tarnopolsky M, Zimmer A, Jeremy P, Safdar A, Aboud A, Pearce E, et al (2007) Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS ONE 2(10):E991. doi:10.1371/journal.pone.0000991
Moya MJ (2007) Use of conjugated linoleic acid in obese children and adolescents. Revista Espanola de Pediatria 63(3):453–457
Close RN, Schoeller DA, Watras AC, Nora EH (2007) Conjugated linoleic acid supplementation alters the 6-mo change in fat oxidation during sleep. Am J Clin Nutr 86:797–804
Syvertsen C, Halse J, Høivik HO, Gaullier J-M, Nurminiemi M, Kristiansen K et al (2007) The effect of 6 months supplementation with conjugated linoleic acid on insulin resistance in overweight and obese. Int J Obes 31(7):1148–1154
Gaullier J-M, Halse J, Høivik HO, Høye K, Syvertsen C, Nurminiemi M et al (2007) Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr 97:550–560
Gaullier J-M, Halse J, Høye K, Kristiansen K, Fagertun H, Vik H et al (2004) Conjugated linoleic acid supplementation for 1 year reduces body fat mass in healthy overweight humans. Am J Clin Nutr 79(6):1118–1125
Larsen TM, Toubro S, Gudmundsen O, Astrup A (2006) Conjugated linoleic acid supplementation for 1 year does not prevent weight or body fat regain. Am J Clin Nutr 83(3):606–612
Racine NM, Watras AC, Carrel AL, Allen DB, McVean JJ, Clark RR, et al (2010) Effect of conjugated linoleic acid on body fat accretion in overweight or obese children. Am J Clin Nutr. doi:10.3945/ajcn.2009.28404
Sluijs I, Plantinga Y, de Roos B, Mennen LI, Bots ML (2010) Dietary supplementation with cis-9, trans-11 conjugated linoleic acid and aortic stiffness in overweight and obese adults. Am J Clin Nutr 91(1):175–183
Watras AC, Buchholz AC, Close RN, Zhang Z, Schoeller DA (2007) The role of conjugated linoleic acid in reducing body fat and preventing holiday weight gain. Int J Obes 31(3):481–487
Whigham LD, O’Shea M, Mohede ICM, Walaski HP, Atkinson RL (2004) Safety profile of conjugated linoleic acid in a 12-month trial in obese humans. Food Chem Toxicol 42:1701–1709
Christian JG, Tsai AG, Bessesen DH (2010) Interpreting weight loss from lifestyle modification trials: using categorical data. Int J Obes 34(1):207–209
Lachim JM (2000) Statistical Considerations in the Intent-to-Treat Principle. Control Clin Trials 21(3):167–169
Hollis S, Campbell F (1999) What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 319(7211):670–674
(1998) Safflower oil consumption does not increase plasma conjugated linoleic acid concentrations in humans. Am J Clin Nutr 67(2):332–337
Raff M, Tholstrup T, Basu S, Nonboe P, Sørensen MT, Straarup EM (2008) A diet rich in conjugated linoleic acid and butter increases lipid peroxidation but does not affect atherosclerotic, inflammatory or diabetic risk markers in healthy young men. J Nutr 138(3):509–514
Avenell A, Sattar N, Lean M (2007) Management: part I-behaviour change, diet, and activity. In: Sattar N, Lean M (eds) ABC of obesity. Blackwell, Malden, pp 8–11
Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P (2005) Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case–control study. Lancet 366(9497):1640–1649
Conflict of interest
IJO has a research fellowship funded by GlaxoSmithKline. The funder had no role in any aspect of this project or the preparation of this manuscript. PPP, LKW, LAD, and EE declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Onakpoya, I.J., Posadzki, P.P., Watson, L.K. et al. The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials. Eur J Nutr 51, 127–134 (2012). https://doi.org/10.1007/s00394-011-0253-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0253-9