Abstract
Conjugated linoleic acid (CLA) is anti-proliferative and anti-inflammatory in the Han:SPRD-cy rat model of kidney disease. We used different doses of CLA and examined effects on renal histological benefit, the renal PPARγ system and hepatic and renal levels of CLA isomers. Male and female offspring of Han:SPRD-cy heterozygotes were fed diets with 0, 1 or 2% CLA isomer mixture for 12 weeks before dual-energy X-ray absorptiometry, harvest of renal and hepatic tissue for histologic and lipid analysis. Both CLA diets reduced body fat content in both genders but did not change lean body mass. CLA produced a dose dependent reduction in female renal cystic change. CLA reduced fibrosis, but this reduction was significantly less with higher dose in males. CLA reduced macrophage infiltration, tissue oxidized LDL content and proliferation of epithelial cells. Serum creatinine rose significantly in female animals fed CLA diets. CLA treatment did not change PPARγ activation. A significant negative correlation with renal content of the 18:2 c9,t11 isomer and the sum of histologic effects was identified. CLA reduces histologic renal injury in the Han:SPRD-cy rat model probably inversely proportionate to c9,t11 renal content. Possible functional CLA toxicity at high dose in female animals warrants further exploration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conjugated linoleic acid (CLA) refers to a group of positional and geometric isomers of linoleic acid produced by bacterial action in the gastrointestinal tract of ruminants [1, 2] that are significant dietary components in humans on hunter-gatherer or primitive agrarian diets, but exposure is much lower in modern urban diets [3]. CLA has been shown to have a dose dependent effect on tumor development or growth in skin, prostate, mammary and colonic tumors [2, 4, 5]. CLA feeding has demonstrated beneficial effects in a murine model of lupus nephritis [6]. CLA produces tissue specific alteration in production of eicosanoids [7, 8], and decreases release of arachidonic acid (ARA) or prostaglandin E2 (PGE2) in different tissue types or cell lines [9–12]. CLA has been associated with improved outcome in experimental porcine colitis [13], and is capable of exerting an anti-inflammatory effect without compromising resistance to infection [14]. CLA has been identified as a ligand for PPARα, through which effects on lipid and carbohydrate metabolism may be modified [15], and PPARγ, through which anti-inflammatory and anti-proliferative effects may be modulated [16].
In a previous study, we demonstrated that dietary supplementation with a mixture of CLA isomers was effective in reducing histologic injury and renal PGE2 production in male animals with the Han:SPRD-cy form of polycystic kidney disease [11]. The Han:SPRD-cy model of polycystic kidney disease (PKD), characterized by autosomal dominant inheritance with marked sexual dimorphism in expression, has proved an excellent system in which to explore the modification of chronic renal injury, including by dietary means. Epithelial proliferation and progressive dilatation of nephrons, marked interstitial inflammation and fibrosis characterize the disease [17]. Oxidant injury has been implicated in its pathogenesis [18]. It can be ameliorated with methylprednisolone [19], angiotensin blockade or converting enzyme inhibition [20, 21], and lovastatin [22]. Amelioration occurs with dietary soy protein substitution [23], protein restriction [21], flaxseed, flax oil or flax lignans [24–28] and citrate supplementation [29].
Although this model is amenable to dietary modification, a recent study we performed with flax derivatives demonstrated that gender influenced the extent of the dietary benefit [27], with female animals with an earlier stage of the disease demonstrating greater beneficial changes in renal histology. This study was therefore undertaken to explore if the observed benefits of CLA supplementation could be increased with a higher level of supplementation and as well as whether this response was influenced by animal gender. As secondary objectives, we explored if histologic benefits were associated with reduction in renal PPARγ activation and whether benefit could be correlated with tissue level changes in specific CLA isomers. We also explored previously reported effects of CLA on body composition to see if they are reproduced in animals with renal disease, and if these showed any relationship to renal histologic and functional change.
Methods
Animals
All animal procedures and care were approved by the University of Manitoba Committee on Animal Use and were within the guidelines of the Canadian Council on Animal Care. Surviving offspring of known Han:SPRD-cy heterozygotes from our own breeding colony were randomly assigned to groups fed 7% by weight corn oil as the lipid source alone, 6% corn oil and 1% CLA concentrate, or 5% corn oil and 2% CLA concentrate ad libitum at weaning at 3 weeks of age. CLA concentrate was a gift of Bioriginal, Saskatoon, Canada, and was diluted into the oil fraction of the diet to achieve the target concentrations. Animals were killed after 12 weeks on the diet and kidney, liver and serum collected for analysis. Animals found not to have PKD at necropsy, approximately one-third of the total, were excluded from further analysis. Diets were based on the formula from prior studies [11, 27] using casein as the protein source (20% by weight) supplemented with 0.3% methionine, corn starch (52% by weight) and dextrose (13% by weight) as the carbohydrate sources and lipid sources as described. The detailed analysis of the dietary fatty acid content is summarized in Table 1. The animals were fed ad libitum, as our prior studies have not found differences in intake related to disease status in animals in the earlier stages of disease progression [11, 23, 28, 30].
DXA Scanning
Animals were anesthetized after 12 weeks of study using sodium pentobarbital (65 mg/kg, i.p.), followed by measurement of whole body weight and body composition including bone mass using dual-energy X-ray absorptiometry and the small animal software (QDR 4500A, Hologic Inc, Waltham, MA). This technology has been validated for use in rodents [31].
Histology and Immunohistochemistry
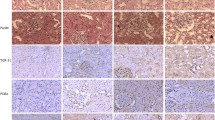
Tissue from the left kidney was processed using our previously described methods [11, 26] for histologic and immunohistochemical analysis. These studies included hematoxylin and eosin, Sirius red staining for fibrosis, PCNA (M 0879, Dako A/S, Glostrup, Denmark) and macrophages (MAB1435, Chemicon International, Temecula, CA). Oxidized LDL (ox-LDL) staining was used as a marker of oxidant injury [27, 32], using a polyclonal antibody (AB3230, Chemicon, Temecula, CA). Animals were classified as affected by one of two experienced observers (N.B.C., M.R.O.), blinded to dietary intervention on the basis of the characteristic cystic and inflammatory pathology of this disease.
Image Analysis
Image analysis procedures were performed with a system consisting of a Spot Junior CCD camera mounted on an Olympus BX60 microscope. The captured images from random stage movement through the sections were subsequently analyzed using Image Pro version 4.5 Package (Media Cybernetics, Silver Spring, MD). The observer was blinded to dietary treatment, although disease status is obvious upon microscopic examination. Raw analysis data were processed as previously described [23] to give objective measures of cystic change, fibrosis, interstitial macrophage infiltration and extent of ox-LDL staining. Measurements of fibrosis and cellular markers were corrected to solid-tissue areas of sections so that the presence of empty cystic areas on the section did not lead to an underestimation of these parameters.
Chemistry
Biochemical measurements were performed by an observer blinded to disease status and dietary intervention (E.N.). Serum and urine creatinine, and serum cholesterol were determined by spectrophotometric methods using Sigma kits (Sigma Chemical Co., St Louis, MO). Urine protein was measured by the brilliant Coomassie Blue method of Bradford.
Gas Chromatography
Lipids were extracted for gas chromatographic analysis using a modified Folch extraction procedure, as we have previously described [23, 26, 33]. Liver tissue was examined in addition to kidney as our previous studies have shown this tissue to be a more sensitive and consistent marker to confirm absorption and incorporation of the ingested PUFA. Prior to analysis, samples were redissolved in 1 ml of dry toluene, mixed with 2.0 ml of 0.5 M sodium methoxide and heated to 50 °C for 10 min, then mixed with 0.1 ml of glacial acetic acid, 5 ml of distilled H2O, and 5 ml of hexane. This method was selected as being reliable for the methylation of the CLA isomers which were primary interest in tissue analysis in this study [34]. After vortexing, the mixture was centrifuged at 2,500×g for 10 min and the hexane fraction removed. Fresh hexane is added to the remaining solution and the previous steps repeated. The hexane fractions were dried under anhydrous sodium sulfate, evaporated under nitrogen and the lipid esters redissolved in 1 ml hexane. Gas chromatography (GC) was performed on a Varian Chrompack 3800 instrument, using a Varian CP-Sil 88 100 meter column (Varian, Walnut Creek, CA). Total ω6:ω3 ratio was calculated from the sum of proportions of linoleic acid (LNA, 18:2 c9,c12), γ-linolenic acid (GLA), 20:3 ω6 and ARA divided by the sum of α-linolenic acid (ALA), 20:3 ω3, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). The ratio of ARA:LNA was calculated as an indicator of Δ-6 desaturase activity. The ratio of 18:1 c9 (oleic acid) to 18:0 (stearic acid) was calculated as an indicator of Δ-9 desaturase activity.
PPARγ Activation
PPARγ activation was measured using a commercial ELISA assay (Panomics EK1211, Fremont, CA) of in-vitro binding to a specific DNA promoter sequence, after extraction of nuclear material according the manufacturer’s specifications. Results were expressed as percentage of activity of a positive control standard provided by the manufacturer.
Statistical Analysis
Results were analyzed using two-way and three-way analyses of variance (ANOVA) with Tukey’s post hoc comparisons, using SAS version 9.1 software packages as appropriate. The model detected differences between main effects by diet, and sex as applicable. The model also reported interaction effects between diet and sex which were considered significant at P < 0.05. Log and square root transformations were done for the variables which were not distributed in a Gaussian fashion. To measure the degree of relationship between histology measures with renal contents of specific fatty acids, canonical correlation analysis was performed using the SAS version 9.1 software package.
Results
A total of 54 female and 47 male animals who were found to be Han:SPRD-cy heterozygotes were included in the analysis. Animal weight was found to be significantly influenced by gender, but not by diet, with females being smaller as expected (Fig. 1). Serum creatinine, as a marker of renal function, was also significantly influenced by gender, again consistent with the known slower progression of the disease in female animals (Fig. 2), but serum creatinine actually increased significantly with increasing dose of CLA in female animals.
When results of all densitometric indices of histologic injury are considered, diet (P < 0.01) and gender (P < 0.001) had significant effect on reducing relative cyst area which was found to be dose dependent for diet, although in post hoc analysis, significant differences between dietary groups in female animals accounted for the observed effect (Fig. 3a). As gender difference is inherent to the model, post hoc comparisons between genders, which were significant for all histologic outcome measures and serum creatinine, are not further reported here. A similar dose dependent effect of diet (P < 0.001) and effect of gender (P < 0.001) was observed with fibrosis (Fig. 3b) Diet also had dose dependent effects on ox-LDL measurement (P < 0.001, Fig. 3c) with marginal gender interactions manifest as lower values in female animals (diet × gender, P = 0.05). Major diet effects were also observed upon macrophage content and PCNA count (P < 0.001, Fig. 4) due entirely to highly significant differences between control and both dietary interventions in post hoc testing. The small differences between the 1 and 2% CLA dietary groups within genders did not achieve significance in post hoc testing for these parameters.
Densitometric analysis of renal cystic change (a) fibrosis (b) and Ox-LDL (c) (fraction image area, fibrosis and ox-LDL are corrected to solid-tissue area). A significant major dietary effect was seen for all histologic measures. a Significant difference from control diet, same gender in post hoc testing. b Significant difference between 1 and 2% CLA, same gender in post hoc testing
Neither diet nor gender had a statistically significant effect on PPARγ activation (Fig. 5).
Diet significantly elevated renal levels of all detected CLA isomers in kidney and liver as expected and decreased levels of LNA in both organs. The levels found in both organs, however, were in different proportions to the diet (Table 2). Both diet and gender were likely to influence fatty acid composition of liver more than that of kidney. Levels of 18:1 c9 were significantly lower in liver but not kidney tissue in both genders, as was Δ-9 desaturase activity. ARA content did not differ with diet in renal and hepatic tissue and Δ-6 desaturase activity was significantly increased in liver from animals of both genders (Table 2). Female animals demonstrated higher hepatic content of the anti-inflammatory PUFA, DHA. Canonical correlation between renal content of CLA isomers and a total histologic score pooled across both genders derived by addition of the numeric values of all histologic variables revealed a significant negative correlation only between the renal content of 18:2 c9, t11 and histologic indices (standardized canonical coefficient = −0.63, P < 0.001). There was no significant correlation between the renal level of any CLA isomer and serum creatinine in the whole group; however, if the analysis was restricted to only female animals with PKD, the correlation between serum creatinine and the renal levels of the t10, t12 isomer approached significance (r 2 = 0.27, P = 0.067).
Gender was associated with expected changes in bone mineral content, body fat and lean body mass (Table 3). Diet only had a significant effect in reducing % body fat in both genders compared to controls of the same gender in post hoc testing. Increases in lean body mass in animals receiving CLA diets did not achieve significance. Lean body mass did not correlate with serum creatinine in either gender (males r 2 = −0.139, females r 2 = 0.299, P = ns).
Discussion
The results reported here reinforce the findings of our previous studies with respect to a possible therapeutic role for CLA in inflammatory renal disease [11]. This study, however, is the first to explore the role of animal gender in the response to CLA in this context, and, as with our recent study with flax derivatives, gender does influence the outcome of this type of dietary intervention. Indeed, we have not been able to identify any prior study exploring gender differences in CLA response in any system.
CLA isomers may compete with linoleic acid at the level of desaturases and elongases, thus influencing ARA content [35]. CLA reduces the expression of the gene for stearoyl-CoA reductase, the initial Δ9-desaturase step in the formation of monoenoic fatty acids [36], a finding confirmed by our results. Δ6-desaturase inhibition by CLA has been reported in a transfected yeast system [37], and by the t10, c12 isomer specifically in human hepatoma cells [38], whereas our study found no such effect in kidney and possibly the opposite effect in liver. In a prior study using a soy protein based diet, we found an association between inhibition of Δ6-desaturase and histologic improvement in this model [23], but this is clearly not the mechanism here. Although found to have benefits in the areas of carbohydrate metabolism, cholesterol metabolism and atherosclerosis [39], the major health related interest in CLA has been as an anti-carcinogen [2]. CLA enhances lymphocyte proliferative responses to phytohemaglutinin, but not to LPS or concanavalin A [40], implying a positive or negative immunomodulatory role under differing circumstances. Our results demonstrating decreased macrophage infiltration and fibrosis might be interpreted as a specific effect on the inflammatory response or may be a non-specific response based on inhibition of cell proliferation.
Most CLA preparations are a mixture of isomers, with the precise mix a function of both the substrate oil and the manufacturing process [41]. Our statistical association of benefit with the renal concentration of the c9-t11 isomer, while a novel approach to the issue of identifying the active lipid, is supported by studies using pure isomers. Jensen et al. [4] have associated antiproliferative effects of CLA with the c9-c11 isomer. Yang and Cook [42] have demonstrated that the release of anti-inflammatory cytokines essential to the innate immune response is inhibited by the c9-t11 isomer. Conversely, the t10-t12 isomer has been associated with pro-inflammatory effects [43], may have adverse effects on lipid metabolism [44], and may play a larger prostaglandin inhibition mediated effects of CLA [38].
Our finding of a different distribution of CLA isomers in tissue compared to the diet and differing proportions in differing organs is consistent with previous reports. Kramer et al. [45] have reported similar findings in a piglet model and speculate that the cause is related to differing metabolism of different isomers, which is supported by Tsuzuki et al. [46] who failed to demonstrate differences in absorption as a basis for such differences in a rat model. The basis for the observed differences remains to be elucidated. In this inflammatory model, the content and role of fatty acids in the inflammatory cell population that may migrate into the kidney from a circulating pool would be extremely difficult to measure and yet may be extremely important in elucidating the precise relationships between this nutritional intervention and histologic change and toxicity.
The role of CLA as a ligand for the PPARγ system suggests a direct pathway that might mediate the observed effects [15]. This pathway has been specifically implicated in the regulation of inflammatory response [16]. Our results do not demonstrate a dietary effect on PPARγ activation, thus cannot at this stage support this as the relevant pathway. It is, however, possible, that as we based our measurements upon whole tissue homogenates, we would not have had sufficient sensitivity to detect activation that is specific to individual cell types such as macrophages and fibroblasts. In-vitro studies of separated cells may offer insight into this question.
The new finding of a rise in serum creatinine in female animals is a serious concern. CLA has been associated with an increase in lean body mass [47–49], which may increase serum creatinine without change in renal function [50]. In this study, however, very small changes in lean body mass that did not achieve significance and were proportionately much less than the change in serum creatinine were observed. In a previous study, we demonstrated that a 1% CLA diet reduced renal PGE2 production [11]. It is plausible that at even higher doses, a more generalized effect of prostaglandin inhibition on glomerular tubular balance, combined with the loss of urinary concentrating ability that is an early clinical feature of this disease my result in disturbed renal function in a manner analogous to the adverse effects of non-steroidal anti-inflammatory drugs [51]. Sullivan et al. [52] have described gender dimorphism with higher expression of prostaglandin synthase and renal production of prostaglandins in female spontaneously hypertensive rats, but the relationship of this observation to renal functional homeostasis is not known. It is possible that their observation implies that more prostaglandin synthesis is required for homeostasis in females; inhibition of the pathway would therefore create a larger functional disturbance. Our finding of a diminution of the antifibrotic effect at higher doses of the CLA mixture in males may also be cautionary and could reflect a shift in the balance of beneficial versus harmful effects of the two predominant isomers [43]. As with an earlier study with flax oil and lignan [27], we did detect relatively subtle qualitative differences in the profile of histologic effects between genders. The magnitude of these differences may not be of clinical significance but the finding underlines the importance of considering gender as a variable in functional food studies.
Proportions of CLA in plasma, adipose tissue and red blood cell membranes are low in adult patients with chronic renal failure managed without dialysis, but increase in dialysis patients [53]. It is likely that changes in dietary intake, with severe restriction of dairy products mandated to maintain serum phosphate in an acceptable range, are a major factor. Our study suggests that CLA, and in particular the c9-t11 isomer, may warrant further exploration as an anti-inflammatory functional food that may influence renal injury. Our results, however, also caution that the unconditional recommendation of CLA supplements of variable composition and quality in this high risk population is not only premature but carries a credible risk of harm.
Abbreviations
- ARA:
-
Arachidonic acid
- ALA:
-
α-linolenic acid
- CLA:
-
Conjugated linoleic acid
- CO:
-
Corn oil
- DHA:
-
Docosahexaenoic acid
- DXA:
-
Dual-energy X-ray absorptiometry
- EPA:
-
Eicosapentaenoic acid
- GC:
-
Gas chromatography
- LNA:
-
Linoleic acid
- ox-LDL:
-
Oxidized low density lipoprotein
- PCNA:
-
Proliferating cell nuclear antigen
- PGE2 :
-
Prostaglandin E2
- PKD:
-
Polycystic kidney disease
- PPAR:
-
Peroxisome proliferator-activated receptor
- PUFA:
-
Polyunsaturated fatty acid
References
Parodi PW (1997) Cows’ milk fat components as potential anticarcinogenic agents. J Nutr 127:1055–1060
Parodi PW (1999) Conjugated linoleic acid and other anticarcinogenic agents of bovine milk fat. J Dairy Sci 82:1339–1349
Cordain L, Watkins BA, Florant GL, Kelher M, Rogers L, Li Y (2002) Fatty acid analysis of wild ruminant tissues: evolutionary implications for reducing diet-related chronic disease. Eur J Clin Nutr 56:181–191
Jensen RG, Lammi-Keefe C (2001) The anticarcinogenic conjugated fatty acid c9, t11–c18:2, or rumenic acid, in human milk: amounts and effects. Adv Exp Med Biol 501:153–156
Cesano A, Visonneau S, Scimeca JA, Kritchevsky D, Santoli D (1998) Opposite effects of linoleic acid and conjugated linoleic acid on human prostatic cancer in SCID mice. Anticancer Res 18:1429–1434
Yang M, Pariza MW, Cook ME (2000) Dietary conjugated linoleic acid protects against end stage disease of systemic lupus erythematosus in the NZB/W F1 mouse. Immunopharmacol Immunotoxicol 22:433–449
Whigham LD, Cook EB, Stahl JL, Saban R, Bjorling DE, Pariza MW, Cook ME (2001) CLA reduces antigen-induced histamine and PGE(2) release from sensitized guinea pig tracheae. Am J Physiol Regul Integr Comp Physiol 280:R908–R912
Whigham LD, Higbee A, Bjorling DE, Park Y, Pariza MW, Cook ME (2002) Decreased antigen-induced eicosanoid release in conjugated linoleic acid-fed guinea pigs. Am J Physiol Regul Integr Comp Physiol 282:R1104–R1112
Harris MA, Hansen RA, Vidsudhiphan P, Koslo JL, Thomas JB, Watkins BA, Allen KG (2001) Effects of conjugated linoleic acids and docosahexaenoic acid on rat liver and reproductive tissue fatty acids, prostaglandins and matrix metalloproteinase production. Prostaglandins Leukot Essent Fatty Acids 65:23–29
Miller A, Stanton C, Devery R (2001) Modulation of arachidonic acid distribution by conjugated linoleic acid isomers and linoleic acid in MCF-7 and SW480 cancer cells. Lipids 36:1161–1168
Ogborn MR, Nitschmann E, Bankovic-Calic N, Weiler HA, Fitzpatrick-Wong S, Aukema HM (2003) Dietary conjugated linoleic acid reduces PGE2 release and interstitial injury in rat polycystic kidney disease. Kidney Int 64:1214–1221
Watkins BA, Seifert MF (2000) Conjugated linoleic acid and bone biology. J Am Coll Nutr 19:478S–486S
Hontecillas R, Wannemeulher MJ, Zimmerman DR, Hutto DL, Wilson JH, Ahn DU, Bassaganya-Riera J (2002) Nutritional regulation of porcine bacterial-induced colitis by conjugated linoleic acid. J Nutr 132:2019–2027
Turnock L, Cook M, Steinberg H, Czuprynski C (2001) Dietary supplementation with conjugated linoleic acid does not alter the resistance of mice to Listeria monocytogenes infection. Lipids 36:135–138
Moya-Camarena SY, Belury MA (1999) Species differences in the metabolism and regulation of gene expression by conjugated linoleic acid. Nutr Rev 57:336–340
Yu Y, Correll PH, Vanden Heuvel JP (2002) Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: evidence for a PPAR gamma-dependent mechanism. Biochim Biophys Acta 1581:89–99
Gretz N, Ceccherini I, Kranzlin B, Kloting I, Devoto M, Rohmeiss P, Hocher B, Waldherr R, Romeo G (1995) Gender-dependent disease severity in autosomal polycystic kidney disease of rats. Kidney Int 48:496–500
Maser RL, Vassmer D, Magenheimer BS, Calvet JP (2002) Oxidant stress and reduced antioxidant enzyme protection in polycystic kidney disease. J Am Soc Nephrol 13:991–999
Gattone VHII, Cowley BD Jr, Barash BD, Nagao S, Takahashi H, Yamaguchi T, Grantham JJ (1995) Methylprednisolone retards the progression of inherited polycystic kidney disease in rodents. Am J Kidney Dis 25:302–313
Keith DS, Torres VE, Johnson CM, Holley KE (1994) Effect of sodium chloride, Enalapril, and Losartan on the development of polycystic kidney disease in. Am J Kidney Dis 24:491–498
Ogborn MR, Sareen S (1995) Amelioration of polycystic kidney disease by modification of dietary protein intake in the rat. J Am Soc Nephrol 6:1649–1654
Gile RD, Cowley BD Jr, Gattone VHII, O’Donnell MP, Swan SK, Grantham JJ (1995) Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. Am J Kidney Dis 26:501–507
Ogborn MR, Nitschmann E, Weiler HA, Bankovic-Calic N (2000) Modification of polycystic kidney disease and fatty acid status by soy protein diet. Kidney Int 57:159–166
Ogborn MR, Nitschmann E, Bankovic-Calic N, Buist R, Peeling J (1998) The effect of dietary flaxseed supplementation on organic anion and osmolyte content and excretion in rat polycystic kidney disease. Biochem Cell Biol 76:553–559
Ogborn MR, Nitschmann E, Bankovic-Calic N, Weiler H, Aukema H (2005) Flax and soy phytoestrogen effects on renal injury and lipid content in experimental polycystic kidney disease. JANA 8:26–32
Ogborn MR, Nitschmann E, Weiler H, Leswick D, Bankovic-Calic N (1999) Flaxseed ameliorates interstitial nephritis in rat polycystic kidney disease. Kidney Int 55:417–423
Ogborn MR, Nitschmann E, Bankovic-Calic N, Weiler HA, Aukema HM (2006) Effects of flaxseed derivatives in experimental polycystic kidney disease vary with animal gender. Lipids 41:1141–1149
Ogborn MR, Nitschmann E, Bankovic-Calic N, Weiler HA, Aukema H (2002) Dietary flax oil reduces renal injury, oxidized LDL content, and tissue n-6/n-3 FA ratio in experimental polycystic kidney disease. Lipids 37:1059–1065
Tanner GA (1998) Potassium citrate/citric acid intake improves renal function in rats with polycystic kidney disease. J Am Soc Nephrol 9:1242–1248
Ogborn M, Bankovic-Calic N, Shoesmith C, Buist R, Peeling J (1998) Soy protein modification of rat polycystic kidney disease, Am J Physiol 274 (Renal Physiol 43):F541–F549
Bertin E, Ruiz JC, Mourot J, Peiniau P, Portha B (1998) Evaluation of dual-energy X-ray absorptiometry for body-composition assessment in rats. J Nutr 128:1550–1554
Bosmans JL, Holvoet P, Dauwe SE, Ysebaert DK, Chapelle T, Jurgens A, Kovacic V, Van Marck EA, De Broe ME, Verpooten GA (2001) Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 1 1/2 years. Kidney Int 59:2346–2356
Uauy RD, Birch DG, Birch EE, Tyson JE, Hoffman DR (1990) Effect of dietary omega-3 fatty acids on retinal function of very-low-birth-weight neonates. Pediatr Res 28:485–492
Kramer JK, Fellner V, Dugan ME, Sauer FD, Mossoba MM, Yurawecz MP (1997) Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids 32:1219–1228
Sebedio J, Juaneda P, Dobson G, Ramilison I, Martin J, Chardigny J, Christie W (1997) Metabolites of conjugated isomers of linoleic acid (CLA) in the rat. Biochim Biophys Acta 1345:5–10
Bretillon L, Chardigny JM, Gregoire S, Berdeaux O, Sebedio JL (1999) Effects of conjugated linoleic acid isomers on the hepatic microsomal desaturation activities in vitro. Lipids 34:965–969
Chuang LT, Thurmond JM, Liu JW, Kirchner SJ, Mukerji P, Bray TM, Huang YS (2001) Effect of conjugated linoleic acid on fungal delta6-desaturase activity in a transformed yeast system. Lipids 36:139–143
Eder K, Slomma N, Becker K (2002) Trans-10, cis-12 conjugated linoleic acid suppresses the desaturation of linoleic and alpha-linolenic acids in HepG2 cells. J Nutr 132:1115–1121
Sebedio JL, Gnaedig S, Chardigny JM (1999) Recent advances in conjugated linoleic acid research. Curr Opin Clin Nutr Metab Care 2:499–506
Wong MW, Chew BP, Wong TS, Hosick HL, Boylston TD, Shultz TD (1997) Effects of dietary conjugated linoleic acid on lymphocyte function and growth of mammary tumors in mice. Anticancer Res 17:987–993
Yang L, Huang Y, Wang H, Chen Z (2002) Production of conjugated linoleic acids through KOH-catalyzed dehydration of ricinoleic acid. Chem Phys Lipids 119:23
Yang M, Cook ME (2003) Dietary conjugated linoleic acid decreased cachexia, macrophage tumor necrosis factor-alpha production, and modifies splenocyte cytokines production. Exp Biol Med (Maywood) 228:51–58
Riserus U, Basu S, Jovinge S, Fredrikson GN, Arnlov J, Vessby B (2002) Supplementation with conjugated linoleic acid causes isomer-dependent oxidative stress and elevated C-reactive protein: a potential link to fatty acid-induced insulin resistance. Circulation 106:1925–1929
Roche HM, Noone E, Sewter C, Mc Bennett S, Savage D, Gibney MJ, O’Rahilly S, Vidal-Puig AJ (2002) Isomer-dependent metabolic effects of conjugated linoleic acid: insights from molecular markers sterol regulatory element-binding protein-1c and LXRalpha. Diabetes 51:2037–2044
Kramer J, Sehat N, Dugan M, Mossoba M, Yurawecz M, Roach J, Eulitz K, Aalhus J, Schaefer A, Ku Y (1998) Distributions of conjugated linoleic acid (CLA) isomers in tissue lipid classes of pigs fed a commercial CLA mixture determined by gas chromatography and silver ion-high-performance liquid chromatography. Lipids 33:549–558
Tsuzuki T, Ikeda I (2007) Slow absorption of conjugated linoleic acid in rat intestines, and similar absorption rates of 9c, 11t-conjugated linoleic acid and 10t, 12c-conjugated linoleic acid. Biosci Biotechnol Biochem 71:2034–2040
Weiler H, Austin S, Fitzpatrick-Wong S, Nitschmann E, Bankovic-Calic N, Mollard R, Aukema H, Ogborn M (2004) Conjugated linoleic acid reduces parathyroid hormone in health and in polycystic kidney disease in rats. Am J Clin Nutr 79:1186S–1189S
Burr LL, Taylor CG, Weiler HA (2006) Dietary conjugated linoleic acid does not adversely affect bone mass in obese fa/fa or lean Zucker rats. Exp Biol Med (Maywood) 231:1602–1609
Kelly GS (2001) Conjugated linoleic acid: a review. Altern Med Rev 6:367–382
Smith SA (1988) Estimation of glomerular filtration rate from the serum creatinine concentration. Postgrad Med J 64:204–208
Taber SS, Mueller BA (2006) Drug-associated renal dysfunction. Crit Care Clin 22:357–374, viii.
Sullivan JC, Sasser JM, Pollock DM, Pollock JS (2005) Sexual dimorphism in renal production of prostanoids in spontaneously hypertensive rats. Hypertension 45:406–411
Lucchi L, Banni S, Melis MP, Angioni E, Carta G, Casu V, Rapana R, Ciuffreda A, Corongiu FP, Albertazzi A (2000) Changes in conjugated linoleic acid and its metabolites in patients with chronic renal failure. Kidney Int 58:1695–1702
Acknowledgments
This work was supported by a grant from Dairy Farmers of Canada and performed in facilities of the Manitoba Institute of Child Health, a division of the Children’s Hospital Foundation of Manitoba, Inc. The assistance of Dr Rasheda Rabbani with the statistical analysis and of Heather Kovacs with the DXA scanning is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ogborn, M.R., Nitschmann, E., Goldberg, A. et al. Dietary Conjugated Linoleic Acid Renal Benefits and Possible Toxicity vary with Isomer, Dose and Gender in Rat Polycystic Kidney Disease. Lipids 43, 783–791 (2008). https://doi.org/10.1007/s11745-008-3211-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3211-4