Abstract
This paper reports the adsorption and interfacial viscoelasticity of gemini surfactants with a hydroxyl-substituted spacer, 2-hydroxyl-propanediyl-α,ω-bis(dimethyldodecylammonium bromide), 2-hydroxyl-butanediyl-α,ω-bis(dimethyldodecylammonium bromide), and 2,3-hydroxyl-butanediyl-α,ω-bis(dimethyldodecylammonium bromide), referred to as 12-3(OH)-12, 12-4(OH)-12, and 12-4(OH)2-12, respectively, at the air/water interface using dropping shape and interface dilational rheology measurements. For comparison, the unsubstituted surfactants were also examined at identical conditions. The results showed that substituted surfactants produced a remarkably higher interfacial elasticity than the corresponding unsubstituted ones. This was attributed to the effect of the intermolecular hydrogen bonding occurring between the hydroxyl-substituted spacers of adsorbed molecules, which resulted in tighter packing of the molecules in the monolayer. Besides, we measured foam stability. The foam produced by the substituted geminis was found to have higher stability than that by the unsubstituted geminis. It was suggested that the foam stability may be related to the limit elasticity of interfacial film at the level of identical surface excesses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Foam is a metastable system consisting of gas bubbles separated by three-dimensional water channels. Control for foam stability is very important in many applications such as floatation, dust control, fire control, tertiary oil recovery, food processing, personal care products, etc. The stability of foam was known to highly relate to the lifetime of the individual thin films that separate the two phases. Surfactants are currently added to stabilize foam by preventing bubbles in the foam from coalescing, which allows the lifetime of film to be greatly increased. Thus, a great deal of effort has been spent in studying the influence of the molecular structure of the surfactant on the film stability.
Gemini surfactant consists of two hydrophilic head groups, two hydrophobic tails, and a spacer linked at or near the head groups [1, 2]. In the past two decades, a great deal of investigations for gemini surfactants have exhibited their excellent properties of self-assembly superior to traditional single-chain surfactants [3]. Even so, however, there are only a few investigations focused on the foamability and foam stability generated by geminis. Kim et al. [4] studied gemini surfactants made from epichlorohydrin, which gives a three-carbon spacer unit with a hydroxyl group at the central carbon. A series of geminis with different lengths of hydrophobic tails were synthesized, and the foaming behavior was compared with that of the single-tail cations. It was found that both the foamability and the foam stability were much higher for the geminis than that for the monomeric surfactants. Dodecyl chains were optimal with respect to both foamability and foam stability. Espert et al. [5] found that the cationic gemini surfactant, ethanediyl-α,ω-bis(dimethyldodecylammonium bromide) (referred to as 12-2-12), efficiently stabilized soap films even at very low concentrations. This behavior was in sharp contrast with that of the corresponding monomer, dodecyltrimethylammonium bromide (C12TABr). Pinazo et al. [6] synthesized a series of arginine-based gemini surfactants and compared the foaming properties with those of the monomeric cationic surfactant. The geminis were found to be much more efficient as foam stabilizers. The effect was ascribed to the very fast surface tension equilibration. Tehrani-Bagha and Holmberg [7] synthesized three cationic gemini surfactants with ester bonds inserted between the hydrocarbon tails and the charged headgroups. They verified that the gemini surfactants can better stabilize foam than the corresponding alkyl esterquat monomer, among which a decyl esterquat with a three-carbon spacer was extremely efficient as a foam stabilizer. The effect was attributed to this surfactant being more susceptible to chemical hydrolysis than the other ester-containing surfactants. Recently, Acharya et al. [8] also reported the effect of a novel gemini surfactant without a spacer (sodium 2,3-didodecyl-1,2,3,4-butane tetracarboxylate, abbreviated as GS) on foam stability. They found that the addition of a small amount of GS in SDS solution increased the foam stability noticeably.
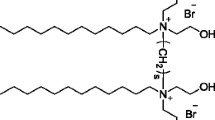
Alkanediyl-bis-(dimethylalkyl-ammonium bromide)—referred to as m-s-m, where m and s represent the number of the carbon atoms in the alkyl tail and in the flexible polymethylenen spacer, respectively—is a common family of cationic gemini surfactants investigated extensively [3, 9]. The derivatives of m-s-m containing a hydroxyl-substituted spacer have been synthesized in several groups [4, 10–15], for instance, 2-hydroxyl-propanediyl-α,ω-bis(dimethyldodecyl- ammonium bromide), 2-hydroxyl-butanediyl-α,ω-bis(dimethyldodecylammonium bromide), and 2,3-hydroxyl-butanediyl-α,ω-bis(dimethyldodecylammonium bromide), referred to below as 12-3(OH)-12, 12-4(OH)-12, and 12-4(OH)2-12, respectively. In a very recent issue, we reported a complementary study for the adsorption and association of 12-3(OH)-12 in aqueous solution [16] in which the intermolecular hydrogen bonding between the substituted spacers of 12-3(OH)-12 was experimentally verified. This led to stronger adsorption and association of 12-3(OH)-12 in comparison with unsubstituted 12-3-12. As a result, 12-3(OH)-12 formed the adsorption film at the air/water interface where the surfactant molecules were tightly packed. Illuminated by this result, we expect that such films would better stabilize the foam by the intermolecular hydrogen bonding interactions. Thus, in the present work, we extended the investigation to the foam systems using 12-3(OH)-12, 12-4(OH)-12, and 12-4(OH)2-12, respectively, as foam stabilizers. To better understand the role of the intermolecular hydrogen bonding, unsubstituted 12-3-12 and 12-4-12 were also examined at an identical level.
It was known that the destabilization of foams generally followed several mechanisms such as drainage and thin-liquid film rupture, both of which were believed to be linked to the interfacial rheology of the dispersion interface [17]. In particular, stress/strain relationships of the adsorbed layers have been measured through shear or dilatation interfacial deformations and subsequently related to individual foam film stability and drainage [18–20], as well as foam stability [21–24], and foam rheology [25, 26]. Therefore, understanding the interfacial rheology of the adsorbed layer is of significance and of great interest for foam stabilization. Several methods can be used to measure the dilatational surface moduli, and the choice depends on the frequency range of interest. For example, the oscillating bubble/drop method probes frequencies between 0.01 and 1 Hz, whereas a capillary wave device probes frequencies from 10 to 104 Hz [27, 28] and surface quasi-elastic light scattering enables one to investigate frequencies up to 106 Hz [29]. The relevant frequency range is dictated by the chemical system under investigation, which determines the time and length scales characterizing the molecular interaction inside the adsorbed layers.
As the adsorbed layers are directly connected with the bulk phase, compressing or expanding the monolayer leads to the desorption or adsorption of the surfactant molecules into or from the bulk to restore the equilibrium interfacial concentration. When the frequency of a sinusoidal perturbation is low, the monolayer has time to reach equilibrium, and there is no resistance to the deformations. When the frequency is high, the monolayer has no time to respond and behaves as if it were insoluble. Therefore, the investigation under the low frequency allows us to understand the relaxation effects coming from the diffusion and adsorption of surfactants [30] and/or conformational changes of the adsorbed molecules [31]. Moreover, a popular model (LVT model) used to describe the viscoelasticity of the adsorbed monolayer has been established on the basis of the low-frequency range [30], which enables us to theoretically analyze the experimental data. Therefore, in the present work, the oscillating drop method was used to study the interfacial viscoelastic properties of the adsorption films. Combining with the equilibrium adsorption, the properties of the adsorption films were characterized. Finally, these properties were related to the foam stability measured by the decay time \( {t_{1/2}} \) for foam height to fall by 50%.
Materials and methods
Materials
Gemini surfactants, 2-hydroxyl-propanediyl-α,ω-bis(dimethyldodecylammonium bromide), 2-hydroxyl-butanediyl-α,ω-bis(dimethyldodecylammonium bromide), 2,3-hydroxyl-butanediyl-α,ω-bis(dimethyldodecylammonium bromide), propanediyl-α,ω-bis(dimethyldodecylammonium bromide), and butanediyl-α,ω-bis(dimethyldodecylammonium bromide), the chemical structures of which are shown in Scheme 1, were synthesized in our laboratory according to the methods reported by Rosen and Song [12] and Zana et al. [32], respectively. All the products were confirmed by 1H NMR and elemental analysis, which are available in Electronic supplementary material (ESM) Supporting Materials. All the solutions were prepared with Milli-Q water (resistivity = 18.2 MΩ cm−1).
Measurements
Equilibrium adsorption of the surfactants at the air/water interface was investigated using surface tension measurements, which were carried out with an optical angle meter, OCA-20, from Dataphysics Instruments GmbH, Germany. A drop of aqueous solution containing different amounts of the surfactant was syringed in a thermostated optical glass cuvette and was hung for 30 min to reach the adsorption equilibrium, which was determined according to the time-dependent curves of interfacial tension shown in ESM Supporting Materials. The drop shape was monitored by a CCD camera and the image was analyzed by the software to obtain interfacial tension data.
Interface dilational rheology was measured by an optical angle meter OCA-20 with oscillating drop accessory ODG-20. The equilibrated interface was disturbed by sinusoidal oscillations. The accessible frequency range was 0.01–1 Hz and the relative area (A) variation was ~6%. These conditions followed the range of linear viscoelasticity. Oscillations caused sinusoidal changes in the surface area and the radius, i.e., in the drop shape. The changes in drop shape were monitored by a CCD camera with a minimum of 50 frames per second. At the end of the experiment, the software retrieved the images and calculated the change in area and respective changes in surface tension for each cycle. Using Fourier transform analysis, the complex dilational modulus (ε*) and phase angle (θ) were determined and the dilational elasticity ε and dilational viscosity η were calculated by the relations
Foam stability was determined using a setup shown in Fig. 1. Gas with a constant flow speed of 68 mL min−1 was bubbled through a porous glass disc fixed at the bottom of a cylindrical glass container (25-mm internal diameter, 140-mm height) in which the test solution of 5 mL was filled. Foam was produced until a height of 40 mm, i.e., a volume of 20 cm3, and then the valve was shut immediately. Foam stability was determined to be the time needed for the collapse of the foam to half its initial height. The temperature was kept at 25 ± 0.1 °C throughout the experiments using a water bath.
Results
Adsorption isotherms of surfactants at air/water interface
Figure 2 illustrates the Frumkin adsorption isotherms of the examined surfactants, which were obtained from the surface tension plots measured experimentally (see ESM Supporting Materials) through fitting the data by Szyszkowski formula and then calculating the surface excess by Gibbs equation [33]. As seen from Fig. 2, all the substituted geminis, regardless of 12-3(OH)-12 and 12-4(OH)-12 or 12-4(OH)2-12, show greatly larger surface excess at the saturation than each of the unsubstituted gemini, 12-3-12 and 12-4-12. As revealed in our previous work, these substituted molecules formed intermolecular hydrogen bonding in their monolayers [16]. Thus, the adsorption molecules are packed tightly, resulting in a large surface excess. Moreover, the addition of a second hydroxyl substitutent has less effect on the surface excess (Fig. 2b), which is well consistent with the observation of Wettig et al. [15]. Comparatively, 12-3(OH)-12 has a larger surface excess than both 12-4(OH)-12 and 12-4(OH)2-12. This is similar to the difference between 12-3-12 and 12-4-12 in adsorption, reflecting the influence of the spacer length [34, 35]. The shorter spacer corresponds to the smaller area of surfactant occupying the air/water interface and thus larger adsorption amount. The results of the equilibrium adsorption provide a basis for discussing the interfacial viscoelasticity and the foam stability in the following sections.
Frequency and concentration dependency of interface dilational viscoelasticity
Figure 3 shows the plots of complex dilational modulus ε*, dilational elasticity ε, dilational viscosity η, and phase angle θ as a function of the frequency of sinusoidal oscillation for a typical system of 12-3(OH)-12 at different concentrations; others are available in ESM Supporting Materials. As seen from Fig. 3, all the characteristic parameters show clear frequency dependence. The increasing disturbance frequency means a decrease in the respond time, over which the surfactant molecules exchange between the interface and the bulk and also move inside the monolayer so as so to restore the equilibrium. At low frequency, the time of surfactant response is sufficient, and thus various relaxation processes coming from diffusion and adsorption of surfactants [30] and/or conformational changes of the adsorbed molecules [31] can occur. At high frequency, the monolayer has no time to respond and behaves as if it were insoluble. The phase angles are always positive for all the gemini samples over the range of examined frequencies, indicating that the phase of interfacial tension oscillation is ahead of that of interfacial area oscillation. The effect of surfactant bulk concentration is also clearly seen (Fig. 4). Comparatively, the frequency dependence is more pronounced at moderate bulk concentrations of the surfactant than at lower or higher concentrations, which agrees with the previous observation in other systems [36] and the theoretical prediction [30, 37].
Experimental plots of complex modulus (ε*) (a), interfacial elasticity (ε) (b), interfacial viscosity (η) (c), and phase angle (θ) (d) as a function of frequency (v), respectively, in 12-3(OH)-12 aqueous solutions at 25 °C. The symbols represent different surfactant concentrations: log(C/mmol L−1) = −1.57 (square), −1.31 (circle), −1.07 (triangle), −0.84 (downturned triangle), −0.60 (diamond), −0.34 (left-pointing triangle)
Semi-logarithmic plots of dilational interfacial elasticity (ε) (a) and interfacial viscosity (η) (b) of 12-3(OH)-12 as a function of the concentration C at different frequencies. The symbols express the frequency of sinusoidal oscillation at 0.010 Hz (square), 0.046 Hz (circle), 0.10 Hz (triangle), 0.46 Hz (downturned triangle), and 1.0 Hz (diamond), respectively
LVT model description for the experimental data
Lucassen–van den Tempel (LVT) model describes the viscoelastic behavior of soluble monolayer [30, 37]. This model assumed that the material transport involved in the adsorption kinetics is governed only by diffusion without energy barriers and considered the instantaneous coupling between the interface rheology and the adsorption kinetics. The model predicted the viscoelastic moduli through the following equations:
with
where ε 0 is the theoretical high-frequency limit of the surface elasticity and ω 0 is the molecular exchange parameter. Figure 5 shows the fitting results for 12-3(OH)-12 using Eqs. 3 and 4 in which the LVT model describes the experimental data very well.
Fitting results of dilational interfacial elasticity (ε) (a) and interfacial viscosity (η) (b) in terms of the LVT model for 12-3(OH)-12 aqueous solutions at 25 °C. Symbol representations are the same as in Fig. 3
According to the LVT model, at low frequencies, the dilational interfacial elasticity is close to zero irrespective of the bulk concentration of surfactant in the solution, as seen in Fig. 5. It can be explained as due to the interfacial tension gradient resulting from interface deformation which almost vanishes during the experimental time. At high frequencies, the dilational elasticity shows little change with further increasing frequency. This states the work frequency far higher than the characteristic frequency of various relaxation processes occurring at and near the interface, and thus, the interface film embodies the characteristic of the insoluble film. In accordance with Eq. 3, one obtains ε = ε 0 for \( \nu \, \to \,\infty \). For the paper at hand, however, ε 0 cannot be experimentally determined since the high-frequency limit is not included. Here, we regard ε 0 and ω 0 as fitting parameters ε 0,fit and ω 0,fit, in accordance with Eqs. 3 and 4. The fitting procedure was carried out so that couples of ε 0,fit and ω 0,fit were determined which best described both the experimental ε(ν, C) and η(ν, C) curves. The fitting parameters for all the systems are listed in Table 1.
Foam stability
According to Tehrani-Bagha and Holmberg’s suggestion [7], foam stability was determined as the time needed for the collapse of the foam to half its initial height, t 1/2. Figure 6 shows semi-logarithmic plots of t 1/2 as a function of surfactant concentration. For all the systems, t 1/2 increases rapidly with increasing the bulk concentration and leaves off beyond the cmc within a reasonable error range. The maximum t 1/2 corresponding to the plateau is significantly larger for the substituted gemini than for the unsubstituted gemini, showing the excellent effect of the former as a foam stabilizer.
Discussion
Role of intermolecular hydrogen bonding in enhancing interfacial elasticity and foam stability
The tight packing of surfactant molecules at the air/water interface generally brings about a high interfacial elasticity of the adsorption film due to the increase in cohesion within the monolayer. For example, in the case of alkyltrimethylammonium bromides, the limiting interfacial elasticity (ε 0) is enhanced from 45 mN m−1 for C10TABr to 61 mN m−1 for C16TABr, which accompanies an increase in the adsorption amount with increasing the length of the alky chain [38]. The addition of small amounts of a long-chain alcohol into the C12TABr solution also greatly improved the stability of the film [38]. Comparatively, gemini surfactants can more popularly produce high interfacial elasticity. Espert et al. [5] obtained a value of ε 0 (62 mN m−1) for 12-2-12 which is greatly higher than that (50 mN m−1) of the corresponding monomer C12TABr and comparable with that of C16TABr, even though each alkyl tail of 12-2-12 has only 12 carbon atoms. The gemini surfactant without a spacer group, GS, even yields ε 0 higher than 100 mN m−1 [8]. Obviously, GS produces a much denser adsorption monolayer than 12-2-12 due to no hindrance of the spacer group. These results indicate that gemini surfactants are better candidates than single-chain surfactants to produce high interfacial elasticity for their adsorption films, in which the nature of the spacer has an important influence on the property of the adsorption monolayer. In the work at hand, we modified the spacer of the bis-quaternary ammonium gemini surfactant with one or two substituted hydroxyl group(s). This leads to higher ε 0 compared with unsubstituted 12-3-12 or 12-4-12, even though the latter has a relatively higher concentration in the bulk solution (Fig. 7) and the ε 0 of 12-3(OH)-12 is even close to 100 mN m−1 at a suitable concentration (Fig. 4). This is mirrored in the large surface excess as seen in Fig. 2. As discussed in the first section, a large surface excess for 12-3(OH)-12, 12-4(OH)-12, or 12-4(OH)2-12 is attributed to the intermolecular hydrogen bonding produced in the monolayer, as verified in our previous work [16]. The intermolecular hydrogen bonding between surfactants can effectively promote their self-assembly. In our recent work, a greatly enhanced ability of 12-3(OH)-12 for adsorption and association was observed under the intermolecular hydrogen bonding interactions [16]. Moreover, as a kind of directive forces, this interaction effectively accelerates the micellar growth, resulting in the formation of wormlike micelles at very low concentrations [16]. Also, Shchipunov [39] and Martin and Ward [40] emphasized the role of intermolecular hydrogen bonding in the construction of supramolecular self-assemblies such as gel or lamellar phase. When this interaction is contributed to the adsorption monolayer, the surfactant molecules pack more tightly at the saturated adsorption, resulting in large surface excess and high interfacial elasticity.
Comparison for the plots of interfacial elasticity vs. frequency between 12-3(OH)-12 (0.027 mmol L−1, (empty circles), 0.049 mmol L−1 (empty squares)) and 12-3-12 (0.039 mmol L−1 (solid circles), 0.057 mmol L−1 (solid squares)) (a), and between 12-4(OH)2-12 (0.056 mmol L−1 (empty circles), 0.10 mmol L−1 (empty squares)) and 12-4-12 (0.074 mmol L−1 (solid circles), 0.16 mmol L−1 (solid squares)) (b)
As seen in Fig. 6, the substituted geminis yield more stable foams compared with the unsubstituted geminis. That is to say that the intermolecular hydrogen bonding interactions occurring within the adsorption monolayer favors stabilizing the foam. This is obviously due to the dense adsorption structure in the monolayer. The present results suggest a valuable approach to enhance the interfacial elasticity of the film generated by gemini surfactants as well as the foam stability, that is, to utilize the intermolecular hydrogen bonding interactions.
Interfacial elasticity and foam stability
Many authors believed that foam stability is relevant to the interfacial rheology of the film separating gas bubbles in the foam system [1–11]. For instance, Joye and coworkers showed that the surface viscosity has a stabilizing effect on the asymmetric drainage of foam films [41, 42]. Fruhner et al. [20] found that the surface dilational viscosity plays an important role in the stability of foam films, but they could not correlate the surface elasticity with the foam stability. Koelsch and Motschmann [43] also suggested the importance of the surface dilational viscosity for the ability of a surfactant system to form a stable foam. Comparatively, the surface elasticity was paid much attention by several groups. Bergeron [38] performed a study on the forces and stability of foam films, suggesting that the role of surface elasticity is more important than that of the forces. Sonin et al. [44] demonstrated that the surface elasticity that plays an important role in the film thinning process is the high-frequency elasticity. Similar conclusions were obtained later by Espert et al. [5] and Stubenrauch et al. [36, 45]. Wang and Yoon [46] determined the surface forces in foam films stabilized with flotation frothers and found that foam stabilities are controlled by both film elasticity and disjoining pressure. At relatively low concentrations, frother dampens the hydrophobic force that is an attractive component of the disjoining pressure and destabilizes foams. At higher concentrations, elasticity plays a more important role in stabilizing foams. Acharya et al. [8] compared the interfacial viscoelasticity with the foam stability using the gemini surfactant without a spacer group, GS, as stabilizer and found that the stability of the wet foam can be correlated with the film elasticity, but the dry foam cannot be explained in terms of film elasticity alone. Typical example is also for cationic alkyltrimethylammonium bromides (CnTABr). For this particular homologous series, a strong increase of ε was observed when the chain length is increased from n = 12 to n = 14 [28, 47]. A further increase of the chain length does not have any significant influence. Comparing these results with the fact that the respective films are only stable for n > 12 [38], they concluded that foam film stability and surface elasticity could be directly correlated. Recently, Georgieva et al. [17] discussed the correlation between the interfacial elasticity and the foam stability in detail and suggested that the Oswald ripening, also called coarsening (transfer of gas/liquid from the smaller bubbles/droplets to the larger ones by diffusion through the continuous phase driven by the difference in the Laplace pressure), can be controlled by the low-frequency elasticity while the bubble coalescence is controlled by the high-frequency elasticity. Our results at hand also indicate that high interfacial elasticity is indeed relevant to high foam stability, as revealed in the above sections.
When the different foam systems are compared, however, the conclusion should be drawn very carefully since the interfacial elasticity depends on both the frequency of disturbance and the concentration of surfactant in bulk solution [30, 36, 37]. To exclude the influence of frequency, the high-frequency elasticity, ε 0, may be an appropriate parameter for comparison [44]. But the effect of surfactant concentration was often not taken into account simultaneously in references, which may be the cause leading to confusing and incomparable results. In fact, the effect of bulk concentration may only be apparent, and on the contrary, the surface excess is a direct factor affecting the property of film. Therefore, the comparison for ε 0 should be carried out at the level of the identical surface excesses. In the work at hand, the high-frequency limit of the elasticity is not included in the measurements and the fitting parameters, ε 0,fit, are adopted. Similarly, ε 0,fit also shows concentration dependence, as seen in Fig. 8. From Fig. 8, one can estimate the limit elasticity at different surface excesses. Table 2 reports the ε 0,fit at the surface excess of 80%, from which remarkably larger ε 0,fit can be seen for substituted surfactants than that for corresponding unsubstituted ones. This well corresponds to the more stable foams for the substituted surfactant systems shown in Fig. 6. Thus, we come to a conclusion that the stability of wet foam can be related to the interfacial elasticity at the identical level of the surface excess.
Conclusion
The adsorption films formed by the hydroxyl-substituted gemini surfactants, 12-3(OH)-12, 12-4(OH)-12 and 12-4(OH)2-12, have a remarkably higher interfacial elasticity than that formed by the corresponding unsubstituted gemini surfactants, 12-3-12 and 12-4-12. This is attributed to the effect of the intermolecular hydrogen bonding interaction since it can produce tighter packing of the adsorbed molecules at the air/water interface. This leads to high stability for the foam as measured experimentally. The present work reveals for the first time that the intermolecular hydrogen bonding can be an effective supplementary contribution to enhance the interfacial elasticity of the adsorption film and, hence, the stability of the foam. For this goal, the surfactant with gemini-type structure may be a valuable candidate since its spacer can be conveniently modified by hydroxyl group(s).
In addition, we evaluate the foam stability by the elasticity of interfacial film and suggest that the wet foam stability may be related to the limit elasticity of interfacial film. If one wishes to evaluate the wet foam stability produced by different surfactants through the limit elasticity of interfacial film, the comparison should be carried out at the level of identical surface excesses.
References
Menger FM, Littau CA (1991) J Am Chem Soc 113:1451–1452
Menger FM, Littau CA (1993) J Am Chem Soc 115:10083–10090
Zana R, Xia JD (2004) Gemini surfactants. Marcel Dekker, New York
Kim TS, Kida T, Nakatsuji Y, Hirao T, Ikeda I (1996) J Am Oil Chem Soc 73:907–911
Espert A, Klitzing RV, Poulin P, Colin A, Zana R, Langevin D (1998) Langmuir 14:4251–4260
Pinazo A, Pérez L, Infante MR, Franses EI (2001) Colloids Surf A 189:225–235
Tehrani-Bagha AR, Holmberg K (2010) Langmuir 26:9276–9282
Acharya DP, Gutiérrez JM, Aramaki K, Aratani K, Kunieda HJ (2005) Colloid Interface Sci 291:236–243
Shukla D, Tyagi VK (2006) J Oleo Sci 55:381–390
Rosen MJ, Liu L (1996) J Am Oil Chem Soc 73:885–890
Song LD, Rosen MJ (1996) Langmuir 12:1149–1153
Rosen MJ, Song LD (1996) J Colloid Interface Sci 179:261–268
Rosen MJ, Mathias JH, Davenport L (1999) Langmuir 15:7340–7346
Mathias JH, Rosen MJ, Davenport L (2001) Langmuir 17:6148–6154
Wettig SD, Nowak P, Verrall RE (2002) Langmuir 18:5354–5359
Pei XM, You Y, Zhao JX, Deng YS, Li EJ, Li ZX (2010) J Colloid Interface Sci 351:457–465
Georgieva D, Cagna A, Langevin D (2009) Soft Matt 5:2063–2071
Joye JL, Miller CA (1992) Langmuir 8:3083–3092
Cohen-Addad S, Di Meglio JM (1994) Langmuir 10:773–778
Fruhner H, Wantke KD, Lunkenheimer K (1999) Colloids Surf A 162:193–202
Wasan DT, Nikolov AD, Lobo LA, Koczo K, Edwards DA (1992) Prog Surf Sci 39:119–154
Sonin AA, Bonfillon A, Langevin D (1993) Phys Rev Lett 71:2342–2345
Prins A (1999) Colloids Surf A 149:467–473
Martin AH, Grolle K, Cohen-Stuart MA, Van Vliet T (2002) J Colloid Interface Sci 254:175–183
Stone H, Koehler SA, Hilgenfeldt S, Durand M (2003) J Phys Condens Matter 15:S283–S290
Hemar Y, Hocquart R, lequeux F (1995) J Phys II Fr 5:1567–1576
Stenvot C, Langevin D (1988) Langmuir 4:1179–1183
Monroy F, Giemanska Kahn J, Langevin D (1998) Colloids Surf A 143:251–260
Hard S, Neuman RD (1987) J Colloid Interface Sci 120:15–29
Lucassen J, van den Tempel M (1972) Chem Eng Sci 27:1283–1291
Monroy F, Rivillon S, Ortega F, Rubio RG (2001) J Chem Phys 115:530–539
Zana R, Benrraou M, Rueff R (1991) Langmuir 7:1072–1075
Rosen MJ (1988) Surfactants and interfacial phenomena, 2nd edn. Wiley, New York
Alami E, Beinert G, Marie P, Zana R (1993) Langmuir 9:1465–1467
Wettig SD, Verrall RE (2001) J Colloid Interface Sci 235:310–316
Stubenrauch C, Miller R (2004) J Phys Chem B 108:6412–6421
Lucassen J, van den Tempel M (1972) J Colloid Interface Sci 41:491–498
Bergeron V (1997) Langmuir 13:3474–3482
Shchipunov YA (2001) Colloids Surf A 183–185:541–554
Martin SM, Ward MD (2005) Langmuir 21:5324–5331
Joye JL, Hirasaki G, Miller CA (1994) Langmuir 10:3174–3179
Joye JL, Hirasaki G, Miller CA (1996) J Colloid Interface Sci 177:542–552
Koelsch P, Motschmann H (2005) Langmuir 21:6265–6269
Sonin AA, Bonfillon A, Langevin D (1994) J Colloid Interface Sci 162:323–330
Santini E, Ravera F, Ferrari M, Stubenrauch C, Makievski A, Krägel J (2007) J Colloids Surf A 298:12–21
Wang L, Yoon RH (2008) Int J Miner Process 85:101–110
Stenvot C (1988) Langmuir 4:1179–1183
Acknowledgments
Support from the National Natural Science Foundation of China (20673021 and 20873024) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 10.5 mb)
Rights and permissions
About this article
Cite this article
Wu, X., Zhao, J., Li, E. et al. Interfacial dilational viscoelasticity and foam stability in quaternary ammonium gemini surfactant systems: influence of intermolecular hydrogen bonding. Colloid Polym Sci 289, 1025–1034 (2011). https://doi.org/10.1007/s00396-011-2425-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-011-2425-9