Abstract
A series of novel cationic gemini surfactants with rigid amido groups inserted as the spacers, named C 12 -PPDA-C 12 , C 14 -PPDA-C 14 and C 16 -PPDA-C 16 , were synthesized by a two-step reaction with dimethyl terephthalate, N,N-dimethyl propylene diamine and alkyl bromide as raw materials. The chemical structures of the prepared compounds were confirmed by IR, 1H and 13C NMR and element analysis. Surface activity properties of the synthesized compounds were investigated by surface tension, electrical conductivity and fluorescence. Increasing the number of carbon atoms in the hydrophobic alkyl chain, decreased the critical micelle concentration (CMC), surface tension at the CMC and the minimum surface area. Other relevant properties including foaming ability and emulsion stability were investigated. The results indicated that the synthesized gemini surfactants possess good surface properties, emulsifying properties and steady foam properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are widely applied in many fields from consumer products to industrial applications. In comparison with conventional single-chain surfactants, gemini surfactants exhibit superior aqueous solution properties, such as lower critical micelle concentration (CMC), higher surface activity, better emulsifying performances, lower Krafft point and unusual aggregation morphologies [1–5]. To identify highly efficient surfactant structures, great efforts have been taken in the design and synthesis of gemini surfactants and their properties have been researched [6–9]. It is well-known that the different spacers [10–12], head groups [13, 14], and hydrophobic groups can make up of different molecular structures of gemini surfactants.
Recently, gemini surfactants with different types of spacers have been developed with different physical and chemical properties [15–17]. Zhu et al. [15] prepared four anionic gemini surfactants with long rigid or semi-rigid spacers, and found that all these gemini surfactants showed lower CMC and C20 values, and that gemini surfactants with rigid spacers preferred to form vesicles. Gao and co-workers [16] reported a series of novel cationic gemini surfactants with diethylammonium headgroups and a diamido spacer. This was the first report of sponge-like aggregates in the cationic gemini surfactant-water binary system which was probably caused by adhesion and fusion of vesicles at high surfactant concentration. Hu et al. [17] synthesized a class of quaternary ammonium salt gemini surfactants with para-xylene as a rigid spacer. These gemini surfactants possessed high surface activity.
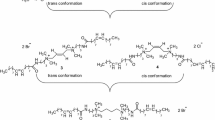
In this paper, we designed and prepared a class of cationic gemini surfactants with rigid amido spacer groups. The hydrophilic radical (amido bond) was selected as the important building block for the rigid spacer, which was hoped to improve the solubility of gemini surfactant in aqueous solution, and further enhance their range of applications. Scheme 1 shows the chemical structures and the synthetic routes used to prepare these gemini surfactants. The surface and physical–chemical properties of these gemini surfactants were investigated, including their surface tension, electrical conductivity, micropolarity, foaming and emulsion properties.
Experimental Section
Materials
Dimethyl terephthalate (AR, Analytical Reagent), 1-bromododecane (CP, Chemical Pure), 1-bromotetradecane (CP), acetonitrile (AR), 1-bromohexadecane (CP), acetone (AR), Diethyl phthalate (CP) and methanol (AR) were purchased from Sinopharm Chemical Reagent Corporation. N, N-dimethyl propylene diamine (AR) and pyrene (AR) were purchased from commercial sources without further purification. Double distilled water was used in all experiments.
1H- and 13C-NMR spectra were recorded on a Bruker AV 400 NMR Spectrometer (Bruker Corporation, Switzerland, 400 or 100 MHz). The infrared (IR) spectra were measured on a Japan FT/IR-430 spectrometer as KBr pellets. Elemental analyses were carried out on a Vario EL-III Spectrometer (Elementer Corporation, Germany).
Synthesis of bis(3-(dimethylamino)propyl)terephthalamide (Compound A)
A mixture of N,N-dimethyl propylene diamine (20.40 g, 0.20 mol) and dimethyl terephthalate (9.70 g, 0.05 mol) was added to a 3-necked flask (150 mL). After stirring for 16 h at reflux (125 °C), the reaction mixture was cooled to room temperature. The excess N,N-dimethyl propylene diamine and methanol were removed under reduced pressure, the white residue was recrystallized from a mixture of ethyl acetate and methanol (4:1) to obtain the white solid product (15.13 g, 90.60 % yield based on the dimethyl terephthalate). 1H NMR (400 MHz, CDCl3, TMS) δ ppm: 1.76–1.78 (m, 4H, 2 NHCH2 CH 2 CH2N), 2.28 (s, 12H, 4CH 3 ), 2.47 (t, J = 6.0 Hz, 4H, 2 NHCH2CH2 CH 2 N), 3.53–3.55 (m, 4H, 2 NHCH 2 CH2CH2N), 7.80–7.81 (s, 4H at the benzene ring), 8.764 (s, 2H, 2 NH). 13C MNR (100 MHz, CDCl3, TMS) δ ppm: 166.47, 136.99, 126.99, 58.89, 45.29, 40.34, 25.38. IR (KBr) cm−1: 3347, (N–H, amide); 1632 (C=O, amide); 2942 (CH3–); 2857 (–CH2–); 1552, 1500, 1457 (benzene ring); 664 (benzene ring hydrogen). Found %: C, 64.80; H, 8.98; N, 16.69. Theory %: C, 64.64; H, 9.04; N, 16.75.
Synthesis of Gemini Surfactant (C16-PPDA-C16)
A mixture of bis(3-(dimethylamino)propyl)terephthalamide (3.34 g, 10 mmol) and 1-bromohexadecane (7.32 g, 24 mmol) in acetone (20 mL) was placed in a dried 3-necked flask (150 mL) equipped with a magnetic stirrer and a condenser closed with a anhydrous calcium chloride drying tube. After stirring for 36 h at reflux (60 °C), the reaction mixture was cooled to room temperature, and a light yellow precipitate was collected by filtration. The crude product was recrystallized twice from a mixture of acetonitrile and acetone (3:1) and dried under reduced pressure to give C 16 -PPDA-C 16 as a white solid (7.60 g, 80.51 % based on bis(3-(dimethylamino)propyl)terephthalamide). 1H MNR (400 MHz, CDCl3, TMS) δ ppm: 0.86 (t, J = 6.0 Hz, 6H, 2(CH2)13 CH 3 ), 1.23–1.30 (m, 52H, 2(CH 2 ) 13 CH3), 1.71 (m, 4H, 2CH 2 (CH2)13CH3), 2.21 (m, 4H, 2 NHCH2 CH 2 CH2N), 3.28 (s, 12H, 2 N(CH 3 ) 2 ), 3.43–3.52 (m, 8H, 2 NCH 2 CH2(CH2)13CH3, 2 NHCH2CH2 CH 2 N), 3.72 (m, 4H, 2 NHCH 2 CH2CH2N), 7.90 (s, 4H at the benzene ring), 8.67 (s, 2H, 2 NH). 13C MNR (100 MHz, CDCl3, TMS) δ ppm: 167.18, 136.16, 127.68, 64,62, 62.83, 50.95, 36.95, 31.88, 29.68, 29.62, 29.50, 29.44, 29.31, 29.22, 26.33, 22.83, 22.63, 14.06. IR (KBr) cm−1: 3482, 3435 (2 N–H, amide); 2919 (CH3–); 2851 (–CH2–); 1644 (C=O, amide); 1539, 1493, 1468 (benzene ring); 727 (benzene ring hydrogen). Anal. Calcd. Found %: C, 63.82; H, 10.26; N, 5.901. Theory %: C, 63.54; H, 10.24; N, 5.928.
Synthesis of Gemini Surfactant (C14-PPDA-C14)
C 14 -PPDA-C 14 was prepared according to the method used for C 16 -PPDA-C 16 by using bis(3-(dimethylamino)propyl)terephthalamide (3.34 g, 10 mmol) and 1-bromotetradecane (6.65 g, 24 mmol) in acetone (20 mL). The crude product was recrystallized twice from a mixture of acetonitrile and acetone (3:1) and dried under reduced pressure to give C 14 -PPDA-C 14 as a white solid (6.80 g, 76.58 % based on bis(3-(dimethylamino)propyl)terephthalamide). 1H MNR (400 MHz, CDCl3, TMS) δ ppm: 0.87 (t, J = 4.0 Hz, 6H, 2(CH2)11 CH 3 ), 1.24–1.30 (m, 44H, 2(CH 2 ) 11 CH3), 1.71 (m, 4H, 2CH 2 (CH2)11CH3), 2.21 (m, 4H, 2 NHCH2 CH 2 CH2N), 3.28 (s, 12H, 2 N(CH 3 ) 2 ), 3.45-3.51 (m, 8H, 2 NCH 2 CH2(CH2)11CH3, 2NHCH2CH2 CH 2 N), 3.74 (m, 4H, 2NHCH 2 CH2CH2N], 7.91 (s, 4H at the benzene ring), 8.67 (s, 2H, 2NH). 13C MNR (100 MHz, CDCl3, TMS) δ ppm: 167.16, 136.17, 127.67, 64.61, 62.81, 50.95, 36.93, 31.86, 29.64, 29.60, 29.48, 29.43, 29.30, 29.21, 26.32, 22.81, 22.62, 14.05. IR (KBr) cm−1: 3481, 3434 (N–H, amide); 2920 (CH3–); 2851 (–CH2–); 1644 (C=O, amide); 1538, 1493, 1469 (benzene ring); 727 (benzene ring hydrogen). Anal. calcd. Found %: C, 61.96; H, 9.949; N, 6.279. Theory %: C, 62.15; H, 9.978; N, 6.302.
Synthesis of Gemini Surfactant (C12-PPDA-C12)
C 12 -PPDA-C 12 was prepared according to the method used for C 16 -PPDA-C 16 by using bis(3-(dimethylamino)propyl)terephthalamide (3.34 g, 10 mmol) and 1-bromododecane (5.98 g, 24 mmol) in acetone (20 mL). The crude product was recrystallized twice from a mixture of acetonitrile and acetone (3:1) and dried under reduced pressure to give C 12 -PPDA-C 12 as a white solid (5.93 g, 71.27 % based on bis(3-(dimethylamino)propyl)terephthalamide). 1H MNR (400 MHz, CDCl3, TMS) δ ppm: 0.86 (t, J = 4.0 Hz, 6H, 2(CH2)9 CH 3 ), 1.23–1.29 (m, 36H, 2(CH 2 ) 9 CH3), 1.70 (m, 4H, 2CH 2 (CH2)9CH3), 2.19 (m, 4H, 2 NHCH2 CH 2 CH2N), 3.26 (s, 12H, 2 N(CH 3 ) 2 ), 3.42–3.51 (m, 8H, 2 NCH 2 CH2(CH2)9CH3, 2NHCH2CH2 CH 2 N), 3.71 (m, 4H, 2NHCH 2 CH2CH2N), 7.91 (s, 4H at the benzene ring), 8.67 (s, 2H, 2NH). 13C MNR (100 MHz, CDCl3, TMS) δ ppm: 167.14, 136.15, 127.64, 64.55, 62.70, 50.94, 36.89, 31.80, 29.58, 29.52, 29.42, 29.38, 29.23, 29.15, 26.27, 22.86, 22.75. 14.01. IR (KBr) cm−1: 3478, 3434 (N–H, amide); 2922 (CH3–); 2852 (–CH2–); 1645 (C=O, amide); 1539, 1493, 1468 (benzene ring); 727 (benzene ring hydrogen). Anal. calcd. Found %: C, 60.29; H, 9.636; N, 6.756. Theory %: C, 60.56; H, 9.681; N, 6.727.
Surface Tension Measurement
The surface tensions of all the gemini surfactant solutions were measured using the du Noüy ring technique (Krüss K20 Tensiometer, Germany) [18], and the value of γ was measured at least three times at 301.0 ± 0.1 K. The surface tension of pure water was measured as being 71.99 ± 0.05 mN/m at 298.0 K. All solutions were prepared with triple-distilled water.
Electrical Conductivity Measurement
The CMC of the gemini surfactants were determined by the electrical conductivity method. The conductivity analyzer (Model DDS-307, Shanghai Precision & Scientific Instrument Corporation) was used to measure the conductivity of the gemini surfactant solutions at 301.0 ± 0.1 K, and each experiment was repeated three times. The concentration at the intersection of the curve of conductivity versus gemini surfactants concentration was taken as the critical micelle concentration [19].
Fluorescence Measurement
Fluorescence measurements were carried out on a F-4600 Fluorescence spectrophotometer (Hitachi High-Technologies Corp, Tokyo Japan). Pyrene was used as the fluorescence probe at 336 nm photoexcitation. The emission spectra were scanned over the spectrum range of 345–460 nm, and the slit widths of excitation and emission were fixed at 5.0 and 2.5 nm, respectively. The ratio of the intensities of the first peak (around 373 nm) to the third peak (around 384 nm) was used as an index of the polarity of the pyrene-solubilizing medium (I 1/I 3) [20]. All solutions were prepared by injecting the appropriate amount of ethanolic pyrene solution (1 × 10−3 M, 0.1 % of the volume of the gemini surfactants solution) into the gemini surfactant solutions, followed by ultrasonication for 2 h. The temperature was kept at 28 °C throughout the experiments.
Foamability and Foam Stability Measurement
A calibrated 100-mL glass cylinder with a stopper and an aqueous solution containing 0.1 % gemini surfactant (20 mL) was used for the measurement of foamability and foam stability. The solution was shaken vigorously for 10 s at room temperature by an inverted cylinder test and the foam height measured [21]. Foam stability was assessed using the time required for the foam to collapse to half its initial height (half-life) [22, 23]. The experiments were repeated at least five times.
Emulsion Stability Measurement
Emulsion stability measurement was performed using a graduated cylinder with a plastic stopper and standard ground-glass joint [21]. Emulsions were prepared by mixing 20 mL of 0.1 % aqueous gemini surfactant solutions with 20 mL of paraffin oil. The stoppered graduated cylinder was inverted up and down five times successively and allowed to equilibrate for 1 min. In order to get a good reproducibility and high reliability, the experiment was repeated five times, and the time for the separation of 10 mL water was recorded in seconds [24]. The measurement was made at room temperature.
Results and Discussion
Synthesis
The synthetic route for preparing the gemini surfactants (C 12 -PPDA-C 12 , C 14 -PPDA-C 14, C 16 -PPDA-C 16 ) is shown in Scheme 1. The three surfactants were easily prepared through a two-step procedure from the starting materials. First, the aminolysis reaction of commercially available dimethyl terephthalate (1 equiv) with an excess molar ratio of N, N-dimethyl propylene diamine (4 equiv) generated the important intermediate bis(3-(dimethylamino)propyl)terephthalamide (Compound A). Subsequently, the target compounds C 12 -PPDA-C 12 , C 14 -PPDA-C 14 and C 16 -PPDA-C 16 were prepared at a high yield of 75.84–80.51 % by quaternization of Compound A with alkyl bromide (1-bromododecane, 1-bromotetradecane, 1-bromohexadecane) in the presence of acetone, respectively. Their chemical structures were confirmed by 1H- and 13C-NMR spectroscopy, infrared spectroscopy and elemental analysis.
The Surface Active Properties of the Gemini Surfactants
The measurement of surface tension is a classical method of studying the surface active properties of surfactants. The critical micelle concentration (CMC) and the surface tension at the CMC (γ CMC) are important parameters for characterizing the surface activity of a surfactant [25]. The curves of the surface tension as a function of the concentration for the new gemini surfactants at 301.15 K are shown in Fig. 1. The CMC and γ CMC values are summarized in Table 1.
As shown in Table 1, the CMC values of C 12 -IPDA-C 12 , C 14 -IPDA-C 14 and C 16 -IPDA-C 16 are 1.17, 0.218 and 0.0601 mmol/L at 301.15 K, respectively, indicating that the gemini surfactants easily form micelles in the bulk solution. Obviously, the gemini surfactants with longer hydrophobic chain length have a lower CMC value. The CMC of conventional ionic surfactants is known to decrease with increasing number of carbon atoms in the hydrophobic groups [26]. For comparison, the CMC values of two cationic single-chain surfactants, CTAB (hexadecyltrimethylammonium bromide) and DTAB (dodecyltrimethylammonium bromide), were also measured as 0.846 and 15.31 mmol/L under the same conditions. Evidently the CMC values for the synthesized gemini surfactants were about one order of magnitude lower than those of the single-chain surfactants with the same number of carbon atom in the hydrophobic groups. In this work, the CMC of the gemini surfactants decrease from 1.17 to 0.0601 mmol/L with increasing number of carbon atoms in the hydrocarbon chain from 12 to 16, which is similar to common surfactants in a homologous serious [27]. The data indicate that the gemini surfactants with rigid spacers have excellent micelle forming ability.
The surface tension at the CMC (γ CMC) can be obtained directly from Fig. 1 and the values are listed in Table 1. The values of γ CMC for these gemini surfactants in aqueous solutions are 36.31, 40.89 and 43.41 mN/m at 301.15 K, respectively. The data showed that the γ CMC values increased from 36.31 to 43.41 mN/m with increasing number of carbon atom in the hydrocarbon chain from 12 to 16. Another important parameter (pC20) of surfactants is also listed in Table 1. The pC20 represents the value of logarithm surfactant concentration required to reduce the surface tension of water by 20 mN/m. The pC20 value indicates the adsorption efficiency of surfactants at the air–water interface, the larger the pC20 value, the more surfactant adsorbed at the air–water interface [28]. As shown in Table 1, the pC20 values of the synthesized gemini surfactants are 3.57, 4.13 and 4.90 at 28 °C, respectively. It is obvious that the adsorption efficiency increases with increasing length of the hydrophobic chain. In other words, the gemini surfactant concentration required for reducing the surface tension of water by 20 mN/m is decreased.
The packing density of surfactants at the air–water interface is important to interpret the surface activities of surfactants [29]. The surface area (A min) occupied by the gemini surfactant molecules at the air–water interface is a measure of their packing density [30]. The surface excess concentration (Γ max) and the area occupied by a surfactant molecule (A min) at the air–water interface were calculated using the Gibbs adsorption Eqs. (1) and (2) [31, 32] as following:
where R is the gas constant (8.314 J/mol K), T is the absolute temperature (K), C is the surfactant concentration (mol/L), dγ/dlogC is the slope below the CMC in the surface tension vs concentration curves, N A is Avogadro’s number (6.02 × 1023 mol−1). The values of n = 2 and 3 (the number of species at the interface whose concentration changes with the surfactant concentration) are used for calculating the Γ max values [16, 33, 34]. The calculation results of Γ max and A min are given in Table 1 for each gemini surfactant.
As shown in Table 1, the values of Γ max of these gemini surfactants decreases with increasing the length of the hydrocarbon chains from 12 to 16 and the values of A min increase, which suggested that the gemini surfactants with the longer hydrophobic chains had lower packing densities at the air–water interface. A possible explanation is that the longer hydrophobic chains are not vertically aligned at the air–water surface and make the A min values larger [29].
The Degree of Counter Ion Dissociation (α) and Thermodynamic Properties of Micellization
The CMC values of the synthesized gemini surfactants in aqueous solutions were also investigated by conductivity measurements at 301.15 ± 0.1 K. As shown in Fig. 2, the CMC values were determined as the sharp break points in the plots of specific conductance (κ) versus gemini surfactants concentration (c) [19], and the degree of counterion dissociation (α) was determined from the pre-micellar slope (S 1 = dκ/dc) to the post-micellar slope (S 2 = dκ/dc), α = S 2/S 1 [35, 36]. The values of CMC and α for the synthesized gemini surfactants are tabulated in Table 1, respectively. As shown in Table 1, the CMC values of the three synthesized gemini surfactants measured by the conductometry are 1.252, 0.26 and 0.0754 mmol/L at 301.15 K, respectively. It should be noted that the CMC results determined by tensiometry are nearly identical those determined by the electrical conductivity method. The data in Table 1 show that the α values of gemini surfactant decrease significantly with increasing hydrophobic tail length for the gemini surfactants. The long hydrophobic tails favor the formation of micelles and result in a decrease in CMC values.
The CMC and α values calculated for all the synthetic gemini surfactants at 28 °C in aqueous solutions have been used to calculate the different thermodynamic parameters of micellization. The standard Gibbs energies of micellization for ionic surfactants \(\Delta G_{m}^{^\circ }\) was calculated using Eq. (3):
where X CMC is the CMC in mole fraction, R is the gas constant and T is temperature on the Kelvin scale. The X CMC is calculated by using the equation of X CMC = CMC/(CMC + number of moles for the solvent).
The \({\Delta G}_{\text{m}}^{\circ}\) values for all the synthetic gemini surfactants in aqueous solutions are also listed in Table 1. The \({\Delta G}_{\text{m}}^{\circ }\) values of C 12 -PPDA-C 12 , C 14 -PPDA-C 14 and C 16 -PPDA-C 16 are −54.54, −65.08 and −76.93 kJ/mol at 301.15 K, respectively, indicating that the process of micellization is spontaneous. The results also revealed that the driving force for the formation of micelles increases with increasing carbon atoms in the hydrocarbon chain, which leads to a \({\Delta G}_{\text{m}}^{\circ }\) value for C 16 -PPDA-C 16 .
Micropolarity and Aggregation
Pyrene is often used as a probe to investigate gemini surfactant aggregates in aqueous solution [28, 37]. The spectral profiles and intensity of the photoluminescence (PL) spectra for pyrene is sensitive to its microenvironment at the site of fluorophore solubilization. The intensity ratio of the first peak to the third peak (I 1/I 3) can be taken as a measure of the microenvironment polarity, which is high in polar media and low in hydrophobic environments. When the gemini surfactant concentration is lower than the CMC, the intensity ratio I 1/I 3 value remains essentially constant, which indicates that surfactant molecules exist as monomers in aqueous solutions, and the microenvironment does not change. When the gemini surfactant concentrations exceeds the CMC, there is an obvious decrease in I 1/I 3 for all the gemini surfactant solutions, which demonstrates that the microenvironment has changed due to the formation of aggregates. When gemini surfactant molecules start to form micelles, the extreme hydrophobic pyrene molecules are transferred from the water environment to the hydrophobic core. As shown in Fig. 3a, the minimum I 1/I 3 values for all three gemini surfactants decrease with increasing length of the hydrocarbon chains, indicating that hydrophobic tail length has a large effect on the aggregate microenvironment. The aggregate micropolarity can be attributed to specific molecular structures. The long and rigid spacer with a certain rigidity and hydrophilicity may increase the steric hindrance of the self-assembly procedure and hinder the gemini surfactant molecules from close packing [16].
As shown in Fig. 3b, the pyrene fluorescent intensities of C 12 -PPDA-C 12 and C 14 -PPDA-C 14 are far greater than that of C 16 -PPDA-C 16 at 0.1 mmol/L, which results from the change in microenvironment due to the micellization of C 16 -PPDA-C 16 in aqueous solution. For C12-PPDA-C12 or C14-PPDA-C14, the concentration is lower than the CMC, so micelles can not form in the bulk. But for C16-PPDA-C16, the concentration exceeds the CMC and great number of micelles and aggregates have been formed, which results in the formation of a hydrophobic microenvironment. The hydrophobic pyrene molecules are solubilized in the hydrophobic microdomain which leads to a decrease in microenvironment polarity [38] and pyrene fluorescence intensity.
Foamability and Foam Stability
Many authors have suggested that foamability and foam stability of gemini surfactants can be attributed to properties of the interfacial film and the interfacial tension [39–41]. When generating foam of the same total surface area, low surface tension benefits foam formation, and it is also good for foam rupture (foam instability). Foam stability is mainly due to elasticity of the interfacial film that separates the two phases. In recent reports [42, 43], we demonstrated that foam stability correlates well with the high limit interfacial elasticity of the interfacial film. This agrees with the finding by Sonin et al. [44] who reported that the high-frequency interfacial elasticity plays an important role in the film thinning process. Under aerated conditions, surfactants adsorb to form bilayers with the hydrophilic head groups forming a hydration layer within the liquid film. Increasing the chain length increases the hydrophobic driving force which increases bilayer adsorption and thus the formation of stable films.
As shown in Table 2, increasing the hydrocarbon chain length from 12 to 14, the initial foam height gradually decreases and the half-life of foam collapse shows a gradual increase. In addition, when the concentration of gemini surfactant increases from 0.5 CMC to 2 CMC, both the initial foam height and the half-life of foam are gradually increased. In comparison with other synthesized gemini surfactants, the lowest foam-producing ability is C 16 -IPDA-C 16 , which is similar to common homologous surfactants [41]. However, foam stability improves as the hydrocarbon chain length increasing from 12 to 16. The low foamability and foam stability of these synthetic gemini surfactants is not desirable. It is probably due to the presence of the rigid spacer linking group, which considerably increases the area per molecule which produces less cohesive force at the interface.
Emulsion Stability
The stability of paraffin oil-in-water emulsions in the presence of the three gemini surfactants was investigated. Emulsification relies on surfactant adsorption forming a protective film at the surface of the dispersed droplets which can delay or prevent coalescence. Cationic gemini surfactants enhance stability by imparting an electrostatic charge on the droplet surface thus reducing the physical contact between the droplets. The amount of surfactant adsorption at the oil–water interface in O/W emulsions depends on the degree to which the hydrophobic groups of the gemini surfactants damage the structure of the water phase. Therefore, the stability of the emulsion increases with increasing length on the hydrophobic chain. As shown in Table 2, for C 12 -PPDA-C 12 , it takes 350 s for separation of the aqueous layer from the emulsion, whereas for C 14 -PPDA-C 14 and C 16 -PPDA-C 16, 586 and 637 s is required, respectively. Thus, the emulsion stability increases with increasing the length of the hydrophobic tail from 12 to 16.
References
Zana R, Talmon Y (1993) Dependence of aggregate morphology on structure of dimeric surfactants. Nature 362:228–230
Patial P, Shaheen A, Ahmad I (2013) Synthesis of ester based cationic pyridinium gemini surfactants and appraisal of their surface active properties. J Surfactants Deterg 16:49–56
Hegazy MA, El-Tabei AS (2013) Synthesis, surface properties, synergism parameter and inhibitive performance of novel cationic gemini surfactant on carbon steel corrosion in 1 M HCl solution. J Surfactants Deterg 16:221–232
Zhang Y, Ding M, Zhou L et al (2012) Synthesis and antibacterial characterization of gemini surfactant monomers and copolymers. Polym Chem 3:907–910
Xie DH, Zhao JX (2013) Unique aggregation behavior of a carboxylate gemini surfactant with a long rigid spacer in aqueous solution. Langmuir 29:545–553
Che YJ, Gao Q, Fang ML et al (2015) Synthesis, characterization and aqueous properties of a new hybrid fluorocarbon cationic surfmer. J Surfactants Deterg 18:233–244
Menger FM, Littau CA (1993) Gemini surfactants: a new class of self assembling molecules. J Am Chem Soc 115:10083–10090
Menger FM, Keiper JS (2000) Gemini surfactants. Angew Chem Int Ed 39:1906–1920
Zana R (2002) Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci 248:203–220
Alcalde MA, Jover A, Meijide F et al (2008) Synthesis and characterization of a new gemini surfactant derived from 3α,12α-dihydroxy-5α-cholan-24-amine (steroid residue) and ethylenediamintetraacetic acid (spacer). Langmuir 24:6060–6066
Lu T, Huang JB (2007) Synthesis and properties of novel gemini surfactant with short spacer. Chin Sci Bull 52:2618–2620
Shankar BV, Patnaik A (2007) Chiral discrimination of a gemini-type surfactant with rigid spacer at the air-water interface. J Phys Chem B 111:11419–11427
Huang X, Cao MW, Wang JB, Wang YL (2006) Controllable organization of a carboxylic acid type gemini surfactant at different pH values by adding copper(II) ions. J Phys Chem B 110:19479–19486
Nieh MP, Kumar SK, Fernando RH, Colby RH, Katsaras J (2004) Effect of the hydrophilic size on the structural phases of aqueous nonionic gemini surfactant solutions. Langmuir 20:9061–9068
Zhu DY, Cheng F, Chen Yu et al (2012) Preparation, characterization and properties of anionic gemini surfactants with long rigid or semi-rigid spacers. Colloids Surf A Physicochem Eng Asp 397:1–7
Zhang Q, Gao ZN, Tai SX et al (2012) Surface tension and aggregation properties of novel cationic gemini surfactants with diethylammonium headgroups and a diamido spacer. Langmuir 28:11979–11987
Hu DH, Guo XF, Jia LH (2013) Synthesis, surface active properties of novel gemini surfactants with amide groups and rigid spacers. J Surfactants Deterg 16:913–919
Cui ZG, Yang LL, Cui YZ, Binks BP (2010) Effects of surfactant structure on the phase inversion of emulsions stabilized by mixtures of silica nanoparticles and cationic surfactant. Langmuir 26:4717–4724
Williams R, Phillips JN, Mysels KJ (1955) The critical micelle concentration of sodium lauryl sulphate at 25 °C. Trans Faraday Soc 51:728–737
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Mao P (1988) Synthetic detergent industrial analysis. Peking: the publishing house of light industry, Beijing, pp 456–462
Tehrani-Bagha AR, Holmberg K (2010) Cationic ester-containing gemini surfactants: physical–chemical properties. Langmuir 26:9276–9282
Zhao JX, Zou WS (2013) Foams stabilized by mixed cationic gemini/anionic conventional surfactants. Colloid Polym Sci 291:1471–1478
Jia W, Rao X, Song Z, Shang S (2009) Microwave-assisted synthesis and properties of a novel cationic gemini surfactant with the hydrophenanthrene structure. J Surfactants Deterg 12:261–267
Zhao GX, Zhu BY (2003) Principles of surfactant action. China Light Industry Press, Beijing, p 66
Yoshimura T, Esumi K (2004) Synthesis and surface properties of anionic gemini surfactants with amide groups. J Colloid Interface Sci 276:231–238
Zana R (2002) Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review. Adv Colloid Interface Sci 97:205–253
Perez L, Pinazo A, Rosen MJ, Infante MR (1998) Surface activity properties at equilibrium of novel gemini cationic amphiphilic compounds from arginine, bis (args). Langmuir 14:2307–2315
Zhou LM, Jiang XH, Li YT, Chen Z, Hu XQ (2007) Synthesis and properties of a novel class of gemini pyridinium surfactants. Langmuir 23:11404–11408
Laschewsky A, Wattebled L, Arotcareìna M, Habib-Jiwan JL, Rakotoaly RH (2005) Synthesis and properties of cationic oligomeric surfactants. Langmuir 21:7170–7179
Alami E, Beinert G, Marie P, Zana R (1993) Alkanediyl-a, x-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air–water interface. Langmuir 9:1465–1467
Song LD, Rosen MJ (1996) Surface properties, micellization, and premicellar aggregation of gemini surfactants with rigid and flexible spacers. Langmuir 12:1149–1153
Devinsky F, Lacko I, Bittererova F, Tomeckova L (1986) Relationship between structure, surface activity, and micelle formation of some new bisquaternary isosteres of 1,5-pentanediammonium dibromides. J Colloid Interface Sci 114:314–322
Li ZX, Dong CC, Thomas RK (1999) Neutron reflectivity studies of the surface excess of gemini surfactants at the air–water interface. Langmuir 15:4392–4396
Zana R, Benraou R, Rueff R (1991) Alkanediyl-a, x-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir 7:1072–1075
Zana R (1996) Critical micellization concentration of surfactants in aqueous solution and free energy of micellization. Langmuir 12:1208–1211
Bhattacharya S, Haldar J (2004) Thermodynamics of micellization of multiheaded single-chain cationic surfactants. Langmuir 20:7940–7947
Yang F, Li G, Liu R, Zhang B, Liu Y, Wang ZX (2009) Synthesis and surface activity properties of cationic surfactants of gemini nonylphenol polyoxyethylene quaternary ammonium chloride. Acta Chim Sin 67:723–728
Acharya DP, Gutiérrez JM, Aramaki K, Aratani K, Kunieda HJ (2005) Interfacial properties and foam stability effect of novel gemini-type surfactants in aqueous solutions. Colloid Interface Sci 291:236–243
Santini E, Ravera F, Ferrari M, Stubenrauch C, Makievski A, Krägel J (2007) A surface rheological study of non-ionic surfactants at the water-air interface and the stability of the corresponding thin foam films. Colloids Surf A 298:12–21
Pinazo A, Pérez L, Infante MR, Franses EI (2001) Relation of foam stability to solution and surface properties of gemini cationic surfactants derived from arginine. Colloids Surf A 189:225–235
Wu XN, Zhao JX, Li EJ, Zou WS (2011) Interfacial dilational viscoelasticity and foam stability in quaternary ammonium gemini surfactant systems: influence of intermolecular hydrogen bonding. Colloid Polym Sci 289(9):1025–1034
You Y, Wu XN, Zhao JX, Ye YZ, Zou WS (2011) Effect of alkyl tail length of quaternary ammonium gemini surfactants on foaming properties. Colloids Surf A 384:164–171
Sonin A, Bonfillon A, Langevin D (1994) Thinning of soap films: the role of surface viscoelasticity. J Colloid Interface Sci 162:323–330
Acknowledgments
We thank the Research Funds of Anhui Science and Technology University (ZRC2014432, AKZDXK2015A01, ZRC2014401) and the Natural Science Foundation of Anhui Province (1508085QB41) for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xu, D., Qi, B., Fang, D. et al. Preparation, Characterization and Properties of Novel Cationic Gemini Surfactants with Rigid Amido Spacer Groups. J Surfact Deterg 19, 91–99 (2016). https://doi.org/10.1007/s11743-015-1752-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1752-0