Abstract
Cotton (Gossypium hirsutum L.) possesses notable inter- and intraspecific salt tolerance variation, but the mechanisms underlying this variation are, more or less, unclear. To explore salt stress responses, Na+/K+ transport and the transcriptional profiles of salinity tolerance candidate genes (e.g., GhSOS1, GhNHX1, and GhAKT1) were evaluated in four cotton cultivars (differing in salt sensitivities) using electrophysiological and qRT-PCR assays. Physiology and lipid peroxidation assays revealed the following pattern of salt tolerance, in decreasing order: CZ91 > CCRI44 > CCRI49 > Z571. Salinity caused ion imbalances in plants, but ion homeostasis was more pronounced in Z571, which accumulated more Na+ and less K+ in leaves. In contrast, salt-tolerant cultivars, especially CZ91, retained less Na+, but more K+ under salt stress. The non-invasive micro-test technique revealed that Z571 exhibited a higher capacity to transport Na+ from roots to shoots, but a lower capacity to extrude Na+ and retain K+ in leaves under salinity. Meanwhile, NaCl-induced changes in Na+ and K+ ion homeostasis were less pronounced in salt-tolerant cultivars, increasing leaf K+/Na+ ratios under salinity. Additionally, NaCl increased the GhSOS1 transcript abundance in hypocotyls, but this increase was more pronounced in salt-sensitive Z571. On the other hand, NaCl significantly enhanced the transcript abundances of GhSOS1 in leaves and/or GhAKT1 in hypocotyls of salt-tolerant cultivars. NaCl also increased the GhNHX1 transcript abundance, but there was no strong correlation between GhNHX1 expression level and salt resistance. The present findings provided an explanation for salt tolerance variation in cotton, illuminating the transcriptional regulation of K+/Na+ transport and the maintenance of the K+/Na+ ratio as underlying attributes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is one of the major environmental factors that severely limits productivity and yield in many areas worldwide (Wang et al. 2001). At present, about 7% of the total land area and 20% of the irrigated arable land are affected by salinity (Parihar et al. 2015). Farmland salinity has become a severe agricultural problem, partly due to unreasonable irrigation and fertilization. As such, enhancing crop salt tolerance could be a putative way to meet the challenge. Salinity tolerance is a complex trait regulated by various mechanisms; hence, a deeper understanding of various protective physiological mechanisms is essential for developing salt-tolerant cultivars (Flowers 2004).
Plants can survive under saline conditions and evolve to better tolerate saline conditions through special strategies utilizing physiological, biochemical, and molecular mechanisms (Munns and Tester 2008; Singh et al. 2015). These special strategies include ion homeostasis, photosynthesis adjustment, antioxidant enzyme induction, hormone overproduction, and combinations of these factors (Parida and Das 2005). Maintaining an optimal cytoplasmic K+/Na+ ratio is an important component of plant salinity tolerance (Wang et al. 2016).
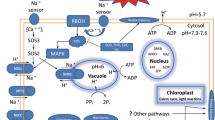
Plants maintain an optimal cytosolic K+/Na+ balance via different strategies for the avoidance of excessive Na+ accumulation by increasing efflux and/or increasing vacuolar sequestration of Na+ (Tester and Davenport 2003). The key salt tolerance gene salt overly sensitive-1 (SOS1) encodes a plasma membrane (PM) Na+/H+ antiporter that can extrude excess Na+ from the cytosol (Shi et al. 2002). Overexpression of SOS1 can significantly improve salt tolerance by enhancing Na+ exclusion from the cytosol to extracellular spaces (Ma et al. 2014; Qiu et al. 2002). Transporting Na+ from the cytoplasm into the vacuole by NHX1, a vacuolar membrane-bound Na+/H+ antiporter, is another effective method of minimizing Na+ concentrations in the cytoplasm (Munns and Tester 2008). Previous studies have shown that the overexpression of NHX1 in transgenic plants can increase salt tolerance (Banjara et al. 2012; Chen et al. 2008). Maintaining cytosolic K+/Na+ homeostasis in tissues can be achieved not only through stronger Na+ exclusion and/or compartmentalization, but also by improved K+ retention (Wu et al. 2011). In plants, K+ acquisition is mainly mediated by K+ transporters and ion channels (Martinez-Cordero et al. 2005; Wang and Wu 2013). Among them, the inward-rectifying potassium channel AKT1 is considered the major route for K+ uptake, and it plays a critical role in maintaining the K+/Na+ balance in salt-stressed plants (Hirsch et al. 1998; Lebaudy et al. 2007). Some reports have also shown that AKT1 transcription levels are up-regulated along with an increase in K+ uptake capacity under salt stress, ultimately contributing to the physiological adaptation to salt stress in plants (Boscari et al. 2009; Chen et al. 2013; Duan et al. 2015).
Cotton (Gossypium hirsutum L.) is a widely grown fiber crop, and about 4 million ha are sown in China annually, accounting for 20% of the world’s cotton production (Dai and Dong et al. 2014). Cotton has been reported to be moderately salt tolerant, but it has shown limited growth and yield in high salinity conditions (Leidi and Saiz 1997). Moreover, a large body of data regarding mechanisms of salt tolerance in cotton is now available (Kong et al. 2012, 2016a; Li et al. 2013; Wang et al. 2016). Plant salinity tolerance is a complex physiological trait exhibiting polygenic inheritance; variation in salt sensitivity occurs not only among species, as even genotypes belonging to a species perform differently under saline conditions (Shabala and Munns 2012). At the same time, however, little information is available on the modulation of key candidate genes that regulate ion homeostasis in cotton genotypes with various salinity tolerance. Thus, it is very important to identify the specific components of protective mechanisms that underlie salt tolerance in cotton.
To further address the above questions, salt tolerance variation in cotton cultivars was comparatively evaluated. This study used atomic absorption spectroscopy to assess the effects of salt stress on the transport and compartmentalization of K+ and Na+ in seedlings of different cotton cultivars. The non-invasive micro-test technique (NMT) was also employed to study the kinetics of stress-induced net Na+ and K+ fluxes and these changes were linked to alterations in the relative abundances of GhSOS1, GhNHX1, and GhAKT1 transcripts. We attempted to characterize and identify the salinity tolerance mechanisms among cultivars varying in K+ and Na+ homeostasis, which is likely linked to the expression of K+ and Na+ transport system under salinity.
Materials and methods
Plant materials and treatment
Four cotton cultivars [Z571 (salt sensitive), CCRI49 (moderately salt tolerant), CCRI44 (moderately salt tolerant), and CZ91 (superior line of Xinluzhong 61, salt tolerant)] were obtained from the Cotton Research Institute at the Chinese Academy of Agricultural Sciences (CCRI), Anyang, Henan, People’s Republic of China. All seeds were surface sterilized in 0.1% (w/v) HgCl2 solution for 10 min and subsequently rinsed several times with tap water to remove traces of HgCl2. The seeds were sown in 10-cm diameter by 10-cm height pots filled with 1000 g of sandy clay soil (sterilized in an autoclave for 20 min). Then, the pots were placed in a growth chamber with a photosynthetic photon flux density, average humidity, and light/dark regime of 500 μmol m−2 s−1, ~80%, and 14/10 h at 28/20 °C, respectively. At the two-leaf stage (20 days after sowing), seedlings exhibiting uniform growth were randomly selected for salt treatment. The NaCl solution was added to the soil to reach a final concentration of 0.3% (w/w) and control plants were supplied with water. The completely randomized design with three replicates (i.e., pots) was used in all experiments. All experiments were separately repeated two times. To maintain soil moisture at field capacity, plants received 100 mL of distilled water every 2 days and 100 mL of Hoagland nutrient solution every 5 days. After 9 days under NaCl stress, physiological and biochemical parameters were assessed.
Measurement of net photosynthetic rate and chlorophyll content
Net photosynthetic rate (P n) of the third fully expanded leaf was determined using a portable photosynthesis open system (Li-6400XT, Li-Cor Inc., USA) between 9:00 and 11:00 am at 28 °C, 60% relative humidity, 400 μmol mol−1 CO2, and 1000 μmol m−2 s−1 quantum flux. Then, these leaves from each plant were detached for the determination of total chlorophyll content. Chlorophyll was extracted from 100 mg of fresh leaf tissues in 5 mL of 80% acetone. The absorbance of the extracts was measured using a spectrophotometer at two wavelengths (645 and 663 nm). Chlorophyll content was calculated according to the method described by Lichtenthaler (1987).
Measurement of relative electric conductivity and malondialdehyde content
After chlorophyll assessment, relative electric conductivity (REC) and malondialdehyde (MDA) content of the third fully expanded leaf were measured. Electrolyte leakage was measured as described by Nayyar et al. (2005) using young segments (0.8-cm diameter) of leaf. Samples were placed in closed vials containing 10 mL of deionized water and incubated at 30 °C on a rotary shaker for 3 h, and the initial electrical conductivity (L 1) of the solution was determined. Samples were then boiled for 30 min at 100 °C and the final electrical conductivity (L 2) was obtained after equilibration at 25 °C. REC was expressed by the formula: REC (%) = (L 1/L 2) × 100. The lipid peroxidation level was determined by assessing the MDA content using the thiobarbituric acid (TCA) test as described by Shi et al. (2010). For MDA extraction, fresh leaf material (0.2 g) was ground in 10% TCA. The mixture was centrifuged at 10,000×g for 20 min, and 2 mL of the supernatant was mixed with 2 mL of 0.6% thiobarbituric acid (TAB). The samples were heated in a boiling water bath for 10 min and then centrifuged at 10,000×g for 5 min, and the absorbance was monitored at 600, 532, and 450 nm, respectively. The MDA content was calculated as follows: C (μmol L−1) = 6.45 × (A532 − A600) − 0.56 × A450.
Measurement of Na+ and K+ fluxes
Net ion fluxes (Na+ and K+) in hypocotyl and leaf mesophyll tissues were measured using NMT (NMT-YG-100, Younger, USA) at Xuyue Sci. & Tech. Co. (Beijing, China). The principle behind this method and the instrument used were described previously (Lei et al. 2014; Wu et al. 2015). The hypocotyl and leaf samples (~5 × 5 mm) were cut from near the cotyledon (Fig. 1a) and the middle part of the third leaf (Fig. 1b), exposing leaf mesophyll tissue. Cut samples were immediately fixed on the bottom of a measuring dish, incubated in the measuring solution, and allowed to equilibrate for 30 min. The measuring solution consisted of 0.1 mM KCl, 0.1 mM CaCl2, 0.1 mM MgCl2, 0.5 mM NaCl, 0.3 mM MES, and 0.2 mM Na2SO4 (pH 6.0, maintained using Tris or HCl). The measuring site was 300 μm from the edge of the samples, into which a vigorous flux of Na+ and K+ ions was measured, having infiltrated the tissues from the fresh measuring solution. The steady-state ion fluxes were continuously recorded for 20–25 min, and the ion flux estimates were calculated using JCal V3.0 (Xuyue Sci. and Tech., China).
Measurement of Na+ and K+ content
After salt treatment, roots were soaked in deionized water for 10 min, and shoots were washed with distilled water to remove surface salts. From each plant, the roots, stems, and leaves were harvested and dried at 80 °C for 72 h. Each sample (0.1 g) was digested in 1.0 mol L−1 HCl solution for 24 h at 30 °C, and then shaken for 60 min. The Na+ and K+ contents were determined using an atomic absorption spectrophotometer (SpectAA-50/55, Varian, Australia).
RNA isolation and quantitative real-time PCR analysis
Under salinity stress, changes of related-gene expression are a relatively quick response (Horie et al. 2012). So, cotton seedlings grown as described earlier were subjected to NaCl stress for time periods of 3, 6, 9, 12, 18, 24, and 48 h. Then, the sampled tissues (hypocotyls near the cotyledon and juvenile leaves) were crushed in liquid nitrogen using a precooled mortar and pestle. Total RNA from the different cotton seedling tissues was extracted using an RNAprep Pure Plant Kit (Tiangen, China) and purified with RNase-free DNase I (Tiangen). A first-strand cDNA fragment was synthesized from total RNA samples using Superscript II reverse transcriptase (Invitrogen, USA). Then, quantitative real-time PCR assays were performed on the 7500 Real-time PCR System (Applied Biosystems, USA) using the reverse-transcribed cDNAs extracted from the different tissues. Gene-specific primers were obtained based on primer sequences for GhSOS1, GhNHX1, and GhAKT1 acquired from NCBI, and UBQ7 was chosen as the reference gene for standardizing RT-PCR experiments (Table 1). According to the manufacturer’s protocol, a melting curve analysis was carried out to determine the specificity of amplification. Relative quantization of gene expression was calculated based on the comparative threshold cycle method using UBQ7 as an internal control and normalized to the gene expression values.
Statistical analysis
The experiment was repeated two times with results showing similar trends and there was no interaction among repetitions of the experiments; hence, the pooled data were presented. All data were statistically analyzed using SPSS 17.0 (SPSS Inc., USA). The differences between treatments were analyzed using analysis of variance (ANOVA) followed by Duncan’s multiple range test, and significance was determined at P < 0.05.
Results
Effect of salinity on leaf photosynthesis
Non-significant differences in the chlorophyll content and P n of the different cotton cultivars were observed under the control conditions (Fig. 2). As shown in Fig. 2, salinity resulted in a significant reduction in chlorophyll content and P n in all cultivars. However, more pronounced effects were observed in the salt-sensitive cultivar Z571, as the chlorophyll content and P n decreased by 55.9 and 65.4%, respectively. The cultivars ranked according to the values of these parameters in seedling leaves, in descending order, were CZ91, CCRI44, CCRI49, and (last) Z571.
Changes in chlorophyll content (a) and photosynthetic rate (b) in the leaves of the four cotton cultivars after treatment with NaCl for 9 days. Different capital letters denote significant differences between treatments in each cultivar; different lowercase letters denote significant differences between cultivars (P < 0.05)
Effect of salinity on relative electric conductivity and malondialdehyde content
Compared with controls, the REC of leaves increased under salinity (Fig. 3a). Less pronounced effects were observed in leaves of CZ91, as its REC increased by only 118.2%. However, in leaves of Z571, CCRI49, and CCRI44, this parameter increased by 232.3, 192.8, and 151.8%, respectively. Additionally, salinity significantly increased the MDA content in cotton leaves (Fig. 3b). The descending order of cultivars according to seedling leaf increases in MDA was Z571, CCRI49, CCRI44, and (last) CZ91, as the MDA contents increased by 258.4, 185.6, 174.4, and 59.3%, respectively.
Changes in relative electric conductivity (a) and malondialdehyde content (b) in the leaves of the four cotton cultivars after treatment with NaCl for 9 days. Different capital letters denote significant differences between treatments in each cultivar; different lowercase letters denote significant differences between cultivars (P < 0.05)
Effect of salinity on Na+ and K+ ion partitioning
As Table 2 shows, salinity produced a significant increase in tissue Na+ concentration, with the highest increase observed in the susceptible cultivar Z571. Among the most sampled tissues, Na+ concentration was lowest in CZ91, especially in CZ91 leaves. Contrasting results were found for K+ concentrations in stems and leaves of the four tested cultivars, which were significantly reduced in response to salt stress (Table 2). Among the cultivars, CZ91, CCRI44, and CCRI49 showed comparatively mild decreases (2.3, 14.7, and 23.1%, respectively) in leaf K+ compared to Z571 (36.8%), while the first three cultivars showed higher decreases (24.1, 25.2, and 12.7%, respectively) in stem K+ concentration than Z571 (5.7%) under salt stress. In contrast to leaves and stems, K+ concentration of root was markedly increased by NaCl treatment, and more pronounced effects were observed in Z571 and CCRI49. Irrespective of the treatment effect, the ratio of K+/Na+ was also found to be considerably reduced across all tissues (Table 2). The descending order of cultivars with respect to their K+/Na+ ratios in leaves was CZ91, CCRI44, CCRI49, and, last, Z571, while no differences among the cultivars were found in roots and stems.
Effect of salinity on Na+ and K+ ion fluxes
To understand how salinity influenced the transport and compartmentalization of Na+ and K+ in cotton seedlings, Na+ and K+ fluxes of the hypocotyls and leaves were assessed. Under control conditions, no significant differences in Na+ and K+ efflux rates were found among cotton cultivars (Fig. 4). As shown in Fig. 4a, b, salt stress induced a marked Na+ efflux in hypocotyls and leaves. Upon salinity exposure, the descending order of cultivars in net Na+ efflux of hypocotyls was Z571, CCRI44, CCRI49, and last, CZ91. However, the net Na+ efflux of leaves exhibited the reverse trend, and a more pronounced effect was observed in CZ91 and CCRI49 (5747 and 4851 pmol cm−2 s−1, respectively) compared with Z571 and CCRI44 (4062 and 3654 pmol cm−2 s−1, respectively). Salinity markedly restrained the net K+ efflux in hypocotyls; a more pronounced effect was recorded in CCRI49 (with a steady-state value of 1962 pmol cm−2 s−1), and a less pronounced effect was observed in CCRI44 (with a steady state value of 3553 pmol cm−2 s−1; Fig. 4c). In contrast to hypocotyls, the net K+ effluxes in leaves were markedly increased by salinity (Fig. 4d). Under salinity, the net K+ effluxes in leaves of Z571 and CCRI44 (1505 and 1117 pmol cm−2 s−1, respectively) were significantly higher than those in leaves of CCRI49 and CZ91 (782 and 700 pmol cm−2 s−1, respectively).
Effect of salinity on SOS1, NHX1, and AKT1 gene expression
To determine whether Na+ and K+ transport-related genes were up-regulated in hypocotyls and leaves under salinity, the expression patterns of GhSOS1, GhNHX1, and GhAKT1 were analyzed by qRT-PCR at 3, 6, 9, 12, 18, 24, and 48 h after salt stress (HAS). The expression of GhSOS1 in the different cultivars increased gradually during the initial salt exposure and then gradually decreased (Fig. 5a, b). Compared with CCRI49 and CZ91, the GhSOS1 expression level in the hypocotyls of Z571 and CCRI44 plants was considerably higher at 12–18 HAS (Fig. 5a). In contrast, the GhSOS1 expression levels in leaves of CZ91 were significantly up-regulated at 12 HAS, and less pronounced effects were observed in leaves of CCRI44 (Fig. 5b). Similar patterns were also observed for GhNHX1 expression levels under salinity (Fig. 5c, d). However, GhNHX1 expression levels in hypocotyls of CCRI44 were significantly higher after exposure to salinity, while the expression level in leaves was lower than that in other cultivars. Irrespective of the treatment effect, Z571 exhibited higher GhNHX1 expression levels at 12–18 HAS in hypocotyls and at 12 HAS in leaves. GhNHX1 expression in leaves of CZ91 was also significantly up-regulated at 9 HAS. Salinity decreased the GhAKT1 expression level in CCRI49, especially the expression level in leaves (Fig. 5e, f). Unlike the GhAKT1 expression pattern in CCRI49, those of Z571, CZ91, and CCRI44 decreased shortly after treatment (3–6 h) and then increased gradually after exposure to salinity, followed by a gradual decrease. Among cultivars, the expression level of GhAKT1 was higher in CCRI44 at 18–24 HAS in hypocotyls and at 12 HAS in leaves. GhAKT1 expression in leaves of Z571 was also significantly up-regulated at 12–18 HAS.
Discussion
Like other cultivated plant species, cotton is relatively tolerant to salinity and possesses a certain degree of variability in salinity tolerance at species level (Dai et al. 2014; Li et al. 2013; Wang et al. 2016). In the present study, distinct differences in salt tolerance among the four cultivars were found. The physiological function assays (Fig. 2) suggested that Z571 was the most sensitive to salinity, exhibiting elevated damage characterized by higher MDA accumulation and REC (Fig. 3). The descending order of salt tolerance among cultivars was CZ91, CCRI44, CCRI49, and last, Z571. To overcome salt-induced limitations, salt-tolerant plants have employed special adaptive mechanisms to cope with salt toxicity, i.e., enhanced Na+ exclusion from shoot tissue, Na+ tissue tolerance, and K+ retention in leaves (Shabala et al. 2015; Wu et al. 2015).
Under salinity, Na+ exerts toxic effects on physiological metabolism and plant growth. Thus, maintaining a low Na+ content is a key factor for plant adaptation to high salinity conditions (Munns and Tester 2008; Kong et al. 2016a). In our study, the four cotton cultivars showed a significant increase in Na+ content under salinity, which is a common response to ionic imbalance in plants under salinity (Chen et al. 2008; Pitann et al. 2013). However, the salt-susceptible cultivar Z571 had the highest Na+ concentration in its tissues under stress, while less pronounced effects of salinity were observed in cultivar CZ91. These results indicated that Z571 exhibited poor regulation of ion transport, which was supported by increased Na+ efflux in hypocotyls with decreased Na+ efflux in leaves under salinity (Fig. 4a, b). These findings are in accordance with previous results showing that salt-tolerant plants often maintain lower Na+ contents by regulating Na+ ion homeostasis (Dai et al. 2014; Wang et al. 2016).
SOS1, an Na+/H+ antiporter, is not only crucial for Na+ exclusion, but also to be involved in long-distance Na+ transport from roots to shoots under salt stress (Ma et al. 2014; Munns and Tester 2008; Shi et al. 2002). Kong et al. (2016b) found that the enhancement of salt tolerance by foliar sodium nitroprusside (SNP) application to cotton can be ascribed to the altered Na+ levels via feedback regulation of GhSOS1 expression. In our study, the addition of NaCl significantly increased the expression of SOS1 (Fig. 5a, b), consistent with previous studies (Ma et al. 2014; Qiu et al. 2002). The GhSOS1 expression level in Z571 was higher in hypocotyls, but lower in leaves than the expression levels in other cultivars, suggesting that salinity induced more Na+ transport from roots to shoots, but less Na+ export from leaves to the phloem (Ma et al. 2014). However, salt-tolerant cultivars possessed a more efficient salt-sensing system that was able to translate changes in saline condition into both transcriptional (Fig. 5a, b) and functional changes (Fig. 4a, b). Thus, it appears that this salt-inducible ability to control GhSOS1 expression partly contributed to the differential salinity tolerance of the cultivars. It has to be noted that the moderately salt-tolerant cultivar CCRI44 also exhibited a higher induction of Na+ transport to its shoots, but lower Na+ export from the leaves (Fig. 4a, b). Thus, multiple adaptive strategies can confer salinity stress tolerance in cotton.
Previous reports on various species have suggested that NHX1 plays a key role in Na+ sequestration into vacuoles, thus minimizing cytoplasmic Na+ concentrations (Chen et al. 2008; Munns and Tester 2008; Pitann et al. 2013). The expression of NHX1 was remarkably induced by salinity (Kong et al. 2016b; Zhang et al. 2016), which is consistent with our findings (Fig. 5c, d). Therefore, the transcriptional activation of GhNHX1 can decrease Na+ accumulation in the cytoplasm via the enhancement of Na+ vacuolar sequestration capabilities. Several studies have also shown that the relative transcription of NHX1 was significantly higher in salt-tolerant genotypes than in salt-sensitive ones after high salinity implementation (Pitann et al. 2013; Saqib et al. 2005). However, there was no strong correlation between GhNHX1 expression levels and salt tolerance in our study and, thus, Na+ sequestration into vacuoles by GhNHX1 might play an important, but non-essential role in the underlying genetic variability of salinity tolerance in cotton.
In general, high Na+ levels can compete with K+ for uptake into cells, leading to a decrease in K+ content, thus impairing metabolic activity and often triggering programmed cell death (Munns and Tester 2008; Shabala et al. 2015). In the present study, salinity lowered K+ concentrations in shoots, but increased K+ concentrations in roots (Table 2), suggesting that salt inhibited K+ transport from roots to shoots. This result was confirmed by measuring K+ efflux of the hypocotyls and leaves under salt stress (Fig. 4c, d). Efficient maintenance of K+ homeostasis has always been considered as one of the most, if not the most prominent feature of halophytes (Munns and Tester 2008; Shabala et al. 2015). Our result is in close agreement with previous findings, as the K+ retention capacity in leaves was, in descending order, CZ91, CCRI44, CCRI49, and Z571, matching the descending order of salt tolerance (Table 2). These results suggested that maintaining K+ homeostasis to provide an optimal cytosolic K+/Na+ ratio could be an essential mechanism underlying salinity tolerance in cotton.
Some studies have indicated that AKT1 is a very likely key factor in controlling the selective absorption capacity of K+ over Na+ under salinity (Ardie et al. 2010; Boscari et al. 2009; Chen et al. 2013). AKT1 transcripts have been reported to be regulated by external Na+ concentrations; however, somewhat contradictory results have been reported (Ardie et al. 2010; Duan et al. 2015; Wang et al. 2015). These differences may be attributed to variation in plant species, tissues, and duration of exposure to salinity (Ardie et al. 2010; Munns and Tester 2008). In our study, GhAKT1 expression patterns were not similar in response to salinity (Fig. 5e, f). There was also a significant correlation between hypocotyl GhAKT1 expression levels (Fig. 5e) and K+ export flux values (Fig. 4c) under salt stress, indicating that GhAKT1 is very likely the key factor in maintaining the selective absorption capacity for K+ from roots to shoots under salinity. Interestingly, however, a significant negative correlation between leaf GhAKT1 expression levels (Fig. 5f) and K+ export flux values (Fig. 4d) was observed in response to saline treatment. This inconsistent result could be attributed to depolarized plasma membranes caused by the massive entry of external Na+, resulting in a K+ loss mediated by AKT1 (Chérel et al. 2014; Wu et al. 2015). However, the specific details of the mechanism underlying this pattern remain obscure and must be examined in follow-up studies.
Conclusion
Salt tolerance in cotton is conferred by at least three complementary physiological mechanisms: (1) Na+ transport from roots to shoots, (2) Na+ export from the leaves, and (3) K+ retention ability resulting from salt stress. These traits may be determined by the efficient transcriptional regulation of key transport genes, i.e., GhSOS1 and GhAKT1. The above mechanisms appear to be coordinated, thus enabling a more optimal K+/Na+ ratio, which is a key mechanism conferring salinity tolerance in cotton. Salinity tolerance in plants is a complex polygenic trait, involving other key genes and mechanisms, and these factors should be addressed in future experiments.
Author contribution statement
GY and QH conceived and designed the experiments. NW, HQ and WQ performed the experiments and analyzed the data. JS, QX and HZ contributed reagents and materials. NW, GY and QH wrote the article. All the authors read and approved the final manuscript.
References
Ardie SW, Liu S, Takano T (2010) Expression of the AKT1-type K+ channel gene from Puccinellia tenuiflora, PutAKT1, enhances salt tolerance in Arabidopsis. Plant Cell Rep 29(8):865–874
Banjara M, Zhu L, Shen G, Payton P, Zhang H (2012) Expression of an Arabidopsis sodium/proton antiporter gene (AtNHX1) in peanut to improve salt tolerance. Plant Biotechnol Rep 6:59–67
Boscari A, Clement M, Volkov V, Golldack D, Hybiak J, Miller AJ, Amtmann A, Fricke W (2009) Potassium channels in barley: cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant Cell Environ 32(12):1761–1777
Chen LH, Zhang B, Xu ZQ (2008) Salt tolerance conferred by overexpression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1 in common buckwheat (Fagopyrum esculentum). Transgenic Res 17:121–132
Chen J, Xiong DY, Wang WH, Hu WJ, Simon M, Xiao Q, Liu TW, Liu X, Zheng HL (2013) Nitric oxide mediates root K+/Na+ balance in a mangrove plant, Kandelia obovata, by enhancing the expression of AKT1-Type K+ channel and Na+/H+ antiporter under high salinity. PLoS One 8(8):1–11
Chérel I, Lefoulon C, Boeglin M, Sentenac H (2014) Molecular mechanisms involved in plant adaption to low K+ availability. J Exp Bot 65:833–848
Dai JL, Dong HZ (2014) Intensive cotton farming technologies in China: achievements, challenges and countermeasures. Field Crops Res 155:99–110
Dai JL, Duan LS, Dong HZ (2014) Improved nutrient uptake enhances cotton growth and salinity tolerance in saline media. J Plant Nutr 37:1269–1286
Duan HR, Ma Q, Zhang JL, Hu J, Bao AK, Wei L (2015) The inward-rectifying K+ channel SsAKT1 is a candidate involved in K+ uptake in the halophyte Suaeda salsa under saline condition. Plant Soil 395:173–187
Flowers TJ (2004) Improving crop salt tolerance. J Exp Bot 55:307–319
Hirsch RE, Lewis BD, Spalding EP, Sussman MR (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280(5365):918–921
Horie T, Karahara I, Katsuhara M (2012) Salinity tolerance mechanisms in glycophytes: an overview with the central focus on rice plants. Rice 5:11
Kong XQ, Luo Z, Dong HZ, Eneji AE, Li WJ (2012) Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J Exp Bot 63(5):2105–2116
Kong XQ, Luo Z, Dong HZ, Eneji AE, Li WJ (2016a) H2O2 and ABA signaling are responsible for the increased Na+ efflux and water uptake in Gossypium hirsutum L. roots in the non-saline side under non-uniform root zone salinity. J Exp Bot 67(8):2247–2261
Kong XQ, Wang T, Li WJ, Tang W, Zhang DM, Dong HZ (2016b) Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol Plant 38(3):61
Lebaudy A, Véry AA, Sentenac H (2007) K+ channel activity in plants: genes, regulations and functions. FEBS Lett 581(12):2357–2366
Lei B, Huang Y, Sun J, Xie JJ, Niu ML, Liu ZX, Fan ML, Bie ZL (2014) Scanning ion-selective electrode technique and X-ray microanalysis provide direct evidence of contrasting Na+ transport ability from root to shoot in salt-sensitive cucumber and salt-tolerant pumpkin under NaCl stress. Physiol Plantarum 152:738–748
Leidi EO, Saiz JF (1997) Is salinity tolerance related to Na+ accumulation in upland cotton (Gossypium hirsutum) seedlings? Plant Soil 190:67–75
Li MY, Li FJ, Yue YS, Tian XL, Li ZH, Duan LS (2013) NaCl-induced changes of ion fluxes in roots of transgenic Bacillus thuringiensis (Bt) cotton (Gossypium hirsutum L.). J Integr Agric 12:436–444
Lichtenthaler H (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Ma Q, Li YX, Yuan HJ, Hu J, Wei L, Bao AK, Zhang JL, Wang SM (2014) ZxSOS1 is essential for long-distance transport and spatial distribution of Na+ and K+ in the xerophyte Zygophyllum xanthoxylum. Plant Soil 374:661–676
Martinez-Cordero MA, Martinez V, Rubio F (2005) High-affinity K+ uptake in pepper plants. J Exp Bot 56(416):1553–1562
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nayyar H, Bainsb TS, Kumar S (2005) Chilling stressed chickpea seedlings: effect of cold acclimation, calcium and abscisic acid on cryoprotective solutes and oxidative damage. Environ Exp Bot 54:275–285
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotox Environ Saf 60:324–349
Parihar P, Singh S, Singh R, Singh VP, Prasad SM (2015) Effect of salinity stress on plants and its tolerance strategies: a review. Environ Sci Pollut Res 22:4056–4075
Pitann B, Mohamed A, Neubert AB, Schubert S (2013) Tonoplast Na+/H+ antiporters of newly developed maize (Zea mays L.) hybrids contribute to salt resistance during the second phase of salt stress. J Plant Nutr Soil Sci 176:148–156
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci 99:8436–8441
Saqib M, Zörb C, Rengel Z, Schubert S (2005) The expression of the endogenous vacuolar Na+/H+ antiporters in roots and shoots correlates positively with the salt resistance of wheat (Triticum aestivum L.). Plant Sci 169:959–965
Shabala S, Munns R (2012) Salinity stress: physiological constraints and adaptative mechanisms. In: Shabala S (ed) Plant stress physiology. CAB International, Oxford, pp 59–93
Shabala S, Bose J, Fuglsang AT, Pottosin I (2015) On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. J Exp Bot 67(4):1015–1031
Shi HZ, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Shi G, Cai Q, Liu C, Li Wu (2010) Silicon alleviates cadmium toxicity in peanut plants in relation to cadmium distribution and stimulation of antioxidative enzymes. Plant Growth Regul 61:45–52
Singh PK, Shahi SK, Singh AP (2015) Effects of salt stress on physico-chemical changes in maize (Zea mays L.) plants in response to salicylic acid. Indian J Plant Sci 4:69–77
Tester M, Davenport RJ (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Wang Y, Wu WH (2013) Potassium transport and signaling in higher plants. Annu Rev Plant Biol 64:451–476
Wang B, Lüttge U, Ratajczak R (2001) Effects of salt treatment and osmotic stress on V-ATPase and V-PPase in leaves of the halophyte Suaeda salsa. J Exp Bot 52:2355–2365
Wang P, Guo Q, Wang Q, Zhou XR, Wang SM (2015) PtAKT1 maintains selective absorption capacity for K+ over Na+ in halophyte Puccinellia tenuiflora under salt stress. Acta Physiol Plant 37(5):100
Wang N, Qi HK, Su GL, Yang J, Zhou H, Xu QH, Huang Q, Yan GT (2016) Genotypic variations in ion homeostasis, photochemical efficiency and antioxidant capacity adjustment to salinity in cotton (Gossypium hirsutum L.). Soil Sci Plant Nutr 62(3):240–246
Wu GQ, Xi JJ, Wang Q, Bao AK, Ma Q, Zhang JL, Wang SM (2011) The ZxNHX gene encoding tonoplast Na+/H+ antiporter from the xerophyte Zygophyllum xanthoxylum plays important roles in response to salt and drought. J Plant Physiol 168:758–767
Wu HH, Zhu M, Shabala L, Zhou MX, Shabala S (2015) K+ retention in leaf mesophyll, an overlooked component of salinity tolerance mechanism: a case study for barley. J Integr Plant Biol 57(2):171–185
Zhang J, Yu HY, Zhang YS, Wang YB, Li MY, Zhang JC, Duan LS, Zhang MC, Li ZH (2016) Increased abscisic acid levels in transgenic maize overexpressing AtLOS5 mediated root ion fluxes and leaf water status under salt stress. J Exp Bot 67(5):1339–1355
Acknowledgements
This work was supported by the Modern Agricultural Industry Technology System in Henan (No. S2013-07-1), the China Agricultural Research System (No. CARS-18-05) and Innovation Project of the Chinese Academy of Agricultural Sciences (No. CAAS-ASTIP-2016-CCRI).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by R. Aroca.
N. Wang and H. Qi have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, N., Qi, H., Qiao, W. et al. Cotton (Gossypium hirsutum L.) genotypes with contrasting K+/Na+ ion homeostasis: implications for salinity tolerance. Acta Physiol Plant 39, 77 (2017). https://doi.org/10.1007/s11738-017-2381-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2381-1