Abstract
Combined stress of salinity and heavy metal is a serious problem for crop production; however, physiological mechanisms of tolerance to such condition remain elusive in cotton. Here, we used two cotton genotypes differing in salt tolerance, to understand their response to salinity (NaCl) and cadmium (Cd) either alone or in combination (Cd + Na) via hydroponics. Results showed that salinity and/or Cd drastically reduced plant growth, chlorophyll content and photosynthesis, with greater effect observed in Zhongmian 41 (sensitive) than Zhong 9806 (tolerant). Although salinity and/or Cd induced malondialdehyde (MDA) accumulation in Zhongmian 41 at 5 and 10 days after treatment, MDA content remained unchanged in Zhong 9806, implying that Zhongmian 41 but not Zhong 9806 faced oxidative stress following exposure to salinity and/or Cd. Differential responses of antioxidant enzymes such as superoxide dismutase, guaiacol peroxidase, catalase and ascorbate peroxidase to Cd, NaCl and Cd + Na indicate genotype- and time course- dependent variations. In both genotypes, Cd content was decreased while Na concentration was increased under combined stress compared with Cd alone. Importantly, NaCl addition in Cd-containing medium caused remarkable reduction in Cd concentration, with the extent of reduction being also dependent on genotypes. The salt-tolerant genotypes had lower Na concentration than sensitive ones. Furthermore, obvious changes in leaf and root ultrastructure was observed under Cd, Na and Cd + Na stress, however Zhong 9806 was less affected compared with Zhongmian 41. These results may provide novel insight into the physiological mechanisms of Cd + Na stress tolerance in various cotton genotypes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil salinity, mainly as a result of high concentration of NaCl, is one of the most important abiotic stresses limiting the distribution and productivity of major crops. Currently, more than 800 Mha of land are salt affected, and the area is still expanding (Munns 2005). As estimated by FAO (2000), every year 0.25–0.50 Mha of land worldwide is lost to agricultural production because of irrigation-induced secondary salinization. Cotton is an important fiber crop and classified as a salt tolerant crop. However, its growth and yield are severely inhibited in higher salinity soil, especially at germination and seedling stages (Ashraf 2002). Meanwhile, as a salt-tolerant crop that could grow in sandy and saline soils, cotton production in China has moved to the marginal saline soils including areas already affected by salinity (e.g. in river valleys and estuaries, coastal zones, etc.), because the traditional cotton-cultivating areas are also the most important food producing areas (Dong et al. 2012). Thus, the development and improvement of cotton production in salt-affected soils are of great significance to sustained cotton production without sacrificing grain production for food security.
In addition to salinity, soils are frequently loaded with potentially toxic heavy elements such as Cd due to increasing anthropogenic activities. Cadmium is one of the most toxic pollutants, due to its high solubility and its carcinogenic, mutagenic, and teratogenic effects in numerous animal species (Barazani et al. 2004). In China, at least 13,330 ha of farmland were contaminated by Cd (Zhang and Huang 2000). Individually, salinity or Cd stress has been the subject of intense research in different plants and well documented in various academic journals (Weggler et al. 2004; Wu et al. 2003). However, the saline depressions of river valleys and estuaries is often constitute sites of accumulation of industrial effluents contaminated by heavy metals due to increasing anthropogenic activities in recent decades (Gabrijel et al. 2009). As a result, soil salinity significantly influenced Cd solubility and thus bioavailability and phytoaccumulation (Weggler et al. 2004). Interestingly, in a survey of durum wheat fields varying in soil pH and salinity, grain Cd concentration of durum wheat was positively correlated with soil salinity (Norvell et al. 2000). Contradictory, Bingham et al. (1984) reported that application of Cl reduced Cd adsorption to soil constituents, and this effect was ascribed to the formation of soluble Cd–Cl complexes. It was also observed that Cd2+ activity decreased with increased Cl level in nutrient solution by forming CdCl 2−nn species, and also reduced Cd uptake by Swiss chard plants. However, there does not appear to be any report on Cd uptake and translocation in cotton under saline conditions. Therefore, a focus on physiological and metabolic aspects of stress combination of salinity and Cd is considerable ecological significance to facilitate the development of cotton cultivars with enhanced tolerance to stress conditions. Meanwhile, it is interesting to examine the possible use of phytoremediation for the reclamation of heavy metal polluted lands affected by salinity using salt-tolerant non-edible plants such as cotton. Therefore, the present study reports, the genotypic difference in antioxidative metabolism, ultrastructure and photosynthetic performance, and nutrient concentration in response to combined stress of salinity and Cd using two cotton genotypes varying in salt tolerance.
Materials and methods
Plant material and growth condition
Greenhouse hydroponic experiments were conducted on Zijingang Campus of Zhejiang University, Hangzhou, China, using two cotton genotypes of Zhong 9806 and Zhongmian 41 being tolerant and sensitive to salt stress (Zhou et al. 2011; Lin et al. 2006), respectively. Seeds of individual genotypes were surface sterilized in H2SO4, rinsed with distilled water, then germinated in moist sand. When seedling grew into two leaves stage, the uniform healthy plants were selected and transplanted to 5 L containers filled up 4.5 basal nutrient solution (BNS) for initial growth. The composition of BNS was as described by Wu et al. (2004). The container was covered with polystyrol plate with 5 evenly spaced holes and 1 plant per hole. On the 7 days after transplanting, Cd as CdCl2 and Na as NaCl were added to the corresponding containers to form 4 treatments: (1) basic nutrition solution (BNS, Control), (2) BNS + 5 µM Cd (Cd), (3) BNS + 150 mM Na, (4) BNS + 5 µM Cd + 150 mM Na (Cd + Na). The experiment was arranged in a split plot design with treatment as the main plot and genotype as the sub plot, and there were five replicates for each treatment. The solution pH was adjusted to 6.5 ± 0.1 with NaOH or HCl, as required. The nutrient solution in the growth container was continuously aerated with pumps and renewed every 5 days.
Growth measurement and metal analysis
At 20 days after treatment, plants were harvested and separated to roots, stems and leaves. The roots were soaked with 20 mM Na2EDTA for 3 h to eliminate the ions on the surface and then wash thoroughly with deionized water. Plant height, root length and fresh weight were measured, and then dry at 80 °C for 72 h and weighed. The dried shoots and roots were powdered and weighted, then ashed at 550 °C for 12 h. The ash was digested with 5 ml 30 % HNO3, and then diluted with deionized water. Metal concentrations of Cd, Na Zn, Fe, Mn, Cu, Ca and Mg were quantified by a flame atomic absorption spectrometry (SHIMADZUAA-6300, Kyoto, Japan).
Measurement of lipid per oxidation and antioxidant enzyme activities
Fresh fully expanded functional leaves (the 3rd or 4th up-most leaves) were sampled at 5, 10, 15 days after treatment, then homogenized in 8 ml of 50 mM phosphate buffer (PBS, pH 7.8) using a pre-chilled mortar and pestle. The homogenates were then centrifuged at 10,000×g for 15 min at 4 °C, and the supernatants were used in an assay to determine the malondialdehyde (MDA) content and activities of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6) and peroxidase (POD, EC 1.11.1.7) according to Wu et al. (2003), and ascorbate peroxidase (APX, EC 1.11.1.11) according to Chen et al. (2010).
Measurement of chlorophyll content, chlorophyll fluorescence and photosynthesis parameters
The chlorophyll content of leaves was determined according to acetone/ethanol mixture method of Chen (1984). Photosynthesis and chlorophyll fluorescence parameters were performed with intact fully expanded functional leaves (the 3rd or 4th up-most leaves). Measurement of net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr) and intercellular CO2 concentration (Ci) were conducted with a portable photosynthesis system LI-6400 (Li-COR, Lincoln, NE, USA). Chlorophyll fluorescence parameters were performed using pulse-modulated chlorophyll fluorometer and ImagingWin software application (IMAGING-PAM, Walz; Effeltrich, Germany). After 20 min dark adaption, leaves were illuminated under a high saturating light pulse with frequency of 0.05 Hz for 260 s. The initial fluorescence (Fo) was determined using a measuring beam (<0.05 μmol m−2 s−1 PAR). The maximal fluorescence (Fm) was determined using saturating pulse (2500 μmol m−2 s−1 PAR). Variable fluorescence (Fv) was calculated from the formula: Fv = Fm − Fo. Maximal photochemical efficiency of PS II (Fv/Fm) was calculated according to Genty et al. (1989) by ImagingWin software automatically. False-color images of Fv/Fm were recorded, stored, and compared with ImagingWin.
Examination of leaf and root ultrastrcture
Leaf/root ultrastructure was performed on the plants after 20 days treatment. Fresh roots (3 mm in length, 2–3 mm behind the apex) and leaves (1 mm2 top middle section of the third fully expanded leaf) were hand-sectioned and fixed for 6–8 h in 100 mM PBS buffer (pH 7.0) containing 2.5 % glutaraldehyde (v/v) and washed three times with the same PBS. Samples were post-fixed in 1 % osmium tetroxide (OsO4) for 1 h and washed in PBS for 1 h, then dehydrated in a graded ethanol series (50, 60, 70, 80, 90, 95, and 100 %) with 15–20 min interval and followed by acetone (100 %) for 20 min and then infiltrated and embedded in Spurr’s resin overnight. Finally, the specimen sections were stained by uranyl acetate and alkaline lead citrate for 15 min, respectively, and ultra-thin sections (80 nm) were prepared and mounted on copper grids and viewed under a transmission electron microscope (JEOL JEM-1230 EX, Japan).
Statistic analysis
All data presented were mean values of each treatment with five replicates. Statistical analysis was performed with the data processing system (DPS) statistical software package (Tang and Feng 1997) using ANOVA followed by the Duncan’s Multiple Range Test (DMRT) to evaluate treatment effects (P < 0.05). Origin Pro version 8.0 (Origin lab corporation, Wellesley Hills, Wellesley, MA, USA) was used to prepare graphs.
Results
Effect of alone or combined stresses of Cd and salinity on growth parameters
The effect of Cd and salinity on plant growth was evaluated by measuring plant height, root length, fresh and dry weight of root, stem and leaf. Cotton plants treated with alone or combined stress of salinity (Na) and Cd showed a significant decrease in plant height, root length, shoot and root dry/fresh weights in the order of Cd > Cd + Na > Na, and with a much severe response in Importantly salt-sensitive genotype Zhongmian 41 (Fig. 1A–H; Supplemental Table S1). On average of the above mentioned 8 growth parameters, plants under Cd, Cd + Na and Na treatments decreased by 56.01, 44.70 and 32.36 % in Zhongmian 41; 43.48, 32.68 and 20.84 % in Zhong 9806, respectively, compared with those of controls (Fig. 1A–H).
Effect of alone and combined stresses of salinity and Cd on growth parameters in the two cotton genotypes after 20 days treatment expressed as the percentage of control (%). Error bars represent SD values, different letters indicate significant differences at (P < 0.05) among the 4 treatments and within the two genotypes of data. Control, Cd, Na, and Cd + Na corresponds to basic nutrition solution (BNS), BNS + 5 µM CdCl2, BNS + 150 mM NaCl, and BNS + 5 µM CdCl2 + 150 mM NaCl, respectively
Photosynthesis parameters
Cotton plants treated with alone or combined stress of salinity and Cd showed a significant decrease in net photosynthetic rate (Pn), stomatal conductance (Gs), transpiration rate (Tr) and intercellular CO2 concentration (Ci), with a larger reduction in Zhongmian 41 over Zhong 9806 (Fig. 2A–D; Table S2.). Meanwhile, addition of NaCl to the solution containing Cd significantly increased the Pn relative to Cd alone treatment. For example, salinity and Cd alone and Cd + Na induced a significant reduction in Pn by 78.91, 51.16 and 60.3 % in Zhongmian; 46.44, 23.07 and 26.36 % in Zhong 9806, respectively (Fig. 2A). In addition, on average of Gs, Ci and Tr, revealed a decrease by 54.21, 32.04 and 44.41 % in Zhongmian; 40.84, 19.62 and 28.89 % in Zhong 9806 under Cd, salinity and Cd + Na, respectively, when compared with their controls (Fig. 2B–D).
Effect of alone and combined stresses of salinity and Cd on photosynthetic parameters in the two cotton genotypes after 20 days treatment expressed as the percentage of control (%). Error bars represent SD values, different letters indicate significant differences (P < 0.05) among the 4 treatments and within the two genotypes of data. Control, Cd, Na, and Cd + Na corresponds to basic nutrition solution (BNS), BNS + 5 µMCdCl2, BNS + 150 mM NaCl, and BNS + 5 µMCdCl2 + 150 mM NaCl, respectively
Chlorophyll content and fluorescence parameters of PSII
Chlorophyll content was significantly decreased in both genotypes under Cd, salinity and Cd + Na (Fig. 3A–D, Table S2), with Zhong 9806 being less affected than Zhongmian 41. Chlorophyll a + b was decreased in Zhongmian 41 under Cd, salinity and Cd + Na by 91.86, 46.59 and 81.97 % respectively, compared to control, however, in Zhong 9806 it was decreased by 55.47, 17.51 and 52.91 % (Fig. 3C). Chlorophyll a/b decreased in Zhongmian 41 under stress of Cd, Na and Cd + Na by 7.91, 42.58 and 61.04 %, respectively, compared to control, while in Zhong 9806 it was increased under Cd and Cd + Na by 53.55 % and 40.46 %, respectively (Fig. 3D).
Effect of alone and combined stresses of salinity and Cd on chlorophyll content in the two cotton genotypes after 20 days treatment expressed as the percentage of control (%). Error bars represent SD values, different letters indicate significant differences (P < 0.05) among the 4 treatments and within the two genotypes of data. Control, Cd, Na, and Cd + Na corresponds to basic nutrition solution (BNS), BNS + 5 µM CdCl2, BNS + 150 mM NaCl, and BNS + 5 µM CdCl2 + 150 mM NaCl, respectively
As shown in Fig. 4A and Supplemental Table S2, Cd, Cd + Na stress caused a significant inhibition in Fv/Fm for both genotypes relative to control. However, it remained unaffected under salinity alone stress in Zhong 9806. Interestingly, NaCl addition in the solution containing Cd levels significantly increased Fv/Fm in both genotypes as compared with Cd alone. The false color image application was useful to understand changes of Fv/Fm induced by different treatments. In comparison with the control, the leaf color shifted from blue to green along with reducing Fv/Fm ratio (Fig. 4B). Images also revealed that Cd alone stress induced the most severe damage, and followed by Cd + Na. While, under salinity alone leaf color was almost blue in Zhong 9806 than in Zhongmian 41.
Effect of alone and combined stresses of salinity and Cd on chlorophyll fluorescence yield as the percentage of control (Fv/Fm; A) and false color images of Fv/Fm (B) of the two cotton genotypes after 20 days treatment expressed. Error bars represent SD values, different letters indicate significant differences (P < 0.05) among the 4 treatments and within the two genotypes of data. Control, Cd, Na, and Cd + Na corresponds to basic nutrition solution (BNS), BNS + 5 µMCdCl2, BNS + 150 mM NaCl, and BNS + 5 µMCdCl2 + 150 mMNaCl, respectively, (B) false color images of Zhongmian 41 (left) and Zhong 9806 (right) treatment from up to bottom control, Cd, Na, Cd + Na, respectively. (Color figure online)
MDA contents and antioxidant enzymes activities
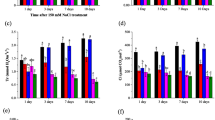
Changes of antioxidant enzyme activities and MDA content in leaves of the two genotypes under Cd and Na alone or Cd + Na are shown in Fig. 5A–H and Table S3. SOD activity showed genotype- and time- dependent variation in response to Cd, Na and Cd + Na (Fig. 5A, B and Supplemental Table S3). The expression patterns of the two genotypes were strikingly different. During Cd and Na alone stress SOD activities of the tolerant Zhong 9806 dropped after 5–10 day, whereas the addition of NaCl in the solution of Cd considerably increased SOD activities in leaves of Zhong 9806. Meanwhile, it increased dramatically in Cd and remained similar with control in salinity after 15 days; while Cd + Na induced significant increase especially after 10–15 days (Fig. 5B). Zhongmian 41 showed significant increase in SOD activity in leaves under the all stress treatment in comparison with the control up to 10 days, whereas the difference was not significant for 15 days except in salinity (Fig. 5A).
Effect of alone and combined stresses of salinity and Cd on SOD (A, B), POD (C, D), CAT (E, F), and APX (G, H) activities in leaves of Zhongmian 41 (left panel) and Zhong 9806 (right panel) at 5, 10 and 15 days after treatment expressed as the percentage of control (%). Error bars represent SD values, different letters indicate significant differences at (P < 0.05) among the 4 treatments and refer to each sampling data. Control, Cd, Na, and Cd + Na corresponds to basic nutrition solution (BNS), BNS + 5 µM CdCl2 BNS + 150 mM NaCl, and BNS + 5 µM CdCl2 + 150 mM NaCl, respectively
The POD activity was markedly increased under Cd, Na and Cd + Na stress in both genotypes (Fig. 5C, D), except for Na stress alone at 15 day in Zhongmian 41 which was no difference relative to control. The CAT activity was significantly increased in both genotypes after 10 and 15 days exposure to Cd, Na and Cd + Na (Fig. 5E, F). However, at day 5 it was decreased under Cd, interestingly NaCl addition in Cd solution (Cd + Na) dramatically increased CAT activity in leaves for both genotypes and in Na alone in Zhongmian 41.
The APX activity differed significantly between the two genotypes. Zhongmian 41 showed lower leaf APX activity in the treatments of Cd stress, relative to the control at day 5 and 10. In addition of NaCl in the solution of Cd significantly increased the APX activity at day 10. However, at day 15 the enzyme activity was increased in all stress treatment as compare with control (Fig. 5G). By contrast, Zhong 9806 showed higher leaf APX activity in the treatment of Cd at day 10 and 15, and no obvious change at day 5, as compared with the control. Interestingly, NaCl addition in the solution containing Cd levels increased APX activity in leaves as compared with the treatment with Cd alone till 15 days with sharply increase at day 10 (Fig. 5H).
The MDA content was measured as an index of lipid peroxidation. The responses of MDA content to Cd stress and Na treatment varied with genotypes (Table S3). On average of 3 sampling data, MDA content in Zhongmian 41 increased by 22.17, 27.33 and 31.84 % under Cd, Na and Cd + Na, respectively, compared with control. Zhongmian 41 had higher leaf MDA contents than Zhong 9806 under Cd, Na and Cd + Na stresses, except at day 15 on average of 5 and 10 days of Cd, Na and Cd + Na stress Zhong 9806 showed lower MDA content by 38.72, 37.04 and 50.74 %, respectively, than Zhongmian 41.
Ultrastructure of chloroplasts and root tip-cells
Under the control conditions, chloroplasts of the two cotton genotypes maintained normal thylakoids arrangement in grana and in membranes interconnecting grana (Fig. 6A, B). Exposure of 5 µM Cd for 20 days resulted in swollen grana/stroma lamellae and loose thylakoid membranes; the number and size of grana stacking per chloroplast decreased significantly compared with control. Furthermore, increased osmiophilic plastoglobuli and cracked chloroplast membrane were observed. The chloroplast ultrastructural deterioration due to Cd stress was more severe in Zhongmian 41 than in Zhong 9806, and starch grain was observed in Zhong 9806 with less osmiophilic plastoglobuli under Cd stress (Fig. 6C, D). Na stress alone, affected ultrastructures of leaf mesophyll cells in Zhongmian 41; disorganized parallel arrangement of lamellae and led to swollen grana/stroma lamellae and loose thylakoid membranes, induced starch grains, increased the number of osmiophilic plastolobulis. While in Zhong 9806 most thylakoids still remained in normal structures though some thylakoids were swelled under Na stress relative to control. Under combined stress (Cd + Na), loose thylakoid membranes, and swollen thylakoids and osmiophilic granule increased significantly in the two genotypes compared with control.
Transmission electron micrograph of chloroplasts of Zhongmian 41 (left panel) and Zhong 9806 (right panel) cultured in basic nutrition (BNS A, B), BNS + 5 µM CdCl2 (C, D), BNS + 150 mM NaCl (E, F), and BNS +5 µM CdCl2 + 150 mM NaCl (G, H). CW cell wall, GL granum lamellae, Os osmiophilic plastolobuli, SG starch grain, SL stroma lamellae. Bar 0.2 µm
The electron micrographs of root tip cells from Cd stressed cotton seedlings revealed obvious ultrastructural changes, characterized by cracked karyotheca and increased vacuolar size and number (Fig. 7C, D). Cracked karyotheca and dissolved nucleolus due to Cd was more severe in Zhongmian 41 than in Zhong 9806. Under Na and Cd + Na stresses affected distortion of cells and nucleus and plasmolysis, characterized by cracked karyotheca and the distortion of cells were observed more in Zhongmian 41 than in Zhong 9806. Meanwhile, under Cd + Na increased vacuole number was more obvious in Zhongmian 41 than in Zhong 9806.
Cd, Na and others mineral element concentrations
Cd concentration in roots, stems and leaves increased markedly when plants were exposed to Cd-containing medium; however, there was no detection of Cd in control plants and under salinity stress. (Table 1). Moreover, Cd + Na treatment had markedly lower Cd concentration than Cd alone treatment, in particular for stems and leaves Cd concentration of Zhong 9806. i.e., Cd concentrations in roots, stems, leaves under Cd + Na treatment was 84.08, 96.99, 94.62 % in Zhongmian 41, and 91.62, 72.04, 83.17 % in Zhong 9806 lower than that in Cd alone stress.
Salinity and Cd + Na stress caused significant increase in Na concentration in both genotypes; however, Zhong 9806 was less affected Cd + Na (Table 1), while under Cd stress there were no significant differences compared with control in the two genotypes. For instance, Na concentration was increased in roots under Na and Cd + Na stress in Zhongmian 41 (increased by 1237.88 and 207.8 %, respectively) and in Zhong 9806 (increased by 531.07 and 57.25 %, respectively) relative to control. Moreover, Na concentration was significantly lower under Cd + Na as relative to salinity (Na) alone. i.e., Na concentrations in roots, stems, leaves in Cd + Na treatment was 76.86, 23.16, 20.06 % in Zhongmian 41, and 75.02, 11.66, 24.38 % in Zhong 9806 lower than that of salinity (Na) alone stress.
Ca concentration was significantly decreased under alone or combined stress of Cd and salinity in both genotypes. In root, Ca concentration was decreased under Cd, Na and Cd + Na in Zhongmian 41 (decreased by 41.07, 52.79 and 35.47 % and in Zhong 9806 (decreased by 34.7, 26.49 and 29.84 %, respectively) compared to control. However, in stem, it was decreased by 45.03, 38.35 and 32.27 % in Zhongmian 41 under Cd, salinity and Cd + Na, whereas in Zhong 9806 by 36.02 and 29.86 % under stress of Cd and Cd + Na, while under stress of salinity there were no significant differences compared to control. Moreover, in leaf, Ca concentration was decreased under stress of Cd, salinity and Cd + Na by 51.81, 34.79 and 37.65 % in Zhongmian 41, respectively, compared to control. However, in Zhong 9806 Ca concentration was decreased by 28.74 and 17.81 % under Cd and Cd + Na, while under salinity stress there were no significant differences compared to control. On the whole, salt tolerant genotype accumulated more Ca in roots, stems and leaves than sensitive genotype when exposed to Na and Cd + Na stresses.
Both salinity alone and Cd + Na stresses led to significant decrease in root Mg concentrations for both cotton genotypes (Table 1), however, the reduction was more in Zhongmian 41. Moreover, more reduction occurred in Cd stress alone than NaCl + Cd stress. The salt tolerant genotypes had more Mg concentrations in leaves than the sensitive ones under NaCl and NaCl + Cd stresses.
Cu concentration was significantly decreased in Zhongmian 41 under alone or combined stress of Cd and salinity; however, in Zhong 9806, it was increased compared to control. Moreover, NaCl addition in the Cd-containing medium led to significant increase of Cu concentration in both stems and leaves except for the root Cu concentration in Zhong 9806 compared to Cd stress alone.
Root Fe concentration in Zhongmian 41 was decreased under Cd, Cd + Na stresses and increased under NaCl stress, compared with the control (Table 1). No significant difference was found in root Fe concentration between the combined stress and the control for Zhong 9806; however, Cd stress decreased root Fe concentration than the control. In stem, Fe concentration was increased under stress of Cd and Cd + Na by 50.08 and 13.84 %, respectively, compared to control in Zhongmian 41, while under stress of salinity, it was decreased by 19.7 %. However, in Zhong 9806, it was decreased under stress of Cd, salinity and Cd + Na by 31.74, 1.05 and 35.81 %, respectively, compared to control. Fe concentration increased in leaves under stress of Cd, salinity and Cd + Na by 20.34, 51.85 and 23.22 % in Zhongmian 41, respectively, however it was decreased under stress of Cd and salinity by 3.04 and 8.24 % in Zhong 9806, respectively, while under combined stress of Cd and salinity, it was increased by 11.75 %.
Mn concentration was significantly decreased in the root of Zhongmian 41 under alone or combined stress of Cd and salinity; however, in Zhong 9806, there were no significant differences compared to control. In stem, Mn concentration was decreased in both genotypes, however, under salinity it was increased in Zhong 9806, while in Zhongmian 41 it was decreased. In leaf, Mn concentration was decreased under stress of Cd, salinity and Cd + Na in both genotypes. On the whole, salt tolerant genotypes had higher in all tissue Mn concentration than sensitive ones when exposed to Cd, salinity and Cd + Na stresses.
Zn concentration was decreased in root of both genotypes under stress of Cd and combined stress of Cd and salinity. Cd stress alone decreased root Zn concentration in all genotypes, with the effect being more severe than Cd + Na stress. However, under stress of salinity; it was increased in Zhong 9806, while in Zhongmian 41 it was decreased. In stem, Zn concentration was decreased in both genotypes under alone or combined stress of Cd and salinity. In leaf, there were no significant differences in zinc concentration under Cd, salinity and Cd + Na compared to control in Zhongmian 41. However, in Zhong 9806 it was increased.
Discussion
Both Cd and NaCl have obvious detrimental effects on plants, such as inhibition of root and shoot growth, nutrients uptake and photosynthesis. Typical salinity values for irrigation water for agriculture often reach up to 180 mM, especially under arid and semi-arid regions (Khoshgoftar et al. 2004), therefore, salinity in agricultural soils is an extensive and important problem. Reports available up to date provided the controversial results on the influence of salinity on Cd stress. It was reported that NaCl would enhance Cd uptake (huang et al. 2006). However, Lefevre et al. (2009) observed that salinity reduced Cd accumulation in Mediterranean halophyte species, and Ghnaya et al. (2007) proved that Cd-induced growth reduction in halophyte Sesuvium portulacastrum was significantly improved by NaCl. Therefore, the interaction between Cd and NaCl is quite complex, being largely affected by many factors, including plant species and growth conditions. Thus, it is interesting to view and understand the interaction between Cd and salt stress in the salt-tolerant cotton plants and its genotypic difference.
In the current study, growth parameters such as plant height and biomass (fresh/dry weight) were reduced in the two cotton genotypes when plants exposed to 5 μM Cd, indicating that Cd stress inhibits plant growth. However, growth inhibition was partly alleviated by NaCl addition (Cd + Na vs. Cd) (Fig. 1A–H; Supplemental Table S1). Furthermore, Cd concentrations in leaves, stems and roots reduced by addition of NaCl to Cd solutions (Cd + Na) compared with Cd alone treatment (Table 1). Indicating that, the plants might be complete for Na+ absorption to Cd2+ under NaCl stress. Generally, reduction in Cd concentration in roots and shoots should be responsible for elevated capacity of Cd tolerance in plants. On the other hand, in view of the results of the present research, there is evidently some potential planting salt-tolerant non-edible cotton plants for fibre, but not for the use of phytoremediation for the reclamation of Cd polluted lands affected by salinity.
Net photosynthesis and chlorophyll content were decreased by Cd, salinity alone and in combination, with less reduction in Zhong 9806 as compared with Zhongmian 41. NaCl and Cd caused reduction in photosynthesis through its adverse impact on gas exchange parameters such as stomatal conductance and photosynthetic rate. The stomatal closure and decrease in leaf conductance would inhibit the diffusion of CO2 to the site of carboxylation and thus reduce photosynthetic uptake. Cd and salt stress drastically reduced photosynthesis by reducing total chlorophyll content and stomatal conductance (Kang et al. 2007). These findings are in agreement with our result that the inhibition of photosynthesis by Cd and salt stress resulted from both of decreased chlorophyll content and stomatal conductance. It was reported that chlorophyll content decreased by salt stress in chickpea cultivars (Garg and Singla 2004), by Cd stress in Phyllanthus amarus and chlorophyll b was more sensitive than chlorophyll a (Rai et al. 2005). These previous reports are in agreement with our results. The ratio of Fv/Fm always used as stress indicator, describing the potential yield of the photochemical reaction. In our result Fv/Fm ratio was found to be decrease under of Cd, salinity alone and combined stress in Zhongmian 41; however, in Zhong 9806 there were no significant difference under salinity stress (Fig. 4A; Supplemental Table S2). In agreement to our results, a reduction in the Fv/Fm ratio has already been reported in rice grown under Cd stress (Pagliano et al. 2006). It was proved that lower Fv/Fm under salt stress conditions, indicating that RUBP regeneration, which needs adequate electron translocation from PSII to electron acceptor, might be disturbed by salinity.
The obvious expected biochemical response during stress is enhanced reactive oxygen species (ROS) production. Rapid increase of ROS result in severe cell biochemical changes during oxidative stress, including lipid peroxidation and damage to proteins and DNA, which may lead to cell death (Dietz 2005). SOD activity is enhanced when plants are subjected to environmental stress. However, the extent and duration of the enhancement varied with stress intensity, species, age of the plants and reduced stress periods (Piquery et al. 2000). In our experiment, SOD activity showed genotype- and time-dependent variation in response to Cd, Na and Cd + Na treatments (Fig. 5A, B; Supplemental Table S3). However, POD activity was markedly increased under Cd, Na alone and combination in both genotypes (Fig. 5C, D), except for salinity alone at 15 days which was no difference relative to control. The higher activity of SOD and POD in the plants subjected to harsh environments is favorable for their tolerance and survival. This finding is in consistent with our result. Salinity induced significantly increased in CAT activity in both genotypes under Cd, salinity alone or in combination after 10 and 15 days. However, after 5 days it was decreased under stress of Cd, but increase under stress of salinity alone and Cd + Na. The variation in CAT activity might be due to Cd stress may be partly attributed to possible variation among species, growth stage, stress intensity, and duration of exposure. Increases in CAT activity have been mentioned by Perez Lopez et al. (2009) after salt exposure. The increase of CAT activity has been reported in certain plant species exposed to heavy metals. These reports are in agreement with our results.
It was reported that APX plays a central role in plant defense against oxidative stress by scavenging H2O2 in chloroplast, ctyosol, mitochondria, and peroxisome of plant cells (Asada 2006). In the present study, the APX activity showed time and genotype dependent variation in response to Cd, salinity alone and Cd + Na. However, in the tolerant genotype Zhong 9806 increased significantly after 5 days salinty and Cd + Na treatments and kept similar in Cd alone with control, remained significantly higher than the control till 15 days (Fig. 5H). In Zhongmian 41, significant inhibition or no effect was detected till 10 days (increase in Na + Cd at day 10), followed by a significant increase at 15 days (Fig. 5G). In parallel to our results, Yu and Liu (2003) observed that APX activity was significantly increased in the leaves of salt tolerant cultivar of soybean plants under salt stress. Variation in APX activity in Zhongmian 41 may be due to the duration of treatment exposure. MDA as the decomposition product of polyunsaturated fatty acids of biomembranes, showed a greater accumulation under stress conditions (Yu and Liu 2003). In our result, Cd, salinity and Cd + Na increased MDA accumulation in Zhongmian 41, and being significantly higher than that of Zhong 9806 (Table S3).
The alterations observed in the ultrastructure of the chloroplasts in leaves of plants exposed to Cd might be due to an increase in the production of reactive oxygen species (ROS) which in high concentration in the cellular environment can cause oxidative damage to cellular structure and function (Choudhury and Panda 2005). In this study, the effect of Cd on the ultrastructure of chloroplasts involved disorganization of the thylakoid system and stroma (Fig. 6C, D). These changes are similar to the disorganization of the thylakoid membranes observed in Nicotiana tabacum cells by Vijaranakul et al. (2001). In addition, Cd can also induce thylakoid distortions and an increase in the number and size of plastoglobuli and peripherical vesicles, as observed in our study, however, Zhong 9806 was less affected and we observed accumulation of starch grains under Cd stress. The accumulation of starch in leaves might be due to either nutrient deficiencies, decrease of the sink force or disturbed vein loading system. The starch accumulation under salt conditions was also observed in NaCl acclimated Citrus cell line and it is tempting to speculate that starch synthesis plays a role in moderating the osmotic condition (Ferreira and Lima-Costa 2008). These results are in agreement with our result in Zhongmian 41 (Fig. 6E). Salinity also induced swelling of the thylakoids which is typical symptom of salinity stress in chloroplast; our result in agreement with previous report in barley (El-Banna and Attia 1999). While in Zhong 9806, most thylakoids still kept in normal structures though some thylakoids were swelling. Under combined stress (Cd + Na), loose thylakoid membranes, and swollen thylakoids and osmiophilic granule increased significantly in the two genotypes compared with control (Fig. 6G, H).
In the present investigation, ultrastructural alterations in root tip cells of both cotton genotypes were mainly concentrated on membranes like plasma and nuclear membrane, vacuoles and nucleoli. Cracked karyotheca, increased vacuolar size and number and dissolved nucleolus due to Cd was more prevalent in Zhongmian 41 than in Zhong 9806. Increased vacuolation in present study might play a significant role in Cd detoxification and tolerance, thus preventing the circulation of free Cd ions in the cytosol and forces them into a limited area. The roots are directly exposed to the soil and affected by salinity. Therefore, this organ must tolerate the salinity stress to keep the whole plant alive and the root tip is also suggested to function as a sensor for different kinds of stress. Thus, the investigation on salinity-induced ultrastructural changes in roots is important. Salinity stress-induced distortion of cells and nucleus and plasmolysis, characterized by cracked karyotheca and the distortion of cells were observed more in Zhongmian 41 than in Zhong 9806. The vacuolation of root tip cells may be an adaptive response to accumulate excess ions under salinity, which protects the cytoplasm from toxic levels of ions. The vacuolation of root cap cells and cortical cells may also function to separate ions from more vulnerable meristematic cells and procambial cells. However, the suppression of mucilage production in the peripheral root cap cells should have adverse effects on the root growth. Vacuolation and vesiculation of root cells are also observed under salinity stress in barley (El-Banna and Attia 1999). These findings are in agreement with our result, under Cd + Na, increased vacuole number was more obvious in Zhongmian 41 than in Zhong 9806 (Fig. 7G, H).
Previous researches about the effect of Cd stress on nutrient uptake and accumulation in plants sorption have provided contradicting results, which might be due to the differences in the culture methods, species, organs, and conditions such as concentration in medium, growth period and temperature. Liu et al. (2003) observed significant positive correlations between Cd and Fe, Cd and Zn, Cd and Cu existed in rice in terms of their concentrations in roots and leaves. In contrast, previous reports indicated that toxic Cd levels inhibited uptake of nutrient elements such as P, K, S, Ca, Zn, Mn, and B by plants in an organ and genotype specific manner in pea (Metwally et al. 2005). In our present work, Na concentrations were significantly lower under Cd + Na as relative to salinity alone in the two cotton genotypes. NaCl addition markedly reduced Cd concentration (Cd + Na vs. Cd; Table 1), which is beneficial to reduced Cd toxicity.
Calcium and Mg concentrations were decreased in plants of both genotypes under Cd, salinity, and alone and combined stresses, and the reduction was more in Zhongmian 41 compared to Zhong 9806. Cd, Na and Cd + Na caused significant decrease in Cu concentration in Zhongmian 41, but increased in Zhong 9806. Fe concentration showed genotypes and organ dependent variation in response to Cd, salinity and Cd + Na stresses. Zn and Mn concentrations in Zhongmian 41 were decreased in the roots and stems. However, in leaves there were no significant differences in Zn concentration compared to control. On the other hand, in Zhong 9806 Mn concentration was increased under salinity in root, while it was decreased under Cd and Cd + Na. Moreover, Zn concentration was decreased in stem under Cd, Na and Cd + Na, while it was increased in leaf. Furthermore, Mn concentration was decreased in Zhong 9806 leaf under Cd, Na and Cd + Na, while in root there were no significant differences. However, in stem it was decreased under Cd and Cd + Na, whereas under stress of salinity it was increased compared to control. Salinity stress may also lead to nutritional disorders in crops because of the effect of salinity on nutrient availability, competitive uptake, transport or partitioning within the plant. The three stresses reduced most nutrient accumulation in both roots and shoots, which was similar to the results of Yang et al. (1998), who reported that addition of Cd to growth medium decreased the accumulation of Fe, Mn, Cu, Ca, and Mg in cabbage, ryegrass, maize, and white clover. Salinity is an important determinant of Cd concentration in crops (Weggler-Beaton et al. 2000); the mechanism has not yet been elucidated. It was reported that Cd uptake was enhanced when plants grew in soils with higher NaCl content. Although, the detailed interaction between salinity and heavy metals, including Cd is still not fully understood. Weggler-Beaton et al. (2000) proved that the effect of salinity in increasing Cd bio-availability is attributable to the formation of Cd-Cl complexes, predominantly the 1:1 (CdCl+) and 1:2 (CdCl2) complexes. These complexes are less strongly sorbed to soil than free Cd2+ ion and hence increase Cd mobility at the soil-root interface. Moreover, these complexes also stimulate transport of Cd across the zone encompassing soil rhizosphere, apoplast, plasma membrane. Thus, increased soil–plant transfer of Cd can occur under salinity. In the present research, nevertheless, we found that addition of NaCl in Cd stressed solution resulted in decrease of Cd concentration in both roots and shoots. The reason for the inconsistency between our results and those of others could be attributable to the different culture conditions. In the experiments with soil media, enhanced Cd uptake was due to the fact that Cl− forms complexes with Cd sorbed to soil originally. While in our experiment using hydroponic solution, Cd is fully dissolved, so formation of Cl-Cd complexes has no distinct effect on Cd bio-availability. Meanwhile, it might be possible that injured roots due to high salinity weaken the capacity of ion uptake, or Na ion competitively inhibits Cd uptake.
In conclusion, the present study demonstrated that Zhong 9806 are more tolerant to salinity and Cd alone and combination stresses than that of Zhongmian 41. NaCl alleviated Cd toxicity in cotton plants. Addition of NaCl to the Cd solution reduced Cd accumulation and translocation, but improved such mineral nutrients as Ca, Mg and Zn uptake and translocation in cotton plants. Addition of NaCl could excite effective antioxidants’ responses to Cd toxicity, especially in the enhancement of SOD, CAT, POD and APX which were beneficial in antagonizing oxidative stress as shown in reduced MDA accumulation. Furthermore, Cd and salinity stresses induced changes in ultrastructure of chloroplasts and in root cells, and Zhong 9806 was less affected than Zhongmian 41. Our data would give insight into the mechanism of combination effects of salinity and Cd toxicity on cotton plants, and it also could provide the effective way to alleviate Cd toxicity for plants.
Abbreviations
- APX:
-
Ascorbate peroxidase
- Cd:
-
Cadmium
- CAT:
-
Catalase
- Ca:
-
Calcium
- Ci:
-
Intercellular CO2 concentration
- Cu:
-
Copper
- Fo, Fm and Fv:
-
Initial, maximal and variable fluorescence
- Fe:
-
Iron
- Fv/Fm :
-
Maximal photochemical efficiency of PSII
- Gs:
-
Stomatal conductance
- MDA:
-
Malondialdehyde
- Mg:
-
Magnesium
- Mn:
-
Manganese
- Na:
-
Sodium
- Pn:
-
Net photosynthesis rate
- POD:
-
Guaicol peroxidase
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- Tr:
-
Transpiration rate
- Zn:
-
Zinc
References
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Ashraf M (2002) Salt tolerance of cotton: some new advances. Crit Rev Plant Sci 21:1–32
Barazani O, Dudai N, Khadka UR, Golan-Goldhirsh A (2004) Cadmium accumulation in Allium choenoprasum L. grown in an aqueous medium. Chemosphere 57:1213–1218
Bingham FT, Sposito G, Strong JE (1984) The effect of chloride on the availability of cadmium. J Environ Qual 13:71–74
Chen FM (1984) Determining the chlorophyll contents of plant leaves by acetone/ethanol mixture assay. For Sci Commun 2:4–8
Chen F, Wang F, Wu FB, Mao WH, Zhang GP, Zhou MX (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48:663–672
Choudhury S, Panda SK (2005) Toxic effects, oxidative stress and ultrastructural changes in moss Taxithelium Nepalense (Schwaegr.) Broth. under chromium and lead phytotoxicity. Water Air Soil Poll 167:73–90
Dietz KJ (2005) Plant thiol enzymes and thiol homeostasis in relation to thiol dependent redox regulation and oxidative stress. In: Smirnoff N (ed) Antioxidants and reactive oxygen species in plants. Blackwell Publishing, Oxford, pp 25–52
Dong H, Li W, Eneji AE, Zhang D (2012) Nitrogen rate and plant density effects on yield and late-season leaf senescence of cotton raised on a saline field. Field Crops Res 126:137–144
El-Banna Y, Attia T (1999) Root tip meristematic cell and leaf chloroplast structure in three barley (H. vulgare L.) genotypes exposed to salinity stress. Cytologia. 64:69–76
FAO (2000) Crops and drops: making the best use of water for agriculture. FAO, Rome
Ferreira AL, Lima-Costa ME (2008) Growth and ultrastructural characteristics of Citrus cells grown in medium containing NaCl. Biol Plant 52:129–132
Gabrijel O, Davor R, Zed R, Marija R, Monika Z (2009) Cadmium accumulation by muskmelon under salt stress in contaminated organic soil. Sci Total Environ 407:2175–2182
Garg N, Singla R (2004) Growth, photosynthesis, nodule nitrogen and carbon fixation in the chickpea cultivars under salt stress. Braz J Plant Physiol. 6(3):137–146
Genty B, Britantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 99:87–92
Ghnaya T, Slama I, Messedi D, Grignon C, Ghorbel MH, Abdelly C (2007) Cd-induced growth reduction in the halophyte Sesuvium portulacastrum is significantly improved by NaCl. J Plant Res 120:309–316
Huang YZ, Zhang GP, Wu FB, Chen JX, Xiao YP (2006) Interaction of salinity and cadmium stresses on antioxidant enzymes, sodium, and cadmium accumulation in four barley genotypes. J Plant Nutr 29(12):2215–2225
Kang W, Shamsi IH, Zhang GP (2007) Synergistic interaction of NaCl and Cd on growth and photosynthetic parameters in soybean genotypes differing in salinity tolerance. J Zhejiang Univ Sci B 8(4):266–271
Khoshgoftar AH, Shariatmadari H, Karimian N, Kalbasi M, van der Zee SEATM, Parker DR (2004) Salinity and zinc application effects on phytoavailability of cadmium and zinc. Soil Sci Soc Am J 68:1885–1889
Lefevre I, Marchal G, Meerts P, Correal E, Lutts S (2009) Chloride salinity reduces cadmium accumulation by the Mediterranean halophyte species Atriplex halimus L. Environ Exp Bot 65:142–152
Lin J, Sun YQ, Li YJ, Zhu S (2006) Studies on the effects of the salinity priming on the NaCl tolerance of transgenic insect resistant cotton (Gossypium hirsutum L). Cotton Sci 18(6):338–341
Liu JG, Li KQ, Xu JK, Liang JS, Lu XL, Yang JC, Zhu QS (2003) Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes. Field Crops Res 83(3):271–281
Metwally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativumL. J Exp Bot 56(409):167–174
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Norvell WA, Wu J, Hopkins DG, Welch RM (2000) Association of cadmium in durum wheat grain with soil chloride and chelate-extractable soil cadmium. Soil Sci Soc Am J 64:2162–2168
Pagliano C, Raviolo M, Vecchia FD, Gabbrielli R, Gonnelli C, Rascio N, Barbato R, Rocca NL (2006) Evidence for PSII donor-side damage and photoinhibition induced by cadmium treatment on rice (Oryza sativa L.). J Photochem Photobiol 84:70–78
Perez Lopez U, Robredo A, Lacuesta M, Sgherri C, Munoz Rueda A, Navari-Izzo F, Mena-Petite A (2009) The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol Plant 135:29–42
Piquery L, Davoine C, Huault C, Billard JP (2000) Senescence of leaf sheaths of ryegrass stubble: changes in enzyme activities related to H2O2 metabolism. Plant Growth Reg 30:71–77
Rai V, Khatoon S, Bisht SS, Mehrotra S (2005) Effect of cadmium on growth, ultramorphology of leaf and secondary metabolites of Phyllanthus amarus Schumm and Thonn. Chem. 61:1644–1650
Tang Q, Feng M (1997) Practical statistics and its DPS statistics software package. China Agriculture Press, Beijing
Vijaranakul U, Jayaswal RK, Nadakavukaren MJ (2001) Alteration in chloroplast ultrastructure of suspension cultured Nicotiana tabacum cells by cadmium. Sci Asia 27:227–231
Weggler K, Mclaughlin MJ, Graham RD (2004) Effect of chloride in soil solution on the plant availability of biosolid-borne cadmium. J Environ Qual 33:496–504
Weggler-Beaton K, Mclaughlin MJ, Graham RD (2000) Salinity increases cadmium uptake by wheat and Swiss chard from soil amended with biosolids. Aust J Soil Res. 38(1):37–45
Wu FB, Zhang GP, Dominy P (2003) Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ Exp Bot 50:67–78
Wu FB, Wu HX, Zhang GP, Bachir DML (2004) Differences in growth and yield in response to cadmium toxicity in cotton genotypes. J Plant Nutr Soil Sci 167:85–90
Yang MG, Lin XY, Yang XE (1998) Impact of Cd on growth and nutrient accumulation of different plant species. Chin J Appl Ecol 19:89–94
Yu BJ, Liu YL (2003) Effects of salt stress on the metabolism of active oxygen in seedlings of annual halophyte Glycine soja. Acta Bot Boreali-Occidentalia Sinica 23:18–22
Zhang JB, Huang WN (2000) Advances on physi-ological and ecological effects of cadmium on plants. Chin J Acta Ecol Sinica 20:514–523
Zhou K, Ye WW, Wang JJ, Wang DL, Fan BX, Wang S (2011) Cloning and salt-tolerance analysis of gene plastid transcriptionally active (GhPTAC) from Gossypium hirsutum L. Acta Agronomica Sinica 37(9):1551–1558
Acknowledgments
This work was financially supported by the National R & D Project of Transgenic Crops of Ministry of Science and Technology of China (Major Program, 2009ZXD8001-027B).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ibrahim, W., Ahmed, I.M., Chen, X. et al. Genotypic differences in photosynthetic performance, antioxidant capacity, ultrastructure and nutrients in response to combined stress of salinity and Cd in cotton. Biometals 28, 1063–1078 (2015). https://doi.org/10.1007/s10534-015-9890-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-015-9890-4