Abstract
New amido-amine-based cationic gemini surfactants with flexible and rigid spacers and different hydrophobic tails were synthesized and characterized. These gemini surfactants were prepared by a modified procedure through amidation of long chain carboxylic acids using 3-(dimethylamino)-1-propylamine followed by treatment with halohydrocarbons. The effect of the trans and cis conformation of the spacer double bond was investigated by means of critical micelle concentration, surface tension reduction, and thermal stability. The short-term thermal stability of the gemini surfactants was assessed using thermogravimetric analysis (TGA) and the long-term thermal stability was examined by a unique approach based on structure characterization techniques including NMR (1H and 13C) and FTIR analysis. TGA results demonstrated excellent short-term thermal stability since no structure degradation was observed up to 200 °C. Structural characterization revealed impressive long-term thermal stability of the gemini surfactants with no structure decomposition after exposing them to 90 °C for 10 days. The critical micelle concentration of gemini surfactants was found to be in the range of 0.77 × 10−4–3.61 × 10−4 mol L−1 and corresponding surface tension (γCMC) ranged from 30.34 to 38.12 mN m−1. The surfactant with the trans conformation of spacer double bond showed better surface properties compared to the surfactant with the cis conformation of spacer double bond. Similarly, increasing surfactant tail length and spacer length resulted in decreasing CMC values. Moreover, bromide counterion showed improved surface properties compared to chloride counterion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants have been widely applied in various oilfield applications including drilling mud, stimulation, completion, and enhanced oil recovery [1]. The primary role of surfactants in oilfield applications is interfacial tension (IFT) reduction and wettability alteration [2,3,4,5]; however, they also act as wetting agents [6] and emulsifiers [7]. Gemini surfactants are composed of more than one hydrophobic tail and head groups joined through a spacer [8, 9]. Due to such unique structure, gemini surfactants are superior to conventional single head single tail surfactants [10] in terms of higher efficiency in IFT reduction, lower critical micelle concentrations (CMC), solubility, viscoelastic behavior, gel formation, wetting properties, and shear thickening [11, 12].
In the past decades, many reports have appeared in the literature and focused on the study of cationic gemini surfactants containing quaternary ammonium head group with different tail and spacer length [13]. Cationic gemini surfactants are extensively applied in medicine [14], household [15], biotechnology [16], pharmacy [17], oilfield [18], and other industries [19]. Ammonium-based cationic gemini surfactants can be economically feasible, environmentally friendly, show excellent water solubility, and have various oil field applications [20]. Cationic gemini surfactants are strong candidates in carbonate reservoirs (which represent almost 60% of the world’s oil reservoirs) where anionic surfactants are not suitable because of high adsorption on to the carbonate rocks [21]. Cationic surfactants change the wettability of carbonate rocks from oil-wet to water-wet resulting in enhanced oil production [22].
In view of such properties, the research is focused more on the synthesis and development of new cationic gemini surfactants with different lengths and nature of the spacers as well as hydrophobic tail groups to investigate the relationship between aggregation behavior and molecular structures [23]. Gemini surfactants aggregate in aqueous media to form micelles and the size and shape of such micelles mainly depend on the spacer group [24]. The nature and the length of the spacer have been found to be the most significant parameter determining the surface properties of gemini surfactants [25, 26]. The spacer can be flexible (hydrophobic methylene group [27] or hydrophilic polyethylene oxide group [28]) and it can be rigid with a double bond [29], triple bond [30], or benzene ring [31].
Previous literature reveals that polyether or phenyl moieties are normally introduced to maximize or minimize the spacer rigidity [32]. However, such polyether and phenyl moieties could also alter the spacer chain length and hydrophobicity. Therefore, the surfactants' aggregation morphologies could be affected by the spacer rigidity, length, hydrophobicity, and π–π stacking interactions between benzene rings [33]. Hence, the selections of gemini surfactants that isolate the other parameters on the aggregation morphologies play an important role.
In this report, six amido-amine-based cationic gemini surfactants (1–6) (Fig. 1) containing different spacers and hydrophobic tails were synthesized from the corresponding carboxylic acids (Scheme 1). The effect of spacer length, spacer rigidity, trans and cis conformation of the spacer double bond, and hydrophobic tail length were studied in terms of thermal stability and surface tension analysis. Three amido-amine based cationic gemini surfactants (3–5) (Fig. 1) have a similar hydrophobic tail group but differ by the rigidity and flexibility of the spacer. Two spacer lengths (C4 and C6) were selected because the noticeable changes in surfactant properties were reported in relatively short spacers [34]. The two sets of gemini surfactants (1, 3 and 2, 4) (Fig. 1) have similar spacer group but differ by the hydrophobic tail groups. The dodecyl group was selected as a hydrophobic tail because it was appropriate to deliver good properties to surfactants [35] and the oleic tail was chosen because it was reported that the CMC decreases with the increase of the chain length [36]. Similarly, we changed the rigidity of spacer in terms of different conformation (trans and cis) of spacer double bond using two sets of gemini surfactants (1–2 and 3–4) (Fig. 1) to investigate the effect of the different conformation of the double bond in spacer on aggregation. The gemini surfactant 6 (Fig. 1) with flexible larger spacer group was synthesized for comparison purposes.
Experimental
Materials
Dodecanoic acid (98%, sigma), oleic acid (92%, biochemical), 3-(dimethylamino)-1-propylamine (99%, GC, Aldrich), 1,4-dibromobutane (99%, Aldrich), trans-1,4-dibromo-2-butene (99%, Aldrich), cis-1,4-dichloro-2-butene (95%, Aldrich), 1,6-dibromohexane (96%, Aldrich), aluminum oxide (Fluka) were utilized as received. Distilled solvents and water were used for the synthesis and purification of gemini surfactants. Deionized water was used to prepare aqueous solutions of surfactants.
Synthesis
Synthesis and Spectral Characterization of Amido-Amine Intermediates (10 and 11)
The synthesis of amido-amine intermediate compound (10) was achieved using an improved method presented by Chu and co-workers [37] as outlined in Scheme 1. Dodecanoic acid (7) (20.00 g, 99.84 mmol), 3-(dimethylamino)-1-propylamine (9) (20.40 g, 200 mmol), and sodium fluoride (NaF) (0.42 g, 9.98 mmol) were added in a 100-mL flask connected with a reflux condenser. The condenser was further connected with a bent distilling adapter filled with Al2O3 in order to absorb the byproduct H2O. The experiment was allowed to continue under reflux at 160 °C for 6-h in an argon atmosphere. After 6 h, further 3-(dimethylamino)-1-propylamine (15.30 g, 150 mmol) was introduced into the flask and the reaction progressed under the same experimental conditions for another 5 h. After completion, the unreacted 3-(dimethylamino)-1-propylamine was separated and the residue was washed three times with a mixture of cold water:acetone (7:93) then dried in a vacuum to achieve intermediate 10 [38].
Intermediate 11 was Prepared by Adopting the Same Method of Intermediate 10
N-Dodecanamidopropyl-N,N-Dimethylamine (10): White solid (25.90 g, 91% yield) 1H NMR (500 MHz, CDCl3) δ (ppm): 0.83 (t, J = 6.7 Hz, 3H), 1.15–1.25 (m, 16 H), 1.51–1.58 (m, 2H), 1.64–1.73 (m, 2H), 2.11 (t, J = 7.6 Hz, 2H), 2.30 (s, 6H), 2.47 (t, J = 6.4 Hz, 2H), 3.29 (pent, J = 5.8 Hz, 2H), 6.99 (s, 1H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.0, 22.6, 25.7, 29.2, 29.3, 29.4, 29.5, 31.8, 36.8, 38.5, 44.8, 58.0, 173.3.
N-Oleamidopropyl-N,N-dimethylamine (11): Pale yellow viscous liquid (22.7 g, 87% yield). 1H NMR (500 MHz, CDCl3) δ (ppm): 0.83 (t, J = 6.7 Hz, 3H), 1.18–1.28 (m, 20 H), 1.52–1.59 (m, 2H), 1.62–1.69 (m, 2H), 1.91–1.98 (m, 4H), 2.10 (t, J = 7.6 Hz, 2H), 2.26 (s, 6H), 2.42 (t, J = 6.4 Hz, 2H), 3.28 (pent, J = 5.8 Hz, 2H), 5.25–5.31 (m, 2H), 7.0 (s, 1H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.0, 22.6, 25.7, 25.8, 27.1, 29.1, 29.2, 29.4, 29.6, 29.7, 31.8, 36.8, 38.7, 44.9, 58.0, 129.7, 129.9, 173.2.
Synthesis and Spectral Characterization of Amido-Amine Cationic Gemini Surfactants (1 and 2)
The amido-amine intermediate compound (10) (10.0 g, 35.15 mmol) was treated with trans-1,4-dibromo-2-butene (12) (3.0 g, 14.06 mmol) in dry ethanol (5 mL) under reflux (80 °C) for 48 h (Scheme 1). After completion, the product was separated and recrystallized using solvent mixture acetone/ethyl acetate to obtain the required gemini surfactant 1 as a white solid [39].
Gemini Surfactant 2 was Synthesized by Adopting the Same Procedure as 1
(E)-Dodecanoic acid [3-({4-[(3-Dodecanoylamino-propyl)-dimethyl-amino]but-2-enyl}-dimethyl-amino)-propyl]-amide dibromide ( 1 ): White solid (9.70 g, 88% yield based on trans-1,4-dibromo-2-butene). 1H NMR (500 MHz, CDCl3) δ (ppm): 0.82 (t, J = 6.7 Hz, 6H), 1.16–1.26 (m, 32 H), 1.52–1.58 (m, 4H), 2.06–2.12 (m, 4H), 2.26–2.33 (m, 4H), 3.31 (s, 12H), 3.32–3.36 (m, 4H), 3.64–3.72 (m, 4H), 4.41–4.49 (m, 4H), 6.77–6.85 (m, 2H), 8.16 (s, 2H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.0, 22.6, 22.8, 25.9, 29.3, 29.4, 29.5, 29.6, 29.7, 31.8, 36.1, 36.6, 51.0, 62.6, 65.0, 130.2, 175.3. FTIR (KBr pellet) υ (cm−1) 3441 (υN–H, secondary amine), 2922 and 2850 (υC–H, aliphatic asymmetric and symmetric respectively), 1641 (amide I band), 1555 (amide II band). Anal. Calcd for C38H78O2N4Br2 (782.86): C, 58.30; H, 10.04; N, 7.16. Found: C, 58.17; H, 10.19; N, 7.08.
(Z)-Dodecanoic acid [3-({4-[(3-dodecanoylamino-propyl)-dimethyl-amino]but-2-enyl}-dimethyl-amino)-propyl]-amide dichloride ( 2 ): White solid (11.85 g, 71% yield based on cis-1,4-dichloro-2-butene). 1H NMR (500 MHz, CDCl3) δ (ppm): 0.83 (t, J = 6.7 Hz, 6H), 1.18–1.28 (m, 32 H), 1.55–1.61 (m, 4H), 2.06–2.12 (m, 4H), 2.28–2.34 (m, 4H), 3.29 (s, 12H), 3.30–3.36 (m, 4H), 3.63–3.71 (m, 4H), 4.67–4.73 (m, 4H), 6.38–6.44 (m, 2H), 8.52 (s, 2H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.1, 22.7, 22.9, 26.0, 29.3, 29.4, 29.5, 29.6, 29.7, 31.9, 35.9, 36.6, 50.5, 60.8, 62.3, 128.1, 175.5. FTIR (KBr pellet) υ (cm−1) 3475 (υN–H, secondary amine), 2924 and 2852 (υC–H, aliphatic asymmetric and symmetric respectively), 1642 (amide I band), 1545 (amide II band). Anal. Calcd for C38H78O2N4Cl2 (693.96): C, 65.77; H, 11.33; N, 8.07. Found: C, 65.65; H, 11.41; N, 8.02.
Synthesis and Spectral Characterization of Amido-Amine Cationic Gemini Surfactants (3–6)
The amido-amine intermediate compound (11) (10.0 g × 27.28 mmol) was treated with trans-1,4-dibromo-2-butene (12) (2.33 g, 10.91 mmol) in dry ethanol (5 mL) for 48 h under reflux (80 °C). After completion, the reaction product was purified using silica gel column chromatography with methanol:acetone (3:7) as eluent to afford the required gemini surfactant 3 [39].
Gemini Surfactants 4–6 of this Series were Synthesized by Adopting the Same Procedure of 3
(E)-Oleic acid [3-({4-[(3-oleamidopropyl)-dimethyl-amino]but-2-enyl}-dimethyl-amino)-propyl]-amide dibromide ( 3 ): Pale yellow viscous oil (8.49 g, 82% yield based on trans-1,4-dibromo-2-butene). 1H NMR (500 MHz, CDCl3) δ (ppm): 0.82 (t, J = 6.7 Hz, 6H), 1.18–1.28 (m, 40H), 1.49–1.55 (m, 4H), 1.91–1.97 (m, 8H), 2.0–2.06 (m, 4H), 2.21 (t, J = 7.6 Hz, 4H), 3.23 (s, 12H), 3.24–3.30 (m, 4H), 3.52–3.58 (m, 4H), 4.27–4.33 (m, 4H), 5.23–5.33 (m, 4H), 6.62–6.68 (m, 2H), 7.79 (s, 2H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.0, 22.6, 23.0, 25.7, 27.2, 29.3, 29.4, 29.5, 29.7, 29.8, 31.8, 36.4, 51.1, 62.6, 64.9, 129.5, 129.9, 130.2, 174.8. FTIR (KBr pellet) υ (cm−1) 3445 (υN–H, secondary amine), 2926 and 28547 (υC–H, aliphatic asymmetric and symmetric respectively), 1643 (amide I band), 1551 (amide II band). Anal. Calcd for C50H98O2N4Br2 (947.15): C, 63.40; H, 10.43; N, 5.92. Found: C, 63.32; H, 10.57; N, 5.82.
(Z)-Oleic acid [3-({4-[(3-oleamidopropyl)-dimethyl-amino]but-2-enyl}-dimethyl-amino)-propyl]-amide dichloride ( 4 ): Pale yellow viscous oil (12.80 g, 80% yield based on cis-1,4-dichloro-2-butene). 1H NMR (500 MHz, CDCl3) δ (ppm): 0.83 (t, J = 6.7 Hz, 6H), 1.17–1.27 (m, 40H), 1.47–1.55 (m, 4H), 1.91–1.97 (m, 8H), 1.99–2.05 (m, 4H), 2.20 (t, J = 7.6 Hz, 4H), 3.23 (s, 12H), 3.24–3.30 (m, 4H), 3.53–3.59 (m, 4H), 4.52–4.58 (m, 4H), 5.22–5.32 (m, 4H), 6.32–6.38 (m, 2H), 8.0 (s, 2H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.0, 22.6, 23.0, 25.9, 27.2, 29.3, 29.4, 29.5, 29.7, 29.8, 31.8, 36.3, 50.6, 60.6, 62.3, 127.7, 129.5, 129.9, 174.8. FTIR (KBr pellet) υ (cm−1) 3431 (υN–H, secondary amine), 2924 and 2853 (υC–H, aliphatic asymmetric and symmetric respectively), 1642 (amide I band), 1552 (amide II band). Anal. Calcd for C50H98O2N4Cl2 (858.24): C, 69.97; H, 11.51; N, 6.53. Found: C, 69.84; H, 11.57; N, 6.63.
Oleic acid [3-({4-[(3-Oleamidopropyl)-dimethyl-amino]butyl}-dimethyl-amino)-propyl]-amide dibromide ( 5 ): Pale yellow gel (9.3 g, 91% yield based on 1,4-dibromobutane). 1H NMR (500 MHz, CDCl3) δ (ppm): 0.83 (t, J = 6.7 Hz, 6H), 1.18–1.28 (m, 40H), 1.50–1.56 (m, 4H), 1.91–1.97 (m, 12H), 1.99–2.05 (m, 4H), 2.21 (t, J = 7.6 Hz, 4H), 3.19 (s, 12H), 3.25-3.31 (m, 4H), 3.44–3.50 (m, 4H), 3.54–3.60 (m, 4H), 5.23–5.33 (m, 4H), 7.81 (s, 2H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.1, 19.6, 22.6, 22.9, 25.9, 27.2, 29.3, 29.4, 29.5, 29.7, 29.8, 31.8, 36.4, 51.4, 62.3, 63.2, 129.6, 129.9, 174.9. FTIR (KBr pellet) υ (cm−1) 3439 (υN–H, secondary amine), 2923 and 2851 (υC–H, aliphatic asymmetric and symmetric respectively), 1632 (amide I band), 1548 (amide II band). Anal. Calcd for C50H100O2N4Br2 (949.16): C, 63.27; H, 10.62; N, 5.90. Found: C, 63.21; H, 10.57; N, 5.87.
Oleic acid [3-({6-[(3-oleamidopropyl)-dimethyl-amino]hexyl}-dimethyl-amino)-propyl]-amide dibromide ( 6 ): White solid (8.7 g, 93% yield based on 1,6-dibromohexane). 1H NMR (500 MHz, CDCl3) δ (ppm): 0.82 (t, J = 6.7 Hz, 6H), 1.17–1.27 (m, 40H), 1.46–1.52 (m, 4H), 1.54–1.60 (m, 4H), 1.91–1.97 (m, 12H), 2.02–2.08 (m, 4H), 2.33 (t, J = 7.6 Hz, 4H), 3.26 (s, 12H), 3.33–3.39 (m, 4H), 3.53-3.59 (m, 4H), 3.69-3.75 (m, 4H), 5.23–5.33 (m, 4H), 8.40 (s, 2H (NH). 13C NMR (125 MHz, CDCl3) δ (ppm): 14.1, 21.6, 22.6, 22.7, 24.6, 25.9, 27.1, 29.1, 29.2, 29.3, 29.4, 29.6, 29.7, 31.8, 35.8, 51.1, 62.3, 65.0, 129.6, 129.9, 175.3. FTIR (KBr pellet) υ (cm−1) 3440 (υN–H, secondary amine), 2924 and 2852 (υC–H, aliphatic asymmetric and symmetric respectively), 1633 (amide I band), 1549 (amide II band). Anal. Calcd for C52H104O2N4Br2 (977.22): C, 63.91; H, 10.73; N, 5.73. Found: C, 63.78; H, 10.80; N, 5.64.
Analytical Equipment
The structures of the amido-amine-based cationic gemini surfactants (1–6) were established by using NMR, FTIR, and elemental analysis. The NMR data was acquired on a 500-MHz NMR instrument (Jeol 1500 model). Deuterated chloroform was used as the solvent, tetramethylsilane (TMS) as an internal standard, and chemical shifts in NMR spectra were recorded in ppm. The FTIR (Fourier Transform Infrared) spectroscopy was carried out using a FTIR spectrophotometer (Perkin-Elmer 16F model). Elemental analysis was obtained using a Perkin Elmer Series 11 (CHNS/O) Analyzer 2400.
Thermogravimetric Analysis
Thermogravimetric analysis (TGA) was conducted using an SDT Q600 apparatus from TA instruments with a constant heating rate of 20 °C/min and in the temperature range 30–500 °C. The experiment was run using an aluminum sample pan and a nitrogen flow rate of 100 mL/min.
Long-Term Thermal Stability
Long-term thermal stability was assessed using a novel approach based on an aging technique. Aqueous solutions of gemini surfactants (1–6) were aged in a sealed tube for 10 days at 90 °C. NMR (1H, 13C) and FTIR instruments were used to identify the changes in the structure of the surfactants after aging.
Surface Properties
Surface tensions were identified with the help of the pendant drop method at 20 °C using Biolin Scientific Attension instrument. Water was used as the solvent for all surface tension experiments. The reported data points of all surface tension measurements are average equilibrium values. The critical micelle concentration was estimated from the break point in the plot of surface tension versus concentration.
Results and Discussion
The synthesis of amido-amine-based cationic gemini surfactants (1–6) was achieved using an improved method outlined in scheme 1 [37]. The condensation of commercially available carboxylic acid (7 and 8) with 3-(dimethylamino)-1-propylamine (9) generated the amide intermediates (10 and 11). The reaction was followed by quaternization reaction with halohydrocarbons (12–15) yielding the desired amido-amine cationic gemini surfactants (1–6) at high yield [38, 39].
The structures of the gemini surfactants and corresponding intermediates were confirmed by characterization techniques such as NMR, FTIR, and elemental analysis.
The six amido-amine-based cationic gemini surfactants exhibited nearly the same peak pattern, therefore, we highlight here the spectral characterization of gemini surfactant (1) and its intermediate (10) as examples. According to 1H-NMR spectra of the intermediate compound (10), the terminal methyl protons [–(CH2)n–CH 3 )] resonated at δ = 0.83 ppm and the methylene protons [–(CH 2)n–CH3)] in the hydrophobic tail resonated at δ = 1.15–1.25 ppm. The disappearance of a hydroxyl proton in the carboxylic acid (–CH2–C = O–OH) (8) at δ = 10.25 ppm and the appearance of the amide proton (–CH2–C = O–NH) (10) at δ = 6.99 ppm were observed. The appearance of the methyl proton directly attached to the tertiary nitrogen [–CH2–N–(CH 3)2] at δ = 2.30 confirmed the formation of intermediate compound (10). According to 13C NMR spectra of intermediate compound (10), the terminal methyl carbon [–(CH2)n–CH3)] resonated at δ = 14.0 ppm and the methylene carbons [–(CH2)n–CH3)] in the hydrophobic tail resonated at δ = 22.6–36.8 ppm. The two methyl carbon directly attached to the tertiary nitrogen [–CH2–N–(CH3)2] resonated at δ = 44.8 ppm. The methylene carbon next to tertiary nitrogen [–CH2–CH2–N–(CH3)2] in compound (10) resonated at δ = 58.0 ppm. The appearance of the carbonyl carbon of fatty acid (7) (–CH2–C = O–OH) resonated at δ = 180.4 ppm and then clear up field shift of the same carbonyl carbon in amide (10) (–CH2–C = O–NH–) at δ = 173.3 ppm confirmed the formation of intermediate (10). According to 1H-NMR spectra of gemini surfactant (1), the terminal methyl protons [–(CH2)n–CH 3)] resonated at δ = 0.82 ppm and the methylene protons [–(CH 2)n–CH3)] in the hydrophobic tail resonated at δ = 1.16–1.26 ppm. The methyl protons directly attached to the nitrogen [–CH2–N–(CH 3)2] that previously appeared at δ = 2.30 ppm in intermediate (10) has shifted downfield to δ = 3.31 ppm in the gemini surfactant (1) [–CH2–N–(CH 3)2–CH2–]. The downfield shift of the amide proton (–CH2–C = O–NH) from δ = 6.99 ppm in compound 10 to δ = 8.16 ppm in gemini surfactant 1 has been also detected. The olefinic protons in the spacer group [–N–CH2–CH = CH–CH2–N–] appeared at δ = 6.77–6.85 ppm which further confirmed the formation of the gemini surfactant 1. According to 13C-NMR spectra of the gemini surfactant (1), the terminal methyl carbon [–(CH2)n–CH3)] resonated at δ = 14.0 ppm and the methylene carbons [–(CH2)n–CH3)] in the hydrophobic tail resonated at δ = 22.6–36.6 ppm. The two methyl carbons of the tertiary nitrogen [–CH2–N–(CH3)2] that were resonated at δ = 44.8 ppm in the intermediate (10) have shifted downfield to δ = 51.0 ppm as evidence of the formation of the gemini surfactant 1. The two new peaks that appeared at δ = 62.6 ppm and 65.0 ppm correspond to 2 methylene groups connected with nitrogen [–CH2–N–(CH3)2–CH2–]. The olefinic carbon in spacer group [–N–CH2–CH = CH–CH2–N–] resonated at δ = 130.2 ppm. The carbonyl carbon (–CH2–C = O–NH–) peak was detected at δ = 175.3 ppm. In general, the NMR (1H and 13C) spectral data appeared to be compatible with the proposed structures of the gemini surfactant 1. In FTIR spectra of the gemini surfactant 1, a disappearance of the hydroxyl group of fatty acid (7) (–CH2–C = O–OH) ranged from 2400 to 3400 cm−1 and an existence of amide (–CH2–C = O–NH) at 3441 cm−1 as well as a shifting of the band of carbonyl stretching (–C = O–) from the region of acid (–CH2–C = O–OH) at 1710 cm−1 to the region of amide (–CH2–C = O–NH–) at 1641 cm−1 were observed. Amide I band resonated at 1641 cm−1 and amide II band resonated at 1555 cm−1 [38]. The two stretching vibrations at 2922 cm−1 (CH aliphatic symmetric) and 2850 cm−1 (CH aliphatic asymmetric) were also detected which confirmed the formation of the gemini surfactant 1 [40,41,42].
Thermal Stability of Gemini Surfactants (1–6)
Thermal stability is an essential property of surfactants for various oilfield applications. A surfactant designed to be used in surfactant flooding should be thermally stable at high reservoir temperatures (≥90 °C) because it may stay inside the oil reservoir for many days. The high temperature in a reservoir can cause surfactant precipitation due to thermal degradation and the surfactant ability to reduce interfacial tension between water and oil can decrease significantly.
Therefore, we investigated the short-term and long-term thermal stability of the synthesized gemini surfactants (1–6). TGA indicates excellent thermal stability of the gemini surfactants (1–6) with no thermal degradation up to 200 °C (Fig. 2). The long-term thermal stability of the gemini surfactants (1–6) was examined using a novel approach based on an aging technique where the aqueous solutions of surfactants were aged in a sealed tube at 90 °C for 10 days. NMR (1H, 13C) and FTIR instruments were used to study the change in the structure of the surfactants after aging after different periods. Only FTIR and NMR spectra of surfactants aged during 10 days are presented here. The six amido-amine cationic gemini surfactants (1–6) exhibited excellent long-term thermal stability with no thermal degradation after 10 days aging. We highlight here the spectral characterization of aged samples of gemini surfactant 1 and 6 as examples. The 1H-NMR spectra of the 10 days aged samples of the gemini surfactants 1 and 6 (Figs. 3, 5) demonstrated the appearance of the protons of the terminal methyl group [–(CH2)n–CH 3)] as well as protons of the methylene group [–(CH 2)n–CH3)] of the surfactant hydrophobic tail. The olefinic protons in the hydrophobic tail of the gemini surfactants 6 (Fig. 5) were also revealed. Similarly, the olefinic protons in the spacer group of 10 days aged sample of gemini surfactants 1 (Fig. 3) were also detected. Likewise, the methylene protons in the spacer group of gemini surfactants 6 (Fig. 5) equally appeared. The protons of methyl group directly attached to quaternary nitrogen [–CH2–N+–(CH 3)2–CH2–] are clearly observed. In addition, the appearance of the amide proton (–CH2–C = O–NH–) confirmed the survival of gemini surfactants 1 and 6 under harsh conditions. An additional peak at δ = 4–5 ppm corresponds to residual water [43]. According to 13C-NMR spectra of the 10 days aged samples of gemini surfactants 1 and 6 (Fig. 4 and 6), the methyl [–(CH2)n–CH3)] and methylene carbon in the hydrophobic tail of gemini surfactants 1 and 6 were clearly identified. The two methyl carbon [–CH2N+–(CH3)2–CH2–] and two methylene carbon [–CH2–N+–(CH3)2–CH2–] directly attached to the quaternary nitrogen were similarly observed in both surfactants (1 and 6) (Figs. 4, 6). The olefinic carbon in hydrophobic tail of gemini surfactant 6 (Fig. 6) as well as the olefinic carbon in spacer group of gemini surfactant 1 (Fig. 4) were also detected. The carbonyl carbon of amide group [–CH2–C = O–NH] was clearly shown in both surfactants. In general, the NMR (1H and 13C) spectra of the aged samples of gemini surfactants (1 and 6) confirmed that no structural changes occurred. According to the FTIR spectra of samples of gemini surfactants aged during 10 days (1 and 6) (Figs. 7, 8), the two clear stretching bands in the region of 2921 and 2850 cm−1 were detected and they correspond to aliphatic symmetric CH and aliphatic-asymmetric CH, respectively. The carbonyl stretching and C–N stretching was also observed which confirmed the structure of gemini surfactants (1 and 6) and demonstrated the thermal stability of amido-amine cationic gemini surfactants. There was no cloudiness and phase separation before and after aging at 90 °C.
Surface Tension Measurements
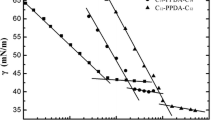
The synthesized gemini surfactants (1–6) showed good water solubility. Figures 9 and 10 show the surface tension of all surfactants at different concentrations; derived surface properties are given in Table 1. The surface tension remarkably decreased with increasing surfactant concentration up to the breakpoint at CMC. Further addition of surfactant above CMC induced no change in the surface tension. The surfactant 2 showed the highest surface tension while the surfactant 6 showed the lowest surface tension at all investigated concentrations. The surface properties can be related to the different chain lengths, spacer lengths, spacer rigidities, and to the presence of different counterions in the gemini surfactants.
Figure 9 shows the effect of the spacer length and rigidity on the surface tension of the gemini surfactants. By comparing the surfactant 5 and 6, it was observed that the surfactant with a larger spacer (6) showed a lower CMC which could be associated with the hydrophobic nature of the longer spacer [44]. The rigidity of spacer is another important parameter in determining the aggregation morphologies of the gemini surfactants. Surfactant 3 and surfactant 5 possess almost similar structures but the spacer of surfactant 3 is more rigid compared to the surfactant 5. The gemini surfactant 3 with more rigid spacer showed a higher CMC and higher surface tension at CMC (γCMC). The CMC and γCMC of gemini surfactant 3 were 1.58 × 10−4 mol L−1 and 35.67 mN m−1, respectively, which is much higher as compared to the surfactant 5 that has a relatively flexible spacer. The gemini surfactants containing rigid spacer usually form vesicles. On the other hand, less rigid gemini surfactants form mixtures of vesicles and micelles [31]. The shape of aggregates depends on the packing parameter of the surfactant.
The surfactant chain length is another critical parameter that affects the surface properties of surfactant. Figure 10 compares the surface tension of the gemini surfactants with different chain lengths and spacer conformations. By comparing the surfactants 1 and 3, the CMC and the corresponding γCMC decrease when the hydrophobic tail is increased from 12 carbons (1) to 18 carbons (3). A similar trend was observed with the surfactants 2 and 4, which also have the same spacer and counterions but differ from each other by the length of the hydrophobic tail.
The double bond in the spacer of the surfactant 1 and 3 was in trans conformation with bromide counterions while the double bond in the spacer of surfactant 2 and 4 was in cis conformation with chloride counterions. The CMC and the γCMC of the surfactants 2 and 4 were higher as compared to the analogous surfactant 1 and 3 respectively. The difference of surface properties between the surfactants 1, 2 and 3, 4 may be associated with the different conformation of the spacer double bond and counters ions. It has been reported previously that the presence of different counterions in the spacer can alter the CMC value [30]. In summary, high CMC and γCMC was observed for the surfactant 2 while the surfactant 6 showed the lowest CMC and γCMC.
The ability of gemini surfactants to lower the surface tension of water (πCMC), the surface access (гmax) at the interface of air–water, as well as the surface area per molecule (Amin) are given in Table 1; these parameters were estimated following the literature [45]. The surface area per molecule decreases by increasing the hydrophobic tail and the spacer length, but it increases with spacer rigidity. The surface access at the air–water interface increases with hydrophobic tail length and the spacer length. The CMC values of the synthesized gemini surfactants ranged from 0.077 mmol/L to 0.36 mmol/L which are comparable with the reported values of a similar class of gemini surfactants (0.008–12.5 mmol/L) [38]. Similarly, γCMC of the synthesized gemini surfactants (30.38–38.12 mN m−1) were in agreement with the reported values (22.76–41.07 mN m−1) [38]. It is noteworthy that the πCMC decreased with increasing rigidity and increased with increasing spacer length (5 and 6). A similar trend was also observed by Chauhan et al. using pyridinium based cationic gemini surfactants [46]. The гmax of the synthesized gemini surfactants were increased by increasing tail length, spacer flexibility, and spacer length. Geng et al. also observed a similar change in гmax by increasing spacer and chain length using di-hydroxyl-sulfate-betaine-type zwitterionic gemini surfactants [47]. The literature values of the surface area per molecule were also found to be in agreement with the synthesized gemini surfactants i.e. Amin decreased with increasing tail length, spacer flexibility, and spacer length [48]. In summary, the lowest CMC and γCMC was observed for surfactant 6 containing a longer tail and large spacer with low rigidity.
Conclusions
Amido-amine-based cationic gemini surfactants (1–6) have been synthesized with excellent yields and high purity from the commercially available carboxylic acids. The short-term and long-term thermal stabilities, as well as the surface tensions, were examined. Thermogravimetric analysis demonstrated excellent thermal stability of the synthesized surfactants (1–6) with no structural degradation up to 200 °C. It was noticed that the thermal stability slightly increased with the surfactant chain length. The long-term thermal stability was assessed using a novel approach based on structure characterization before and after aging. The NMR and FTIR results revealed excellent long-term thermal stability of the gemini surfactants (1–6) with no change in the structures even after aging for 10 days at 90 °C. The highest CMC and γCMC was observed for gemini surfactant 2 and lowest CMC and γCMC was obtained for gemini surfactant 6. Moreover, the surfactants with trans conformation showed better surface properties as compared to surfactants with cis conformation. The great tolerance to high temperature and unique surface activities of the synthesized gemini surfactants (1–6) make them attractive for several oilfield applications. The rheological investigation, adsorption studies, and interfacial tension measurements of the synthesized gemini surfactants are currently underway.
References
Sheng JJ. Status of surfactant EOR technology. Petroleum. 2015;1(2):97–105. doi:10.1016/j.petlm.2015.07.003.
Sheng JJ. Comparison of the effects of wettability alteration and IFT reduction on oil recovery in carbonate reservoirs. Asia-Pac J Chem Eng. 2013;8(1):154–61.
Kamal MS, Hussain SS, Sultan AS. Development of novel amidosulfobetaine surfactant-polymer systems for EOR applications. J Surfactants Deterg. 2016;19(5):989–97.
Kamal MS, Sultan AS, Hussein IA. Screening of amphoteric and anionic surfactants for cEOR applications using a novel approach. Colloid Surface A. 2015;476:17–23.
Al-Amodi AO, Al-Mubaiyedh UA, Sultan AS, Kamal MS, Hussein IA. Novel fluorinated surfactants for enhanced oil recovery in carbonate reservoirs. Can J Chem Eng. 2016;94(3):454–60.
Musselman SW, Chander S. Wetting and adsorption of acetylenic diol based nonionic surfactants on heterogeneous surfaces. Colloids Surf A. 2002;206(1–3):497–513. doi:10.1016/S0927-7757(02)00055-9.
Ebrahimpour B, Yamini Y, Moradi M. Application of ionic surfactant as a carrier and emulsifier agent for the microextraction of fluoroquinolones. J Pharm Biomed Anal. 2012;66:264–70. doi:10.1016/j.jpba.2012.03.028.
Hait S, Moulik S. Gemini surfactants: a distinct class of self-assembling molecules. Curr Sci Bangalore. 2002;82(9):1101–11.
Kamal MS. A review of gemini surfactants: potential application in enhanced oil recovery. J Surfactants Deterg. 2016;19(2):223–36.
Pozniak BP, Kuliszewska E. Intracluster reactions in negatively charged aggregates of diquaternary amines – Gemini surfactants with bromide and formate counterions. Int J Mass Spectrom. 2014;359:44–54. doi:10.1016/j.ijms.2013.12.016.
Alimohammadi MH, Javadian S, Gharibi H, reza Tehrani-Bagha A, Alavijeh MR, Kakaei K. Aggregation behavior and intermicellar interactions of cationic Gemini surfactants: effects of alkyl chain, spacer lengths and temperature. J Chem Thermodyn. 2012;44(1):107–15.
Deng M, Li J, Liu J, Ma X, Wang Y. Abnormal interfacial tension behavior of alkanediyl-α, ω-bis(dodecyldimethylammonium bromide) gemini surfactants. Colloids Surf A. 2010;356(1–3):97–103. doi:10.1016/j.colsurfa.2009.12.032.
Shukla D, Tyagi V. Cationic gemini surfactants: a review. J Oleo Sci. 2006;55(8):381–90.
Kumar N, Tyagi R. Industrial applications of dimeric surfactants: a review. J Dispersion Sci Technol. 2014;35(2):205–14.
Gerba CP. Quaternary ammonium biocides: efficacy in application. Appl Environ Microbiol. 2015;81(2):464–9.
Akram M, Bhat IA, Anwar S, Kabirud D. Biophysical analysis of novel oxy-diester hybrid cationic gemini surfactants (Cm-E2O-Cm) with xanthine oxidase (XO). Process Biochem. 2016;. doi:10.1016/j.procbio.2016.05.014.
Madunić-Čačić D, Sak-Bosnar M, Galović O, Sakač N, Matešić-Puač R. Determination of cationic surfactants in pharmaceutical disinfectants using a new sensitive potentiometric sensor. Talanta. 2008;76(2):259–64. doi:10.1016/j.talanta.2008.02.023.
Ma K, Cui L, Dong Y, Wang T, Da C, Hirasaki GJ, Biswal SL. Adsorption of cationic and anionic surfactants on natural and synthetic carbonate materials. J Colloid Interface Sci. 2013;408:164–72. doi:10.1016/j.jcis.2013.07.006.
Abd El-Lateef HM, Abo-Riya MA, Tantawy AH. Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros Sci. 2016;108:94–110. doi:10.1016/j.corsci.2016.03.004.
Negm NA, Aiad IA. Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surfactants Deterg. 2007;10(2):87–92.
Joonaki E, Erfani Gahrooei HR, Ghanaatian S. Experimental study on adsorption and wettability alteration aspects of a new chemical using for enhanced oil recovery in carbonate oil reservoirs. J Unconv Oil Gas Resour. 2016;15:11–21. doi:10.1016/j.juogr.2016.05.001.
Sheng JJ (2011) Chapter 7—Surfactant Flooding. In: Modern Chemical Enhanced Oil Recovery. Gulf Professional Publishing, Boston, pp 239–335. doi: 10.1016/B978-1-85617-745-0.00007-3
Wang X, Wang J, Wang Y, Yan H, Li P, Thomas RK. Effect of the nature of the spacer on the aggregation properties of gemini surfactants in an aqueous solution. Langmuir. 2004;20(1):53–6.
Manne S, Schäffer T, Huo Q, Hansma P, Morse D, Stucky G, Aksay I. Gemini surfactants at solid-liquid interfaces: control of interfacial aggregate geometry. Langmuir. 1997;13(24):6382–7.
Zana R. Dimeric (gemini) surfactants: effect of the spacer group on the association behavior in aqueous solution. J Colloid Interface Sci. 2002;248(2):203–20. doi:10.1006/jcis.2001.8104.
Parikh K, Mistry B, Jana S, Gupta S, Devkar RV, Kumar S. Physico-biochemical studies on cationic gemini surfactants: role of spacer. J Mol Liq. 2015;206:19–28. doi:10.1016/j.molliq.2015.01.055.
Du X, Lu Y, Li L, Wang J, Yang Z. Synthesis and unusual properties of novel alkylbenzene sulfonate gemini surfactants. Colloids Surf A. 2006;290(1):132–7.
Bendjeriou-Sedjerari A, Derrien G, Charnay C, Zajac J, De Menorval LC, Lindheimer M. Contribution of 1H NMR to the investigation of the adsorption of cationic Gemini surfactants with oligooxyethylene spacer group onto silica. J Colloid Interface Sci. 2009;331(2):281–7. doi:10.1016/j.jcis.2008.12.007.
Zhang Z, Wang H, Zheng P, Shen W. Effect of spacer rigidity on the aggregations of ester containing Gemini surfactants in aqueous solutions: a study of density and fluorescence. Colloids Surf A. 2013;421:193–200.
Menger F, Keiper J, Azov V. Gemini surfactants with acetylenic spacers. Langmuir. 2000;16(5):2062–7.
Zhu D-Y, Cheng F, Chen Y, Jiang S-C. Preparation, characterization and properties of anionic gemini surfactants with long rigid or semi-rigid spacers. Colloid Surface A. 2012;397:1–7.
Mivehi L, Bordes R, Holmberg K. Adsorption of cationic gemini surfactants at solid surfaces studied by QCM-D and SPR: effect of the rigidity of the spacer. Langmuir. 2011;27(12):7549–57.
Zhang Z, Zheng P, Guo Y, Yang Y, Chen Z, Wang X, An X, Shen W. The effect of the spacer rigidity on the aggregation behavior of two ester-containing gemini surfactants. J Colloid Interface Sci. 2012;379(1):64–71.
Laschewsky A, Lunkenheimer K, Rakotoaly RH, Wattebled L. Spacer effects in dimeric cationic surfactants. Colloid Polym Sci. 2005;283(5):469–79.
Huang Z, Zhong H, Wang S, Xia L, Zou W, Liu G. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: ethane-1,2-bis(dimethyl-dodecyl-ammonium bromide). Chem Eng J. 2014;257:218–28. doi:10.1016/j.cej.2014.07.057.
Lee M-T, Vishnyakov A, Neimark AV. Calculations of critical micelle concentration by dissipative particle dynamics simulations: the role of chain rigidity. J Phys Chem B. 2013;117(35):10304–10.
Chu Z, Feng Y. A facile route towards the preparation of ultra-long-chain amidosulfobetaine surfactants. Synlett. 2009;16:2655–8.
Ghumare AK, Pawar BV, Bhagwat SS. Synthesis and antibacterial activity of novel amido-amine-based cationic gemini surfactants. J Surfactants Deterg. 2013;16(1):85–93.
Zana R, Benrraou M, Rueff R. Alkanediyl-. alpha., omega.-bis (dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree. Langmuir. 1991;7(6):1072–5.
Dardir M, Mohamed D, Farag A, Ramdan A, Fayad M. Preparation and evaluation of cationic bolaform surfactants for water-based drilling fluids. Egypt J Petrol. 2017;26(1):67–77.
Shaban SM, Aiad I, Fetouh HA, Maher A. Amidoamine double tailed cationic surfactant based on dimethylaminopropylamine: synthesis, characterization and evaluation as biocide. J Mol Liq. 2015;212:699–707.
El-Lateef HMA, Abo-Riya MA, Tantawy AH. Empirical and quantum chemical studies on the corrosion inhibition performance of some novel synthesized cationic gemini surfactants on carbon steel pipelines in acid pickling processes. Corros Sci. 2016;108:94–110.
Fulmer GR, Miller AJ, Sherden NH, Gottlieb HE, Nudelman A, Stoltz BM, Bercaw JE, Goldberg KI. NMR chemical shifts of trace impurities: common laboratory solvents, organics, and gases in deuterated solvents relevant to the organometallic chemist. Organometallics. 2010;29(9):2176–9.
Chavda S, Bahadur P, Aswal VK. Interaction between nonionic and gemini (cationic) surfactants: effect of spacer chain length. J Surfactants Deterg. 2011;14(3):353–62.
Hussain SS, Animashaun MA, Kamal MS, Ullah N, Hussein IA, Sultan AS. Synthesis, characterization and surface properties of amidosulfobetaine surfactants bearing odd-number hydrophobic tail. J Surfactants Deterg. 2016;19(2):413–20.
Chauhan V, Singh S, Kamboj R, Mishra R, Kaur G. Self-assembly, DNA binding and cytotoxicity trends of ether functionalized gemini pyridinium amphiphiles. J Colloid Interface Sci. 2014;417:385–95.
Geng XF, Hu XQ, Xia JJ, Jia XC. Synthesis and surface activities of a novel di-hydroxyl-sulfate-betaine-type zwitterionic gemini surfactants. Appl Surf Sci. 2013;271:284–90.
Pérez L, Pinazo A, Pons R, Infante M. Gemini surfactants from natural amino acids. Adv Coll Interface Sci. 2014;205:134–55.
Acknowledgements
This work was supported by the Center for Integrative Petroleum Research (CIPR), King Fahd University of Petroleum and Minerals (KFUPM), Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hussain, S.M.S., Kamal, M.S., El Ali, B. et al. Synthesis and Evaluation of Novel Amido-Amine Cationic Gemini Surfactants Containing Flexible and Rigid Spacers. J Surfact Deterg 20, 777–788 (2017). https://doi.org/10.1007/s11743-017-1969-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-017-1969-1