Abstract
New pyridinium gemini surfactants have been synthesized by esterification of halogenated carboxylic acids with long chain fatty alcohols furnishing respective esters (dodecyl-2-chloroacetate, tetradecyl-2-chloroacetate, hexadecyl-2-chloroacetate, dodecyl-2-bromoacetate, tetradecyl-2-bromoacetate and hexadecyl-2-bromoacetate) followed by their subsequent treatment with 4,4′-trimethylene dipyridine resulting in the formation of title Gemini surfactants: 4,4′-(propane-1,3-diyl)bis1-{2-(dodecyloxy)-2-oxoethyl}; 4,4′-(propane-1,3-diyl)bis{1-(2-(tetradecyloxy)-2-oxoethyl}; 4,4′-(propane-1,3-diyl)bis{1-(2-(hexadecyloxy)-2-oxoethyl} dipyridinium chlorides; 4,4′-(propane-1,3-diyl)bis{1-(2-(dodecyloxy)-2-oxoethyl}; 4,4′-(propane-1,3-diyl)bis{1-(2-(tetradecyloxy)-2-oxoethyl} and 4,4′-(propane-1,3-diyl)bis{1-(2-(hexadecyloxy)-2-oxoethyl} dipyridinium bromides. Their identifications are based on IR, 1H-NMR, 13C-NMR, DEPT, COSY and Mass spectral studies. Their surface active properties were also evaluated on the basis of surface tension and conductivity measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of surfactants based on natural renewable resources is a concept that is gaining recognition in the cosmetic and detergent industries. A new class of gemini cationic surfactants is a response to the increasing consumer demand for the products that are both greener and more efficient. In order to achieve these objectives, it is necessary to use renewable low-cost materials that are available in large quantities and to design molecular structures that show improved performance, favorable properties and reduced environmental impact [1]. Several series of new surfactants have been designed using environmentally friendly processes and their production permits us to attain various products and by-products of the oleochemical industries or ones derived from marine resources [2, 3].
A wide range of original surfactants derived from renewable resources have been developed with potential applications notably, in detergent and cosmetic industries. The production of these entirely natural molecules may substitute the surfactants conventionally used.
Cationic surfactants, with almost 7 % of the total surfactant market, have many applications such as fabric softeners, asphalt additives, corrosion inhibitors, biocides, and textile auxiliaries. They adsorb strongly onto a wide variety of materials by an ion exchange mechanism [4–6]. However, cationic surfactants have higher aquatic toxicity than other surfactants and are also more irritating to the skin and to the eyes. The toxicity of these surfactants is believed to result from their tendency to adsorb on to negatively charged surfaces [6, 7]. Different approaches are taken to overcome this problem. One approach is to introduce an easily cleavable bond into the surfactant structure. The search for novel surfactants with higher efficiency and effectiveness gave birth to the concept of gemini surfactants. Gemini surfactants are made up of two monomeric surfactant molecules with their head groups chemically bonded together by a spacer [8, 9]. It was found that the surfactant properties of gemini-type surfactants, such as a low critical micelle concentration (CMC) value and surface tension lowering, were superior to those of the corresponding single-type surfactants [10–17]. Since the term was coined by Menger in 1991, many different types of gemini surfactants have been synthesized, and their physicochemical properties have been investigated [18, 19].
Keeping in view the past work and perception on cationic gemini surfactants, we have attempted to synthesize them from renewable raw materials like fatty alcohols and halo-carboxylic acids. Here we have chosen the greener approach to make the process environmentally friendly and cost effective too. The purpose of this work was to prepare and characterize the cationic gemini surfactants with ester bonds inserted between the hydrocarbon tails and the positively charged head groups and to evaluate their surface active properties.
Experimental Section
Materials, Methods and Instrumentation
Chloroacetic acid, bromoacetic acid and 4,4′-trimethylene dipyridine were purchased from Sigma-Aldrich Chemical Co. USA. Lauryl alcohol (dodecyl alcohol), Myristyl alcohol (tetradecyl alcohol), cetyl alcohol (hexadecyl alcohol) and silica gel for T.L.C. were purchased from S. D. Fine Chemicals Ltd; Mumbai, India. Sulfuric acid was purchased from Merck, Germany. IR spectra were recorded as a thin film on KBr Pellet on a Shimadzu 8,400 s FT-IR (Kyoto, Japan) instrument. Mass spectra were recorded on a Waters Q-T of Micro mass using ESI as an ion source at sophisticated analytical instrumentation facility (SAIF), Panjab University, Chandigarh. 1H-NMR, DEPT (Distortionless enhanced polarization Transfer), COSY (correlation spectroscopy) and 13C-NMR spectra were recorded on a JEOL AL-300 (JEOL, Japan) and (Bruker Advance II 400 NMR spectrometer) system as a solution in CDCl3, using tetramethylsilane (TMS) as an internal standard.
Synthesis of Dodecyl/Tetradecyl/Hexadecyl 2-Chloro/Bromo Acetate
The preparation of these esters from halogenated fatty acids has earlier been reported [20]. However, we herein report the modified procedure for their preparation with excellent yield, that too in a short time. Chloro/bromo acetic acids (0.01 mol; 0.945 g for chloroacetic acid and 0.1389 g for bromo acetic acid) was added in a fatty alcohol (0.01 mol; dodecyl 1.863 g; tetradecyl 2.143 g; hexadecyl 2.422 g) followed by the addition of catalytic amount of sulfuric acid. The contents of the flask were then stirred for 2–3 h at 60 °C. The progress of the reaction was monitored by thin layer chromatography [silica gel G coated (0.25 mm thick) glass plates using hexane/ethyl acetate (98:2) as the mobile phase, the spots were visualized in iodine]. The reaction was completed in 3 h. In each case the crude reaction mixture was extracted with 50 ml of chloroform and washed repeatedly (3 × 25 ml) with water and then dried over sodium sulfate. Chloroform was removed from crude reaction mixture under reduced pressure in a rotary flash evaporator at 40 °C. The individual crude product was then purified using aqueous methanol i.e. (the crude compound was taken in a separating funnel and 10 ml of methanol was added with 2–3 drops of water which led to the settling of product at the bottom in its purest form). The yields of resulting esters are reported in parenthesis {hexadecyl-2-chloroacetate (1, 92 %), tetradecyl-2-chloroacetate (2, 92 %), dodecyl-2-chloroacetate (3, 91 %), hexadecyl-2-bromoacetate (4, 90.1 %), tetradecyl-2-bromoacetate (5, 94.4 %) and) dodecyl-2-bromoacetate (6, 92 %)}.
Synthesis of Gemini Surfactants (7–12)
Each resulting ester (1–6) was immediately reacted with 4,4′-trimethylene dipyridine in 2:1 molar ratio (0.01 mol) at 60 °C for 1 h (for chloro esters) and 30 min (for bromo esters) i.e. for 1, 6.378 g; for 2, 5.817 g; for 3, 5.256 g; for 4, 7.267 g; for 5, 6.706 g; for 6, 6.145 g and 4,4′-trimethylene dipyridine (1.982 g) were taken. In each case the resulting crude product was crystallized with ether and subsequently recrystallized in cold acetone to get the pure compounds (7-12) which were characterized on the basis of IR, 1H-NMR, 13C-NMR, COSY and DEPT experiments and mass spectral analysis as 4,4′-(propane-1,3-diyl) bis(1-(2-(hexadecyloxy)-2-oxoethyl) dipyridinium chloride (7); (4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecyloxy)-2-oxoethyl)dipyridinium chloride (8); 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecyloxy)-2-oxoethyl)dipyridinium chloride (9); 4,4′-(propane-1,3-diyl) bis(1-(2-(hexadecyloxy)-2-oxoethyl) dipyridinium bromide (10); 4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecyloxy)-2-oxoethyl)dipyridinium bromide (11) and 4,4′-(propane-1,3-diyl)bis(1-(2-dodecyloxy)-2-oxoethyl)dipyridinium bromide (12) (Scheme 1).
Conductivity Measurements [21, 22]
The critical micelle concentrations (CMC) of these surfactants (7–12) were determined by the conductivity method. The conductance as a function of surfactant concentration was measured at 25 °C. Measurements were performed with an Equiptronic Conductometer (Auto temperature conductivity meter model E.Q.661) with stirring to control the temperature. The solutions were thermostated in the cell at 25 °C. For each series of measurements, an exact volume of 25 ml Millipore water (resistivity 18 MΩ) was introduced into the vessel and the specific conductivity of the water was measured. For the determination of CMC, adequate quantities of concentrated stock surfactant solutions in water (i.e. 0.25, 2, 3, 0.25, 2 and 3 mM for surfactants 7, 8, 9, 10, 11 and 12, respectively) were added in order to change the surfactant concentration from concentrations well below the critical micelle concentration (CMC) and repeated to verify our results. The intersection point of specific conductivity versus surfactant concentration was taken as the CMC. The degree of counterion binding (β) was calculated as (1−α), where α = Smicellar/Spremicellar, i.e. ratio of the slope before and after CMC.
Surface Tension Measurements
Surface tension values were used to calculate CMC using a CSC Du Nouy interfacial tensiometer (Central Scientific Co., Inc.) equipped with platinum-iridium ring (circumference 5.992 cm) at 25 °C. The tensiometer was calibrated using triple distilled water. For the determination of CMC and surface tension, adequate quantities of a concentrated stock solution (i.e. 0.25, 2, 3, 0.25, 2 and 3 mM, stock solution for surfactants 7, 8, 9, 10, 11 and 12, respectively) were used. The data of this determination is presented in Table 1.
Where X = Cl, Br and R = CH3 (CH2)11-; CH3(CH2)13- and CH3(CH2)15-.
Results and Discussion
The structures of ester based gemini pyridinium surfactants (7–12) were established by IR, 1H-NMR, 13C-NMR, COSY, DEPT and mass spectral data.
Spectral Results
The IR spectra of the pyridinium gemini surfactants (7–12) showed the absorption bands in the region at 2,915–2,849 cm−1 indicating the presence of methylene groups. The absorptions at 1,741–1,730 cm−1 indicate the presence of ester carbonyl group whereas other absorptions at 1,641–1,620 cm−1 indicate the presence of C–N. The band at 1,570–1,540 cm−1 very well established the presence of aromatic C=C in (7–12). The two terminal methyl protons of these gemini surfactants (7–12) are observed as a distorted triplet at δ 0.61–0.87 in their 1H NMR spectra. Broad doublets in (7–12) are observed at δ 1.07–1.26 accountable for methylene protons of chain. Multiplet signals are observed at δ 1.29–1.65 due to presence of methylene protons next to terminal methyl groups. Multiplets are observed at δ 1.48–2.17 due to sandwiched methylene protons of spacer (PyCH2 CH 2 CH2Py). Triplets of a second type are observed at 2.67–2.99 due to α methylene proton of the spacer (PyCH 2 CH2 CH 2 Py). Triplets of a third type are observed at δ 4.01–4.21 due to α methylene proton. A doublet is observed at δ 5.84–6.04 due to methylene protons attached to nitrogen of pyridine. The two sets of ring protons of pyridine methine are observed as a doublet at δ 7.88–8.04 and δ 8.97–9.12. 13C/DEPT NMR spectra displayed sp3 carbon of terminal methyl group at δ 14.00–14.08. The carbons next to terminal methyl groups are observed in the range of δ 22.58–22.66. The carbons (COOCH2 CH 2 ) are observed at 25.63–25.69. The middle carbon of spacer i.e. (PyCH2 CH2CH2Py) is observed at 28.31–28.35. The chain carbons are observed at 29.10–29.90. The methylene carbons i.e. (CH2CH2CH3) are observed at 31.83–31.89. The α methylene carbons of spacer i.e. (PyCH2CH2 CH2Py) are observed at 34.89–35.00. The methylene carbons attached to pyridine nitrogen are observed at δ 60.36–60.58. Other signals are observed at δ 67.26–67.35 due to methylene carbons α to carbonyl groups. Other structure revealing signals are observed at δ 127.78–127.90 due to ring carbons located β to nitrogen of pyridine. Other structure revealing signals are observed at δ 145.96–146.15 due to quaternary carbon joined to the methylene group of the spacer. More significant signals are observed at δ 162.74–162.85 due to ring carbons attached α to nitrogen of pyridine. The carbonyl carbons are observed at δ 166.61–166.78. All these data are in almost comparable with the previous report [23]. On all these accounts the structures of (7–12) are deduced as 4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecyloxy)-2-oxoethyl)dipyridinium chloride (7); (4,4′-(propane-1,3-diyl) bis(1-(2-(tetradecyloxy)-2-oxoethyl) dipyridinium chloride (8); 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecyloxy)-2-oxoethyl) dipyridinium chloride (9); 4,4′-(propane-1,3-diyl)bis(1-(2-(hexadecyloxy)-2-oxoethyl)dipyridinium bromide (10); 4,4′-(propane-1,3-diyl)bis(1-(2-(tetradecyloxy)-2-oxoethyl)dipyridinium bromide (11) and 4,4′-(propane-1,3-diyl)bis(1-(2-(dodecyloxy)-2-oxoethyl)dipyridinium bromide (12). The structures of these gemini surfactants (7–12) are further consolidated by ESI–MS (positive ion) mass spectral data. Important peaks in these spectra are found at m/z 763.3, 764.4, 765.5, 707.4, 708.4, 651, 652.4, 763.3, 764.4, 765.5, 707.4, 708.4, 651, 652.4. These ion peaks account for the loss of proton and two chloride/bromide ions from the molecule leading to the formation of positively charged parent ion {M-2Cl)−1)}+/{M-2Br)−1)}+ and direct loss of two chloride/bromide ions from the molecule leading to the formation of (M-2Cl)+/(M-2Br)+ positively charged ions.
(4,4′-(Propane-1,3-diyl)bis(1-(2-(hexadecyloxy)-2-oxoethyl)dipyridinium chloride(7). Brown solid, Yield is 92 %. Melting point 128 °C. IR (KBr pellet, cm−1) 2,910, 1,744, 1,643, and 1,570. 1H NMR (δ ppm CDCl3): 0.67 (t, 6H, 2XCH3), 1.07 (d, chain 56H, 2X(–CH2–)14), 1.46(m, 4H, 2XCH2 next to terminal methyl groups), 1.48 (m, 2H, PyCH2 CH 2 CH2Py), 2.82 (t, 4H, PyCH 2 CH2 CH 2 Py), 4.01 (t, 4H 2XCO2 CH 2 ), 5.84(d, 4H, 2XNCH 2 CO2), 7.88 (d, 4H, 4XCH ring protons β to nitrogen of pyridine), 8.97 (d, 4H, 4XCH ring protons α to nitrogen of pyridine). 75 MHz 13C/DEPT NMR (δ ppm CDCl3): 14.05(terminal methyl carbons), 22.63(CH3 CH2), 25.67(CH 2CH2CO2), 28.33(PyCH2 CH2CH2Py), 29.31(chain methylene carbons), 31.86(CH2CH2CH3), 34.99(PyCH2CH2 CH2Py), 60.36(CH2CO2), 67.26(CO2 CH2), 127.79 (methine carbon β to nitrogen of pyridine), 145.96 (quaternary carbon of pyridine nucleus bearing spacer), 162.74 (methine carbon α to nitrogen of pyridine), 166.61(carbonyl carbon). ESI–MS positive ions m/z 763.3(100 %) {(M-2Cl)-1)}+, 764.4 (90 %) (M-2Cl)+.
(4,4′-(Propane-1,3-diyl)bis(1-(2-(tetradecyloxy)-2-oxoethyl)dipyridinium chloride (8). Brown solid, Yield, 95.5 %. Melting point 122 °C. IR (KBr pellet, cm−1) 2871, 1743, 1643, and 1570. 1H NMR (δ ppm CDCl3): 0.87 (t, 6H, 2XCH3), 1.26 (d, chain 48H, 2X(–CH2–)12), 1.65 (m, 4H, 2XCH2 next to terminal methyl groups), 1.99 (m, 2H, PyCH2 CH 2 CH2Py), 2.97 (t, 4H, PyCH 2 CH2 CH 2 Py), 4.19 (t, 4H 2XCO2 CH 2 ), 6.04 (d, 4H, 2XCH 2 CO2), 7.96 (d, 4H, 4XCH ring protons β to nitrogen of pyridine), 9.12 (d, 4H, 4XCH ring protons α to nitrogen of pyridine). 75 MHz 13C/DEPT NMR (δ ppm CDCl3): 14.05 (terminal methyl carbons), 22.63 (CH3 CH2), 25.67 (CH2CH2CO2), 28.35 (PyCH2 CH2CH2Py), 29.31 (chain methylene carbons), 31.87 (CH2CH2CH3), 34.89 (PyCH 2 CH2 CH2Py), 60.39 (CH2CO2), 67.24(CO2 CH 2 ), 127.89 (methine carbon β to nitrogen of pyridine), 145.85 (quaternary carbon of pyridine nucleus bearing spacer), 162.87 (methine carbon α to nitrogen of pyridine), 166.66 (carbonyl carbon). ESI–MS positive ions m/z 707.4 (100 %) {(M-2Cl)-1)}+, 708.4 (51 %) (M-2Cl)+.
4,4′-(Propane-1,3-diyl)bis(1-(2-(dodecyloxy)-2-oxoethyl)dipyridinium chloride (9): Brown sticky solid, Yield, 92.5 %. Melting point 117 °C. The IR (KBr Pellet, cm−1): 2915, 1746, 1639, and 1570. 1H NMR (δ ppm, CDCl3): 0.61 (t, 6H, 2XCH3), 1.10 (d, chain 40H, 2X(–CH2–)10), 1.29 (m, 4H, 2XCH2 next to terminal methyl groups), 1.69 (m, 2H, PyCH2 CH 2 CH2Py), 2.67 (t, 4H, PyCH 2 CH2 CH 2 Py), 4.19 (t, 4H, 2XCO2 CH 2 ), 5.92 (d, 4H, 2X CH 2 CO2), 7.75 (d, 4H, 4XCH ring protons β to nitrogen of pyridine), 8.98 (d, 4H, 4XCH ring protons α to nitrogen of pyridine). 75 MHz 13C/DEPT NMR (δ ppm, CDCl3): 14.08 (terminal methyl carbons), 22.66 (CH3 CH2), 25.69 (CH2CH2CO2), 28.34 (PyCH2 CH2CH2Py), 29.33 (chain methylene carbons), 31.89 (CH2CH2CH3), 34.95 (PyCH2CH2 CH2Py), 60.40 (CH2CO2), 67.30 (CO2 CH 2 ), 127.78 (methine carbon β to nitrogen of pyridine), 146.01 (quaternary carbon of pyridine nucleus bearing spacer), 162.68 (methine carbon α to nitrogen of pyridine), 166.62 (carbonyl carbon). ESI–MS positive ions at m/z (relative intensity %) 651.4 (100 %) {(M-2Cl)-1)}+, 652.4 (60 %) (M-2Cl)+.
(4,4′-(Propane-1,3-diyl)bis(1-(2-(hexadecyloxy)-2-oxoethyl)dipyridinium bromide (10). Brown solid, Yield, 95.5 %. Melting point 145 °C, IR (KBr pellet, cm−1) 2870, 1741, 1641, 1570 and 1514. 1H NMR (δ ppm CDCl3): 0.79 (t, 6H, 2XCH3), 1.20 (d, chain 56H, 2X(–CH2–)14), 1.57 (m, 4H, 2XCH2 next to terminal carbons), 1.82 (m, 2H, PyCH2 CH 2 CH2Py), 2.83 (t, 4H, PyCH 2 CH2 CH 2 Py), 4.11 (t, 4H, 2XCO2 CH 2 ), 5.98 (d, 4H, 2XCH 2 CO2), 7.93 (d, 4H, 4XCH ring protons β to nitrogen of pyridine), 9.06 (d, 4H, 4XCH ring protons α to nitrogen of pyridine). 75MHz13C/DEPTNMR (δ ppm CDCl3): 14.01 (terminal methyl carbons), 22.58 (CH3 CH2), 25.63 (CH2CH2CO2), 28.31 (PyCH2 CH2CH2Py), 29.46 (chain methylene carbons), 31.83 (CH2CH2CH3), 34.91 (PyCH2CH2 CH2Py), 60.50 (CH2CO2), 67.36 (CO2 CH2), 127.88 (methine carbon β to nitrogen of pyridine), 145.70 (quaternary carbon of pyridine nucleus bearing spacer), 163.05 (methine carbon α to nitrogen of pyridine), 166.23 (carbonyl carbon). ESI–MS Positive ions m/z 763.3 (20 %) {(M-2Br)-1)}+, 764.4 (10 %) (M-2Br)+.
(4,4′-(Propane-1,3-diyl)bis(1-(2-(tetradecyloxy)-2-oxoethyl)dipyridinium bromide (11). Brown solid, Yield 93.4 %, Melting point 139 °C. IR (KBr pellet, cm−1) 2,860, 1,742, 1,642 and 1,560. 1H NMR (δ ppm CDCl3,) 0.87 (t, 6H, 2XCH3), 1.26 (d, chain 48H, 2X(–CH2–)12), 1.64 (m, 4H, 2XCH2 next to terminal methyl groups), 2.17 (m, 2H, PyCH2 CH 2 CH2Py), 2.99 (t, 4H, PyCH 2 CH2 CH 2 Py), 4.21 (t, 4H, 2XCO2 CH 2 ), 6.01(d, 4H, 2XCH 2 CO2), 8.04 (d, 4H, 4XCH ring protons β to nitrogen of pyridine), 9.12 (d, 4H, 4XCH ring protons α to nitrogen of pyridine). 75 MHz 13C/DEPT NMR (δ ppm CDCl3): 14.06 (terminal methyl carbons), 22.64 (CH3 CH2), 25.68 (CH2CH2CO2), 28.35 (PyCH2 CH2CH2Py), 29.21 (chain methylene carbons), 31.87 (CH2CH2CH3), 35.01 (PyCH2CH2 CH2Py), 60.55 (CH2CO2), 67.43 (CO2 CH2), 127.90 (methine carbon β to nitrogen of pyridine), 145.79 (quaternary carbon of pyridine nucleus bearing spacer), 163.01 (methine carbon α to nitrogen of pyridine), 166.25 (carbonyl carbon). ESI–MS positive ions m/z 707.4 (100 %) {(M-2Br)-1)}+ 708.4 (40 %) (M-2Br)+.
(4,4′-(Propane-1,3-diyl)bis(1-(2-(dodecyloxy)-2-oxoethyl)dipyridinium bromide (12). Brown sticky solid, Yield 93.4 %, Melting point 131 °C. IR (KBr pellet, cm−1) 2910, 1746, 1640 and 1574. 1H NMR (δ ppm CDCl3): 0.68 (t, 6H, 2XCH3), 1.09 (d, chain 40H, 2X(–CH2–)10), 1.49 (m, 4H, 2XCH2 next to terminal methyl groups), 1.83 (m, 2H, PyCH2 CH 2 CH2Py), 2.72 (t, 4H, PyCH 2 CH2 CH 2 Py), 4.02 (t, 4H, 2XCO2 CH 2 ), 5.94 (d, 4H, 2XCH 2 CO2), 7.87 (d, 4H, 4XCH ring protons β to nitrogen of pyridine), 9.01 (d, 4H, 4XCH ring protons α to nitrogen of pyridine) 0.75 MHz 13C/DEPT NMR (δ ppm CDCl3): 14.05 (terminal methyl carbons), 22.63 (CH3 CH2), 25.67 (CH2CH2CO2), 28.34 (PyCH2 CH2CH2Py), 29.30 (chain methylene carbons), 31.86 (CH2CH2CH3), 34.98 (PyCH2CH2 CH2Py), 60.52 (CH 2CO2), 67.41 (CO2 CH2), 127.89 (methine carbon β to nitrogen of pyridine), 145.76 (quaternary carbon of pyridine nucleus bearing spacer), 163.01 (methine carbon α to nitrogen of pyridine), 166.24 (carbonyl carbon). ESI–MS positive ions m/z 651.4 (50 %) {(M-2Br)-1)}+, 652.4 (40 %) (M-2Br)+.
Critical Micelle Concentration
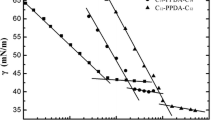
Gemini surfactants have astonishingly low CMC values, much lower than the corresponding single tail surfactants [24]. Only a few reports are available regarding synthesis and CMC values of gemini pyridinium surfactants [25, 26]. Critical micelle concentration and the degree of counter ion binding of these new pyridinium amphiphiles have been determined by the conductivity method. These new gemini pyridinium amphiphiles have low CMC values. It has been found that the CMC of these gemini amphiphiles decreases with increasing chain length. The values of CMC and degrees of counter ion binding are given in Table 1. The graphs of the specific conductivity versus concentration have been plotted (Figs. 1, 2). It is found that the pyridinium gemini surfactants with bromine as a counter ion have low values of CMC as compared to the pyridinium gemini surfactants with chlorine as a counter ion [27].
The Degree of Counterion Binding (β)
The ratio of the slopes of the specific conductivity versus concentration curve above and below CMC gives degree of counterion dissociation α (i.e. α = Smicellar/Spremicellar) and (1−α) gives the degree of counterion binding, β. It is an important parameter because it manifests the counterions that are contained in the Stern layer to counterbalance the electrostatic force that opposes micelle formation. Quagliotto et al. [22] determined the β value for a series of gemini bispyridinium bromides having different spacers where they had shown that a different spacer is responsible for a different β value. The β value signifies the ability of counter ion to bind micelles. We, in our study, on new series of gemini pyridinium surfactants (7–12), have found that the β value decreases with increases in hydrophobicity [22].
Surface Tension Measurements
The CMC of new pyridinium gemini surfactants were calculated by using surface tension measurements. The graphs of the surface tension versus concentration are shown for gemini surfactants (7–12). A clear break is observed in all the pyridinium gemini surfactants (Figs. 3, 4). It is observed from the graphs that pyridinium gemini surfactants having bromine as a counter ion have low CMC values as compared to the pyridinium gemini surfactants having chlorine as a counter ion. The CMC values are reported in Table 1 for all the gemini surfactants. The values of CMC found by both the conductivity method and surface tension method correspond well with each other.
Conclusions
In the present study we have described a new protocol for the synthesis of six new ester based cationic gemini pyridinium surfactants starting from renewable long chain fatty alcohols through an environmentally friendly, energy saving and cost effective process. All the gemini cationic surfactants (7–12) are produced in excellent yields and these surfactants have been examined and are found to have good surface active properties. The results show that gemini pyridinium surfactants with longer hydrophobic chains have lower CMC values than those with shorter hydrophobic chains. Also it is found that gemini surfactants having bromine as a counter ion have lower CMC values as compared to gemini surfactants having chlorine as a counter ion. In a previous report [28] ester-containing gemini surfactants are more readily degradable than their non-ester counterparts. In addition, pyridinium cationic gemini surfactants may show good antimicrobial properties, DNA binding capability if tested properly.
References
Benvegnu T, Plusquellec D, Lemiegre L, Belgacem MN, Gandini A (2008) Monomers, polymers and composites from renewable resources. Elsevier, Amsterdam
Hatanaka K, Hiryana T (1993) Liquid cleaning cosmetics containing acidic polymers and nitrogen heterocyclic cationic surfactants. Japanese patent JP 2005187338
Kenji N, Nakamura K August (12, 2003) Antimicrobial and deodorant cosmetic brush and method of producing the same. US Patent 6604531
Steichen DS, Holmberg K, (2001) Handbook of applied surface and colloid chemistry, vol 1. Wiley, New York, pp 309–314
Steichen DS, Gadberry JF, Karsa DR (1998) New products and applications in surface technology. Sheffield Academic Press, Sheffield, UK, p 59
Cross J, Singer EJ (1994) Cationic surfactants. Marcel Dekker, New York, p 3
Huber L, Nitschke L, Holmberg. K (2002) Handbook of applied surface and colloid chemistry, vol 1. Wiley, Chichester UK, pp 509–516
Stjerndahl M, Landberg D, Holmberg K (2003) Novel surfactants, preparation, application and biodegradability. Marcel Dekker, New York, pp 317–321
Tehrani-Bagha AR, Holmberg K (2007) Cleavable surfactants. Curr Opin J Colloid Interface Sci 12:81–84
Rosen MJ (1993) Geminis: a new generation of surfactants. Chem Tech 23:30–33
Menger FM, Littau CA (1993) Gemini surfactants a new class of self-assembling molecule. J Am Chem Soc 115:10083–10090
Zana R (2002) Dimeric gemini surfactants effect of the spacer group on the associate behavior in aqueous solution. J Colloid Interface Sci 248:203–220
Zana R (2002) Dimeric and oligomeric Surfactants. Behavior at interfaces and in aqueous solution a review. Adv Colloid Interface Sci 97:205–253
Aisaka T, Oida T, Kawase T (2007) A novel synthesis of succinic acid type gemini surfactant by the function group interconversion of corynomicolic acid. J Oleo Sci 56:633–644
Bunton CA, Robinson L, Schaak J, Stam MF (1971) Catalysis of nucleophilic substitution by micelles of dicationic detergents. J Org Chem 36:2346–2350
Devinsky F, Masarova L, Lacko I (1985) Surface activity and micelle formation of some new bis quaternary ammonium salts. J Colloid Interface Sci 105:235–239
Devinsky F, Lacko I, Bitterova F, Tomeckova L (1986) Relationship between structure, surface activity and micelle formation of some new bis quaternary isosteres of 1,5-pentane diammonium dibromides. J Colloid Interface Sci 114:314–322
Menger FM, Keiper JS (2000) Gemini Surfactants. Angew Chem Int Ed 39:1906–1907
Zana R, Xia J (2004) Gemini surfactants. Marcel Dekker, New York, pp 301–302
Kanjilal S, Sunitha S, Reddy SP, Kumar PK, Upadyayula SNM, Rachapudi BNP (2009) Synthesis and evaluation of micellar properties and antimicrobial activities of imidazole based surfactants. Eur J Lipid Sci Technol 111:941–948
Rodrguez A, Graciani MM, Moya ML (2007) Effects of ethylene glycol addition on the aggregation and micellar growth of cationic dimeric surfactants. Langmuir 23:11496–15505
Viscardi G, Quagliotto P, Barolo C, Savarino P, Barni E, Fisicaro E (2000) Gemini pyridinium surfactants synthesis and conductometric study of novel class of amphiphiles. J Org Chem 65:8197–8203
Chauhan V, Singh S, Bhadani A (2012) Synthesis characterization and surface properties of long chain β-hydroxy-γ-alkyloxy-N-methylimidazolium surfactants. Colloids Surf A 395:1–9
Singh S, Bhadani A, Kataria H, Kaur G, Kamboj R (2009) Synthesis of glycerol based pyridinium surfactants and appraisal of their properties. Ind Eng Chem Res 48:1673–1677
Quagliotto P, Viscardi G, Barolo C, Barni E, Bellinvia S, Fisicaro E, Compari C (2003) Gemini pyridinium surfactants synthesis and conductometric study of novel class of amphiphiles. J Org Chem 68:7651–7660
Zhou l, Jiang X, Li Y, Chen Z, Hu X (2007) Synthesis and properties of a novel class of gemini pyridinium surfactants. Langmuir 23:11404–11408
Nan J, Li P, Wang Y, Wang J, Yan H, Thomas KR (2004) Thermodynamics of dissymmetric gemini surfactants in aqueous solutions. J Phys Chem B 108:15385–15391
Tehrani-bagha AR, Holmberg K (2008) Cationic ester containing gemini surfactants. Langmuir 24:6140–6145
Acknowledgments
Pankaj Patial is thankful to the UGC (University Grant Commission, India) for providing the research grant for this work and Sophisticated Analytical Instrumentation Facility (SAIF), Panjab University, Chandigarh for the mass spectral and NMR analysis of the compounds. We are thankful to Dr Sukhprit Singh and Mr. Avinash Bhadani for their help and fruitful discussions.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Patial, P., Shaheen, A. & Ahmad, I. Synthesis of Ester Based Cationic Pyridinium Gemini Surfactants and Appraisal of Their Surface Active Properties. J Surfact Deterg 16, 49–56 (2013). https://doi.org/10.1007/s11743-012-1380-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1380-x