Abstract
There are several existing methods for synthesizing metallic nanoparticles, with the most commonly applied being chemical reduction methods. Recently, the biogenic synthesis of noble metal nanoparticles has been developed. These methods involve biological systems such as bacteria, fungus, and plant extracts. Green and biological methods are economical, eco-friendly, and non-toxic methods of obtaining nanoparticles for possible biomedical applications. Here, we present a short overview of the biogenic synthesis of platinum nanoparticles using plants, plant extracts, bacteria, fungi, and other substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
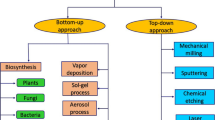
There are multiple methods of obtaining platinum nanoparticles, which can be divided into two strategies: top to bottom (top-down) and bottom to top (bottom-up) (Habibullah et al. 2021; Loza and Epple 2019). The first consists of structurally decomposing a large metal (that is, the bulk material), which has some drawbacks, such as the high energy cost of the equipment, limited control over the size or shape of adjustments, reduced heterogeneity, and increased material homogeneity. Examples of this strategy include photolithography, electron beam lithography, milling techniques, anodizing, and etching with ions and plasma. These processes can produce ligand-free nanoparticles (Figure 1).
Although bottom-up is one of the most common techniques for preparing platinum nanoparticles, which consists of self-assembly of the particles using wet chemical methods, this results in greater reliability in morphology and size. However, one of the most frequently mentioned drawbacks is the presence of impurities from the use of toxic inorganic and organic chemicals that remain in the reaction mixture. As the production of these colloidal metals requires chemical reactions, which involve the reduction of a Pt (II) precursor, such as potassium tetrachloroplatinate \(({\text{K}}_{2} {\text{PtCl}}_{4} )\) or platinum (II) bis(acetylacetonate); or a Pt (IV) precursor such as potassium (IV) hexachloroplatinate \(({\text{K}}_{2} {\text{PtCl}}_{6} )\) (other examples: chloroplatinic acid \(\left( {{\text{H}}_{2} {\text{PtCl}}_{6} } \right)\), platinum (II) chloride \(\left( {{\text{PtCl}}_{2} } \right)\), tetraammineplatinum(II) nitrate \(\left( {{\text{Pt}}\left( {{\text{NH}}_{3} } \right)_{4} \left( {{\text{NO}}_{3} } \right)_{2} } \right)\), or tetraammineplatinum(II) hydroxide hydrate \(\left( {{\text{Pt}}\left( {{\text{NH}}_{3} } \right)_{4} \left( {{\text{OH}}} \right)_{2} \cdot x{\text{H}}_{2} {\text{O}}} \right.\), tetraammineplatinum (II) chloride hydrate \(\left. {\left( {{\text{Pt}}\left( {{\text{NH}}_{3} } \right)_{4} {\text{Cl}}_{2} \cdot x{\text{H}}_{2} {\text{O}}} \right),\,etc.} \right)\) (Chen and Holt-Hindle 2010; Hikosaka et al. 2008; Kankala et al. 2020; Mironava et al. 2013; Shim et al. 2017; Zheng et al. 2013) dissolved in appropriate solvents in the presence of reducing agents (e.g., hydrogen, carbon monoxide, sodium borohydride, ethylene glycol, glycerol, etc.), tensioactive materials or surfactants (polyvinyl alcohol (Asharani et al. 2010), Brij-58 (Shim et al. 2017), cetyl trimethyl ammonium bromide (Lee et al. 2006), sodium dodecyl sulfate (Mohammadi et al. 2013), etc.), ligands (e.g., peptides, proteins, nucleic acids, small molecules, etc.), or stabilizing/coating polymers (e.g. polyvinylpyrrolidone (Herricks et al. 2004; Koebel et al. 2008), hyaluronic acid (Zhu et al. 2017), alginic acid, thiols, etc.) that help reduce dispersion and prevent aggregation and may or may not endanger complex biological systems. Thus, we must carefully consider the design depending on the objectives we want to achieve since the biological and chemical applications are diverse (Fig. 2) (Liu et al. 2014; Stepanov et al. 2014; Yamada et al. 2015).
Among these strategies, we find a set of methods such as chemical or electrochemical precipitation, sol–gel, laser-induced pyrolysis, chemical vapor deposition (CVD), synthesis by plasma, or flame spraying (Habibullah et al. 2021). These ideas, however, have not remained stagnant; rather, they have formed new links with biological studies with the aim of obtaining environmentally friendly syntheses since living beings interact with the environment (Mohanpuria et al. 2008; Pedone et al. 2017; Siddiqi and Husen 2016). Modification and optimization have been used to obtain high-quality products, directing responses through product safety with the help of bio-assisted synthesis and the use of green chemistry. The sizes and shapes of the nanoparticles are diverse, since all syntheses use variables such as pH, temperature, reducing agents (chemical or biological), and the concentration of the platinum precursor compound, making endless products with different characteristics (Jameel et al. 2020).
Due to their unique physicochemical properties, e.g., catalytic, magnetic, and optical properties, Pt nanoparticles have potential technological interest (Chen and Holt-Hindle 2010; Elder et al. 2007). The latter are of utmost importance for biomedical use since, from the catalytic perspective, platinum is inert and does not corrode within the human body, endowing it with the ability to inhibit cell division in mammals and in some bacteria (Jan et al. 2021; Puja and Kumar 2019). Regarding optical properties, platinum nanoparticles are distinguished by the presence of a very distinctive localized surface plasmon resonance in the UV–Vis region, a characteristic that is not evident in the bulk material. On the other hand, we find the efficiency of heat generation is higher than in other nanoparticles (del Valle et al. 2020; Sadrolhosseini et al. 2019; San et al. 2013). Therefore, Pt nanoparticles could be an alternative treatment in hyperthermia therapy (Fang et al. 2020; Zhao et al. 2019). These properties have made platinum nanomaterials significant candidates for biomedical applications in catalysts, sensors, nanomedicine, anti-inflammatories, enzymes, and pharmaceutical immobilization, among others (Madlum et al. 2021; Naseer et al. 2020). Recently, the effect of Pt nanoparticles on human cells for cancer therapy was investigated (Almeer et al. 2018; Gurunathan et al. 2019, 2020; Ismail and Al-Radadi 2017; Kankala et al. 2020;). The increased number of biological and biomedical applications has necessitated the development of new synthesis methods.
Biological synthesis methods
Methods for the green synthesis of nanoparticles are ecological routes that represent an alternative to chemical and physical methods. These methods eradicate or reduce the generation of dangerous substances. Various metal nanoparticles have been synthesized using plant phytochemicals as reducing agents and stabilizers without the use of costly and toxic chemicals. This green synthesis involves biological methods using plants, enzymes, biomolecules, agricultural and industrial waste products, microorganisms, or algae (Alshatwi et al. 2015; Ojo et al. 2021). Nanobiotechnology is the application and use of nanotechnology in life sciences, including in molecular diagnostics, drug discovery, drug delivery, and the development of nanomedicine (Jain 2005; Rahman et al. 2019). Green nanobiotechnology should be defined as synthesizing nanoparticles or nanomaterials using biological routes with the help of various biotechnological tools (Patra and Baek 2014).
Biological or bio-assisted synthesis methods are very diverse as they require biological organisms (unicellular or multicellular), which act as “bio-laboratories” or “nano-factories” for producing biogenic nanoparticles (Jan et al. 2021; Jeyaraj et al. 2019; Pedone et al. 2017; Puja and Kumar 2019). Metallic nanoparticles are synthesized through metabolic pathways or using derivatives (e.g., extracts, wastes, animal products) of these organisms. Syntheses can occur intracellularly or extracellularly (Ali et al. 2015; Puja and Kumar 2019; Sharma et al. 2019). Using these methods, safer, more ecological, and more environmentally friendly protocols have been designed. However, these methods also have disadvantages (Jameel et al. 2020; Narayanan and Sakthivel 2010; Sharma et al. 2019). Particular disadvantages include the fact that this method is not easy to control in terms of designing the shape, size, crystal growth, and stability, and the possibility of finding endotoxins when the reaction has been completed (Jameel et al. 2020). However, the advantages are that biological organisms can be cultured easily and can manifest high intracellular absorption of metallic salts, and the fact that it is easier to manage the biomass and waste that these may generate than the toxic reagents derived from the chemical pathways described previously (Fahmy et al. 2020; Naseer et al. 2020; Rai and Duran 2011).
Biological synthesis methods are frequently used to prepare a wide variety of nanoparticles, the most common among them being Au and Ag (Cardoso-Avila et al. 2021; Kalimuthu et al. 2020; Tarannum et al. 2019; Tepale et al. 2019; Zamiri et al. 2011). Other metallic and oxide nanoparticles are also reported with these methods, like Pd, Cu, Fe, CuO, Fe3O4, ZnO TiO2, NiO, CeO2, etc. (Ishak et al. 2019; Marouzi et al. 2021; Singh et al. 2018; Sabouri et al. 2019, 2020a, b).
Here, we briefly review the use of microorganisms such as bacteria, fungi, and algae and complex systems including plants, along with products derived from all of these, for the synthesis of platinum nanoparticles (Fig. 3, Table 1).
Plant-mediated synthesis
These biogenic pathways can be targeted in two ways. The first is by intracellular synthesis, where metallic nanoparticles can bioaccumulate. The second is extracellular synthesis, where platinum accumulation is mediated by plant biomass (e.g., agro-industrial waste) so that all the components of the plant from the roots, stem, bark, leaves, flowers, fruits, and even peels and bio-derivatives (e.g., extracts, gums, etc.) obtained from these components are employed. All parts of the plant have biomolecules to a greater or lesser extent, for example, proteins, enzymes, flavonoids, polyphenols, cannabinoids, terpenoids, glycosides, sugars, alcohols, aldehydes, amines, carbonyls, etc., that aid in the reduction of platinum salts and their stabilization for the formation of metal nanoparticles. An advantage of this method is that it eliminates elaborate stages in the maintenance process that occur in cell cultures. Thus, the use of plants can be suitably scaled up for large-scale synthesis.
Many living plants have been shown to function as aids in the formation of nanoparticles by absorbing metal ions; the first report of this occurrence was in 2002 by Gardea-Torresdey et al. when it was demonstrated that gold nanoparticles had formed in the roots and shoots of alfalfa plants that grew in an environment rich in potassium tetrachloroaurate \(({\text{KAuCl}}_{4} )\), thus intracellularly initiating the bioreduction of the metal salt to form insoluble compounds (e.g., nanoparticles) (Gardea-Torresdey et al. 2002). It was later shown that alfalfa could also form silver nanoparticles when exposed to a solid medium rich in silver salts (Gardea-Torresdey et al. 2002, 2003). Therefore, Bali et al. (2010) selected two facultative metallophyte plants (Medicago sativa and Brassica juncea), as it had previously been shown that both species accumulated precious metals; thus, the distribution of Pt in vivo in the different tissues of these organisms was determined for the first time using proton-induced X-ray emissions. In both plants, Pt concentration increased by raising aqueous substrate concentration, prolonging exposure time, and lowering pH to 2 and 3 for Medicago sativa and Brassica juncea, respectively. Pt nanoparticles between 3 and 100 nm with varied morphology were formed due to the action of local metabolites (Bali et al. 2010). Despite this, information on the intracellular biosynthesis of Pt nanoparticles in living plants is still scant, but it has been possible to accumulate information about synthesis by using various products and by-products of plants, as presented in Table 1 (Naseer et al. 2020).
The first study to focus on the green chemistry of Pt nanoparticles was that of Song et al. who discussed the biosynthesis of this metal with plant extracts in 2010 (Song et al. 2010) using an extract from a Diospyros kaki leaf that functioned as a medium and a reducing agent in the ecological extracellular synthesis of biogenic Pt nanoparticles in an aqueous solution of \({\text{K}}_{2} {\text{PtCl}}_{6} \cdot 6{\text{H}}_{2} {\text{O}}\) with a conversion from platinum ions to platinum nanoparticles of over 90% and a concentration of foliar biomass > 10% at 95 °C. The microscopic studies reported for transmission electron microscopy (TEM) showed oscillations between 2 and 20 nm, giving a mixture of shapes that included spheres and disks, whereas the Fourier transformed infrared spectroscopic studies revealed the presence of metabolites such as terpenoids, where the reduction process is not an enzyme-mediated process because the nanoparticle formation temperature exceeds 95 °C. Therefore, these techniques show how the modification of incubation temperature, concentration of foliar extract, and metallic ions influence the resulting nanoparticles’ yield, size, and shape.
Plant extracts from Taraxacum laevigatum, which is a medicinal plant, had a high content of phenolic biomolecules that were the principle reducing and stabilizing agents of Pt nanoparticles (Tahir et al. 2017). The obtained Pt nanoparticles had high antibacterial activity against two bacterial species, B. subtilis and P. aeruginosa.
A leaf extract from Ocimum sanctum (tulsi) has also been used as a reducing agent in the synthesis of Pt nanoparticles; tulsi leaves had abundant tannins, such as gallic acid and chlorogenic acid, and alkaloids, glycosides, and saponins (Soundarrajan et al. 2012).
Another plant with abundant phytoconstituents is the Carica papaya, which contains phenolic compounds, tocopherol, ascorbic acid, flavonoids, and reducing sugars. A leaf extract of this plant has been used in the green synthesis of Pt nanoparticles and bimetallic aurium@platinum nanoparticles. The metabolites in C. papaya leaf extract played a crucial role in the bioreduction of precursor metals, especially polyphenolic compounds that were also responsible for stabilizing and capping the nanoparticles (Olajire and Adesina, 2017). The proposed reaction mechanism is presented in Fig. 4.
Proposed mechanism for the bioreduction of Pt4+ to Pt atom by a typical polyphenolic compound in C. papaya (Olajire and Adesina 2017. Reproduced under Creative Commons Attribution CC-BY 4.0)
In addition to the extracts, the powder of dried leaves has also been used in the synthesis of platinum nanoparticles, as reported by Sheny et al. (2013). Dried leaf powder of Anacardium occidentale was used, and the effect of different amounts of leaf powder (50, 100, 200, 300, and 400 mg) on the formation of nanoparticles was investigated. The amount of leaf powder determined the size of the particles; a smaller amount of powder seems to be adequate for the formation of small rod-shaped Pt nanoparticles (Sheny et al. 2013).
The variation in pH and temperature also has consequences on the size and shape of the nanoparticles. The synthesis of Pt nanoparticles using herbal Bidens tripartita extract has been reported. The reaction was maintained at 90 °C for 8 hours with a pH of 8 to ensure the reduction of Pt4+ to Pt0. This pH facilitated the formation of more stable Pt nanoparticles; the particles showed an irregular rod shape with a size of 4 nm (Dobrucka, 2016b).
Algae-mediated synthesis of platinum nanoparticles
The use of algae for the green synthesis of nanoparticles has the advantages that it is a low-cost raw material, has a large number of secondary metabolites, and is free of secondary contamination. The green synthesis of platinum nanoparticles has been reported using the brown algae Padina gymnospora, which is abundant on the coasts of the Ramanathapuram district of the state of Tamil Nadu, India (Shiny et al. 2014). The obtained Pt nanoparticles had a spherical shape in the size range of 5 to 20 nm. Ramkumar et al. (2017) also used this alga for the production of platinum nanoparticles with a truncated octahedral shape and a size range of 5 to 50 nm.
Stable palladium and platinum nanoparticles were produced using extracts of the green alga Botryococcus braunii (Arya et al. 2020). The green-synthesized nanoparticles exhibited antimicrobial activity against Gram-positive and Gram-negative bacterial strains, antifungal activity against a fungus, and antioxidant activity.
Synthesis of Pt nanoparticles mediated by fungi
Among living organisms, fungi are now estimated to account for approximately 0.8 to 1.5 million species on the planet, of which 100,000 have been described (Rai et al. 2009; Tedersoo et al. 2014). We have taken particular advantage of their secondary metabolites, which are associated with different structures (e.g., terpenoids, alkaloids, quinones, xanthones, peptides, steroids, flavonoids, phenols, and phenolic compounds), meaning that interest has turned to considering them as an alternative method for the production of nanoparticles, on both small and large scales in laboratories and industries, respectively (Argumedo-Delira et al. 2020; Srivastava 2019). Fungi have the ability to bioaccumulate and tolerate metals thanks to their large secretions of proteins and enzymes. Thus, we were able to use them to synthesize metallic nanoparticles, avoiding agglomerations of particles by using either extracellular or intracellular processes (dependent on metabolism) and the fact that these species require only simple means for supervenience and proliferation, making subsequent biomass processing easy—which is of great economic advantage (Argumedo-Delira et al. 2020; Subashini and Bhuvaneswari 2018).
In 2006, T. Riddin et al. began work on the first protocol for obtaining platinum nanoparticles using Fusarium oxysporum fungi (Riddin et al. 2006). The fungal strain was evaluated and found to be successful for the inter and extracellular production of Pt nanoparticles in a size range of 10–100 nm with varying shapes (circular, triangular, hexagonal, square, and rectangular) according to TEM studies. The effects of temperature and concentration of hexachloroplatinic acid \(\left( {{\text{H}}_{2} {\text{PtCl}}_{6} } \right)\) and pH during the synthesis of the material were studied. Compared to intracellular synthesis, extracellular synthesis is more advantageous due to the simple post-processing techniques involved. Furthermore, intracellular synthesis requires advanced instruments to extract nanoparticles from biomass (Riddin et al. 2006). Syed and Ahmad (2012) reported the extracellular synthesis of stable Pt nanoparticles using the same microorganism. They showed that when working at room temperature, morphology remains spherical and size ranges between 15 and 30 nm, as shown by TEM analysis. It is therefore apparent that temperature plays an important role in the shape, as already mentioned (Syed and Ahmad 2012). Thus, production of nanoparticles depends mostly on the type of fungus involved and consequently on abiotic factors (temperature, pH, metal ions, and time) (Jameel et al. 2020).
Castro-Longoria et al. (2012) reported the use of the Neurospora crassa fungus for the intracellular synthesis of nanoparticles (4–35 nm) at room temperature. The material described formed quasi-spherical and monocrystalline nanoaggregates with a mean size between 20 and 110 nm. Similar results were obtained using fungal extracts to produce Pt nanoaggregates at a range of 17–76 nm, so it can be used as a reducing and stabilizing agent for the synthesis of Pt nanoparticles (Castro-Longoria et al. 2012).
In 2018, Subramaniyan et al. synthesized Pt nanoparticles using the free cell culture of Penicillium chrysogenum as a reducer, which was treated in two different environments (normal gravity and micro-gravity), obtaining spheres with a diameter of 15 nm and 8.5 nm, respectively, and evaluating its toxic effect on the mouse myoblast cell line (C2C12). Results showed that cytotoxicity depends on the concentration of the Pt nanoparticles; a decrease in cell viability (apoptosis) is caused by surface stress and the release of ions, which causes an increase in the generation of reactive oxygen species (Subramaniyan et al. 2018). In this way, in 2019 Gupta and Chundawat analyzed the antimicrobial potential and antioxidant activity of Pt nanoparticles synthesized from extracts of Fusarium oxysporum, where the hydrogenase enzyme, which behaves as an electron shuttle with excellent redox properties, is already known to be present. This fungus is thus able to achieve this nanoeffect on metal ions (Gupta and Chundawat 2019), where they show good to moderate antibacterial activity against various pathogens, affirming that biosynthesized Pt nanoparticles are non-toxic (Nida and Khan 2017; Durán et al. 2005). Argumedo-Delira et al. (2020) used a set of filamentous fungi with the aim of evaluating the effect of groups of precious metals on them. Platinum specifically was shown not to have secondary effects on their growth, and so Pt nanoparticles can be considered synthetic alternatives for possible biotechnological or biomedical applications. Notably, however, many organisms of this type can still be considered pathogens as they release mycotoxins, phytotoxins, etc., that can cause side effects in animals and humans (Argumedo-Delira et al. 2020).
Bacterium-mediated synthesis
It is well known that many organisms, both unicellular and multicellular, produce inorganic materials intracellularly or extracellularly (Bhattacharya and Gupta 2005; Singh and Singh 2019). Therefore, over the past two decades, research has focused on bacteria as a potential alternative for nanomaterial biosynthesis, employing their natural defense mechanisms that have evolved over time under extreme environmental conditions (Riddin et al. 2010; Solak et al. 2017). These stress conditions have allowed these microorganisms to develop survival mechanisms to overcome particular problems, including the toxicity caused by high concentrations of xenobiotic ions or metals (e.g., Au, Ag, Cd, Sn, Hg, Pb, etc.) by means of an active process (reductase/hydrogenase enzymes) or a passive process (metallothioneins) (Bertini et al. 2007). In each of these examples, processes may be mediated by various types of resistance mechanisms encoded by plasmids (or transposons) that can be activated not only in their offspring but also in other bacteria of the same or different species, triggering the mobilization of enzymatic pathways in order to initiate detoxification and maintain survival (Rai and Duran 2011; Rouch et al. 1995). Consequently, nanotechnology has used these pathways in order to bioreduce metal ions to form more stable metal particles (Beveridge et al. 1996; Carpentier et al. 2003; Rouch et al. 1995; Silver 1996) (Fig. 5).

adapted from Bloch et al. 2021)
Extracellular and intracellular bacterial synthesis of nanoparticles (
These mechanisms include efflux systems and alterations in solubility and toxicity due to changes in the redox state of metal ions, complexation and chelation reactions, precipitation of metals either extracellularly or intracellularly or in the periplasmic space, and the lack of specific systems for transport of metals (Carpentier et al. 2003; Rai and Duran 2011). In 2005, Oleg A. Zadvorny and collaborators (Zadvorny et al. 2005) demonstrated that two phototrophic purple sulfur bacteria (PSB) (Thiocapsa roseopersicina and Lamprobacter modestohalophilus) can reduce Ni (II), Pt (IV), and Pd (II) due to the action of hydrogenase with an electron donor. These hydrogenases have valuable theoretical and practical utility as a type of “metal oxidoreductase.” Thus, by 2006 Maggy F. Lengke et al. (Lengke et al. 2006) were already offering the first viable alternative method to standard chemical methods for developing Pt nanoparticles. In this study, the synthesis of Pt nanoparticles by interacting platinum (IV) chloride \(\left( {{\text{PtCl}}_{4} } \right)\) with a filamentous cyanobacterium from the species Plectonema boryanum (UTEX 485 strain) was investigated in order to react and precipitate these as amorphous spherical nanoparticles (≤0.3 µm), both intracellularly and extracellularly. The presence of intracellular platinum suggested that platinum entered the cells as \({\text{PtCl}}_{4}\) and, according to complementary analyses, the Pt (IV) complex was reduced to Pt (II) and then to Pt (0) due to its interaction with sulfur, phosphorus, and nitrogen, They thus deduced a staged reaction as shown below (Brayner et al. 2007; Lengke et al. 2006):
where Pt (II) forms a type of organometallic complex, although this is still not very clear. However, three years later they proposed, with experimental evidence based on an uncharacterized consortium of sulfate-reducing bacteria (SRB), a two-stage enzymatic process for the bioreduction of Pt (IV) to Pt (0) by means of hydrogenases with interference from two electrons (Riddin et al. 2009).
The first “fast” stage from Pt (IV) to Pt (II) occurred in the cytoplasm through a hydrogenase redox system, whereas Pt (II) begins the second stage in a “slow” way, diffusing into the periplasm and then reducing to Pt (0) (Govender et al. 2009; Siddiqi and Husen 2016):
by one or more oxygen-sensitive periplasmic hydrogenase. However, this mechanism remains debatable and will be clarified by greater understanding of the platinum bioreduction mechanism (Govender et al. 2010).
Furthermore, this finding confirms the studies and hypotheses proposed in 2007 by two research groups involving hydrogenase enzymes and reducing bacteria, Shewanella algae and a mixed consortium of SRB, respectively, where it was demonstrated that the platinum salts were bioreduced and the metallic Pt deposited in the periplasm (Konishi et al. 2007; Rashamuse and Whiteley 2007). Additionally, unlike Zadvorny et al. (2005), in the research of Riddin et al. (2009) Pt (IV) ions were shown to bioreduce to Pt (0) in the presence or absence of an exogenous electron donor, while Zadvorny only used an electron donor (methyl viologen). In 2008, the potential of the purified enzyme to recover platinum from wastewater was investigated, demonstrating the industry application (Rashamuse, et al. 2008). Riddin et al. (2010) show that, like chemical syntheses, the concentration of platinum salt and proteins plays an important role in the control of size and shape, as their previous studies showed that when using only cells, amorphous deposits of Pt (0) were obtained, but when the cells are eliminated with variations of salt and protein extracts, the morphology of the nanoparticles may vary (Riddin et al. 2009; Brayner et al. 2007).
To date, researchers have relied primarily on known freshwater bacteria and very little research has focused on other bacteria. Five years ago, however, Maes et al. (2016) showed a radical change when using halophilic bacterial cultures from the Halomonadaceae, Bacillaceae, and Idiomarinaceae families, which managed to recover > 98% of Pt (II) and > 97% of Pt (IV) at pH 2 over a period of between 3 and 21 hours \(\left( {453\;{\text{mg}}\;Pt_{{\text{recovered }}} {\text{h}}^{ - 1} {\text{g}}^{ - 1} {\text{biomass}}} \right)\) from solutions of \({\text{K}}_{2} {\text{Pt}}\left( {{\text{II}}} \right){\text{Cl}}_{4}\) and \({\text{K}}_{2} {\text{Pt}}\left( {{\text{IV}}} \right){\text{Cl}}_{6}\) at \(100\;{\text{mg}}\;{\text{L}}^{ - 1}\). Based on previous studies, we can better select the microorganisms to be adapted to industrial conditions that we require today, as not all bacteria are able to reduce platinum (Konishi et al. 2007; Maes et al. 2016; Yong et al. 2002).
Baskaran et al. proposed a mechanism for the extracellular production of Pt nanoparticles in Streptomyces sp. The chloride reductase enzyme is involved in the nitrogen cycle and is responsible for the reduction of chloride to chlorine. The nicotinamide adenine dinucleotide-dependent chloride reductase enzyme is known to be an important factor in the biogenic synthesis of nanoparticles. A possible mechanism is the electron shuttle enzymatic metal reduction process (Baskaran et al. 2017):
Due to their ability to reduce metals, sulfate-reducing bacteria are not only used to remove toxic metals but also for the biogenic synthesis of platinum nanoparticles in which the Acinetobacter calcoaceticus and Desulfovibrio vulgaris strains have been used (Gaidhani et al. 2014; Martins et al. 2017).
Synthesis mediated by other microorganisms or substances
As observed in the previous sections, protocols have been developed to obtain monodispersed and stable platinum nanoparticles by bio-synthetic methods, where derivatives of plants, bacteria, and fungi are the main promoters of platinum nanoparticle synthesis. However, there are other methods using biogenic elements.
One of the first reports on the use of elements of this nature was made by Nadagouda and Varma (2006), who used one of the most frequent organic cofactors in nature, vitamin B2, with a density-assisted self-assembly method in different solvent media. Additionally, obtaining platinum nanoparticles without the intervention of reducing agents in the system to transform the precursor of platinum is being considered—something that many end up happening even with the use of friendly materials (Cai et al. 2009; Deng et al. 2009). Benaissi et al. (2010) used cotton nanocrystalline cellulose alone to do all the reduction and stabilization work, thanks to the functional groups at the surface of the biopolymer. It is well known that cellulose (Benaissi et al. 2010), lignin (Coccia et al. 2012), and hemicelluloses (Lin et al. 2016) are the main constituents of the cell wall in plants, so wood becomes interesting due to the electron-rich nature of hydroxyl and ether groups, which can act as high-speed reducers of metal ions that are suitable for the preparation of Pt nanoparticles (Lin et al. 2011). However, we can also find other interesting derivatives such as fulvic acid (Coccia et al. 2012), humic extract, and bacterial cellulose matrixes (Aritonang et al. 2014). Information about biogenic substances is accumulating slowly, but safe approaches are being worked on, as these do not require toxic reagents to obtain this material, thus preventing the release of toxic substances that may play an important role in the immune system. This has led to more complex substances such as sheep's milk, quail egg yolk, and honey being used in an attempt to maintain mild conditions (Gholami-Shabani et al. 2016; Nadaroglu et al. 2017; Venu et al. 2011). These are often used as a mixture of simultaneous reducing and stabilizing agents. In this sense, the bio-manufacture of these elements is developing as a modern and appropriate technique for improving the shape, size, and stability of Pt nanoparticles with the help of techniques that are apt for clarifying their nanoscopic levels (Jeyaraj et al. 2019). Because these are new synthetic strategies, work to establish training mechanisms has been limited. However, these techniques are particularly useful, as by using renewable resources of this type one saves on independent reducing and stabilizing agents. But applications continue to be focused on catalysis, neglecting the possible applications related to anticancer, antimicrobial, and antifungal activity.
Conclusion
All these synthetic strategies reveal an effort to improve the ecological conditions that surround us by using less toxic synthesis methods. These constructive syntheses are controlled by the platinum precursor, temperature, and use of reagents or organisms that will affect the shape and size of the platinum nanoparticles, playing a decisive role in safety and in producing successful applications in the biomedical context. Due to the complexity of the biological environment, where each organism produces materials that result in a corresponding reaction, devising a universal strategy seems unlikely. However, results still show discrepancies. Thus, platinum nanoparticles are undergoing rigorous nanotoxicological investigations with the aim of outperforming their more commonly available analogs (nickel, gold, silver, iron oxide, gadolinium, and titanium dioxide) and their predecessors (cisplatin, carboplatin, oxaliplatin, etc.), demonstrating an improved cytotoxic and pharmacokinetic profile (Almarzoug et al. 2020; Gurunathan et al. 2020, 2019; Kankala et al. 2020; Labrador-Rached et al. 2018; Ma et al. 2019; Shatokhina et al. 2020; Zhang et al. 2018).
References
Ali J, Zainab S, Ali N (2015) Green synthesis of metal nanoparticles by microorganisms a current prospective. Journal of Nanoanalysis 2:32–38. https://doi.org/10.22034/jna.2015.1.004
Almarzoug MHA, Ali D, Alarifi S, Alkahtani S, Alhadheq AM (2020) Platinum nanoparticles induced genotoxicity and apoptotic activity in human normal and cancer hepatic cells via oxidative stress-mediated Bax/Bcl-2 and caspase-3 expression. Environ Toxicol 35(9):930–941. https://doi.org/10.1002/tox.22929
Almeer RS, Ali D, Alarifi S, Alkahtani S, Almansour M (2018) Green platinum nanoparticles interaction with HEK293 cells: cellular toxicity, apoptosis, and genetic damage. Dose-Response 16(4):1–11. https://doi.org/10.1177/1559325818807382
Al-Radadi NS (2019) Green synthesis of platinum nanoparticles using Saudi’s dates extract and their usage on the cancer cell treatment. Arab J Chem 12(3):330–349. https://doi.org/10.1016/j.arabjc.2018.05.008
Al-Radadi NS, Adam SIY (2020) Green biosynthesis of Pt-nanoparticles from Anbara fruits: toxic and protective effects on CCl4 induced hepatotoxicity in Wister rats. Arab J Chem 13(2):4386–4403. https://doi.org/10.1016/j.arabjc.2019.08.008
Alshatwi AA, Athinarayanan J, Vaiyapuri Subbarayan P (2015) Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J Mater Sci: Mater Med 26:7. https://doi.org/10.1007/s10856-014-5330-1
Argumedo-Delira R, Gómez-Martínez MJ, Uribe-Kaffure R (2020) Fungal tolerance: an alternative for the selection of fungi with potential for the biological recovery of precious metals. Appl Sci 10(22):8096. https://doi.org/10.3390/app10228096
Aritonang HF, Onggo D, Ciptati C, Radiman CL (2014) Synthesis of platinum nanoparticles from K2PtCl4 solution using bacterial cellulose matrix. J Nanopart 2014:285954. https://doi.org/10.1155/2014/285954
Arya A, Gupta K, Chundawat TS (2020) In vitro antimicrobial and antioxidant activity of biogenically synthesized palladium and platinum nanoparticles using Botryococcus braunii. Turk J Pharm Sci 17(3):299–306. https://doi.org/10.4274/tjps.galenos.2019.94103
Asharani PV, Xinyi N, Hande MP, Valiyaveettil S (2010) DNA damage and P53-mediated growth arrest in human cells treated with platinum nanoparticles. Nanomedicine 5(1):51–64. https://doi.org/10.2217/nnm.09.85
Aygun A, Gülbagca F, Ozer LY, Ustaoglu B, Altunoglu YC, Baloglu MC, Atalar MN, Alma MH, Sen F (2020) Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J Pharm Biomed Anal 179:112961. https://doi.org/10.1016/j.jpba.2019.112961
Bali R, Siegele R, Harris AT (2010) Biogenic Pt uptake and nanoparticle formation in Medicago sativa and Brassica juncea. J Nanopart Res 12:3087–3095. https://doi.org/10.1007/s11051-010-9904-7
Baskaran B, Muthukumarasamy A, Chidambaram S, Sugumaran A, Ramachandran K, Manimuthu TR (2017) Cytotoxic potentials of biologically fabricated platinum nanoparticles from Streptomyces Sp. on MCF-7 breast cancer cells. IET Nanobiotechnol 11:241–246. https://doi.org/10.1049/iet-nbt.2016.0040
Benaissi K, Johnson L, Walsh DA, Thielemans W (2010) Synthesis of platinum nanoparticles using cellulosic reducing agents. Green Chem 12:220–222. https://doi.org/10.1039/b913218j
Bendale Y, Bendale V, Paul S, Bhattacharyya SS (2012) Gren synthesis, characterization and anticancer potential of platinum nanoparticles Bioplatin. J Chin Integr Med 10(6):681–689
Bertini I, Gray HB, Stiefel EI, Valentine JS (2007) Biological inorganic chemistry: structure and reactivity. University Science Books, Sausalito
Beveridge TJ, Hughes MN, Lee H, Leung KT, Poole RK, Savvaidis I, Silver S, Trevors JT (1996) Metal-microbe interactions : contemporary approaches. In: Poole RK (ed) Advances in microbial physiology. Elsevier, pp 177–243
Bhattacharya D, Gupta RK (2005) Nanotechnology and potential of microorganisms. Crit Rev Biotechnol 25:199–204. https://doi.org/10.1080/07388550500361994
Bloch K, Pardesi K, Satriano C, Ghosh S (2021) Bacteriogenic platinum nanoparticles for application in nanomedicine. Front Chem 9:624344. https://doi.org/10.3389/fchem.2021.624344
Brayner R, Barberousse H, Hemadi M, Djedjat C, Yéprémian C, Coradin T, Livage J, Fiévet F, Couté A (2007) Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J Nanosci Nanotechnol 7:2696–2708. https://doi.org/10.1166/jnn.2007.600
Cai J, Kimura S, Wada M, Kuga S (2009) Nanoporous cellulose as metal nanoparticles support. Biomacromolecules 10:87–94. https://doi.org/10.1021/bm800919e
Cardoso-Avila PE, Patakfalvi R, Rodríguez-Pedroza C, Aparicio-Fernández X, Loza-Cornejo S, Villa-Cruz V, Martínez-Cano E (2021) One-pot green synthesis of gold and silver nanoparticles using Rosa canina L. extract. RSC Adv 11:14624–14631. https://doi.org/10.1039/D1RA01448J
Carpentier W, Sandra K, De Smet I, Brigé A, De Smet L, Van Beeumen J (2003) Microbial reduction and precipitation of vanadium by Shewanella oneidensis. Appl Environ Microbiol 69:3636–3639. https://doi.org/10.1128/AEM.69.6.3636
Castro L, Blázquez ML, González F, Muñoz JA, Ballester A (2015) Biosynthesis of silver and platinum nanoparticles using orange peel extract : characterisation and applications. IET Nanobiotechnol 9(5):252–258. https://doi.org/10.1049/iet-nbt.2014.0063
Castro-Longoria E, Moreno-Velásquez SD, Vilchis-Nestor AR, Arenas-Berumen E, Avalos-Borja M (2012) Production of platinum nanoparticles and nanoaggregates using Neurospora crassa. J Microbiol Biotechnol 22(7):1000–1004. https://doi.org/10.4014/jmb.1110.10085
Chen A, Holt-Hindle P (2010) Platinum-Based nanostructured materials : synthesis, properties, and applications. Chem Rev 110:3767–3804. https://doi.org/10.1021/cr9003902
Chen R, Wu S, Meng C (2021) Size-tunable green synthesis of platinum nanoparticles using chlorogenic acid. Res Chem Intermed 47:1775–1787. https://doi.org/10.1007/s11164-020-04377-4
Coccia F, Tonucci L, Bosco D, Bressan M, d’Alessandro N (2012) One-pot synthesis of lignin-stabilised platinum and palladium nanoparticles and their catalytic behaviour in oxidation and reduction reactions. Green Chem 14:1073–1078. https://doi.org/10.1039/c2gc16524d
Dauthal P, Mukhopadhyay M (2014) Biofabrication, characterization, and possible bio-reduction mechanism of platinum nanoparticles mediated by agro-industrial waste and their catalytic activity. J Ind Eng Chem 22:185–191. https://doi.org/10.1016/j.jiec.2014.07.009
del Valle AC, Yeh CK, Huang YF (2020) Near infrared-activatable platinum-decorated gold nanostars for synergistic photothermal/ferroptotic therapy in combating cancer drug resistance. Adv Healthc Mater 9:1–15. https://doi.org/10.1002/adhm.202000864
Deng QY, Yang B, Wang JF, Whiteley CG, Wang XN (2009) Biological synthesis of platinum nanoparticles with apoferritin. Biotechnol Lett 31:1505–1509. https://doi.org/10.1007/s10529-009-0040-310.1007/s10529-009-0040-3
Dobrucka R (2016) Synthesis and structural characteristic of platinum nanoparticles using herbal Bidens Tripartitus extract. J Inorg Organomet Polym 26:219–225. https://doi.org/10.1007/s10904-015-0305-3
Dobrucka R (2016) Biofabrication of platinum nanoparticles using Fumariae herba extract and their catalytic properties. Saudi J Biol Sci 26:31–37. https://doi.org/10.1016/j.sjbs.2016.11.012
Dobrucka R, Romaniuk-Drapa A, Kaczmarek M (2019) Evaluation of biological synthesized platinum nanoparticles using Ononidis radix extract on the cell lung carcinoma A549. Biomed Microdevices 21:2–10. https://doi.org/10.1007/s10544-019-0424-7
Durán N, Marcato PD, Alves OL, de Souza GIH, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol 3:8. https://doi.org/10.1186/1477-3155-3-8
Elder A, Yang H, Gwiazda R, Teng X, Thurston S, He H, Oberdörster G (2007) Testing nanomaterials of unknown toxicity: an example based on platinum nanoparticles of different shapes. Adv Mater 19:3124–3129. https://doi.org/10.1002/adma.200701962
Fahmy SA, Preis E, Bakowsky U, Azzazy HME-S (2020) Platinum nanoparticles: green synthesis and biomedical applications. Molecules. https://doi.org/10.3390/molecules25214981
Fang T, Zhang J, Zuo T, Wu G, Xu Y, Yang Y, Yang J, Shen Q (2020) Chemo-photothermal combination cancer therapy with ros scavenging, extracellular matrix depletion, and tumor immune activation by telmisartan and diselenide-paclitaxel prodrug loaded nanoparticles. ACS Appl. Mater. Interfaces 12(28):31292–308. https://doi.org/10.1021/acsami.0c10416
Gaidhani SV, Yeshvekar RK, Shedbalkar UU, Bellare JH, Chopade BA (2014) Bio-reduction of hexachloroplatinic acid to platinum nanoparticles employing Acinetobacter calcoaceticus. Process Biochem 49(12):2313–2319. https://doi.org/10.1016/j.procbio.2014.10.002
Gama-Lara SA, Natividad R, Vilchis-Nestor AR, López-Castañares R, García-Orozco I, Gonzalez-Pedroza MG, Morales-Luckie RA (2019) Ultra-small platinum nanoparticles with high catalytic selectivity synthesized by an eco-friendly method supported on natural hydroxyapatite. Catal Lett 149:3447–53. https://doi.org/10.1007/s10562-019-02919-z
Ganaie SU, Abbasi T, Abbasi SA (2018) Biomimetic synthesis of platinum nanoparticles utilizing a terrestrial weed Antigonon leptopus. Part Sci Technol 36:681–688. https://doi.org/10.1080/02726351.2017.1292336
Gardea-Torresdey JL, Parsons JG, Gomez E, Peralta-Videa J, Troiani HE, Santiago P, Yacaman MJ (2002) Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Letters 2:397–401. https://doi.org/10.1021/nl015673+
Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, Parsons JG, Troiani H, Yacaman MJ (2003) Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir 19:1357–1361. https://doi.org/10.1021/la020835i
Gholami-Shabani M, Shams-Ghahfarokhi M, Gholami-Shabani Z, Akbarzadeh A, Riazi G, Razzaghi-Abyaneh M (2016) Biogenic approach using sheep milk for the synthesis of platinum nanoparticles: the role of milk protein in platinum reduction and stabilization. Int J Nanosci Nanotechnol 12:199–206
Ghosh S, Nitnavare R, Adewle N, Tomar GB, Chippalkatti R, More P, Kitture R, Kale S, Bellare J, Chopade BA (2015) Novel platinum-palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: anticancer and antioxidant activities. Int J Nanomed 10:7477–7490. https://doi.org/10.2147/IJN.S91579
Govender Y, Riddin TL, Gericke M, Whiteley CG (2009) Bioreduction of platinum salts into nanoparticles : a mechanistic perspective. Biotechnol Lett 31:95–100. https://doi.org/10.1007/s10529-008-9825-z
Govender Y, Riddin TL, Gericke M, Whiteley CG (2010) On the enzymatic formation of platinum nanoparticles. J. Nanopart Res 12:261–271. https://doi.org/10.1007/s11051-009-9604-3
Gupta K, Chundawat TS (2019) Bio-inspired synthesis of platinum nanoparticles from fungus Fusarium oxysporum: its characteristics, potential antimicrobial, antioxidant and photocatalytic activities. Mater Res Express 6:1050d6. https://doi.org/10.1088/2053-1591/ab4219
Gurunathan S, Jeyaraj M, Kang MH, Kim JH (2019) The effects of apigenin-biosynthesized ultra-small platinum nanoparticles on the human monocytic THP-1 cell line. Cells 8:1–23. https://doi.org/10.3390/cells8050444
Gurunathan S, Jeyaraj M, La H, Yoo H, Choi Y, Do JT, Park C, Kim JH, Hong K (2020) Anisotropic platinum nanoparticle-induced cytotoxicity, apoptosis, inflammatory response, and transcriptomic and molecular pathways in human acute monocytic leukemia cells. Int J Mol Sci 21:1–32. https://doi.org/10.3390/ijms21020440
Habibullah G, Viktorova J, Ruml T (2021) Current strategies for noble metal nanoparticle synthesis. Nanoscale Res Lett 16:47. https://doi.org/10.1186/s11671-021-03480-8
Henam PS, Heikham FD, Henam SD (2018) Sustainable synthesis of ultrasmall biogenic platinum nanoparticles for selective aqueous phase conversion of glucose and effective hydrogen peroxide decomposition. Ind Eng Chem Res 57:5190–5194. https://doi.org/10.1021/acs.iecr.7b05347
Herricks T, Chen J, Xia Y (2004) Polyol synthesis of platinum nanoparticles: control of morphology with sodium nitrate. Nano Letters 4:2367–2371. https://doi.org/10.1021/nl048570a
Hikosaka K, Kim J, Kajita M, Kanayama A, Miyamoto Y (2008) Platinum nanoparticles have an activity similar to mitochondrial NADH:ubiquinone oxidoreductase. Colloids Surf B Biointerfaces 66:195–200. https://doi.org/10.1016/j.colsurfb.2008.06.008
Ishak NAIM, Kamarudin SK, Timmiati SN (2019) Green synthesis of metal and metal oxide nanoparticles via plant extracts: an overview. Mater Res Express 6:112004. https://doi.org/10.1088/2053-1591/ab4458
Ishak NAIM, Kamarudin SK, Timmiati SN, Basri S, Karim NA (2021) Exploration of biogenic Pt nanoparticles by using agricultural waste (Saccharum officinarum L. Bagasse extract) as nanocatalyst for the electrocatalytic oxidation of methanol. Mater Today: Proc 42:138–147. https://doi.org/10.1016/j.matpr.2020.10.499
Ismail EH, Al-Radadi NS (2017) An eco-friendly synthesis of platinum nanoparticles and their applications on the cancer cell treatments. J Comp Theor Nanosci 14:6044–6052. https://doi.org/10.1166/jctn.2017.6550
Jain KK (2005) Role of nanobiotechnology in developing personalized medicine for cancer. Technol Cancer Res Treat 4:645–650. https://doi.org/10.1177/153303460500400608
Jameel MS, Aziz AA, Dheyab MA (2020) Green synthesis: proposed mechanism and factors influencing the synthesis of platinum nanoparticles. Green Process Synth 9:386–398. https://doi.org/10.1515/gps-2020-0041
Jan H, Gul R, Andleeb A, Ullah S, Shah M, Khanum M, Ullah I, Hano C, Abbasi BH (2021) A detailed review on biosynthesis of platinum nanoparticles (PtNPs), their potential antimicrobial and biomedical applications. J Saudi Chem Soc 25:101297. https://doi.org/10.1016/j.jscs.2021.101297
Jeyapaul U, Kala MJ, Bosco AJ, Piruthiviraj P, Easuraja M (2018) An eco-friendly approach for synthesis of platinum nanoparticles using leaf extracts of Jatropa Gossypifolia and Jatropa Glandulifera and its antibacterial activity. Oriental J Chem 34:783–790. https://doi.org/10.13005/ojc/340223
Jeyaraj M, Gurunathan S, Qasim M, Kang MH, Kim JH (2019) A Comprehensive review on the synthesis, characterization, and biomedical application of platinum nanoparticles. Nanomaterials 9(12):1719. https://doi.org/10.3390/nano9121719
Jha B, Rao M, Chattopadhyay A, Bandyopadhyay A (2018) Punica granatum fabricated platinum nanoparticles: a therapeutic pill for breast cancer. AIP Conf Proc 1953:030087. https://doi.org/10.1063/1.5032422
Kalimuthu K, Cha BS, Kim S, Park KS (2020) Eco-friendly synthesis and biomedical applications of gold nanoparticles: a review. Microchem J 152:104296. https://doi.org/10.1016/j.microc.2019.104296
Kankala RK, Liu CG, Yang DY, Wang SB, Chen AZ (2020) Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem Eng J 383:123138. https://doi.org/10.1016/j.cej.2019.123138
Karim NA, Rubinsin NJ, Burukan MAA, Kamarudin SK (2019) Sustainable route of synthesis platinum nanoparticles using orange peel extract. Int J Green Energy 16:1518–1526. https://doi.org/10.1080/15435075.2019.1671422
Karthik R, Sasikumar R, Chen SM, Govindasamy M, Kumar JV, Muthuraj V (2016) Green synthesis of platinum nanoparticles using quercus glauca extract and its electrochemical oxidation of hydrazine in water samples. Int J Electrochem Sci 11:8245–8255. https://doi.org/10.20964/2016.10.62
Ko YL, Krishnamurthy S, Yun YS (2015) Facile synthesis of monodisperse Pt and Pd nanoparticles using antioxidants. J Nanosci Nanotech 15:412–417. https://doi.org/10.1166/jnn.2015.8375
Koebel MM, Jones LC, Somorjai GA (2008) Preparation of size-tunable, highly monodisperse PVP-protected pt-nanoparticles by seed-mediated growth. J Nanopart Res 10:1063–1069. https://doi.org/10.1007/s11051-008-9370-7
Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H, Takahashi Y, Uruga T (2007) Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotech 128:648–653. https://doi.org/10.1016/j.jbiotec.2006.11.014
Kumar NM, Govindh B, Annapurna N (2017) Green synthesis and characterization of platinum nanoparticles using Sapindus mukorossi Gaertn. fruit pericarp. Asian J Chem 29:2541–2544. https://doi.org/10.14233/ajchem.2017.20842A
Kumar PV, Kala SMJ, Prakash KS (2019) Green synthesis derived Pt-nanoparticles using Xanthium strumarium leaf extract and their biological studies. J Environ Chem Eng 7(3):103146. https://doi.org/10.1016/j.jece.2019.103146
Labrador-Rached CJ, Browning RT, Braydich-Stolle LK, Comfort KK (2018) Toxicological implications of platinum nanoparticle exposure : stimulation of intracellular stress, inflammatory response, and akt signaling in vitro. J Toxicol 2018:1367801. https://doi.org/10.1155/2018/1367801
Lee H, Habas SE, Kweskin S, Butcher D, Somorjai GA, Yang P (2006) Morphological control of catalytically active platinum nanocrystals. Angew Chem Int Ed 45:7824–7828. https://doi.org/10.1002/anie.200603068
Lengke MF, Fleet ME, Southam G (2006) Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial biomass with a palladium(II) chloride complex. Langmuir 22:7318–23. https://doi.org/10.1021/la7012446
Leo J, Oluwafemi OS (2017) Plant-mediated synthesis of platinum nanoparticles using water hyacinth as an efficient biomatrix source-an eco-friendly development. Mater Lett 196:141–44. https://doi.org/10.1016/j.matlet.2017.03.047
Li Y, Zhang J, Gu J, Chen S (2017) Biosynthesis of polyphenol-stabilised nanoparticles and assessment of anti-diabetic activity. J Photochem Photobiol B 169:96–100. https://doi.org/10.1016/j.jphotobiol.2017.02.017
Lin X, Wu M, Wu D, Kuga S, Endo T, Huang Y (2011) Platinum nanoparticles using wood nanomaterials: eco-friendly synthesis, shape control and catalytic activity for p-nitrophenol reduction. Green Chem 13:283–87. https://doi.org/10.1039/c0gc00513d
Lin X, Zhao J, Wu M, Kuga S, Huang Y (2016) Green synthesis of gold, platinum and palladium nanoparticles by lignin and hemicellulose. RR J Microbiol Biotechnol 5:14–18
Liu NA, Wang Z, Ma Z (2014) Platinum porous nanoparticles for the detection of cancer biomarkers: what are the advantages over existing techniques. Bioanalysis 6:903–905. https://doi.org/10.4155/bio.14.32
Loza K, Epple M (2019) Synthesis of Metallic and Metal Oxide Particles. In: Gehr P, Zellner R (eds) Biological responses to nanoscale particles. NanoScience and technology. Springer, Cham. https://doi.org/10.1007/978-3-030-12461-8_1
Ma Z, Wu L, Han K, Han H (2019) Pt nanozyme for O2 self-sufficient, tumor specific oxidative damage and drug resistance reversal. Nanoscale Horiz 4:1124–1131. https://doi.org/10.1039/C9NH00088G
Madlum KN, Khamees EJ, Abdulridha SA, Naji RA (2021) Antimicrobial and cytotoxic activity of platinum nanoparticles synthesized by laser ablation technique. J Nanostruct 11:13–19. https://doi.org/10.22052/JNS.2021.01.002
Maes S, Props R, Fitts JP, De Smet R, Vilchez-Vargas R, Vital M, Pieper DH, Vanhaecke F, Boon N, Hennebel T (2016) Platinum recovery from synthetic extreme environments by halophilic bacteria. Environ Sci Technol 50:2619–2626. https://doi.org/10.1021/acs.est.5b05355
Marouzi S, Sabouri Z, Darroudi M (2021) Greener synthesis and medical applications of metal oxide nanoparticles. Ceram Int 47:19632–19650. https://doi.org/10.1016/j.ceramint.2021.03.301
Martins M, Mourato C, Sanches S, Noronha JP, Crespo MTB, Pereira IAC (2017) Biogenic platinum and palladium nanoparticles as new catalysts for the removal of pharmaceutical compounds. Water Res 108:160–168. https://doi.org/10.1016/j.watres.2016.10.071
Mavukkandy MO, Chakraborty S, Abbasi T, Abbasi SA (2016) A clean-green synthesis of platinum nanoparticles utilizing a pernicious weed lantana (Lantana camara). Am J Eng Appl Sci 9:84–90. https://doi.org/10.3844/ajeassp.2016.84.90
Mironava T, Simon M, Rafailovich MH, Rigas B (2013) Platinum folate nanoparticles toxicity: cancer vs. normal cells. Toxicol in Vitro 27:882–889. https://doi.org/10.1016/j.tiv.2013.01.005
Mohammadi H, Abedi A, Akbarzadeh A, Mokhtari MJ, Shahmabadi HE, Mehrabi MR, Javadian S, Chiani M (2013) Evaluation of synthesized platinum nanoparticles on the MCF-7 and HepG-2 cancer cell lines. Int Nano Lett 3:3–7. https://doi.org/10.1186/2228-5326-3-28
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10:507–517. https://doi.org/10.1007/s11051-007-9275-x
Nadagouda MN, Varma RS (2006) Green and controlled synthesis of gold and platinum nanomaterials using vitamin B2: density-assisted self-assembly of nanospheres, wires and rods. Green Chem 8:516–518. https://doi.org/10.1039/b601271j
Nadaroglu H, Gungor AA, Ince S, Babagil A (2017) Green synthesis and characterisation of platinum nanoparticles using quail egg yolk. Spectrochim Acta A 167:43–47. https://doi.org/10.1016/j.saa.2016.05.023
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1–13. https://doi.org/10.1016/j.cis.2010.02.001
Naseer A, Ali A, Ali S, Mahmood A, Kusuma HS, Nazir A, Yaseen M (2020) Biogenic and eco-benign synthesis of platinum nanoparticles (Pt NPs) using plants aqueous extracts and biological derivatives: environmental, biological and catalytic applications. J Mater Res Technol 9:9093–9107. https://doi.org/10.1016/j.jmrt.2020.06.013
Nellore J, Pauline C, Amarnath K (2013) Bacopa monnieri phytochemicals mediated synthesis of platinum nanoparticles and its neurorescue effect on 1-methyl 4-phenyl 1,2,3,6 tetrahydropyridine-induced experimental parkinsonism in zebrafish. J Neurodegener Dis 2013:8. https://doi.org/10.1155/2013/972391
Nguyen TKL, Nguyen ND, Dang VP, Phan DT, Tran TH, Nguyen QH, Mai HD (2019) Synthesis of platinum nanoparticles by gamma Co-60 ray irradiation method using chitosan as stabilizer. Adv Mat Sci Eng 2019:1–6. https://doi.org/10.1155/2019/9624374
Nida TK, Khan MJ (2017) Biogenic nanoparticles : an introduction to what they are and how they are produced. Int J Biotech Bioeng 3:66–70. https://doi.org/10.25141/2475-3432-2017-3.0066
Ojo OA, Olayide II, Akalabu MC, Ajiboye BO, Ojo AB, Oyinloye BE, Ramalingam M (2021) Nanoparticles and their biomedical applications. Biointerface Res Appl Chem 11:8431–8445. https://doi.org/10.33263/BRIAC111.84318445
Olajire AA, Adesina EO (2017) Green approach to synthesis of Pt and bimetallic Au@Pt nanoparticles using Carica papaya leaf extract and their characterization. J. Nanostruct 7:338–344
Patra JK, Baek K-H (2014) Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomat 2014:417305. https://doi.org/10.1155/2014/417305
Pedone D, Moglianetti M, De Luca E, Bardi G, Pompa PP (2017) Platinum nanoparticles in nanobiomedicine. Chem Soc Rev 46:4951–4975. https://doi.org/10.1039/c7cs00152e
Porcel E, Liehn S, Remita H, Usami N, Kobayashi K, Furusawa Y, Sech CL, Lacombe S (2010) Platinum nanoparticles: a promising material for future cancer therapy? Nanotechnology 21:1–7. https://doi.org/10.1088/0957-4484/21/8/085103
Prabhu N, Gajendran T (2017) Green synthesis of noble metal of platinum nanoparticles from Ocimum sanctum (Tulsi) plant-extracts. J Biotech Biochem 3:107–112. https://doi.org/10.9790/264X-0301107112
Puja P, Kumar P (2019) A perspective on biogenic synthesis of platinum nanoparticles and their biomedical applications. Spectrochim Acta A 211:94–99. https://doi.org/10.1016/j.saa.2018.11.047
Rahman K, Khan SU, Fahad S, Chang MX, Abbas A, Khan WU, Rahman L, Haq ZU, Nabi G, Khan D (2019) Nano-biotechnology: a new approach to treat and prevent malaria. Int J Nanomedicine 14:1401–1410. https://doi.org/10.2147/IJN.S190692
Rai M, Duran N (2011) Metal Nanoparticles in microbiology. Edited by Mahendra. Rai and Nelson. Duran. Deblik. Springer, New York. https://doi.org/10.1007/978-3-642-18312-6
Rai M, Yadav A, Bridge P, Gade A (2009) Myconanotechnology: a new and emerging science. In: Rai M, Yadav A, Bridge P, Gade A (eds) Applied mycology, 14th ed., 258–67. New York. https://doi.org/10.1079/9781845935344.0258
Rajasekharreddy P, Rani PU (2014) Biosynthesis and Characterization of Pd and Pt nanoparticles using Piper betle L. plant in a photoreduction method. J Cluster Sci 25:1377–1388. https://doi.org/10.1007/s10876-014-0715-3
Ramkumar VS, Pugazhendhi A, Prakash S, Ahila NK, Vinoj G, Selvam S, Kumar G, Kannapiran E, Rajendran RB (2017) Synthesis of platinum nanoparticles using seaweed padina gymnospora and their catalytic activity as PVP/PtNPs nanocomposite towards biological applications. Biomed Pharmacother 92:479–490. https://doi.org/10.1016/j.biopha.2017.05.076
Rashamuse KJ, Whiteley CG (2007) Bioreduction of Pt (IV) from aqueous solution using sulphate-reducing bacteria. Appl Microbiol Biotechnol 75:1429–1435. https://doi.org/10.1007/s00253-007-0963-3
Rashamuse KJ, Mutambanengwe CCZ, Whiteley CG (2008) Enzymatic recovery of platinum (IV) from industrial wastewater using a biosulphidogenic hydrogenase. Afr J Biotechnol 7(8):1087–95. https://doi.org/10.4314/ajb.v7i8.58626
Raut RW, Haroon ASM, Malghe YS, Nikam BT, Kashid SB (2013) rapid biosynthesis of platinum and palladium metal nanoparticles using root extract of Asparagus racemosus Linn. Adv Mater Lett 4:650–654. https://doi.org/10.5185/amlett.2012.11470
Riddin TL, Gericke M, Whiteley CG (2006) Analysis of the inter-and extracellular formation of platinum nanoparticles by Fusarium Oxysporum f. sp. lycopersici using response surface methodology. Nanotechnology 17:3482–3489. https://doi.org/10.1088/0957-4484/17/14/021
Riddin TL, Govender Y, Gericke M, Whiteley CG (2009) Two different hydrogenase enzymes from sulphate-reducing bacteria are responsible for the bioreductive mechanism of platinum into nanoparticles. Enzyme Microb Technol 45:267–273. https://doi.org/10.1016/j.enzmictec.2009.06.006
Riddin TL, Gericke M, Whiteley CG (2010) Biological synthesis of platinum nanoparticles: effect of initial metal concentration. Enzyme Microb Technol 46:501–505. https://doi.org/10.1016/j.enzmictec.2010.02.006
Rokade SS, Joshi KA, Mahajan K, Tomar G, Dubal DS, Singh V, Kitture R, Bellare J, Ghosh S (2017) Novel anticancer platinum and palladium nanoparticles from Barleria prionitis. Glob J Nanomed 2:1–9. https://doi.org/10.19080/GJN.2017.02.555600
Rokade SS, Joshi KA, Mahajan K, Patil S, Tomar G, Dubal DS, Parihar VS, Kitture R, Bellare JR, Ghosh S (2018) Gloriosa superba mediated synthesis of platinum and palladium nanoparticles for induction of apoptosis in breast cancer. Bioinorg Chem Appl 2018:1–9. https://doi.org/10.1155/2018/4924186
Rouch DA, Lee BTO, Morby AP (1995) Understanding cellular responses to toxic agents: a model for mechanism-choice in bacterial metal resistance. J Ind Microbiol 14:132–141. https://doi.org/10.1007/BF01569895
Sabouri Z, Akbari A, Hosseini HA, Hashemzadeh A, Darroudi M (2019) Bio-based synthesized NiO nanoparticles and evaluation of their cellular toxicity and wastewater treatment effects. J Mol Struct 1191:101–109. https://doi.org/10.1016/j.molstruc.2019.04.075
Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M (2020a) Tragacanth-mediate synthesis of NiO nanosheets for cytotoxicity and photocatalytic degradation of organic dyes. Bioprocess Biosyst Eng 43:1209–1218. https://doi.org/10.1007/s00449-020-02315-7
Sabouri Z, Sabouri M, Amiri MS, Khatami M, Darroudi M (2020b) Plant-based synthesis of cerium oxide nanoparticles using Rheum Turkestanicum extract and evaluation of their cytotoxicity and photocatalytic properties. Mater Technol 2020:1–14. https://doi.org/10.1080/10667857.2020.1863573
Sadrolhosseini AR, Habibiasr M, Shafie S, Solaimani H, Lim HN (2019) Optical and thermal properties of laser-ablated platinum nanoparticles graphene oxide composite. Int J Mol Sci 20:6153. https://doi.org/10.3390/ijms20246153
Sahin M, Gubbuk IH (2019) Green synthesis of antioxidant silver and platinum nanoparticles using ginger and turmeric extracts and investigation of their catalytic activity. J Turk Chem Soc Sect A Chem 6(3):403–10. https://doi.org/10.18596/jotcsa.497440
Sahin B, Aygün A, Gündüz H, Sahin K, Demir E, Akocak S, Sen F (2018) Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloid Surf B Biointerface 163:119–124. https://doi.org/10.1016/j.colsurfb.2017.12.042
San BH, Moh SH, Kim KK (2013) Investigation of the heating properties of platinum nanoparticles under a radiofrequency current. Int J Hyperth 29:99–105. https://doi.org/10.3109/02656736.2012.760137
Sanchez-Mendieta V, Vilchis-Nestor AF (2012) Green synthesis of noble metal (Au, Ag, Pt) nanoparticles, assisted by plant-extracts. In: Su Y-H (ed) Noble metals. IntechOpen, London, pp 391–408. https://doi.org/10.5772/34335
Sarkar J, Acharya K (2017) Alternaria alternata culture filtrate mediated bioreduction of chloroplatinate to platinum nanoparticles. Inorg Nano-Met Chem 47:365–369. https://doi.org/10.1080/15533174.2016.1186062
Sathiyaraj G, Vinosha M, Sangeetha D, Manikandakrishnan M, Palanisamy S, Sonaimuthu M, Manikandan R, You SG, Prabhu NM (2021) Bio-directed synthesis of Pt-nanoparticles from aqueous extract of red algae Halymenia dilatata and their biomedical applications. Colloids Surf A 618:126434. https://doi.org/10.1016/j.colsurfa.2021.126434
Selvi AM, Palanisamy S, Jeyanthi S, Vinosha M, Mohandoss S, Tabarsa M, You SG, Kannapiran E, Prabhu NM (2020) Synthesis of Tragia involucrata mediated platinum nanoparticles for comprehensive therapeutic applications: antioxidant, antibacterial and mitochondria-associated apoptosis in HeLa cells. Process Biochem 98:21–33. https://doi.org/10.1016/j.procbio.2020.07.008
Sharma D, Kanchi S, Bisetty K (2019) Biogenic synthesis of nanoparticles: a review. Arab J Chem 12:3576–3600. https://doi.org/10.1016/j.arabjc.2015.11.002
Shatokhina SN, Filippov AG, Aleksandrin VV, Uvarova DS, Kubatiev AA, Shabalin VN (2020) Effect of platinum nanoparticles on the structure of protein molecules and regulation of the tone of brain vessels in experimental animals. Biotechnologies 168:781–84. https://doi.org/10.1007/s10517-020-04801-0
Sheny DS, Philip D, Mathew J (2013) Synthesis of platinum nanoparticles using dried Anacardium Occidentale leaf and its catalytic and thermal applications. Spectrochim Acta A 114:267–271. https://doi.org/10.1016/j.saa.2013.05.028
Shim K, Kim J, Heo YU, Jiang B, Li C, Shahabuddin M, Wu KCW, Hossain MSA, Yamauchi Y, Kim JH (2017) Synthesis and cytotoxicity of dendritic platinum nanoparticles with HEK-293 cells. Chem Asian J 12:21–26. https://doi.org/10.1002/asia.201601239
Shiny PJ, Mukherjee A, Chandrasekaran N (2014) Haemocompatibility assessment of synthesised platinum nanoparticles and its implication in biology. Bioprocess Biosyst Eng 37:991–997. https://doi.org/10.1007/s00449-013-1069-1
Siddiqi KS, Husen A (2016) Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res Lett. https://doi.org/10.1186/s11671-016-1695-z
Silver S (1996) Bacterial resistances to toxic metal ions-a review. Gene 179:9–19. https://doi.org/10.1016/S0378-1119(96)00323-X
Singh VK, Singh AK (2019) Role of microbially synthesized nanoparticles in sustainable agriculture and environmental management. Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology. Elsevier Inc. https://doi.org/10.1016/B978-0-12-817004-5.00004-X
Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumaret P (2018) ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16:84. https://doi.org/10.1186/s12951-018-0408-4
Solak K, Mavi A, Ergon T, Sezen A, Algu OF (2017) Nanomaterials and the microbial sphere. Nanosci Nanotech Lett 9:609–623
Song JY, Kwon E-Y, Kim BS (2010) Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst 33:159–64. https://doi.org/10.1007/s00449-009-0373-2
Soundarrajan C, Sankari A, Dhandapani P, Maruthamuthu S, Ravichandran S, Sozhan G, Palaniswamy N (2012) Rapid biological synthesis of platinum nanoparticles using Ocimum sanctum for water electrolysis applications. Bioprocess Biosyst Eng 35:827–33. https://doi.org/10.1007/s00449-011-0666-0
Srivastava AK (2019) The role of fungus in bioactive compound production and nanotechnology. In role of plant growth promoting microorganisms in sustainable agriculture and nanotechnology, edited by Akhileshwar Kumar Srivastava, 145–162. Elsevier, Negev, Israle. https://doi.org/10.1016/B978-0-12-817004-5.00009-9.
Stepanov AL, Golubev AN, Nikitin SI, Osin YN (2014) A review on the fabrication and properties of platinum nanoparticles. Rev Adv Mat Sci 38:160–75
Subashini G, Bhuvaneswari S (2018) Nanoparticles from fungi (myconanoparticles). In: Subashini G, Bhuvaneswari S (eds) Fungi and their role in sustainable development: current perspectives. Springer, Berlin, pp 753–778. https://doi.org/10.1007/978-981-13-0393-7_39
Subramaniyan SA, Sheet S, Vinothkannan M, Yoo DY, Lee YS, Belal SA, Shim KS (2018) One-Pot facile synthesis of Pt nanoparticles using cultural filtrate of microgravity simulated grown P. chrysogenum and their activity on bacteria and cancer cells. J Nanosci Nanotech 18:3110–3125. https://doi.org/10.1166/jnn.2018.14661
Syed A, Ahmad A (2012) Extracellular biosynthesis of platinum nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B 97:27–31. https://doi.org/10.1016/j.colsurfb.2012.03.026
Tahir K, Nazir S, Ahmad A, Li B, Khan AU, Khan ZUH, Khan FU, Khan QU, Khan A, Rahman AU (2017) Facile and green synthesis of phytochemicals capped platinum nanoparticles and in vitro their superior antibacterial activity. J Photochem Photobiol B 166:246–251. https://doi.org/10.1016/j.jphotobiol.2016.12.016
Tarannum N, Divya S, Gautam YK (2019) Facile green synthesis and applications of silver nanoparticles: a state-of-the-art review. RSC Adv 9:34926–34948. https://doi.org/10.1039/c9ra04164h
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Ruiz LV (2014) Global diversity and geography of soil fungi. Science 346:1078. https://doi.org/10.1126/science.aaa1185
Tepale N, Fernández-Escamilla VVA, Carreon-Alvarez C, González-Coronel VJ, Luna-Flores A, Carreon-Alvarez A, Aguilar J (2019) Nanoengineering of gold nanoparticles: green synthesis, characterization, and applications. Crystals 9:612. https://doi.org/10.3390/cryst9120612
Thirumurugan A, Aswitha P, Kiruthika C, Nagarajan S, Christy AN (2016) Green synthesis of platinum nanoparticles using Azadirachta indica—An eco-friendly approach. Mater Lett 170:175–178. https://doi.org/10.1016/j.matlet.2016.02.026
Ullah S, Ahmad A, Wang A, Raza M, Jan AU, Tahir K, Rahman AU, Qipeng Y (2017) Bio-fabrication of catalytic platinum nanoparticles and their in vitro efficacy against lungs cancer cells line (A549). J Photochem Photobiol B 173:368–375. https://doi.org/10.1016/j.jphotobiol.2017.06.018
Velmurugan P, Shim J, Oh B-T (2016) Prunus x yedoensis tree gum mediated synthesis of platinum nanoparticles with antifungal activity against phytopathogens. Mater Lett 174:61–65. https://doi.org/10.1016/j.matlet.2016.03.069
Venu R, Ramulu TS, Anandakumar S, Rani VS, Kim CG (2011) Bio-directed synthesis of platinum nanoparticles using aqueous honey solutions and their catalytic applications. Colloids Surf A 384:733–738. https://doi.org/10.1016/j.colsurfa.2011.05.045
Vinod VTP, Saravanan P, Sreedhar B, Devi DK, Sashidhar RB (2011) A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium). Colloids Surf B 83:291–298. https://doi.org/10.1016/j.colsurfb.2010.11.035
Yamada M, Foote M, Prow TW (2015) Therapeutic gold, silver, and platinum nanoparticles. WIREs Nanomed Nanobiotechnol 7:428–445. https://doi.org/10.1002/wnan.1322
Yong P, Rowson NA, Farr JPG, Harris IR, Macaskie LE (2002) Bioaccumulation of palladium by Desulfovibrio desulfuricans. J Chem Technol Biotechnol 77:593–601. https://doi.org/10.1002/jctb.606
Zadvorny OA, Zorin NA, Gogotov IN (2005) Transformation of metals and metal ions by hydrogenases from phototrophic bacteria. Arch Microbiol 184:279–285. https://doi.org/10.1007/s00203-005-0040-1
Zamiri R, Azmi BZ, Darroudi M, Sadrolhosseini AR, Husin MS, Zaidan AW, Mahdi MA (2011) Preparation of starch stabilized silver nanoparticles with spatial self-phase modulation properties by laser ablation technique. Appl Phys A 102:189–194. https://doi.org/10.1007/s00339-010-6129-7
Zhang H, Jiang X, Cao G, Zhang X, Croley TR (2018) Environmental carcinogenesis and ecotoxicology reviews effects of noble metal nanoparticles on the hydroxyl radical scavenging ability of dietary antioxidants. J Environ Sci Health C 36:84–97. https://doi.org/10.1080/10590501.2018.1450194
Zhao W, Li Z, Yang H, Ren C, Lv F, Gao S, Ma H (2019) Mesoporous platinum nanotherapeutics for combined chemo-photothermal cancer treatment. ACS Appl Bio Mater 2:3269–3278. https://doi.org/10.1021/acsabm.9b00250
Zheng B, Kong T, Jing X, Odoom-Wubah T, Li X, Sun D, Lu F, Zheng Y, Huang J, Li Q (2013) Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism. J Colloid Interface Sci 396:138–145. https://doi.org/10.1016/j.jcis.2013.01.021
Zhu Y, Li W, Zhao X, Zhou Z, Wang Y, Cheng Y, Huang Q, Zhang Q (2017) Hyaluronic acid-encapsulated platinum nanoparticles for targeted photothermal therapy of breast cancer. J Biomed Nanotech 13:1457–1467. https://doi.org/10.1166/jbn.2017.2446
Acknowledgements
Ramiro Muñiz-Diaz and Sagrario Yadira Gutiérrez de la Rosa thank CONACyT for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muñiz-Diaz, R., Gutiérrez de la Rosa, S.Y., Gutiérrez Coronado, Ó. et al. Biogenic synthesis of platinum nanoparticles. Chem. Pap. 76, 2573–2594 (2022). https://doi.org/10.1007/s11696-021-01970-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-021-01970-8