Abstract
Electrochemical and gravimetric measurements were performed to investigate the effectiveness of a synthesized cationic gemini surfactant as corrosion inhibitor for carbon steel in 1 M HCl solution. The inhibition efficiency obtained from all methods are in good agreement. Potentiodynamic polarization measurements showed that, the prepared surfactant hinders both anodic and cathodic processes, i.e. acted as mixed-type inhibitor. It was found that the adsorption of the synthesized inhibitor followed the Langmuir adsorption isotherm and showed a mixed physical and chemical adsorption. Kinetic parameters were calculated and discussed. The inhibitor exhibited a synergistic effect with Sn2+ more than with Cu2+. The synthesized cationic gemini surfactant exhibited good surface properties.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrochloric acid is widely used in industry, the most important fields of application being acid pickling, industrial acid cleaning, acid descaling and oil well acidizing. It is also used for removal of oxide from metallic parts before applying coatings. The use of acid solutions during industrial applications leads to a corrosive attack on metal. Therefore, inhibitors are commonly used to minimize metal dissolution and acid consumption.
Most of the efficient inhibitors used in industry are organic compounds which mainly contain oxygen, sulfur, nitrogen atoms and multiple bonds in the molecule through which they are adsorbed on metal surfaces [1–8]. Moreover, many N-heterocyclic compounds have been proved to be effective inhibitors for the corrosion of metals and alloys in aqueous media [9–16].
Surfactants are molecules composed of a polar hydrophilic group, the “head”, attached to a nonpolar hydrophobic group, the “tail”. Generally, in aqueous solution, the inhibitory action of surfactant molecules may also be due to physical (electrostatic) adsorption or chemisorption onto the metallic surface, depending on the charge of the solid surface and the free energy change of transferring a hydrocarbon chain from water to the solid surface. The adsorption of a surfactant markedly changes the corrosion resisting property of a metal.
Research during the last decade reveals that cationic gemini surfactants have been used as corrosion inhibitors. Accordingly, this work deals with the study of the corrosion inhibition properties of the a newly synthesized cationic gemini surfactant. The choice of this compound was based on the consideration that this compound contains an ammonium group, a bromide ion, and an aliphatic chain, The aldimine compound group and heteroatoms such as N and O, induce greater adsorption of the inhibitor molecule onto the surface of carbon steel compared with compounds containing only nitrogen, oxygen or a non-surface active agent.
The aim of this work is to determine the inhibition efficiency of 4,4′-(3,3′-(1Z,1′Z)-pentane-1,5-diylidenebis(azan-1-yl-1-ylidene)bis(propane-3,1-diyl))bis(4-dodecylmorpholin-4-ium bromide) as a novel inhibitor for the corrosion of carbon steel in 1 M HCl, to calculate the surface parameters of the prepared surfactant and to study the synergistic effect between the synthesized inhibitor with Sn2+ and Cu2+. The inhibition efficiency was determined using three different techniques: (a) electrochemical impedance spectroscopy (EIS), (b) potentiodynamic polarization and (c) gravimetric measurements.
Materials and Experimental Methods
Material Preparation
Carbon steel specimens of the following chemical composition (wt.%) were used in the experiment: C- 0.21; Si- 0.38; P- 0.09; Mn- 0.05; S- 0.05; Al- 0.01; and Fe- balance.
A pre-treatment was carried out prior to each experiment, in which the specimen surface was mechanically ground with 340, 400, 600, 800, 1,000 and 1,200 grades of emery paper, rinsed with bidistilled water, degreased in ethanol and dried at room temperature before use.
Synthesis of Novel Cationic Gemini Surfactant
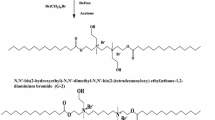
The novel cationic surfactant used in this study was synthesized as shown in Scheme 1. This process was carried out in two steps as follow:
-
1.
Aldimine reaction of 3-morpholinopropan-1-amine (2.88 g, 0.02 mol) and glutaraldehyde (1.00 g, 0.01 mol) in ethanol at 70 °C for 8 h to produce 4,4′-(3,3′-(1Z,1(N,N’Z,N,N’Z)-N,N’-(pentane-1,5-diylidene)bis(3-morpholinopropan-1-amine). The mixture was allowed to cool-down. Then, the obtained precipitate was further purified by diethyl ether then recrystallized by ethanol.
-
2.
Quaternization reaction of the above product (3.52 g, 0.01 mol) and 1-bromododecane (2.49 g, 0.01 mol) in ethanol at 70 °C for 48 h to production of 4,4′-(3,3′-(1Z,1′Z)-pentane-1,5-diylidenebis(azan-1-yl-1-ylidene)bis(propane-3,1-diyl))bis(4-dodecylmorpholin-4-ium bromide). The mixture was allowed to cool-down. Then, the obtained precipitate was further purified by diethyl ether then recrystallized by ethanol.
The chemical structure of the synthesized compound (Scheme 1) was confirmed by FTIR, 1H-NMR and mass spectroscopy analysis.
Solutions
The aggressive solution, 1 M HCl was prepared by dilution of analytical grade 37 % HCl with distilled water. The concentration range of the synthesized inhibitor used varied from 5 × 10−5 to 5 × 10−3 M for corrosion measurements and from 1 × 10−6 to 1 × 10−2 M for both surface tension and conductivity measurements, while the concentration range of Sn2+ and Cu2+ varied from 10 to 100 ppm without and with 1 × 10−4 M of the synthesized inhibitor for the synergistic effect measurements. Double distilled water was used for preparing test solutions in all measurements.
Electrochemical Measurements
Electrochemical experiments were carried out using a conventional three-electrode cell with a platinum counter electrode (CE) and a saturated calomel electrode (SCE) as a reference electrode. The working electrode (WE) was a rod of carbon steel embedded in a PVC holder using epoxy resin so that the flat surface was the only exposed surface in the electrode. Before each measurement, the electrode was immersed in a test solution at open circuit potential (OCP) for 30 min, until a steady state was reached. Electrochemical measurements were recorded by a Voltalab 40 Potentiostat PGZ 301 and a personal computer was used with Voltamaster 4 software at 25 °C. Each experiment was repeated at least three times to check the reproducibility.
Potentiodynamic polarization measurements were obtained by changing the electrode potential automatically from −800 to −300 mV versus SCE with a scan rate 2 mV s−1 at 25 °C.
Electrochemical impedance spectroscopy (EIS) measurements were carried out as described elsewhere [17]. A small alternating voltage perturbation (5 mV) was imposed on the cell over the frequency range of 100 kHz to 30 mHz at 25 °C.
Gravimetric Measurements
Carbon steel sheets of 7 cm × 3 cm × 0.5 cm were accurately weighed with an analytical balance (A&D Model: HR 200, readability: 0.1 mg and accuracy: ±0.2 mg) and then immersed for 24 h in a solution containing 1.0 M HCl with and without different concentrations of the synthesized inhibitor. The steel specimens were taken out after 24 h. Then they were rinsed with distilled water twice and degreased with acetone. Then specimens were immersed in 1 M HCl solution for 10 s (chemical method for cleaning rust products), rinsed twice with distilled water, finally dried, and accurately weighted. The temperature for weight loss measurements was controlled by water bath provided with thermostat control ±0.5 °C. In order to get good reproducibility, experiments were carried out in triplicate. The average weight loss of three parallel carbon steel sheets was obtained.
Surface Tension Measurements
Surface tension was measured for different concentrations of the synthesized cationic gemini surfactant with a Du Nouy Tensiometer (Krüss Type 6). All solutions were prepared in double distilled water with a surface tension equal to 72 mN m−1 at 25 °C.
Conductivity Measurements
An electrical conductivity meter (Type 522; Crison Instrument, S.A.) was used to measure the conductivity of the different surfactant solutions.
Results and Discussion
Structure Confirmation of the Synthesized Inhibitor
Structure Confirmation of the Synthesized Aldimine Compound
FTIR Spectroscopy
The FTIR spectrum of the synthesized aldimine compound showed the following absorption bands at 753.37 cm−1 ((CH2) n rocking), 1,366.64 cm−1 (CH3 symmetric bending), 2,859.24 cm−1 (CH symmetric stretching), 2,945.10 cm−1 (CH asymmetric stretching), 1,659.87 cm−1 (C = N), 1,118.75 cm−1 (C–O–C) and the disappearance of-NH2 band at 3,300 cm−1. The FTIR spectrum confirmed the expected functional groups in the synthesized aldimine compound.
Mass Spectroscopy
The mass spectrum of the synthesized aldimine compound (Fig. 1) illustrated a molecular ion peak at m/z 352 (26.98 %) together with a base peak at 97 (100.00 %, C5H8N2). Fragmentation as follows: 127(45.16 % H2C = CHCH2NCH2CH2OCH2CH2), 129(40.08 % CH3CH2CH2NCH2CH2OCH2CH2) and 172(11.11 % CH2CH2OCH2CH2N2CH2CH2OCH2CH2).
Data of mass spectrum confirmed the chemical structure of the synthesized aldimine compound.
Structure Confirmation of the Synthesized Cationic Gemini Surfactant
FTIR Spectroscopy
The FTIR spectrum of the synthesized cationic gemini surfactant showed the following absorption bands at 716 cm−1 ((CH2)n rocking), 1,367 cm−1 (CH3 symmetric bending), 2,853 cm−1 (CH symmetric stretching), 2,921 cm−1 (CH asymmetric stretching), 3,171, 1,011 cm−1- (C–N+), 1,625 cm−1 (C = N) and 1,117 cm−1 (C–O–C). The FTIR spectrum (supplementary material, Fig. 1) confirmed the expected functional groups in the synthesized cationic gemini surfactant.
1H-NMR Spectroscopy
The 1H-NMR (DMSO-d6) spectrum of the synthesized cationic gemini surfactant showed different peaks at δ = 0.81 ppm (t, 3H, NCH2CH2(CH2)8CH2CH 3); δ = 1.33 ppm (t, 2H, NCH2CH2(CH2)8CH 2CH3); δ = 1.20 ppm (m, 16H, NCH2CH2(CH 2)8CH2CH3); δ = 1.73 ppm (m, 2H, NCH2CH 2(CH2)8 CH2CH3); δ = 3.11 ppm (t, 2H, NCH 2CH2(CH2)8CH2CH3); δ = 3.11 ppm (t, 2H, NCH 2CH2CH2N); δ = 1.86 ppm (t, 2H, NCH2CH 2CH2N); δ = 1.55 ppm (t, 2H, NCH2CH2 CH 2N); δ = 8.12 ppm (t, 1H, N = CHCH2CH2CH2CH = N); δ = 1.55 ppm (t, 6H, N = CHCH 2CH 2CH 2CH = N); δ = 3.47 ppm (t, 4H, 3,5-morpholine nucleus); δ = 3.67 ppm (t, 4H, 2,6-morpholine nucleus). 1H-NMR spectrum of the synthesized inhibitor is shown in Fig. 2. The data of 1H-NMR spectrum confirmed the expected hydrogen proton distribution in the synthesized cationic gemini surfactant.
Mass spectroscopy
The mass spectrum of the synthesized cationic gemini surfactant illustrated a molecular ion peak at m/z 850 (7.29 %) together with a base peak at 364 (100.00 % Br−N+ (C4H8O)(C3H7)(C12H25)) and 123(11.0 % CH2NCH(CH2)3CHNCH2).
Data of the mass spectrum (supplementary material, Fig. 2) confirmed the chemical structure of the synthesized cationic gemini surfactant.
Potentiodynamic Polarization Measurements
Figure 3 shows Tafel plots for the carbon steel electrode in 1 M HCl in the absence and presence of the prepared inhibitor.
The percentage inhibition efficiency (η P) was calculated using the following equation [18]:
where i corr and i ocorr are the uninhibited and inhibited corrosion current densities, respectively, which were determined by extrapolation of cathodic and anodic Tafel lines to the corrosion potential.
The values of corrosion current density (i corr), corrosion potential (E corr), cathodic Tafel slope (β c), and anodic Tafel slope (β a) as functions of the synthesized inhibitor concentrations, were calculated from the curves in Fig. 3 and listed in Table 1.
Inspection of Table 1 revealed that the presence of inhibitor leads to the following:
-
1.
Values of i corr were decreased, indicating the inhibiting effect.
-
2.
Values of E corr were slightly shifted toward both negative and positive potential, which means that the studied inhibitor was the mixed type.
-
3.
Values of β a and β c were slightly changed in the presence as in the absence of the inhibitor, which indicated that the added inhibitor does not change the mechanism of iron dissolution and hydrogen evolution reactions, and that it decreases both reactions by surface coverage.
-
4.
The inhibition efficiency of the tested inhibitor increased with its rise in concentration with a maximum value of 97 % at 5 × 10−3 M.
The average of the standard deviation for the three replicates was 0.35.
Electrochemical Impedance Spectroscopy Measurements
Nyquist plots of the carbon steel electrode in 1 M HCl in the absence and presence of the prepared inhibitor are shown in Fig. 4a.
a Nyquist plots for carbon steel in 1 M HCl in the absence and presence of different concentrations of inhibitor. (1) 1 M HCl, (2) 5 × 10−5 M, (3) 1 × 10−4 M, (4) 5 × 10−4 M, (5) 1 × 10−3 M, (6) 2.5 × 10−3 M and (7) 5 × 10−3 M. b Bode plots for carbon steel in 1 M HCl in the absence and presence of different concentrations of inhibitor
The inhibition efficiency (η I) was calculated from the values of R ct using the following equation [19]:
where, R ct and R oct are the charge transfer resistance values in the absence and presence of the inhibitor, respectively.
The double layer capacitance (C dl) was calculated from the following equation [19]:
where f max is the frequency at a maximum imaginary component of the impedance.
Various parameters such as charge transfer resistance (R ct), double layer capacitance (C dl) and inhibition efficiency (η I) were calculated and listed in Table 2.
From Table 2 it is clear that the presence of inhibitor leads to the following:
-
1.
Charge transfer resistance, R ct, values in the presence of the inhibitor are always greater than their values in the absence of the inhibitor, which indicated a reduction in the steel corrosion rate.
-
2.
Charge transfer resistance, R ct, values increased with increasing the concentration of the inhibitor.
-
3.
The double layer capacitance, C dl, values are decreased in the presence of the inhibitor due to a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer, suggesting that the inhibitor molecules acted by adsorption at the metal/solution interface. Addition of synthesized inhibitor provides lower C dl values, probably as a consequence of replacement of water molecules by inhibitor molecules at the electrode surface. Also, the inhibitor molecules may reduce the capacitance by increasing the double layer thickness according to the Helmholtz model [20]:
where ε is the dielectric constant of the medium, ε o is the vacuum permittivity, A is the electrode surface area and δ org is the thickness of the protective layer.
The value of C dl is always smaller in the presence of the inhibitor than in its absence, which may be resulting from the effective adsorption of the synthesized inhibitor.
For more complicated system, Bode plots can give more information. The Bode plots of the prepared inhibitor are presented in Fig. 4b. The low frequency impedance modulus Z mod is one of the parameters which can be easily used to compare corrosion resistance of different samples. A larger Z mod demonstrates a better protection performance [21]. In Fig. 4b, it is shown that Z mod increases as a function of the concentration for the prepared inhibitor. These phenomena can be explained as follows; the high frequency phase angle range (105–104 Hz) of the impedance spectra corresponds to the properties of an outer layer, the middle frequency range (104–102 Hz) reflects the properties of an inner barrier layer, while the low frequency range (less than 102 Hz) corresponds to the properties of the double-electrical layer information [22]. Therefore, the high frequency phenomenon may due to the thickness increase of the outer porous layer, and the middle frequency phenomenon can be attributed to the penetration of active chloride ions and water through the defect of the prepared inhibitor inner barrier layer, though the whole effect induced the increase of Z mod. Figure 4b shows that each impedance diagram consists of a large capacitive loop with one capacitive time constant in the Bode-phase diagrams. The shape of the capacitive loops suggests that the charge-transfer controls the corrosion of carbon steel at E corr [23].
Data were fitted with the equivalent circuit as shown in supplementary material (Fig. 3) which was described before [24] to obtain the fitting parameters shown in Table 2. The average of the standard deviation for the three replicates is 0.41.
Gravimetric Measurements
The mass loss is determined after removing the corrosion products from the metal surface in accordance with the procedures recommended by the ASTM G1-67 standard. Then the corresponding corrosion inhibition efficiency (η w) is calculated by the following equation [25]:
where W and W o are the weight loss in the absence and presence of inhibitor, respectively.
The corrosion rate (k) of carbon steel is calculated by the following equation [25]:
where ΔW and S represent the weight loss and the exposure area of specimen, t is the testing period.
Effect of Synthesized Inhibitor Concentration on Inhibition Efficiency
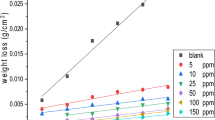
Values of the inhibition efficiency (η w) and corrosion rate (k) are obtained by the weight loss method at different concentrations of inhibitor are summarized in Table 3. It is found that the inhibition efficiency increases with rising concentration (supplementary material, Fig. 4). The maximum inhibition efficiency of the tested inhibitor is 97.2 % at 5 × 10−3 M. The corrosion rate values decreases with the rise in inhibitor concentration. The average of the standard deviation for the three replicates is 0.55.
Effect of Temperature on Inhibition Efficiency
The influence of solution temperature on the inhibition efficiency was studied at 25, 40 and 60 °C (supplementary material, Fig. 4). It was observed that the inhibition efficiency decreased with the temperature increasing from 25 to 60 °C. The reduction in inhibition efficiency with temperature may be attributed to desorption of the inhibitor molecules from the metal surface at higher temperature [26].
Adsorption Isotherm
Basic information on the interaction between the inhibitor molecules and metal surfaces was provided by the adsorption isotherms. The values of surface coverage (θ) were calculated from the following equation [27] and obtained from weight loss measurements at 25, 40 and 60 °C, respectively:
where W and W o are the weight loss in the absence and presence of inhibitors, respectively.
A correlation between θ and inhibitor concentration in the corrosive medium was represented by the Langmuir adsorption isotherm [27, 28]:
where K ads is the equilibrium constant of the inhibitor adsorption process and C is the inhibitor concentration.
Plotting of C/θ versus C yielded a straight line (supplementary, Fig. 5) with slope close to 1 which suggested that the adsorption of the prepared inhibitor on metal surface obeyed Langmuir adsorption isotherm model at different temperatures (25–60 °C). This isotherm assumes that the adsorbed molecules occupied only one site and there was no interaction with other molecules adsorbed. The linear regression coefficients (r 2) and the slopes parameter variations with different temperatures are shown in Table 4. All correlation coefficient (r 2 > 0.9993) indicate that the corrosion inhibition of carbon steel by the synthesized inhibitor is attributed to its adsorption on the metal surface. However, the slopes of the C/θ versus C plots were close to 1 which means ideal simulating [29] and obeying the Langmuir adsorption isotherm.
K ads values could be calculated from the intercepts of the straight lines on the C/θ-axis, the K ads was related to the standard free energy of adsorption, ΔG oads , according to the following equation:
The value 55.5 in the above equation is the molar concentration of water in solution in mol dm−3 [29].
The adsorption heat, ΔH oads , can be calculated according to the Van’t Hoff equation [30]:
To obtain the adsorption heat, the relationship between ln K ads and 1/T is plotting (supplementary material, Fig. 6). The adsorption heat could be approximately regarded as the standard adsorption heat, ΔH oads , under the experimental conditions [31].
According to the thermodynamic basic equation, the standard adsorption entropy ΔS oads could be calculated from the following equation:
All the obtained thermodynamic parameters are listed in Table 4. Generally, values of ΔG oads up to −20 kJ mol−1, the adsorption type is regarded as physisorption, the inhibition act due to the electrostatic interactions between the charged molecules and the charged metal, while the values around—40 kJ mol−1 or smaller are associated with chemisorption due to sharing or transferring of electrons from organic molecules to the metal surface to form a coordinate type of bond [32, 33]. ΔG oads values in our measurements ranged from −33.99 to −35.85 kJ mol−1. Thus the adsorption mechanism of the inhibitor on steel in 1 M HCl was typical mixed physical and chemical adsorption [32]. From Table 4 it was found that ΔG oads became more negative with increasing temperature; this result indicated that the adsorption of the synthesized inhibitor on the carbon steel surface was favorable to the experimental temperature.
The negative sign of ΔH oads value indicated that the adsorption of inhibitor molecules was an exothermic process, and the positive values of ΔS oads was attributed to the increase of disorder due to the adsorption of only one surfactant molecule by desorption of more water molecules.
Kinetic Parameters
The adsorption phenomenon can be successfully explained by the thermodynamic parameter; to further elucidate the inhibition properties the kinetic model was another useful tool to explain the mechanism of corrosion inhibition.
The activation parameters for the corrosion process were calculated from Arrhenius equation [34]:
where, E a represents the apparent activation energy, R represents the gas constant, A represents the pre-exponential factor and k represents the corrosion rate, obtained from the weight loss method.
Arrhenius plots for the corrosion rate of carbon steel were plotted (supplementary material, Fig. 7). Values of E a for carbon steel in 1 M HCl without and with inhibitor at different concentrations were calculated by linear regression between ln k and 1/T and results were listed in Table 5.
An alternative formulation of the Arrhenius equation is [35]:
where h is Planck’s constant, N A is Avogadro’s number, R is the universal gas constant, ΔH* is the enthalpy of the activation and ΔS* is the entropy of activation.
Plotting the ln (k/T) against 1/T (supplementary material, Fig. 8) gave straight lines with a slope (−ΔH*/R) and an intercept of (ln (R/NAh) + (ΔS*/R)) from which the values of ΔH* and ΔS* have been calculated and listed in Table 5. From Table 5, it seemed that E a and ΔH* varied in the same fashion. Values of E a of the inhibited solutions were higher than those of the uninhibited solutions, and the values of E a were enhanced with the increase in the inhibitor concentration from 5 × 10−5 to 5 × 10−3 M (the increase of E a decelerated the corrosion rate of steel). The positive signs of ΔH* reflected the endothermic nature of the carbon steel dissolution process. Values of ΔS* for the inhibited solution were higher than those for the uninhibited solution. This suggested that an increase in randomness occurred on going from reactants to the activated complex. This might be a result of the adsorption of organic inhibitor molecules from the acidic solution that could be regarded as a quasi-substitution process between the organic compound in the aqueous phase and water molecules at electrode surface [36]. In this situation, the adsorption of organic inhibitor was accompanied by desorption of water molecules from the surface. Thus the increasing in entropy of activation was attributed to the increasing solvent entropy [37].
Synergism Parameter
All the experimental results suggested that the addition of Sn2+ or Cu2+ to the inhibited solutions increases the inhibition efficiency and the degree of surface coverage (θ). This behavior was attributed to a synergistic effect between Sn2+ or Cu2+ and the synthesized cationic gemini surfactant.
The synergism parameter, S I, was calculated from the data of gravimetric measurements using the following equation [38]:
where I 1+2 = I 1 + I 2, I 1 is the inhibition efficiency of the cation, I 2 is the inhibition efficiency of synthesized inhibitor and I ′1+2 is the measured inhibition efficiency for synthesized inhibitor in combination with the cation.
The synergism parameter values are listed in Table 6. It is clear that the S I values are more than unity, thereby suggesting that the enhanced inhibition efficiency caused by the addition of Sn2+ or Cu2+ to the inhibitor is due to the synergistic effecting. S I values for the addition of Sn2+ more than Cu2+ is due to Sn being higher than Cu in the electrochemical series.
In order to explain this phenomenon, the following synergism mechanism is proposed. Sn2+ and Cu2+ belong to the transition elements in periodic table which have a vacant orbit (3d). The synthesized cationic gemini surfactant contains nitrogen and oxygen atoms which have lone-pair of electrons. When the synthesized cationic gemini surfactant and Sn2+ or Cu2+ were mixed, the new complex of Sn2+—cationic gemini surfactant or Cu2+—cationic gemini surfactant was easily produced. The complex plays an important role in the enhancement of the protection of carbon steel against corrosion. Stabilization of adsorbed Sn2+ or Cu2+ with the inhibitor lead to great surface coverage and thereby greater inhibition efficiency.
The complex was able to be adsorbed onto the steel surface by van der Waals forces to form a denser and more tightly protective film, which drastically decreases the steel surface corrosion. In this case, cationic gemini surfactant molecules may play a role in bridging the access of rare earth Sn2+ or Cu2+ to the metal surface from solution. Consequently, Sn2+ or Cu2+ and cationic gemini surfactant molecules exhibit a strong synergistic inhibition effect for carbon steel in 1 M HCl.
Surface Parameters
Critical Micelle Concentration (CMC) Figure 5a shows the dependence of surface tension on surfactant concentrations. From which we derived the CMC and the surface tension at the CMC. The results demonstrate that the synthesized inhibitor has surface active properties and can significantly lower the surface tension.
Effectiveness (π cmc) Surface pressure at CMC, which is defined as the effectiveness of a surfactant in reducing surface tension [39], are calculated from the following equation:
where γ o is the surface tension measured for pure water at the appropriate temperature and γ cmc is the surface tension at critical micelle concentration.
CMC values of the prepared surfactant are listed in Table 7. The data showed that the synthesized surfactant is considered as a strong surface active agent.
Surface Excess (Γ max) The surface excess, Γ max, is an effective measure of the surfactant adsorption at the air/water interface. The concentration of the surfactant was always higher at the surface phase than that in the bulk solution. The surface excess (Γ max) values are calculated by the Gibbs adsorption equation [40]:
where, dγ/d ln C is the slope in each case, R is the gas constant, T is the absolute temperature, and C is the surfactant concentration, respectively.
The value of “n” for the Gibbs equation is the number of species whose concentration at the interface varied with the surfactant bulk phase concentration and was taken as 3 for the synthesized cationic gemini surfactant [19].
The high value of Γ max suggested that the surfactant molecules at air/water interface are closely packed.
Minimum Surface Area (A min) Values of minimum area per molecule (A min) are calculated by the Gibbs adsorption equation [41, 42]:
where NA is the Avogadro number.
A min values are listed in Table 7. The low value of A min suggested that the surfactant molecules at air/water interface are close-packed; therefore, the orientation of the surfactant molecule at the interface was almost perpendicular to the interface leading to the low surface tension in CMC.
Conductivity Measurements Conductivity measurements were performed for the cationic gemini surfactant at 25 °C in order to evaluate the CMC and the degree of counter ion dissociation, β. It is known that the specific conductivity is linearly correlated to the surfactant concentration in both the premicellar and in the postmicellar regions, and the slope in the premicellar region is greater than that in the postmicellar region [19, 43]. The intersection point between the two straight lines gives the CMC, while the ratio between the slopes of the postmicellar region to that of the premicellar region gives the counter ion dissociation β. Specific conductivity versus concentration plots of the prepared surfactant in water at 25 °C are presented in Fig. 5b. The values of CMC and the degrees of counter ion dissociation β, are listed in Table 8. The CMC value determined using electrical conductivity is in agreement with the one obtained using surface tension variation.
Standard Free Energy of Micelle Formation (ΔG omic ) The standard free energy of micellization, a measure of the tendency of the synthesized cationic gemini surfactant to form micelles, is calculated from the following equation [19]:
where R is the gas constant, T is the temperature and CMC is expressed in the molarity of the surfactant. ΔG omic value of the prepared cationic gemini surfactant is listed in Table 8.
Conclusion
-
1.
4,4′-(3,3′-(1Z,1′Z)-pentane-1,5-diylidenebis(azan-1-yl-1-ylidene)bis(propane-3,1-diyl))bis(4-dodecylmorpholin-4-ium bromide) was synthesized by aldimine compound and quaternization reactions, purified and characterized by FTIR, 1H-NMR and mass spectroscopy.
-
2.
Polarization curves indicated that the novel inhibitor acted as a mixed inhibitor for carbon steel in 1 M HCl.
-
3.
The impedance measurements showed that the adsorbed protective layer on the metal surface obstructs the anodic dissolution of iron. The maximum charge transfer resistance of the synthesized inhibitor is attributed to the formation of a compact adsorbed layer.
-
4.
Gravimetric measurements results confirmed that the synthesized novel inhibitor has a perfect inhibitive performance on carbon steel in 1 M HCl solution. The inhibitive performance of the novel inhibitor is improved by increasing the concentration and decreasing temperature, it showed a maximum inhibiting power at 5 × 10−3 M is 97.2 % at 25 °C.
-
5.
The adsorption mechanism of the prepared inhibitor on the carbon steel obeyed the Langmuir adsorption isotherm model.
-
6.
S I values for the addition of Sn2+ more than Cu2+ is related to the fact that Sn is higher than Cu in electrochemical series.
-
7.
The synthesized cationic gemini surfactant exhibited good surface properties.
References
Hegazy MA, Ahmed HM, El-Tabei AS (2011) Investigation of the inhibitive effect of p-substituted 4-(N, N, N-dimethyldodecylammonium bromide)benzylidene-benzene-2-yl-amine on corrosion of carbon steel pipelines in acidic medium. Corros Sci 53:671–678
Lebrini M, Lagrenée M, Vezin H, Gengembre L, Bentiss F (2005) Electrochemical and quantum chemical studies of new thiadiazole derivatives adsorption on mild steel in normal hydrochloric acid medium. Corros Sci 47:485–505
Dehri İ, Özcan M (2006) The effect of temperature on the corrosion of mild steel in acidic media in the presence of some sulphur-containing organic compounds. Mater Chem Phys 98:316–323
Ehteshamzade M, Shahrabi T, Hosseini MG (2006) Inhibition of copper corrosion by self-assembled films of new aldimine compounds and their modification with alkanethiols in aqueous medium. Appl Surf Sci 252:2949–2959
Atta AM, El-Azabawy OE, Ismail HS, Hegazy MA (2011) Novel dispersed magnetite core–shell nanogel polymers as corrosion inhibitors for carbon steel in acidic medium. Corros Sci 53:1680–1689
El-Etre AY, Abdallah M, El-Tantawy ZE (2005) Corrosion inhibition of some metals using lawsonia extract. Corros Sci 47:385–395
Larabi L, Harek Y, Benali O, Ghalem S (2005) Hydrazide derivatives as corrosion inhibitors for mild steel in 1 M HCl. Prog Org Coat 54:256–262
Galal A, Atta NF, Al-Hassan MHS (2005) Effect of some thiophene derivatives on the electrochemical behavior of AISI 316 austenitic stainless steel in acidic solutions containing chloride ions: I. Molecular structure and inhibition efficiency relationship. Mater Chem Phys 89:38–48
Bentiss F, Gassama F, Barbry D, Gengembre L, Vezin H, Lagrenée M, Traisnel M (2006) Enhanced corrosion resistance of mild steel in molar hydrochloric acid solution by 1,4-bis(2-pyridyl)-5H-pyridazino[4,5-b]indole: electrochemical, theoretical and XPS studies. Appl Surf Sci 252:2684–2691
Ouchrif A, Zegmout M, Hammouti B, Dafali A, Benkaddour M, Ramdani A, Elkadiri S (2005) New synthesized diamine derivatives as corrosion inhibitors of steel in 0.5 M H2SO4. Prog Org Coat 53:292–296
Lebrini M, Bentiss F, Vezin H, Lagrenée M (2006) The inhibition of mild steel corrosion in acidic solutions by 2,5-bis(4-pyridyl)-1,3,4-thiadiazole: structure–activity correlation. Corros Sci 48:1279–1291
Bouklah M, Benchat N, Hammouti B, Aouniti A, Kertit S (2006) Thermodynamic characterization of steel corrosion and inhibitor adsorption of pyridazine compounds in 0.5 M H2SO4. Mater Lett 60:1901–1905
Wang L (2006) Inhibition of mild steel corrosion in phosphoric acid solution by triazole derivatives. Corros Sci 48:608–616
Bouklah M, Ouassini A, Hammouti B, EL Idrissi A (2006) Recrystallization behavior of high-fluence N+-implanted GaAs studied by Raman spectroscopy. Appl Surf Sci 252:2178–2185
Mihit M, El Issami S, Bouklah M, Bazzi L, Hammouti B, Addi EA, Salghi R, Kertit S (2006) The inhibited effect of some tetrazolic compounds towards the corrosion of brass in nitric acid solution. Appl Surf Sci 252:2389–2395
Abboud Y, Abourriche A, Saffaj T, Berrada M, Charrouf M, Bennamara A, Cherquaoui A, Takky D (2006) The inhibition of mild steel corrosion in acidic medium by 2,2′-bis(benzimidazole). Appl Surf Sci 252:8178–8184
Hegazy MA, Zaky MF (2010) Inhibition effect of novel nonionic surfactants on the corrosion of carbon steel in acidic medium. Corros Sci 52:1333–1341
Badawi AM, Hegazy MA, El-Sawy AA, Ahmed HM, Kamel WM (2010) Novel quaternary ammonium hydroxide cationic surfactants as corrosion inhibitors for carbon steel and as biocides for sulfate reducing bacteria (SRB). Mater Chem Phys 124:458–465
Hegazy MA (2009) A novel aldimine compound-based cationic gemini surfactants: synthesis and effect on corrosion inhibition of carbon steel in hydrochloric acid solution. Corros Sci 51:2610–2618
Oguzie EE, Li Y, Wan FH (2007) Effect of 2-amino-3-mercaptopropanoic acid (cysteine) on the corrosion behaviour of low carbon steel in sulphuric acid. Electrochim Acta 53:909–914
Lamaka SV, Zheludkevich ML, Yasakau KA, Serra R, Poznyak SK, Ferreira MGS (2007) Nanoporous titania interlayer as reservoir of corrosion inhibitors for coatings with self-healing ability. Prog Org Coat 58:127–135
Duan H, Du K, Yan C, Wang F (2006) Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim Acta 51:2898–2908
Morad MS (2000) An electrochemical study on the inhibiting action of some organic phosphonium compounds on the corrosion of mild steel in aerated acid solutions. Corros Sci 42:1307–1326
Hegazy MA, Abdallah M, Ahmed H (2010) Novel cationic gemini surfactants as corrosion inhibitors for carbon steel pipelines. Corros Sci 52:2897–2904
Hosseini SMA, Azimi A (2009) The inhibition of mild steel corrosion in acidic medium by 1-methyl-3-pyridin-2-yl-thiourea. Corros Sci 51:728–732
Li X, Deng S, Fu H (2009) Synergism between red tetrazolium and uracil on the corrosion of cold rolled steel in H2SO4 solution. Corros Sci 51:1344–1355
Tao Z, Zhang S, Li W, Hou B (2009) Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros Sci 51:2588–2595
Badawy WA, Ismail KM, Fathi AM (2006) Corrosion control of Cu–Ni alloys in neutral chloride solutions by amino acids. Electrochim Acta 51:4182–4189
Flis J, Zakroczymski T (1996) Impedance study of reinforcing steel in simulated pore solution with tannin. J Electrochem Soc 143:2458–2464
Tang L, Mu G, Liu G (2003) The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid. Corros Sci 45:2251–2262
Zhao T, Mu V (1999) The adsorption and corrosion inhibition of anion surfactants on aluminium surface in hydrochloric acid. Corros Sci 41:1937–1944
Szklarska-Smialowska Z, Mankowski J (1978) Crevice corrosion of stainless steels in sodium chloride solution. Corros Sci 18:953–960
Yurt A, Ulutas S, Dal H (2006) Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some aldimine compounds. Appl Surf Sci 253:919–925
Shukla SK, Quraishi MA (2009) 4-Substituted anilinomethylpropionate: new and efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 51:1990–1997
Hegazy MA, El-Tabei AS, Bedair AH, Sadeq MA (2012) An investigation of three novel nonionic surfactants as corrosion inhibitor for carbon steel in 0.5 M H2SO4. Corros Sci 54:219–230
Sahin M, Bilgic S, Yilmaz H (2002) The inhibition effects of some cyclic nitrogen compounds on the corrosion of the steel in NaCl mediums. Appl Surf Sci 195:1–7
Ateya BG, El-Anadouli BE, El-Nizamy FM (1984) The adsorption of thiourea on mild steel. Corros Sci 24:509–515
Ebenso EE (2003) Synergistic effect of halide ions on the corrosion inhibition of aluminium in H2SO4 using 2-acetylphenothiazine. Mater Chem Phys 79:58–70
Rosen MJ (1981) Purification of surfactants for studies of their fundamental surface properties. J Colloid Interface Sci 79:587–588
Samakande A, Chaghi R, Derrien G, Charnay C, Hartmann PC (2008) Aqueous behaviour of cationic surfactants containing a cleavable group. J Colloid Interface Sci 320:315–320
Gambo C, Ole AF, Gambo C (2006) Association of cationic surfactants to humic acid: effect on the surface activity. Colloids Surf A 278:241–245
Al-Sabagh AM, Kandil NGh, Bdawi AM, El-Sharkawy H (2000) Surface activity and thermodynamic of micellization and adsorption for isooctylphenol ethoxylates, phosphate esters and their mixtures with N-diethoxylated perfluorooctanamide. J. Colloid Surf A 170:127–136
Shehata HA, Abd El-wahab, Abd Allah, Hafiz A, Aiad I, Hegazy M (2008) Syntheses and characterization of some cationic surfactants. J Surf Deterg 11:139–144
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Hegazy, M.A., El-Tabei, A.S. Synthesis, Surface Properties, Synergism Parameter and Inhibitive Performance of Novel Cationic Gemini Surfactant on Carbon Steel Corrosion in 1 M HCl Solution. J Surfact Deterg 16, 221–232 (2013). https://doi.org/10.1007/s11743-012-1388-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1388-2