Abstract
Salinity is a major environmental stress that affects agricultural productivity worldwide. One approach to improving salt tolerance in crops is through high expression of the Arabidopsis gene AtNHX1, which encodes a vacuolar sodium/proton antiporter that sequesters excess sodium ion into the large intracellular vacuole. Sequestering cytosolic sodium into the vacuoles of plant cells leads to a low level of sodium in cytosol, which minimizes the sodium toxicity and injury to important enzymes in cytosol. In the meantime, the accumulation of sodium in vacuoles restores the correct osmolarity to the intracellular milieu, which favors water uptake by plant root cells and improves water retention in tissues under soils that are high in salt. To improve the yield and quality of peanut under high salt conditions, AtNHX1 was introduced into peanut plants through Agrobacterium-mediated transformation. The AtNHX1-expressing peanut plants displayed increased tolerance of salt at levels up to 150 mM NaCl. When compared to wild-type plants, AtNHX1-expressing peanut plants suffered less damage, produced more biomass, contained more chlorophyll, and maintained higher photosynthetic rates under salt conditions. These data indicate that AtNHX1 can be used to enhance salt tolerance in peanut.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity is a soil condition characterized by a high concentration of soluble salts. About 20% of the world’s cultivated land and nearly half of the irrigated lands are unsuitable for growing crops because of contamination with high levels of salt (Rhoades and Loveday 1990). High salinity in the soil makes it harder for plant roots to extract water, and a high concentration of salt within plant cells can be toxic to the cellular enzymes (Munns and Tester 2008). Most salt-affected land arises through natural causes, from the accumulation of salts over long periods of time in arid and semiarid zones (Rengasamy 2002). Sodium chloride is an abundant and soluble salt released in soil by the degradation of native saline rocks (Szabolcs 1989).
Peanut (Arachis hypogaea L.) is a glycophytic plant whose growth and yield are severely inhibited by high salinity. It is the twelfth most valuable cash crop in the United States, with a farm value of over one billion US dollars. Texas is ranked second among the peanut-growing states, and it accounts for approximately a quarter of all peanuts grown in the United States (http://www.peanutsusa.com). Salinity and drought are the two major abiotic stresses that limit agricultural productivity in the southwestern USA, including Texas. Increasing peanut tolerance of salt stress will likely improve its yield, which will have a major impact on the economy of Texas as well as peanut production areas in other arid and semi-arid regions of the world.
Salinity tolerance is a complex multigenic trait that may involve a large number of genes, and these genes are likely responsible for (1) encoding proteins to sequester Na+ into the vacuole or efflux Na+ to the apoplast, (2) synthesizing compatible solutes, and (3) radical-scavenging enzymes (Parida and Das 2005). Excess sodium ion (Na+) in cytosol may be excluded to the apoplastic space via the plasma membrane (Zhu 2003; Shi et al. 2002) or sequestered into the large intracellular vacuole via the tonoplast membrane (Apse et al. 1999; Blumwald 2000) by active mechanisms. Accumulation of ions in the vacuole acts as an osmoticum to maintain water flow into the cell (Glenn et al. 1999; Gaxiola et al. 2002). Apse et al. (1999) demonstrated that overexpressing a single Arabidopsis gene, AtNHX1, which encodes a vacuolar membrane-bound sodium/proton (Na+/H+) antiporter, could confer a high salt tolerance on transgenic plants. It was thought that the proton electrochemical gradient (PEG) across the tonoplast membrane is the driving force for Na+ to flow into vacuoles via the activity of the Na+/H+ antiporter, which is likely the molecular basis for the AtNHX1 overexpression that leads to increased salt tolerance in transgenic plants (Blumwald 2000). However, recent studies indicate that increased K+ homeostasis, cytosolic pH regulation, vesicle trafficking, and protein targeting are all likely to contribute to the increased salt tolerance in AtNHX1-overexpressing plants (Rodríguez-Rosales et al. 2009; Leidi et al. 2010). Transgenic plants that express AtNHX1, such as Arabidopsis (Apse et al. 1999), tomato (Zhang and Blumwald 2001), rapeseed (Zhang et al. 2001), cotton (He et al. 2005), tobacco (Duan et al. 2009), tall fescue (Tian et al. 2006), Petunia hybrida (Xu et al. 2009), and soybean (Li et al. 2010), all displayed increased salt tolerance. Since overexpression of AtNHX1 in native and heterologous systems leads to the same phenotype—increased salt tolerance, this approach should work in most plants, including peanut. Therefore, we expressed AtNHX1 in peanut in order to increase its salt tolerance. As we expected, AtNHX1-expressing peanut plants produced higher biomass in greenhouse conditions than wild-type plants under 150 mM NaCl treatment. Also, the physiological and biochemical parameters of transgenic peanut plants are better than wild-type control plants grown under greenhouse conditions, indicating that AtNHX1 can indeed increase salt tolerance in peanut.
Materials and methods
Peanut transformation
The recombinant vector 35S-AtNHX1 (He et al. 2007) was introduced into the Agrobacterium tumefaciens strain GV3101 and used to transform wild-type peanut (Flavor runner 458). The transformation protocol described by Sharma and Anjaiah (2000) was followed.
Isolation of genomic DNAs and polymerase chain reaction analysis
The Powerplant™ DNA isolation kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA) was used to isolate genomic DNA from fresh leaves of greenhouse-grown peanut plants. The concentration and purity of the genomic DNA were determined by a NanoDrop® (ND-1000 spectrophotometer, NanoDrop Technologies, Wilmington, DE, USA).
Polymerase chain reaction (PCR) was carried out with a thermocycler (Mastercycler Gradient, Eppendorf, Hamburg, Germany) to amplify a fragment of the AtNHX1 gene using EcnoTaq® DNA polymerase (Lucigen Corporation, Middleton, WI, USA). Primers NHX-1 and NHX-2 were designed based on the known sequences of AtNHX1. The 25 μl PCR reaction mixture contained 5 μl genomic DNA (0.1 μg/μl), 2.5 μl 10× EconoTaq reaction buffer [10 mM Tris–HCl (pH 9.0), 500 mM KCl, 15 mM MgCl2, and 1% Triton X-100], 2 μl dNTP (2.5 mM concentration for each of the four different deoxyribonucleotides), 1 μl forward primer NHX-1 (0.1 μg/μl), 1 μl reverse primer NHX-2 (0.1 μg/μl), 0.25 μl thermotolerant EconoTaq DNA polymerase enzyme, and 13.25 μl H2O. The amplification reactions were carried out under the following conditions: an initial denaturation at 94°C for 4 min, followed by 35 denaturation cycles at 94°C for 1 min, annealing at 52°C for 30 s, and extension at 72°C for 30 s, with a final extension at 72°C for 10 min. The amplified products were segregated by electrophoresis on 0.8% agarose gels with ethidium bromide and visualized under UV light. The nucleotide sequences of NHX-1 and NHX-2 are as follows: NHX-1, 5′-TGATTGGGCTAGGCACTG-3′; NHX-2, 5′-CAGCTTCGTGGTTTAGGTGA-3′.
Isolation of total RNAs and reverse-transcription PCR analysis
About 0.1 g of fresh leaf tissues from each peanut plants was ground into a fine powder using a mortar and pestle in liquid N2, then mixed with 1 ml of TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in a 1.5 ml microcentrifuge tube. After incubating the sample at room temperature for 5 min, 200 μl chloroform were added, followed by shaking and vortexing. The samples were again incubated at room temperature for 3 min, then the supernatant was transferred to a new tube and centrifuged at 3,800×g for 20 min at 4°C. An equal volume of isopropyl alcohol was mixed with the sample and placed at −20°C for 45 min, and then centrifuged for 20 min at 13,100×g and 4°C. The supernatant was discarded, and after washing the pellet with 1 ml of 75% ethanol, the sample was air dried. The pellet was dissolved in 90 μl of 0.5% sodium dodecyl sulfate (SDS), applying heat for 10 min at 55°C. The samples were then centrifuged at 1,200×g for 2 min at room temperature, and the supernatant was collected and transferred to a fresh tube. 1/10 of the sample volume of 3 M sodium acetate along with three times ice-cold 100% alcohol were then added. The sample was mixed by inversion and placed at −80°C for 2 h before being centrifuged at 13,100×g for 20 min at 4°C. The pellet was washed in 75% alcohol, air dried, and re-suspended in H2O. It was re-dissolved by heating at 55°C for 10 min, then centrifuged for 2 min at 1, 200×g, and the supernatant was placed in a new sterile microcentrifuge tube. The RNA concentration and purity were measured using the NanoDrop® (ND-1000 spectrophotometer).
The cDNAs were synthesized from 1.0 μg of DNase-treated total RNAs using Superscript reverse transcriptase (SuperScript®VILOTM cDNA synthesis kit, Invitrogen). The AtNHX1-specific primers, NHX-3 and NHX-4, were used for reverse transcription (RT)-PCR. Amplification conditions were: 3 min at 95°C initially, followed by 40 cycles of 1 min at 95°C, 45 s at 60°C, 45 s at 72°C. The amplified products were segregated by electrophoresis on 0.8% agarose gels and visualized under UV light. The actin gene was used as the internal standard (the oligonucleotides for the actin gene are Actin-F1 and Actin-R1). The sequences of the oligonucleotides used are shown below: NHX-3, 5′-CAGGGTTTCAAGTAAAAAAG-3′; NHX-4, 5′-CATACCAAACACCACTGTG-3′; Actin-F1, 5′-GAGGAGAAGCAGAAGCAAGTTG-3′; Actin-R1, 5′-AGACAGCATATCGGCACTCATC-3′.
Salt tolerance tests
Peanut seeds of both AtNHX1-expressing plants and wild-type plants were germinated and grown in a greenhouse for 21 days prior to the salt treatment, which was conducted in incremental manner, starting with 30 mM NaCl for 6 days, and then applying 60, 90, 120, and 150 mM for 6 days, respectively (Fig. 2). The temperature and humidity were maintained at 28 ± 2°C and 50 ± 10%, respectively, throughout the growth period in the greenhouse. The experiment was repeated twice.
Plant growth and biomass measurement under salt treatment in a greenhouse
The fresh aboveground and underground biomasses of transgenic and wild-type control plants were determined after the 150 mM NaCl treatment. Roots were analyzed after gently flushing the soil with water and drying it with a paper towel. Subsequently, the dry aboveground and underground biomasses were weighed after drying the shoots and roots for 15 days under light in the greenhouse.
Leaf chlorophyll measurement
The relative amount of chlorophyll was measured using a portable chlorophyll meter (SPAD-502PLUS, Konica Minolta, Japan), which calculates a numerical SPAD value upon measuring the absorbance of leaves in the red and near-infrared regions that is proportional to the amount of chlorophyll present in the leaves. At each evaluation, the content was measured at the center of each leaflet of every primary leaf, and the average was used for analysis.
Gas-exchange measurements
Photosynthesis, stomatal conductance, and transpiration were measured using a portable photosynthesis system (LI-COR 6400, Li-Cor, Inc., Lincoln, NE, USA) at an ambient CO2 concentration of 400 μmol mol−1, 60% relative humidity, 28°C, and a light intensity of 1,500 μmol m−2 s−1. The instrument was stabilized as per the manufacturer’s guidelines. Before taking any measurements, the steady state levels of the reference CO2 and the reference H2O were observed, and the infra-red gas analyzers (IRGAs) were matched manually. The mean of five measurements obtained for each sample was used for analysis.
AC i curve measurement and estimation of derived parameters
The same photosynthesis system (LI-COR 6400, Li-Cor, Inc.) was used to measure the response of the CO2 assimilation rate (A) to changes in the internal CO2 concentration (C i) in order to characterize the photosynthetic performance of AtNHX1-expressing and wild-type peanut plants. The AC i curves were plotted at a saturated photosynthetic photon flux density (PPFD) of 2,000 μmol m−2 s−1, and the reference and sample IRGAs were matched automatically before taking each measurement. The curves were initiated at 0 Pa CO2 and increased step-wise to 200 Pa CO2, as described in He et al. (2007). Photosyn Assistant software (ver. 1.1.2; Dundee Scientific, Dundee, UK) was used to estimate the photosynthetic parameters such as V cmax (the maximum rate of carboxylation by Rubisco) and J max (the light-saturated rate of maximum electron transport). Afterward, these parameters were used to estimate A sat (net photosynthesis at saturating PPFD) and A max (photosynthetic capacity at saturating PPFD and saturating atmospheric CO2), based on the description provided in van Gestel et al. (2005).
Statistical analysis
Microsoft® Office Excel 2007 was used for all statistical analysis, which was performed using Student’s t test, considering one-tailed, two-sample unequal variance. Significance levels of P < 0.05 and P < 0.01 (i.e., the null hypothesis is rejected at the 5% or 1% level, respectively) were used in hypothesis testing. P values were calculated from the comparison between wild-type plants and transgenic lines.
Results
Creation and molecular analysis of AtNHX1-expressing peanut plants
The 35S-AtNHX1 construct (He et al. 2007) was introduced into the genome of peanut through the Agrobacterium-mediated transformation (Sharma and Anjaiah 2000). Eighty independent transgenic lines were generated from tissue culture. To confirm the insertion of AtNHX1 into the genome of the putative transgenic peanut plants, genomic DNA was extracted from young leaves of 12 independent putative transgenic lines and analyzed using the PCR technique. Our data indicated that 11 independent transgenic lines contained the introduced AtNHX1 transgene in their genome (Fig. 1a). Subsequently, RT-PCR was performed to identify the transgene transcript in three selected transgenic plants. A PCR product was amplified from all three putative transgenic peanut plants, but not from the wild-type peanut (Fig. 1b), indicating that the AtNHX1 transgene was indeed expressed in these transgenic plants.
Molecular analysis of wild-type and AtNHX1-expressing peanut plants. a Analysis of putative AtNHX1-expressing peanut plants by PCR. Genomic DNAs from the wild-type plant and 12 putative transgenic lines were amplified by PCR using AtNHX1-specific primers. WT wild-type; 4–12, 14 and 15 eleven independent transgenic peanut lines harboring AtNHX1; 13, a peanut line deriving from tissue culture that does not contain AtNHX1. b RT-PCR analysis of wild-type and AtNHX1-expressing plants. WT wild-type; 4, 7, and 11 three independent transgenic lines expressing AtNHX1 transcripts. The actin gene was used as the internal control
AtNHX1-expressing peanut plants are more salt tolerant than wild-type plants
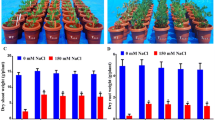
To test the salt tolerance of AtNHX1-expressing peanut plants, we selected transgenic lines 7 and 11 for detailed study under greenhouse conditions. The salt treatment was designed as follows: NaCl was added to peanut plants on day 22 at 30 mM (Fig. 2), then the concentration was increased incrementally every 6 days. When the salt concentration reached 150 mM NaCl on day 42, the phenotypic differences between the wild-type and AtNHX1-expressing peanut plants were noticeable. The AtNHX1-expressing peanut plants were bigger than the control wild-type plants (Fig. 3); also, there were large numbers of chlorotic leaves in wild-type peanut plants in comparison to AtNHX1-expressing transgenic plants (Fig. 3). On the other hand, when grown under normal conditions, there were no apparent phenotypic differences between the wild-type and AtNHX1-expressing peanut plants (data not shown).
To quantify the phenotypic differences between wild-type and AtNHX1-expressing lines grown under saline conditions, the fresh and dry biomasses of the aboveground and underground portions of the peanut plants were measured. The fresh shoot masses of AtNHX1-expressing peanut plants were higher than those of the wild-type plants, and the differences in the fresh root masses were even larger between the transgenic and control plants (Fig. 4a). In addition to the fresh masses, dry mass analysis also confirmed that the AtNHX1-expressing lines exhibited better shoot and root growth than the wild-type peanut plants (Fig. 4b).
Biomasses of wild-type and AtNHX1-expressing peanut plants after 150 mM NaCl treatment. a Fresh shoot weight and fresh root weight. b Dry shoot weight and dry root weight. WT wild-type; 7 and 11 two independent AtNHX1-expressing peanut lines. Values are mean ± SD (n = 4 plants). Double stars indicate statistical significance at P < 0.01
To measure the relative chlorophyll content in peanut leaves, we used a chlorophyll meter to analyze the SPAD values (Fig. 5). The SPAD reading indicates the plant nitrogen status, which is an indicator of healthy growth and development, protein synthesis, and potential good yields of plants (Evans 1983). Our data indicate that the leaves of NaCl-treated AtNHX1-expressing peanut plants have a higher nitrogen content and hence more chlorophyll than wild-type peanut plants.
AtNHX1-expressing peanut plants maintain higher photosynthetic rates than wild-type plants under salinity conditions
The photosynthetic performance of AtNHX1-expressing peanut plants was analyzed under normal and salt stress conditions. Net CO2 assimilation rate, stomatal conductance, and transpiration rate were examined using a LI-COR 6400 photosynthesis system. There were no significant differences in photosynthetic parameters between wild-type peanut plants and transgenic lines expressing AtNHX1 under normal conditions (data not shown), while AtNHX1-expressing peanut plants showed better photosynthetic rates (Fig. 6a), stomatal conductance (Fig. 6b), and transpiration rates (Fig. 6c) under salt conditions.
Gas-exchange performances of wild-type and AtNHX1-expressing peanut plants after 150 mM NaCl treatment. a Photosynthetic rate measurement. b Stomatal conductance measurement. c Transpiration rate measurement. WT wild-type; 7 and 11 two independent AtNHX1-expressing peanut lines. Values are mean ± SD (n = 4 plants). Double stars indicate statistical significance at P < 0.01
To study the effects of salinity on photosynthetic parameters, CO2 assimilation rates (A) with respect to internal CO2 concentrations (C i) were obtained from AtNHX1-expressing lines and wild-type plants grown under salt conditions (Table 1). To estimate the photosynthesis-limiting parameters V cmax (maximum rate of carboxylation by Rubisco) and J max (light-saturated rate of maximum electron transport), the data from the CO2 response curves were passed to the Photosyn Assistant software (ver. 1.1.2; Dundee Scientific). Next, photosynthesis on wild-type and transgenic plants was characterized by using the data derived from Photosyn Assistant software to estimate A sat (net photosynthesis at saturating PPFD) and A max (photosynthetic capacity at saturating PPFD and saturating atmospheric CO2). The transgenic peanut lines grown under 150 mM NaCl had significantly higher V cmax, J max, A sat, and A max values than wild-type plants (Fig. 7). The higher V cmax value for AtNHX1-expressing peanut plants indicates that under low CO2 conditions, the photosynthesis of the transgenic lines was limited to a lesser degree by the Rubisco activity than the wild-type plants. Similarly, the higher J max value indicates that at high C i, the photosynthesis of the transgenic lines was less limited by the rate of regeneration of the CO2 acceptor and ribulose bisphosphate compared to wild-type plants.
Estimation of photosynthetic parameters and characteristics for wild-type and AtNHX1-expressing peanut plants after 150 mM NaCl treatment. a Maximum carboxylation rate by Rubisco (V cmax). b Light-saturated rate of maximum electron transport (J max). c CO2-saturated photosynthesis (A sat). d Maximum potential photosynthesis (A max). WT wild-type; 7 and 11 two independent AtNHX1-expressing peanut lines. Values are mean ± SD (n = 3 plants). Double stars indicate statistical significance at P < 0.01; stars indicates statistical significance at P < 0.05
Discussion
Salinity is a major environmental stress that reduces crop yields, yet the increasing population and warming environment are increasing demands for improvements in agricultural productivity, which makes it necessary to generate salt-tolerant crops that can produce the increasing amounts of food required. Along with traditional breeding strategies, genetic engineering can be employed to improve crop yield and quality. Advances in plant biotechnology have made it possible to identify genes that may be valuable for improving crops (Wang et al. 2003; Chinnusami et al. 2004). AtNHX1 is one of these genes, as it can be used to improve salt tolerance in crops (Zhang and Blumwald 2001; Zhang et al. 2001; He et al. 2005; Duan et al. 2009; Tian et al. 2006; Xu et al. 2009; Li et al. 2010).
In this study, we aimed to improve salt tolerance in peanut by introducing AtNHX1 into peanut through a Agrobacterium-mediated transformation (Sharma and Anjaiah 2000). Eighty independent putative transgenic lines were generated, and PCR analysis of 12 putative lines confirmed the incorporation of AtNHX1 into the genomes of 11 transgenic peanut lines (Fig. 1a), indicating a high transformation efficiency of this transformation protocol. RT-PCR data indicated that the AtNHX1 transcript was expressed in all three transgenic lines tested (Fig. 1b). Two of these three AtNHX1-expressing lines, 7 and 11, were selected for detailed physiological studies. When compared to wild-type controls, AtNHX1-expressing peanut plants displayed better growth under salt conditions. For example, they suffered less damage (Fig. 3) and produced more biomass (Fig. 4) after 150 mM NaCl treatment. The leaf area and number of leaves of AtNHX1-expressing peanut plants are larger than those of wild-type plants after salt treatment (data not shown). In addition to the increased photosynthetic surface area, transgenic lines had higher chlorophyll content than wild-type plants under salt conditions (Fig. 5). Furthermore, AtNHX1-expressing peanut plants maintained higher photosynthetic rates, higher stomatal conductances, and higher transpiration rates (Fig. 6).
While CO2 assimilation rate (A) was measured using internal CO2 concentration (C i) values, A values for AtNHX1-expressing peanut plants showed greater improvements than those of wild-type plants grown at 150 mM NaCl (Table 1). Carboxylation efficiency by Rubisco and electron transport rate are two photosynthetic characteristics that were enhanced for the AtNHX1-expressing plants at low and high CO2, respectively (Fig. 7a, b). Also, the transgenic peanut lines showed improved photosynthesis under saturated light and saturated CO2 conditions than wild-type plants under salt-stressed conditions (Fig. 7c, d). Therefore, we believe that the increased biomass produced by AtNHX1-expressing plants is likely due to the multiple effects of large photosynthetic surface area, better photosynthetic rate, and higher chlorophyll content. Overall, our data are consistent with other AtNHX1-overexpression studies in Arabidopsis, rapeseed, tomato, cotton, tobacco, tall fescue, Petunia hybrid, and soybean (Apse et al. 1999; Zhang and Blumwald 2001; Zhang et al. 2001; He et al. 2005; Duan et al. 2009; Tian et al. 2006; Xu et al. 2009; Li et al. 2010).
Since PEG is generated by vacuolar adenosine triphosphatase (V-ATPase) and H+-pyrophosphatase (Apse et al. 1999; Gaxiola et al. 2002), increased activities of these two enzymes should lead to increased salt tolerance. Indeed, increased expression of AVP1, which encodes an H+-pyrophosphatase on the vacuolar membrane, increased salt tolerance in transgenic Arabidopsis (Gaxiola et al. 2001). We also confirmed this by overexpressing AVP1 in cotton, and obtained increased salt tolerance in transgenic cotton plants (Pasapula et al. 2011). However, based on our observations, and others (Gaxiola et al. 2002), it appears that the increase in salt tolernace caused by AtNHX1 overexpression is between 150 and 200 mM NaCl. To further improve salt tolerance in transgenic plants, it may be necessary to overexpress both AVP1 and AtNHX1 (Gaxiola et al. 2002). We should test this idea to generate higher salt tolerance in peanut in the future.
References
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Blumwald E (2000) Salt transport and salt resistance in plants and other organisms. Curr Opin Cell Biol 12:431–434
Chinnusami V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signaling in plants. J Exp Bot 55:225–236
Duan XG, Song YJ, Yang AF, Zhang JR (2009) The transgenic pyramiding tobacco with betaine synthesis and heterologous expression of AtNHX1 is more tolerant to salt stress than either of the tobacco lines with betaine synthesis or AtNHX1. Physiol Plant 135:281–295
Evans JR (1983) Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol 72:297–302
Gaxiola RA, Li J, Undurraga S, Dang LM, Allen GJ, Alper SL, Fink GR (2001) Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc Natl Acad Sci USA 98:11444–11449
Gaxiola RA, Fink GR, Hirschi KD (2002) Genetic manipulation of vacuolar proton pumps and transporters. Plant Physiol 129:967–973
Glenn EP, Brown JJ, Blumwald E (1999) Salt tolerance and crop potential of halophytes. Crit Rev Plant Sci 18:227–256
He C, Yan J, Shen G, Fu L, Holaday AS, Auld D, Blumwald E, Zhang H (2005) Expression of an Arabidopsis vacuolar sodium/proton antiportor gene in cotton improves photosynthetic performance under salt conditions and increases fiber yield in the field. Plant Cell Physiol 46:1848–1854
He C, Shen G, Pasapula V, Venkataramani S, Qiu X, Kuppu S, Kornyeyev D, Holaday AS, Auld D, Blumwald E, Zhang H (2007) Ectopic expression of AtNHX1 in cotton (Gossypium hirsutum L.) increases proline content and enhances photosynthesis under salt stress conditions. J Cotton Sci 11:266–274
Leidi EO, Barragan V, Rubio L, El-Hamdaoui A, Ruiz MT, Cubero B, Fernandez JA, Bressan RA, Hasegawa PM, Quintero FJ, Pardo JM (2010) The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J 61:495–506
Li TX, Zhang Y, Liu H, Wu YT, Li WB, Zhang HX (2010) Stable expression of Arabidopsis vacuolar Na+/H+ antiporter gene AtNHX1, and salt tolerance in transgenic soybean for over six generations. Chin Sci Bull 55:1127–1134
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:645–681
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plant: a review. Ecotoxicol Environ Saf 60:324–349
Pasapula V, Shen G, Kuppu S, Paez-Valencia J, Mendoza M, Hou P, Chen J, Qin X, Zhu L, Zhang X, Auld D, Blumwald E, Zhang H, Gaxiola R, Payton P (2011) Expression of an Arabidopsis vacuolar H+-pyrophosphatase gene (AVP1) in cotton improves drought- and salt tolerance and increases fiber yield in the field conditions. Plant Biotechnol J 9:88–99
Rengasamy P (2002) Transient salinity and subsoil constraints to dry land farming on Australian sodic soils: an overview. Aust J Exp Agric 42:351–361
Rhoades JD, Loveday J (1990) Salinity in irrigated agriculture. In: Stewart BA, Nielsen DR (eds) Irrigation of agricultural crops (Agron Monogr 30). ASA/CSSA/SSSA, Madison, pp 1089–1142
Rodríguez-Rosales MP, Gálvez FJ, Huertas R, Aranda MN, Baghour M, Cagnac O, Venema K (2009) Plant NHX cation/proton antiporters. Plant Signal Behav 4:265–276
Sharma KK, Anjaiah V (2000) An efficient method for the production of transgenic plants of peanut (Arachis hypogaea L.) through Agrobacterium tumefaciens-mediated genetic transformation. Plant Sci 159:7–19
Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporters SOS1 controls long distance Na+ transporter in plants. Plant Cell 14:465–477
Szabolcs I (1989) Salt-affected soils. CRC, Boca Raton
Tian L, Huang C, Yu R, Liang R, Li Z, Zhang L, Wang Y, Zhang X, Wu Z (2006) Overexpression of AtNHX1 confers salt-tolerance of transgenic tall fescue. Afr J Biotechnol 5:1041–1044
van Gestel NC, Nesbit AD, Gordon EP, Green C, Pare PW, Thompson L, Peffley EB, Tissue DT (2005) Continuous light may induce photosynthetic down-regulation in onion—consequences for growth and biomass partitioning. Physiol Plant 125:235–246
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Xu K, Hong P, Luo L, Xia T (2009) Overexpression of AtNHX1, a vacuolar Na+/H+ antiporter from Arabidopsis thaliana, in Petunia hybrid enhances salt and drought tolerance. J Plant Biol 52:453–461
Zhang HX, Blumwald E (2001) Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nat Biotechnol 19:765–768
Zhang HX, Hodson JN, Williams JP, Blumwald E (2001) Engineering salt-tolerant Brassica plants: characterization of yield and seed oil quality in transgenic plants with increased vacuolar sodium accumulation. Proc Natl Acad Sci USA 98:12832–12836
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6:441–445
Acknowledgments
We thank Natasja van Gestel for help with using the LI-COR instrument and Dr. Megha N. Parajulee for providing the chlorophyll meter. We thank Yinfeng Zhu, Sundaram Kappu, Rongbin Hu, Qiang Gu, Hua Qin, Jian Chen, and Xiaoyun Qiu for help and guidance with tissue culture, molecular analysis, and physiological and biochemical studies. This project was supported by grants from the Texas Peanut Producers Board and the National Peanut Board.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banjara, M., Zhu, L., Shen, G. et al. Expression of an Arabidopsis sodium/proton antiporter gene (AtNHX1) in peanut to improve salt tolerance. Plant Biotechnol Rep 6, 59–67 (2012). https://doi.org/10.1007/s11816-011-0200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-011-0200-5